Abstract
Squalene, a linear triterpene compound characterized by its distinctive isoprenoid backbone with six transisoprene units, is widely used in the medicinal, nutraceutical, and cosmetic industries. The escalating global demand for squalene, coupled with growing ethical concerns over shark-derived sources and the inherent limitations of plant extraction (low yield) and chemical synthesis (environmental burden), has propelled microbial biosynthesis as a sustainable alternative. While substantial progress has been made in elucidating the mevalonate pathway and regulatory mechanisms of squalene biosynthesis, achieving industrially viable titers through microbial platforms remains an unresolved challenge. This review systematically summarizes recent advances in squalene biosynthesis using yeast chassis, with a focus on metabolic engineering strategies implemented in Saccharomyces cerevisiae and Yarrowia lipolytica. Furthermore, we elaborated on how squalene yields a diverse array of downstream derivatives through intricate enzymatic reactions. These derivatives—including triterpenoid saponins, triterpenoid acids, and steroids—exhibit significant applications in the pharmaceutical, nutraceutical, and cosmetic sectors. By integrating systems metabolic engineering with emerging synthetic biology tools, this work provides a roadmap for advancing strain engineering toward economically feasible squalene biomanufacturing.
1. Introduction
Squalene, also known as triacontahexaene (C30H50), is a polyunsaturated hydrocarbon characterized by six isoprene double bonds, classifying it as a triterpene. It was first isolated from shark liver oil in 1916, and its name originates from the Latin word squalus, meaning shark [1]. This compound is naturally present in plants, fungi, insects, animals, and humans, among other organisms. Owing to its diverse biological activities—such as moisturizing, antioxidant, anti-aging, anti-fatigue, anti-inflammatory, and anti-tumor effects—squalene exhibits broad applicability [2,3,4,5]. It also functions as an immune enhancer, potentiating the immune response to specific antigens, and is employed as an adjuvant in vaccines, including those against influenza [6,7,8,9]. Given these versatile properties, the demand for squalene is increasing across various sectors such as cosmetics, food, medicinal health supplements, and personal care products (Figure 1) [4,7,10,11,12,13]. According to estimates by Mordor Intelligence, the global market value of squalene is projected to reach $167.15 million by 2024, with a compound annual growth rate of 5.96%, potentially climbing to $223.28 million by 2029 [14]. Thus, squalene demonstrates substantial potential in terms of both applications and market value.
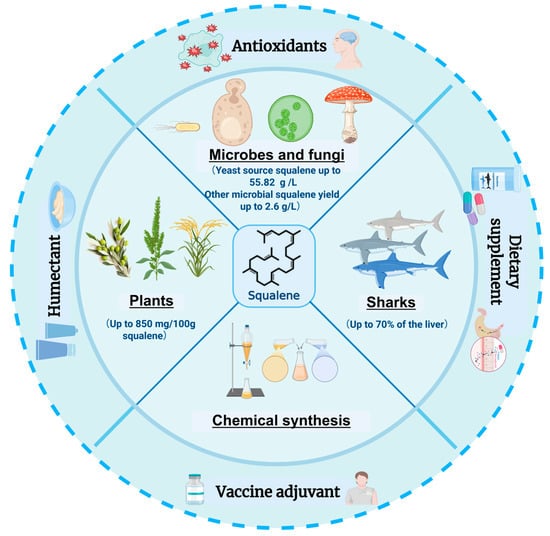
Figure 1.
Applications and sources of squalene [4,15,16,17,18,19,20,21,22,23,24,25]. This figure was created using Biorender.com. The inner circle of the figure depicts the possible sources of squalene and its production, ranging from unicellular microorganisms such as yeast and bacteria, to multicellular fungi, plants, deep-sea sharks, and synthetic methods. The outer ring describes the main applications of squalene, including as antioxidants, moisturizers, vaccine adjuvants and dietary supplements.
In humans, squalene accounts for approximately 13% of sebum [26,27]. While it comprises less than 0.5% of the epidermis, it makes up 10% of surface lipids—a characteristic that underscores its efficient role in the lipid layer of the skin surface [28]. Squalene is primarily synthesized in the liver and secreted via the sebaceous glands [29,30]. The daily secretion of squalene varies between individuals due to dietary and genetic factors, with levels ranging from 125 to 475 mg per day [31]. Clinical studies have demonstrated that 60% to 85% of orally ingested squalene is effectively absorbed and subsequently distributed to various tissues [32]. For the skin in particular, it shields the skin against oxidative stress and free radicals [33]. However, squalene levels in the body start to decline after the age of 30, rendering supplementation—typically at a dose of 500 mg per day—advantageous for maintaining overall health and well-being [30,34,35].
Generally, squalene has been extracted from shark liver oil, with 3000 sharks required to obtain one ton of squalene [7]. The growing demand for squalene is endangering one-third of the world’s shark species, raising concerns about resource depletion and sustainability. Plant-derived squalene has attracted attention as a possible alternative. However, the long cultivation periods of plants, their low squalene content, and complex separation processes imply high costs and the need for abundant arable land [36,37,38,39]. In 1970, Johnsons et al. attempted the synthesis of squalene starting with 2,5-dimethoxy tetrahydrofuran, which was hydrolyzed from bis-diacetone alcohol [18,40]. The reaction route consisted of up to seven steps, and required complex conditions. In 1980, Scott et al. synthesized squalene from carvone, but the excessive complexity and number of steps meant that these attempts had little industrial value [41]. In recent years, microbial synthesis has emerged as an environmentally friendly, efficient, and sustainable alternative [42]. Yeast, which serves as a chassis cell for many bio-products, offers multiple advantages for the synthesis of squalene: its metabolic pathway is similar to that of eukaryotes, and it has a complete endoplasmic reticulum and secretion system for the accumulation of fat-soluble compounds. In fact, oleaginous yeasts are naturally rich in lipids and, thus, ideal for squalene production. Moreover, model yeasts such as S. cerevisiae are relatively easy to edit genes due to their high natural homologous recombination ability. The gene editing of Yarrowia lipolytica is more difficult than that of S. cerevisiae. However, efficient editing can now be achieved through CRISPR and homologous recombination optimization, enabling metabolic engineering strategies to optimize the biosynthesis of squalene [43,44,45]. These strains (i.e., Y. lipolytica and S. cerevisiae) are also suitable for stable, large-scale production due to their rapid growth, high cultivation density, and tolerance to industrial fermentation conditions, such as osmotic pressure and pH fluctuations. Several strategies have been demonstrated to be effective in enhancing squalene production in yeast, encompassing promoter engineering, organelle engineering, cofactor engineering, augmentation of precursor supply, knockout of competitive pathways, mitigation of squalene consumption, improvement of strain tolerance, promotion of extracellular secretion, and application of computer-aided design [46,47,48,49]. Yeasts show flexible metabolism by being able to use different carbon sources, including renewable materials like glucose and glycerol, which lowers costs in bioprocessing [50,51]. In addition, they are resistant to bacterial contamination, requiring fewer cleaning steps, making the industrial fermentation process easier [52,53]. With the rise of artificial intelligence (AI) and machine learning (ML), it has added a new dimension to bioengineering. The design of microbial cell factories relies on a large number of experimental trial and error, and the metabolic network is complex, with many variables, and the efficiency of manual screening is low. Through the innovation of AI and ML, the paradigm shift from “trial and error experiment” to “rational design” has been realized. Through enhanced ML, enzyme classification, enzyme or substrate properties prediction, optimal microenvironment prediction, and discovery of new enzymes or enzyme combinations with enhanced catalytic activities (e.g., AlphaFold2, RoseTTAFold2, CLEAN, Prekcat, etc.) were performed to achieve target product yield improvement [54]. In addition, AI deep learning was used to achieve reasonable metabolic pathway prediction and design. By using KEGG, MetaCyc, BIGG, KBase and other databases, biological information from multiple sources, such as genetic, molecular, physical and chemical, is integrated to make them available for metabolic pathway prediction. Computer simulations have been performed to enhance heterologous terpenoid production in S. cerevisiae using self-regulated gene knockdown strategies [55].
This review provides a comprehensive overview of the current knowledge regarding the biosynthesis of squalene in engineered yeasts, with particular emphasis on recent advancements involving S. cerevisiae and Y. lipolytica. These developments lay a theoretical foundation for future research in squalene biosynthesis. Given its wide range of existing application, yeast offers a mature platform for sustainable large-scale production of squalene.
2. Sources of Squalene
2.1. Squalene from Animal and Plant Sources
Traditionally, shark liver has been the primary source of squalene, accounting for 40–70% of its organ mass [4]. The rising global demand, however, has led to an estimated annual mortality of 3 to 6 million deep-sea sharks, posing severe risks to marine ecosystems. In response, the Convention on International Trade in Endangered Species of Wild Fauna and Flora has imposed strict bans on animal-derived squalene extraction, stimulating research into plant-based and microbial alternatives.
As shown in Table 1, which summarizes the squalene content from different plant sources. Olive oil contains 250–850 mg of squalene per 100 g of biomass, making it one of the richest plant sources available [17]. Amaranth seeds contain approximately 7% oil, while the proportion of squalene in oil can reach 6% to 8%., and amaranth seeds produce up to 470 mg/100 g of squalene [16,20,24]. Cold-pressed pumpkin seed oil usually has a lot of squalene, with levels up to 747 mg/100 g [15,21]. Argan oil has high levels (313 mg/100 g), as do traditional medicinal plants like tea leaves (28.9–368.2 mg/100 g) [19,23]. The amount of squalene in rice bran oil was 320 mg/100 g [22]. Nevertheless, plant-based extraction faces considerable limitations. Cultivation cycles for both plants and animals typically require six months to a year, rendering such sources relatively inefficient for scalable production. Moreover, the extraction process itself is not only costly but also environmentally detrimental, involving multiple steps and generating hazardous waste [56].

Table 1.
Squalene content from different plant sources.
2.2. Chemical Synthesis of Squalene
The chemical synthesis of squalene encompasses multiple steps and reagents [18,40,41]. The starting material is succinaldehyde, this seven-step reaction involves multiple chemical transformation steps to progressively synthesize the target molecule through esterification, reduction, and oxidation reactions. In the first step, a nucleophilic addition reaction with the strong nucleophile 2-propenyllithium with succinaldehyde is used to attack one of the aldehyde groups in the succinaldehyde molecule, forming a new carbon-carbon bond and generating an intermediate. This was followed by esterification at 130 °C by using the MeC (OEt)3/AcOH; Aldehydes or ketones were then reduced to alcohols using LiAlH4 at −5 °C. Low temperature conditions were used in this step to ensure high selectivity of the reduction reaction and to reduce side reactions. Then, the alcohols were oxidized to aldehydes or ketones by the CrO3/pyridine, and the esterification, reduction and oxidation reactions were repeated. Finally, the molecular structure was fine adjusted through successive oxidation and reduction steps, and the target molecule was finally obtained. The whole process is synthesized through multiple functional group transformation and structural modification (Figure 2). The process is complicated. It has low yield and high cost. It also produces byproducts with similar structures. These factors make chemical synthesis of squalene more expensive. The product must undergo additional purification steps, which raises the cost even more. Hence, chemical synthesis remains at a disadvantage in terms of market competitiveness.
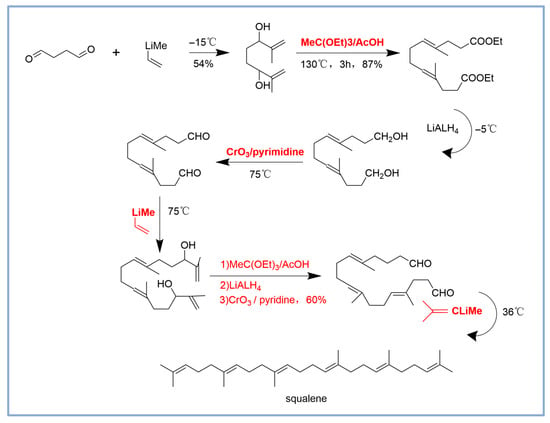
Figure 2.
Chemical synthesis of squalene [18,40,41]. The parts marked in red and bold denote the key reagents and conditions employed in the reaction. They play specific roles at each step. As a strong nucleophile, 2-propenyllithium is utilized for the nucleophilic addition. MeC (OEt)3/AcOH is applied in esterification reactions to convert the enol into a diester compound. LiAlH is employed in the reduction of esters, converting the diester compound to a diol. CrO3/pyridine oxidizes alcohols to aldehydes or ketones.
2.3. Squalene from Microbial Synthesis
In recent years, squalene has been successfully synthesized using S. cerevisiae, Y. lipolytica, and Escherichia coli [42,49,57,58,59,60,61,62,63,64,65,66]. While E. coli offers advantages, such as ease of genetic manipulation, a short growth cycle, and strong synthetic capacity, it has notable drawbacks. In particular, E. coli is unable to perform post-translational modifications on proteins after their synthesis—for instance, the attachment of sugar molecules (a process known as glycosylation). These post-translational modifications are essential for proteins from other species to fold into their correct conformation and exert proper biological functions. Additionally, the endotoxins produced by E. coli complicate the isolation and purification of squalene, making it less suitable for use in food and pharmaceuticals.
In contrast, the eukaryotic model organism S. cerevisiae presents several advantages for industrial production. These include robustness, safety, ease of genetic manipulation, low nutrient requirements, straightforward culture processes, and resistance to phage infections. Importantly, S. cerevisiae utilizes a process called the mevalonate (MVA) pathway. This pathway provides many building blocks, also called precursors, that are needed to make squalene. Examples of these precursors include isopentenyl diphosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). Y. lipolytica may not be a traditional yeast, but it has a strong ability to break down fatty acids into acetyl-CoA, which is important in the production of certain substances. Additionally, Y. lipolytica is able to survive in salty water and extreme pH levels, as well as when it is in contact with organic compounds. The strong activity of its tricarboxylic acid cycle and pentose phosphate pathway supports the production of acetyl-CoA, ATP and NADPH, making it an excellent host for squalene synthesis.
3. Biosynthesis of Squalene in Yeast
The biosynthesis of squalene in yeast uses several enzymes. It starts with acetyl-CoA, a key building block in many cell processes. The first step combines two acetyl-CoA molecules, a reaction helped by the enzyme ERG10, to form acetoacetyl-CoA. This middle compound then reacts with another acetyl-CoA molecule, assisted by the enzyme ERG13, to create HMG-CoA. Finally, HMG-CoA is turned into MVA by the enzyme HMGR, using two NADPH energy molecules.
Next, the MVA pathway undergoes a series of phosphorylations and decarboxylations, resulting in the production of IPP. IPP is then isomerized to DMAPP, followed by the condensation of DMAPP and IPP to produce farnesyl pyrophosphate (FPP) in a reaction catalyzed by the enzyme ERG20. In the final step, the squalene synthase ERG9 located in the endoplasmic reticulum, catalyzes the conversion of FPP and NADPH into squalene.
In the subsequent oxidation steps, squalene epoxidase ERG1 converts squalene into 2,3-oxidosqualene, which is eventually becomes ergosterol through a series of reactions (Figure 3). The biosynthesis of squalene in yeast begins in the cytoplasm, with the production of FPP via the MVA pathway; The process of converting FPP into squalene is primarily located within the endoplasmic reticulum. This spatial separation reflects the compartmentalization of metabolic pathways within yeast cells and the membrane-associated properties of the participating enzymes.
There are notable differences in enzyme structure, metabolic regulation, and flux between S. cerevisiae and Y. lipolytica. In the former, the MVA pathway is used solely for the synthesis of endogenous metabolites such as ergosterol [67]. In contrast, Y. lipolytica exhibits greater metabolic flexibility, enabling the MVA pathway to efficiently utilize various carbon sources and adapt to diverse growth conditions [56,64]. These differences highlight the distinct metabolic optimization strategies of the two yeasts, although the effectiveness of each pathway ultimately depends on the specific target product and the engineering strategies employed.
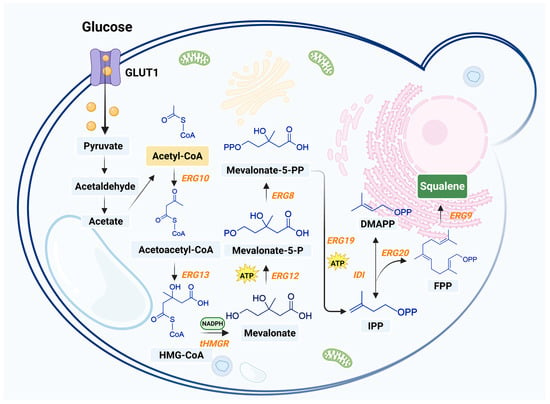
Figure 3.
Biosynthesis of squalene in yeast [68,69,70,71]. This figure was created using Biorender.com. Black arrows denote endogenous pathways. Enzymes involved in squalene biosynthesis are listed in orange. The orange spheres represent glucose. The yellow box points to acetyl-CoA, a key precursor for squalene biosynthesis; the blue boxes represent various intermediates; and the green box denotes the target product (squalene). ERG10, acetyl-CoA C-acetyltransferase; ERG13, 3-hydroxy-3-methylglutaryl-CoA synthase; tHMGR, truncated 3-hydroxy-3-methylglutaryl-CoA reductase; ERG12, mevalonate kinase; ERG8, phosphomevalonate kinase; ERG19, diphosphomevalonate decarboxylase; IDI, isopentenyl-diphosphate delta-isomerase; ERG20, bifunctional (2E,6E)-farnesyl diphosphate synthase; ERG9, squalene synthase.
4. Metabolic Engineering Strategies for the Biosynthesis of Squalene in Yeast
In recent years, researchers have tried different ways to make squalene using yeast as a cell factory [47]. Common strategies include enhancing precursor supply, knocking out competitive pathways, alleviating consumption, cofactor engineering, enhancing strain tolerance, extracellular secretion and computer-aided design (Figure 4). As shown in Supplementary Material, which summarizes recent reports on the biosynthesis of squalene in yeast.
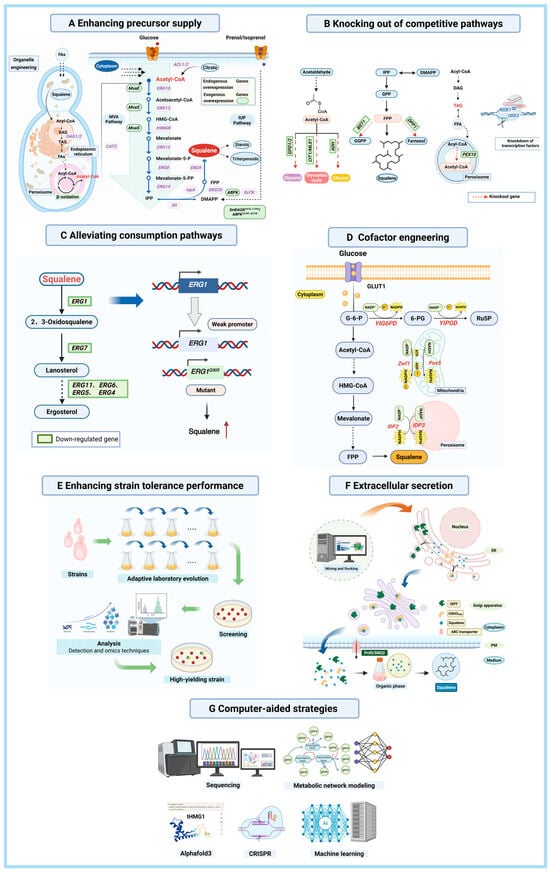
Figure 4.
Metabolic engineering strategies for the biosynthesis of squalene in yeast [48,58,68,72,73,74,75,76,77]. This figure was created using Biorender.com. (A) Enhancing precursor supply. The glycolytic pathway generates acetyl-CoA, which enters the mevalonate (MVA) pathway. The supply of key precursors (acetyl-CoA, IPP, and DMAPP) is enhanced through endogenous gene overexpression, heterologous gene introduction, and organelle engineering. Glucose and isoprenol/prenol substrates are represented by red and purple spheres, respectively. (B) Knocking out competitive pathways. Byproduct formation is reduced by knocking out key genes in competing pathways (ethanol, glycerol, and glyoxylate cycle), thereby optimizing acetyl-CoA utilization. Squalene accumulation is further enhanced through regulation of transcription factors or knockdown of competing pathway genes. (C) Alleviating consumption. The consumption of squalene is limited by down-regulating or knocking down the related gene via weak promoters or mutations. Red arrow represents yield improvement (D) Cofactor engineering. The supply of cofactors, such as NADPH and ATP, can be ameliorated via overexpression of genes, such as YIPGD or YIG6PD, associated with cofactor production. (E) Enhancing strain tolerance. High-tolerance, high-yield strains are developed through adaptive laboratory evolution, involving random mutagenesis, domestication, high-throughput screening, and omics-assisted phenotypic optimization. (F) Extracellular secretion. Abbreviations: tSPF refers to the lipid-binding domain (amino acids 1–278) of transcription factor SPF, which participates in intracellular lipid transport and storage; OSH3ORD denotes a fusion of OSH3 (an oxysterol-binding protein involved in lipid transport and ER membrane stability) and the ORD domain (which binds oxidized steroids and aids in cholesterol metabolism regulation); ABC transporters are transmembrane proteins that utilize ATP to transport substrates across membranes, with Pdr5 being a prominent plasma membrane ABC transporter in Saccharomyces cerevisiae known for drug efflux, and SNQ2 representing a potential endogenous ABC transporter in Yarrowia lipolytica involved in the efflux of lipids, metabolites, drugs, and toxic compounds. (G) Computer-aided strategies encompass computer simulation and AI tools to optimize metabolic engineering design. Tools include sequencing for genome and metabolic pathway profiling, Metabolic network modeling to help predict the optimal path, and ML (e.g., AlphaFold3) to predict protein structure or CRISPR-assisted gene editing.
4.1. Enhancing Precursor Supply
Squalene and its precursors are predominantly synthesized via the MVA pathway. Consequently, enhancing the supply of these precursors represents a crucial strategy for increasing squalene yields [77]. A critical regulatory step in this pathway is the reduction of HMG-CoA to MVA, catalyzed by HMGR. In yeast, HMGR is encoded by two isozymes, HMG1 and HMG2, which share functional similarity but differ in subcellular localization and regulation. A widely adopted approach to enhance squalene production is the overexpression of HMG1 or its truncated form, tHMG1 [61,70,72,78,79,80]. Furthermore, combinatorial expression of HMG1 with other enzymatic genes has been employed to augment precursor flux. For example, Kwak et al. overexpressed tHMG1 and ERG10 in conjunction with xylose feeding, achieving a squalene titer of 150 mg/L [81].
The transcription factor UPC2 helps to control the ERG genes that make ergosterol in yeast [82]. The UPC2-1 version has a small change (G888D) in its activation domain, and this change greatly boosts the production of terpenes [83,84,85]. The bacterial enzyme ispA, which is involved in isoprenoid biosynthesis, catalyzes the polymerization of isoprene units to IPP or FPP [76,86,87]. Co-expression of ispA with tHMG1 in S. cerevisiae has resulted in 400 mg/L of squalene [86]. The metabolic capacity of S. cerevisiae for the MVA pathway can be expanded by catalyzing enzymes associated with lipid metabolism and stress response, such as the diacylglycerol acyltransferase (DGA1) and carnitine CIS acetyltransferase (CAT2). Wang et al. co-overexpressed HMGR, ERG9, and ERG20, followed by further co-overexpression of DGA1 and CAT2, which increased squalene production in Y. lipolytica to 1381.4 mg/L [64]. Additionally, overexpression of ACL1 and ACL2, which encode citrate ATP lyase from Y. lipolytica, significantly increased squalene production by boosting acetyl-CoA biosynthesis in S. cerevisiae [65].
In addition to modulating endogenous pathways, the introduction of exogenous pathways can effectively enhance precursor availability. Ma et al. introduced MvaE and MvaS from Enterococcus faecalis to enhance the metabolic flux through the upstream MVA pathway in Y. lipolytica, thereby increasing squalene content [69]. The same group also introduced an exogenous isopentenol utilization pathway into S. cerevisiae, which significantly shortened the biosynthesis route to IPP and DMAPP and established an efficient platform for terpenoid production [88]. Other researchers further improvements to this pathway came from molecular docking and enzyme-substrate structure analysis, which led to the co-overexpression of SmDAGKS47A,L124A and AtIPKS270P,A272R, which increased the accumulation of squalene by 152.95% [89].
In a different approach, the human hypoxia-inducible factor complex was introduced into S. cerevisiae, triggering a Warburg effect similar to that observed in cancer cells. This complex enhances the accumulation of pyruvate in the cytoplasm, and because yeast lacks lactate dehydrogenase, the increased pyruvate promotes the pyruvate dehydrogenase bypass, ultimately boosting acetyl-CoA biosynthesis. This metabolic shift resulted in a 2.7-fold increase in squalene production compared to the control strain [90].
Organelle engineering has emerged as a key strategy to improve squalene biosynthesis by optimizing the structure and function of organelles, enhancing metabolic flow, and improving energy utilization. Kim et al. overexpressed INO2, a key regulator of endoplasmic reticulum size in S. cerevisiae, resulting in a larger reticulum and greater squalene production [91]. At the same time, Liu et al. increased the activity of several enzymes, including ERG10, ERG13, tHMG1, ERG12, ERG8, MVD1, IDI1, ERG20, ERG9, acetyl-CoA synthase (ACS1), and citrate lyase (ACL). They did this in peroxisomes to increase the production of acetyl-CoA [92]. Similarly, some researchers overexpressed these same genes in mitochondria [71]. Another method of used cytoplasm-peroxisome linkage engineering, which incorporates peroxisome signal peptide screening, was used to overexpress peroxisomal genes POT1, PXA1/2, and POX1/2. This approach helped optimize carbon flux and prevent metabolic drain from the MVA pathway [70]. Notably, Tang et al. implemented simultaneous engineering of multiple organelles, achieving a remarkable squalene concentration of 55.8 g/L in S. cerevisiae—the highest titer reported to date [25].
4.2. Knockout of Competitive Pathways
Disrupting competing metabolic pathways is a highly effective strategy to increase squalene production. Knockout of ERG6 and ERG11, which are involved in ergosterol biosynthesis, has been shown to increase squalene production to 43 mg/g DCW [48,74]. Some researchers also combined knockout of downstream genes about ergosterol biosynthesis pathway along with that of ADH, which is involved in ethanol production, resulted in 304.49 mg/L squalene [93]. Wei et al. induced lipid accumulation and increased squalene accumulation by knocking out the peroxisomal membrane E3 ubiquitin ligase PEX10 and URE2, a transcriptional regulator responsible for the inhibition of nitrogen catabolism in Y. lipolytica [65]. Additionally, acetyl-CoA consumption has been reduced through knockout of CIT2 and MLS1 [64]. Furthermore, deletion of the ROX1 gene, which encodes a transcriptional repressor of ergosterol biosynthesis, has been shown to enhance both the MVA pathway and terpenoid synthesis in S. cerevisiae by derepressing the overall metabolic flux [94,95].
4.3. Alleviating Consumption Pathways
Reducing metabolic flux through competing consumption pathways is a crucial strategy for improving the yield and efficiency of target metabolites. By optimizing these pathways, metabolic flux could be redirected toward the desired product, thus enhancing overall biosynthetic efficiency.
An effective strategy involves converting ethanol to acetyl-CoA. Li et al. demonstrated that overexpressing ADH2 (alcohol dehydrogenase) in S. cerevisiae, along with heterologous expression of the transcriptional activator ADA from Dickeya zeae, promoted the conversion of acetaldehyde to acetyl-CoA [58]. ADA does not require ATP, alleviating the metabolic burden on the cell and enhancing the efficiency of squalene biosynthesis.
In the native metabolic pathways of S. cerevisiae, squalene is formed mainly as an intermediate in ergosterol biosynthesis. It is quickly converted into 2,3-oxidosqualene by ERG1 and then further metabolized to produce ergosterol. In unmodified wild-type strains, squalene levels are typically undetectable due to its swift diversion to ergosterol biosynthesis. Because ergosterol is a necessary component of the yeast cell membrane and is essential for cell growth and reproduction, simply knocking out genes involved in ergosterol synthesis is not a viable option [96]. To overcome this issue, promoter engineering can be employed to regulate ERG1 expression. Kamimura et al. achieved a squalene yield of 5 mg/g DCW by introducing random mutations into ERG1, effectively reducing the flux through the ergosterol biosynthesis pathway [97]. Zhou et al. further engineered pERG1 in S. cerevisiae by replacing the SRE sequence with the marO sequence and inserting marO after the TATA box. This modification reduced ERG1 expression and promoted squalene accumulation. Additionally, they introduced the ERG1G30S mutant to further minimize squalene consumption [98]. Lu et al. replaced the endogenous ERG1 promoter with a homolog from Phaffa rhodozyma to down-regulate ERG1, whereas Manzoor et al. used a weaker pERG1 [61,99]. ERG1 expression can be modulated also by transcription factors. Jorda et al. found that knocking out the ROX1 transcriptional repressor or the post-transcriptional repressors CTH1 and CTH2, increased ERG1 expression. Conversely, downregulating these repressors had the opposite effect [100].
In summary, a combination of genetic modifications and metabolic engineering strategies, such as promoter engineering, gene knockout, and transcription factor modulation, can effectively alleviate consumption pathways and redirect metabolic flux toward the production of valuable metabolites such as squalene.
4.4. Cofactor Engineering
Cofactor engineering is essential for optimizing biosynthetic processes, particularly in improving the efficiency and yield of target metabolites. Cofactors such as NAD (P)H and ATP mediate the activity and stability of metabolic pathways [73]. Introduction or overexpression of key genes such as NADH-HMG-CoA reductase (NADH-HMGR) to alleviate cofactor stress in the metabolic pathway boosts the concentration of target metabolites including squalene [101,102]. For instance, Li et al. tackled the limited availability of NADPH in the MVA pathway by introducing NADH-HMGR from Silicibacter pomeroyi. This alleviated cofactor pressure and resulted in 11.05% higher squalene production [58]. Paramasivan et al. enhanced Pos5 and Zwf1 expression in S. cerevisiae [103]. And then co-expressed them with other genes to boost the NADPH supply, eventually achieving 27.5-fold more squalene compared to control strains [79]. Similarly, Liu et al. overexpressed G6PD and PGD—two key pentose phosphate pathway genes—in Y. lipolytica, significantly elevating squalene synthesis [78]. Additionally, Liu et al. improved squalene titers by optimizing the supply of both NADPH and acetyl-CoA [60]. Beyond central carbon metabolism, the expression of specific isocitrate dehydrogenases such as IDP2 and IDP3 can also support NADPH generation required for mitochondrial β-oxidation of fatty acids [92,104].
4.5. Enhancing Strain Tolerance
Enhancing strain tolerance represents a pivotal metabolic engineering strategy, enabling sustainable synthesis of target molecules and facilitating the rational design of robust microbial cell factories [76]. In S. cerevisiae, a key approach to boosting squalene production is by reducing the squalene epoxidase activity. This can be achieved using non-competitive inhibitors such as terbinafine, an antifungal drug whose structure resembles squalene. Terbinafine competes with squalene for binding to the enzyme ERG1, thereby inhibiting the formation of 2,3-oxidosqualene, a precursor in the squalene biosynthetic pathway [105,106]. This specific inhibition mechanism enables terbinafine to serve as a selective pressure during adaptive laboratory evolution, enriching for engineered S. cerevisiae exhibiting enhanced squalene flux and improved accumulation [42]. Such approaches are crucial for advancing the adaptive evolution of S. cerevisiae strains designed for efficient squalene production, as well as for cultivating more robust, high-yield strains. In a complementary approach, using another approach, Son et al. adapted cells to elevated squalene levels by reinforcing the yeast cell wall and minimizing the dose-dependent inhibitory effect of squalene on growth [107]. While the use of ethanol as a carbon source during fermentation has been shown to increase squalene accumulation, high ethanol concentrations can negatively affect both strain growth and product accumulation. To address this conundrum, researchers have implemented adaptive evolution under progressively increasing ethanol stress, successfully obtaining strains with enhanced ethanol tolerance and higher squalene yields [108].
4.6. Extracellular Secretion
Squalene is an extremely hydrophobic compound. Given its pronounced hydrophobicity and considerable molecular size, the lipid bilayer of the cell membrane effectively restricts its passive diffusion. In yeast, efficient transport of squalene across the cell membrane is challenging, preventing natural extracellular secretion. Additionally, intracellular accumulation of squalene can disrupt the fluidity and stability of the cell membrane, further complicating its extracellular transport. Excessive buildup of squalene inside cells may also have toxic effects, damaging cellular structure and function.
To overcome these limitations, specialized transport systems or binding proteins are typically required to facilitate squalene’s passage through the cell membrane. Utilizing appropriate signal peptides or binding proteins can enhance the interaction between squalene and transport proteins, improving both binding and transport efficiency. This promotes efficient secretion of squalene from the intracellular to the extracellular environment, preventing excessive intracellular accumulation and reducing metabolic stress on the cells. Ultimately, this approach aims to increase overall squalene production. Son et al. enhanced squalene production by systematically integrating Suc2-tSPF into the original production strain, achieving a yield of 69 mg/g DCW—nearly three orders of magnitude higher than the wild-type strain [109]. Liu et al. identified the ATP-binding cassette transporter Pdr5 and the oxysterol-binding protein Osh3 as key facilitators of squalene efflux. Through a comprehensive “mining-docking-construction-validation” process, they demonstrated that strains overexpressing Pdr5 and Osh3 showed a 141.1-fold greater squalene production compared to control strains [110]. Chai et al. screened that the most significant secretion of squalene was observed when SNQ2 was bound to OSH3. Additionally, they developed a carrier protein-mediated metabolite transport system by fusing the OSH3 binding region to a secreted signal peptide, further enhancing squalene efflux [68].
4.7. Computer-Aided Strategies
Computer-aided strategies offer powerful methods for modeling metabolic networks in yeast, identifying bottlenecks, and targeting modifications to enhance squalene production [75]. Zhang et al. designed a gene silencing strategy based on autonomous oscillation, integrating computational and synthetic biology. By combining genome-scale metabolic models of S. cerevisiae with advanced algorithms such as flux balance analysis and OptKnock, they optimized the production of pentacyclic triterpenoids [55]. Liu et al. utilized databases such TransportDB2 to identify potential squalene transporters in S. cerevisiae [110]. Systems- and model-based metabolic engineering can enhance product formation by predicting gene knockdown and overexpression targets. Using the novel metaheuristic tool FOCuS to predict some key gene knockout targets, including LYS1, GAP1, AAT1, and PDC1, it was possible to increase squalene synthesis by 2.23-fold and 4.24-fold, respectively, compared with the control. These findings offer important strategies for overcoming squalene production bottlenecks, which is a critical challenge in microbial cell factory design and natural product biosynthesis [75].
5. Applications
As a natural triterpenoid, squalene is the precursor of many important bioactive compounds. Its unique chemical structure generates a variety of downstream products through complex enzymatic reactions in vivo (Table 2). Triterpenoids and steroids are important derivatives of squalene, with applications in medicine, healthcare, and cosmetics (Figure 5).

Table 2.
The downstream applications and production of squalene.
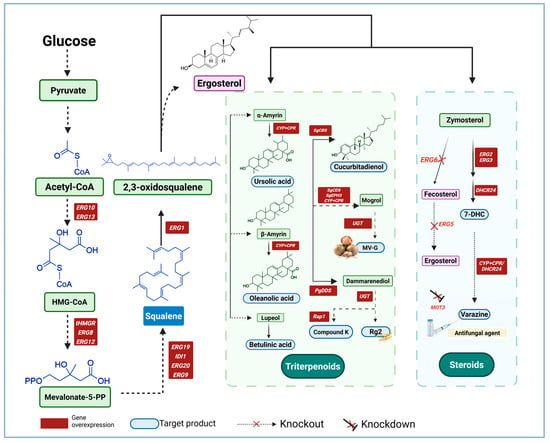
Figure 5.
Downstream applications of squalene [111,112,113,115,118,119,120,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142]. This section mainly mentions the application of squalene in the biosynthesis of Terpenoids and steroids, and the main enzymes involved in the reaction process.
5.1. Triterpenoids
Squalene is a precursor for the synthesis of triterpenoids, such as ursolic acid, oleanolic acid, ginsenoside and mogroside V (MG-V), etc. Ursolic and oleanolic acids possess anti-cancer, antioxidant, and so on [143,144]. The synthesis of them starts with squalene, and is followed by a series of oxidation and carboxylation reactions. These biologically active compounds have significant clinical value as therapeutics against inflammation, tumors, liver diseases, and hypoglycemia, and are widely used in botanicals and nutraceuticals. In synthetic biology, it is common to overexpress key enzyme genes, optimize the expression efficiency of heterologous genes, and knock out genes of competitive metabolic pathways to enhance the flux of target metabolites [135,138]. These steps are coupled with the optimization of fermentation processes (e.g., adjusting the initial sugar concentration and implementing fed-batch fermentation) to further augment the yield of target products. Lu et al. introduced amyrin C-28 oxidase and cytochrome P450 reductase to optimize the titers and ratios of ursolic and oleanolic acids. Through a glucose and ethanol fed-batch fermentation strategy, the final titers of the two acids reached 123.27 and 155.58 mg/L, respectively, with yields that were 4.77 and 4.95-fold higher than in the original strain [111]. Jin et al. ultimately achieved titers of 692.3 mg/L and 253.4 mg/L for ursolic and oleanolic acids in shake flasks, and 1132.9 mg/L and 433.9 mg/L in 3 L bioreactor through lipid droplet compartmentalization of CrAO and AtCPR1, which improved NADPH regeneration [112]. Jia et al. obtained 2.33 g/L ursolic acid in a 5 L bioreactor by screening efficient CYP450 and compatible CPR, as well as by optimizing cofactors and metabolic pathways. The resulting yield was 70-fold higher than in the original strain [113]. Recently, by constructing a heterologous synthesis module, optimizing the mevalonate, carbon flow balance and so on, the yield of the engineered strain reached 1083.62 mg/L in shake flask culture, and increased to 8.59 g/L in a 5 L bioreactor. This is the highest microbial synthesis ever recorded [114]. Moreover, the yield of oleanolic acid has been successfully increased by a range of different methods. Some sought to optimize electron transfer efficiency through fusion protein expression, achieving a fed-batch fermentation yield of 540.7 mg/L oleanolic acid in a 5 L bioreactor [115]. Moreover, a 7.6-fold increase in oleanolic acid production was attained by reconstructing metabolic pathways or the galactose regulatory network, thereby enhancing the expression of heterologous genes to dynamically balance cellular metabolism [138]. Another study employed computational biology to design a gene autonomous oscillatory silencing strategy based on global metabolic flux simulation and applied it in S. cerevisiae to promote stable oleanolic acid production [55]. A comprehensive comparison of different β-amyrin synthases and CYP716A subfamilies, provides a reference for future engineering of both S. cerevisiae and Y. lipolytica [136]. In addition, there have been some studies on betulinic acid (BA) biosynthesis. BA is a lupane-type triterpenoid that exhibits remarkable anti-cancer and anti-HIV properties. In the innovative study of S. cerevisiae, the researchers cleverly established a second synthesis pathway in the peroxisome, successfully realized the synergistic regulation of the two pathways, by further optimizing the fed-batch fermentation process, the yield of BA was finally increased to 682.29 mg/L, which was 48.7-fold higher than the initial level [116]. What is more, the dual engineering strategy of combining peroxisomes and lipid droplets has been studied to optimize the synthesis and storage of hydrophobic BA. The enzymes BPLO and ATR1 were used to construct BA synthesis pathway. By optimizing the expression of BPLO and ATR1 linker peptide, BA production reached 205.74 mg/L in fed-batch fermentation in 5 L bioreactor [117].
Rare-types ginsenosides are triterpenoid saponins extracted from ginseng. Unlike common ginsenosides, rare ginsenosides have unique glycosylation structures, usually containing some special sugar groups or linkages to these groups, which confer specific biological activity. Rare ginsenosides have anti-fatigue, anti-aging, anti-cancer, and immunoregulatory properties, which could be exploited in clinical practice for the treatment of cancer, cardiovascular disease, and aging [145,146,147,148]. Metabolic engineering, enzyme modification, and fermentation process optimization have significantly improved the production of Rh2 and other rare ginsenosides [139,140]. Specific engineering steps involve increase the supply of the precursor protopanaxiadol and improving glycosylation efficiency by optimizing the MVA pathway and the expression of key UDP-glycosyltransferases (UGTs). A maximum Rh2 yield of 2.25 g/L in a 10 L fermenter has been achieved through modular optimization and UGTPg45 modification [118]. Similarly, boosting the catalytic efficiency of UGT51 by approximately 1800-fold through semi-rational design, in combination with glycosylation and precursor optimization, has led to 300 mg/L Rh2 in a 5 L bioreactor [119]. Zhang et al. constructed a heterologous xylose metabolic pathway, using xylose and mixed sugar medium, with which they achieved 1.47 g/L Rh2 in a fermenter [120]. Their results opened the way for the utilization of renewable carbon sources in steroid biosynthesis. A yield of 354.69 mg/L Rh2 and a glycosylation ratio of 60.4% were reported in culture flasks following screening for efficient UGTs and heterologous expression [141]. In addition, researchers increased the production of Dammarenediol-II by 37.5-fold by enhancing the expression of ERG1 gene to increase the supply of 2,3-oxidosqualene, down-regulated the expression of ERG7 gene to reduce competitive consumption, and exogenously supplemented squalene, which provided an optimized platform for the efficient biosynthesis of ginsenosides [121]. Moreover, overexpression of Rap1 increased the production of Compound K, a downstream product of Dammarenediol-II, by 4.5-fold [122]. What is more, MG-V, as a cucurbitane triterpene saponins, is 400-fold sweeter than sucrose, and has anti-inflammatory, neuroprotective and other medicinal values. De novo biosynthesis of triterpene saponins MG-V was achieved by the addition of two glycosyltransferases to a modified S. cerevisiae strain. The yield reached 10.25 mg/L in shake flask and 28.62 mg/L in a 5 L bioreactor [123]. The accumulation of cucurbitadienol as a precursor for MG-V was increased to 6.19 /L in a 5 L bioreactor through a multi-module strategy, which is the highest titer reported so far [124]. These studies have demonstrated innovative strategies for the industrial production of ginsenosides and other rare triterpenoid saponins through systematic optimization.
5.2. Steroids
7-Dehydrocholesterol (7-DHC) is a sterol found in animals and precursor for synthesizing cholesterol and also the ecdysone hormone, it can be directly converted into vitamin D3 after exposure to ultraviolet light [128]. 7-DHC is widely used in the food and pharmaceutical industries [132]. We can increase how much 7-DHC we get by using genetic engineering and metabolic optimization. For example, by optimizing the inducible GAL promoter, integrating multiple copies of key enzyme genes and knockout NEM1, the yield of 7-DHC reached 1.07 g/L [125]. Another study achieved 360.6 mg/L 7-DHC yield in shake flask by compartmentalization and modular metabolic remodeling [126]. Moreover, blocking the competing pathways by knocking out the ERG5 and ERG6 genes and integrating two copies of the DHCR24 gene from Gallus cucurbitacin, along with knockout of MOT3 and the overexpression of key enzymes, elevated the yield of 7-DHC to 2.0 g/L [128]. A modular metabolic remodeling strategy, whereby the metabolic pathway was divided into central metabolism, MVA pathway, squalene pathway, and 7-DHC synthesis module, coupled with endoplasmic reticulum compartmentalization, successfully raised the 7-DHC production in a fermenter to 2.87 g/L [129]. In another study, a 7-DHC yield of 1.328 g/L was achieved by dynamically suppressing the ERG6 gene and using the Ty1 transposon to increase the copy numbers of ERG1 and DHCR24 [127]. Furthermore, Bi et al. achieved 640.77 mg/L in shake flask and 4.28 g/L through strategies such as peroxidase body localization and enhancement of NADPH [131]. Some researchers also rebalancing the redox state and integrating multiple copies of key enzyme genes increased the 7-DHC yield in a shake flask to 867.6 mg/L [130]. Finally, Xiu et al. directed the squalene posterior pathway towards lipid droplets and further alleviated the alleviating redox imbalance, increasing the yield of 7-DHC to 5.1 g/L, which is the highest yield reported to date [132]. Taken together, these approaches increase the prospects for the efficient microbial production of 7-DHC at industrial scale.
Additional promise comes from the development of production platforms for 7-DHC derivatives. For instance, blocking competing pathways, overexpressing key MVA pathway genes, manipulating lipid metabolism, and enhancing the supply of the cofactor 3-phosphoadenosine-5 phosphosulfate, raised the cholesterol sulfate yield to 545 mg/L in fed-batch fermenters [133]. Interesting, 7-DHC was used also as a precursor to reconstruct the five-step verazine biosynthesis pathway. The procedure required heterologous expression of eight enzymes from seven different species, and yielded 83 μg/L verazine, a potent antifungal agent [134].
6. Summary and Outlook
Shark liver oil restrictions and the limited yields from plant sources have accelerated the shift toward microbial production of squalene. However, several challenges remain, including low titers, cellular toxicity, and difficulties in scaling up processes. This review systematically summarizes recent advances in yeast-based squalene biosynthesis, focusing on seven key engineering strategies: enhancing precursor supply, blocking competitive pathways, cofactor engineering, reducing metabolic consumption, improving strain tolerance, promoting extracellular secretion, and computer-aided design. We also highlight applications in sustainable manufacturing and associated environmental benefits. Machine learning integration with metabolic engineering and omics data aids strain optimization, but high-throughput method gaps limit efficiency. Standardizing biological datasets via knowledge engineering can train models to predict gene–enzyme dose effects and identify robust cell factories. Coupling ML with responsive biosensors may revolutionize squalene and triterpenoid biosynthesis, advancing sustainable microbial production. In recent years, AI-driven biomanufacturing is a hot spot, and some progress has been made. For example, Bo Wang’s team from the University of Toronto recently proposed a model MFM that can be predicted and trained in genomics, transcriptomics, etc., and can be applied to a variety of downstream tasks through transfer learning. The continuous development of these technologies lays a solid foundation for future intelligent production, making the realization of a fully self-driven laboratory just around the corner! Nevertheless, the integration of AI into biological design faces several hurdles, including the need for large, high-quality datasets and interdisciplinary expertise. Technical barriers, regulatory frameworks, and data privacy issues also present challenges that require collaborative efforts across academia and industry to overcome. In summary, AI and ML will push biomaterial from “experience driven” to “data driven” and reshape the industrial ecology through agents, multimodal models, and self-evolving systems!
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13112422/s1, Table S1. Research progress in the biosynthesis of squalene in yeast. References [25,68,69,70,71,92,110,149,150,151] are cited in the Supplementary Materials.
Author Contributions
J.L.: Conceptualization, Funding acquisition, Writing—original draft. F.F.: Project administration, Supervision. S.Z. (Shasha Zuo): Writing—original draft, Writing—review and editing. X.T., J.M., F.H., J.C., L.F., Q.L., Y.Z., Y.W., Z.X., S.Z. (Siqi Zhang) and Y.S.: Writing—editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research were funded by the Key Research Project of Dongting Laboratory (2024-DTZD-001), Changsha Municipal Natural Science Foundation (kq2502098), the National Key Research and Development Program of China (2022YFD2100804) and Agricultural Science and Technology Innovation Fund of Hunan Province (2024CX02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tsujimoto, M. A highly unsaturated hydrocarbon in shark liver oil. J. Ind. Eng. Chem. 1916, 8, 889–896. [Google Scholar] [CrossRef]
- Cárdeno, A.; Aparicio-Soto, M.; Montserrat-de la Paz, S.; Bermudez, B.; Muriana, F.J.G.; Alarcón-de-la-Lastra, C. Squalene targets pro- and anti-inflammatory mediators and pathways to modulate over-activation of neutrophils, monocytes and macrophages. J. Funct. Foods 2015, 14, 779–790. [Google Scholar] [CrossRef]
- Huang, Z.R.; Lin, Y.K.; Fang, J.Y. Biological and pharmacological activities of squalene and related compounds: Potential uses in cosmetic dermatology. Molecules 2009, 14, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Morgan, N.R.; Magalingam, K.B.; Radhakrishnan, A.K.; Arumugam, M.; Jamil, A.; Bhuvanendran, S. Explicating the multifunctional roles of tocotrienol and squalene in promoting skin health. Skin Health Dis. 2024, 4, e448. [Google Scholar] [CrossRef]
- Zhang, Y.; Bejaoui, M.; Linh, T.N.; Arimura, T.; Isoda, H. A novel amphiphilic squalene-based compound with open-chain polyethers reduces malignant melanoma metastasis in-vitro and in-vivo. Cell Commun. Signal. 2024, 22, 437. [Google Scholar] [CrossRef]
- Fisher, K.J.; Shirtcliff, L.; Buchanan, G.; Thompson, A.W.; Woolard, F.X.; LaMunyon, D.H.; Marshall, J.L.; Baranouskas, M.B.; Voelker, R.B.; Lusk, J.S.; et al. Kilo-Scale GMP synthesis of renewable semisynthetic vaccine-grade squalene. Org. Process Res. Dev. 2023, 27, 2317–2328. [Google Scholar] [CrossRef]
- Mendes, A.; Azevedo-Silva, J.; Fernandes, J.C. From sharks to yeasts: Squalene in the development of vaccine adjuvants. Pharmaceuticals 2022, 15, 265. [Google Scholar] [CrossRef]
- Reddy, L.H.; Couvreur, P. Squalene: A natural triterpene for use in disease management and therapy. Adv. Drug Deliv. Rev. 2009, 61, 1412–1426. [Google Scholar] [CrossRef]
- Tateno, M.; Stone, B.J.; Srodulski, S.J.; Reedy, S.; Gawriluk, T.R.; Chambers, T.M.; Woodward, J.; Chappell, J.; Kempinski, C.F. Synthetic Biology-derived triterpenes as efficacious immunomodulating adjuvants. Sci. Rep. 2020, 10, 17090. [Google Scholar] [CrossRef]
- Najafi, A.; Heidary, M.; Martinez, R.M.; Baby, A.R.; Morowvat, M.H. Microalgae-based sunscreens as green and sustainable cosmetic products. Int. J. Cosmet. Sci. 2025, 47, 213–222. [Google Scholar] [CrossRef]
- Shalu, S.; Karthikanath, P.K.R.; Vaidyanathan, V.K.; Blank, L.M.; Germer, A.; Balakumaran, P.A. Microbial Squalene: A Sustainable alternative for the cosmetics and pharmaceutical industry—A review. Eng. Life Sci. 2024, 24, e202400003. [Google Scholar] [CrossRef]
- Van Nguyen, A.; Deineka, V.I.; Vu, A.T.N.; Le, T.D.; Tran-Trung, H.; Nguyen, T.A. Inclusion complexes of squalene with beta-cyclodextrin and methyl-beta-cyclodextrin: Preparation and characterization. Turk. J. Chem. 2023, 47, 294–306. [Google Scholar] [CrossRef]
- Yarkent, Ç.; Oncel, S.S. Recent progress in microalgal squalene production and its cosmetic application. Biotechnol. Bioprocess Eng. 2022, 27, 295–305. [Google Scholar] [CrossRef]
- Mordor Intelligence Research & Advisory (2024 JSMSSA-GTaF-MIRD). 2024. Available online: https://www.mordorintelligence.com/zh-CN/industry-reports/squalene-market (accessed on 16 December 2024).
- Akin, G.; Arslan, F.; Karuk Elmas, S.; Yilmaz, I. Cold-pressed pumpkin seed (Cucurbita pepo L.) oils from the central Anatolia region of Turkey: Characterization of phytosterols, squalene, tocols, phenolic acids, carotenoids and fatty acid bioactive compounds. Grasas Aceites 2018, 69, e232. [Google Scholar] [CrossRef]
- Chmelík, Z.; Šnejdrlová, M.; Vrablík, M. Amaranth as a potential dietary adjunct of lifestyle modification to improve cardiovascular risk profile. Nutr. Res. 2019, 72, 36–45. [Google Scholar] [CrossRef]
- Hernández, M.L.; Muñoz-Ocaña, C.; Posada, P.; Sicardo, M.D.; Hornero-Méndez, D.; Gómez-Coca, R.B.; Belaj, A.; Moreda, W.; Martínez-Rivas, J.M. Functional characterization of four olive squalene synthases with respect to the squalene content of the virgin olive oil. J. Agric. Food. Chem. 2023, 71, 15701–15712. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.S.; Werthemann, L.; Bartlett, W.R.; Brocksom, T.J.; Li, T.-T.; Faulkner, D.J.; Petersen, M.R. Simple stereoselective version of the Claisen rearrangement leading to trans-trisubstituted olefinic bonds. Synthesis of squalene. J. Am. Chem. Soc. 1970, 92, 741–743. [Google Scholar] [CrossRef]
- Khallouki, F.; Younos, C.; Soulimani, R.; Oster, T.; Charrouf, Z.; Spiegelhalder, B.; Bartsch, H.; Owen, R.W. Consumption of argan oil (Morocco) with its unique profile of fatty acids, tocopherols, squalene, sterols and phenolic compounds should confer valuable cancer chemopreventive effects. Eur. J. Cancer Prev. 2003, 12, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Krulj, J.; Brlek, T.; Pezo, L.; Brkljača, J.; Popović, S.; Zeković, Z.; Bodroža Solarov, M. Extraction methods of Amaranthus sp. grain oil isolation. J. Sci. Food Agric. 2016, 96, 3552–3558. [Google Scholar] [CrossRef]
- Rabrenović, B.B.; Dimić, E.B.; Novaković, M.M.; Tešević, V.V.; Basić, Z.N. The most important bioactive components of cold pressed oil from different pumpkin (Cucurbita pepo L.) seeds. LWT-Food Sci. Technol. 2014, 55, 521–527. [Google Scholar] [CrossRef]
- Rukmini, C.; Raghuram, T.C. Nutritional and biochemical aspects of the hypolipidemic action of rice bran oil: A review. J. Am. Coll. Nutr. 1991, 10, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.Y.; Xiang, J.; Wang, K.R.; Li, Z.Y.; Li, K.; Lu, J.L.; Ye, J.H.; Liang, Y.R.; Zheng, X.Q. Extraction of squalene from tea leaves (Camellia sinensis) and its variations with leaf maturity and tea cultivar. Front. Nutr. 2022, 9, 755514. [Google Scholar] [CrossRef] [PubMed]
- Szabóová, M.; Záhorský, M.; Gažo, J.; Geuens, J.; Vermoesen, A.; D’Hondt, E.; Hricová, A. Differences in seed weight, amino acid, fatty acid, oil, and squalene content in γ-irradiation-developed and commercial amaranth varieties (Amaranthus spp.). Plants 2020, 9, 1412. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Gao, W.; Guo, Q.; Wei, D.; Wang, F.Q. Orchestrating multiple subcellular organelles of Saccharomyces cerevisiae for efficient production of squalene. Bioresour. Technol. 2025, 424, 132294. [Google Scholar] [CrossRef]
- Kim, S.K.; Karadeniz, F. Biological importance and applications of squalene and squalane. Adv. Food Nutr. Res. 2012, 65, 223–233. [Google Scholar] [CrossRef]
- Passi, S.; De Pità, O.; Puddu, P.; Littarru, G.P. Lipophilic antioxidants in human sebum and aging. Free Radic. Res. 2002, 36, 471–477. [Google Scholar] [CrossRef]
- Nicolaides, N. Skin lipids: Their biochemical uniqueness. Science 1974, 186, 19–26. [Google Scholar] [CrossRef]
- Bhattacharyya, A.K.; Connor, W.E.; Lin, D.S. The origin of plant sterols in the skin surface lipids in humans: From diet to plasma to skin. J. Investig. Dermatol. 1983, 80, 294–296. [Google Scholar] [CrossRef]
- Popa, O.; Băbeanu, N.E.; Popa, I.; Niță, S.; Dinu-Pârvu, C.E. Methods for obtaining and determination of squalene from natural sources. Biomed Res. Int. 2015, 2015, 367202. [Google Scholar] [CrossRef]
- Nikkari, T.; Schreibman, P.H.; Ahrens, E.H., Jr. In vivo studies of sterol squalene secretion by human skin. J. Lipid Res. 1974, 15, 563–573. [Google Scholar] [CrossRef]
- Gabás-Rivera, C.; Barranquero, C.; Martínez-Beamonte, R.; Navarro, M.A.; Surra, J.C.; Osada, J. Dietary squalene increases high density lipoprotein-cholesterol and paraoxonase 1 and decreases oxidative stress in mice. PLoS ONE 2014, 9, e104224. [Google Scholar] [CrossRef]
- Ryu, A.; Arakane, K.; Koide, C.; Arai, H.; Nagano, T. Squalene as a target molecule in skin hyperpigmentation caused by singlet oxygen. Biol. Pharm. Bull. 2009, 32, 1504–1509. [Google Scholar] [CrossRef][Green Version]
- Ibrahim, N.; Naina Mohamed, I. Interdependence of Anti-Inflammatory and Antioxidant Properties of Squalene-Implication for Cardiovascular Health. Life 2021, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, T.A.; Vanhanen, H. Serum concentration and metabolism of cholesterol during rapeseed oil and squalene feeding. Am. J. Clin. Nutr. 1994, 59, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Barp, L.; Miklavčič Višnjevec, A.; Moret, S. Analytical determination of squalene in extra virgin olive oil and olive processing by-products, and its valorization as an ingredient in functional food-a critical review. Molecules 2024, 29, 5201. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liu, G.; Yang, M.; Wang, X.; Chen, X.; Chen, F.; Yang, Y. Isolation and functional analysis of squalene synthase gene in tea plant Camellia sinensis. Plant Physiol. Biochem. 2019, 142, 53–58. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, H.; Chen, X.; Köllner, T.G.; Jia, Q.; Wymore, T.W.; Wang, F.; Chen, F. Volatile squalene from a nonseed plant Selaginella moellendorffii: Emission and biosynthesis. Plant Physiol. Biochem. 2015, 96, 1–8. [Google Scholar] [CrossRef]
- Vriet, C.; Russinova, E.; Reuzeau, C. From squalene to brassinolide: The steroid metabolic and signaling pathways across the plant kingdom. Mol. Plant 2013, 6, 1738–1757. [Google Scholar] [CrossRef]
- Werthemann, L.; Johnson, W.S. Application of the chloro ketal claisen reaction to the total synthesis of squalene. Proc. Natl. Acad. Sci. USA 1970, 67, 1465–1467. [Google Scholar] [CrossRef]
- Scott, J.M.W.; Valentine, D.J. Facile catalytic syntheses of squalane. Org. Prep. Proced. Int. 1980, 11, 7–11. [Google Scholar] [CrossRef]
- Paramasivan, K.A.A.; Gupta, N.; Mutturi, S. Adaptive evolution of engineered yeast for squalene production improvement and its genome-wide analysis. Yeast 2021, 38, 424–437. [Google Scholar] [CrossRef]
- Cui, Z.; Zheng, H.; Zhang, J.; Jiang, Z.; Zhu, Z.; Liu, X.; Qi, Q.; Hou, J. A CRISPR/Cas9-mediated, homology-independent tool developed for targeted genome integration in Yarrowia lipolytica. Appl. Environ. Microbiol. 2021, 87, e02666-20. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Y.; Hou, J. Optimizing the CRISPR/Cas9 system for gene editing in Yarrowia lipolytica. Eng. Microbiol. 2025, 5, 100193. [Google Scholar] [CrossRef]
- Schwartz, C.; Frogue, K.; Ramesh, A.; Misa, J.; Wheeldon, I. CRISPRi repression of nonhomologous end-joining for enhanced genome engineering via homologous recombination in Yarrowia lipolytica. Biotechnol. Bioeng. 2017, 114, 2896–2906. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Che, J.; Qi, Q.; Hou, J. Metabolic engineering for squalene production: Advances and perspectives. J. Agric. Food. Chem. 2024, 72, 27715–27725. [Google Scholar] [CrossRef] [PubMed]
- Paramasivan, K.; Mutturi, S. Progress in terpene synthesis strategies through engineering of Saccharomyces cerevisiae. Crit. Rev. Biotechnol. 2017, 37, 974–989. [Google Scholar] [CrossRef]
- Paramasivan, K.; Rajagopal, K.; Mutturi, S. Studies on squalene biosynthesis and the standardization of its extraction methodology from Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 2019, 187, 691–707. [Google Scholar] [CrossRef]
- Zhang, T.-L.; Yu, H.-W.; Ye, L.-D. Metabolic engineering of Yarrowia lipolytica for terpenoid production: Tools and strategies. ACS Synth. Biol. 2023, 12, 639–656. [Google Scholar] [CrossRef]
- Nonaka, K.; Nishimura, K.; Uesaka, K.; Mishiro-Sato, E.; Fukase, M.; Kato, R.; Okumura, F.; Nakatsukasa, K.; Obara, K.; Kamura, T. Snf1 and yeast GSK3-β activates Tda1 to suppress glucose starvation signaling. EMBO Rep. 2025, 26, 2910–2930. [Google Scholar] [CrossRef]
- Klein, M.; Swinnen, S.; Thevelein, J.M.; Nevoigt, E. Glycerol metabolism and transport in yeast and fungi: Established knowledge and ambiguities. Environ. Microbiol. 2017, 19, 878–893. [Google Scholar] [CrossRef]
- Lopes, D.R.; Santos, L.O.; Prentice-Hernández, C. Antioxidant and antibacterial activity of a beverage obtained by fermentation of yerba-maté (Ilex paraguariensis) with symbiotic kombucha culture. J. Food Process. Preserv. 2021, 45, e15101. [Google Scholar] [CrossRef]
- Rima, H.; Steve, L.; Ismail, F. Antimicrobial and probiotic properties of yeasts: From fundamental to novel applications. Front. Microbiol. 2012, 3, 421. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, S.; Wang, Y.; Liang, Q.; Luo, W. Artificial intelligence technology assists enzyme prediction and rational design. J. Agric. Food. Chem. 2025, 73, 7065–7073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, X.; Zhou, Q.; Zhang, Y.; Lv, B.; Hu, B.; Li, C. Self-controlled in silico gene knockdown strategies to enhance the sustainable production of heterologous terpenoid by Saccharomyces cerevisiae. Metab. Eng. 2024, 83, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-B.; Chen, H.; Song, Z.-Q.; Pan, Y.-Q.; Hu, P.-C.; Yang, X.-N.; Lu, X.-Y.; Tian, Y.; Liu, H.-H. Cost-effective production of squalene using Yarrowia lipolytica via metabolic engineering and fermentation engineering. Biotechnol. Lett. 2025, 47, 49. [Google Scholar] [CrossRef]
- Katabami, A.; Li, L.; Iwasaki, M.; Furubayashi, M.; Saito, K.; Umeno, D. Production of squalene by squalene synthases and their truncated mutants in Escherichia coli. J. Biosci. Bioeng. 2015, 119, 165–171. [Google Scholar] [CrossRef]
- Li, T.; Liu, G.-S.; Zhou, W.; Jiang, M.; Ren, Y.-H.; Tao, X.-Y.; Liu, M.; Zhao, M.; Wang, F.-Q.; Gao, B.; et al. Metabolic engineering of Saccharomyces cerevisiae to overproduce squalene. J. Agric. Food. Chem. 2020, 68, 2132–2138. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, X.; Ji, Z.; Fan, B.; Chen, Y.; Wu, Y.; Gan, Y.; Li, Z.; Shang, Y.; Duan, L.; et al. Metabolic engineering of Saccharomyces cerevisiae for high-level production of (+)-ambrein from glucose. Biotechnol. Lett. 2024, 46, 615–626. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Deng, L.; Xu, P. Genetic and bioprocess engineering to improve squalene production in Yarrowia lipolytica. Bioresour. Technol. 2020, 317, 123991. [Google Scholar] [CrossRef]
- Lu, S.; Zhou, C.; Guo, X.; Du, Z.; Cheng, Y.; Wang, Z.; He, X. Enhancing fluxes through the mevalonate pathway in Saccharomyces cerevisiae by engineering the HMGR and β-alanine metabolism. Microb. Biotechnol. 2022, 15, 2292–2306. [Google Scholar] [CrossRef]
- Meng, Y.; Shao, X.; Wang, Y.; Li, Y.; Zheng, X.; Wei, G.; Kim, S.W.; Wang, C. Extension of cell membrane boosting squalene production in the engineered Escherichia coli. Biotechnol. Bioeng. 2020, 117, 3499–3507. [Google Scholar] [CrossRef]
- Tang, W.-Y.; Wang, D.-P.; Tian, Y.; Fan, X.; Wang, C.; Lu, X.-Y.; Li, P.-W.; Ji, X.-J.; Liu, H.-H. Metabolic engineering of Yarrowia lipolytica for improving squalene production. Bioresour. Technol. 2021, 323, 124652. [Google Scholar] [CrossRef]
- Wang, S.; Sun, X.; Han, Y.; Li, Z.; Lu, X.; Shi, H.; Zhang, C.-y.; Wong, A.; Yu, A. Sustainable biosynthesis of squalene from waste cooking oil by the yeast Yarrowia lipolytica. Metab. Eng. Commun. 2024, 18, e00240. [Google Scholar] [CrossRef]
- Wei, L.-J.; Cao, X.; Liu, J.-J.; Kwak, S.; Jin, Y.-S.; Wang, W.; Hua, Q. Increased accumulation of squalene in engineered Yarrowia lipolytica through Deletion of PEX10 and URE2. Appl. Environ. Microbiol. 2021, 87, e00481-21. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yao, J.; Liu, L.; Ma, X.; Li, W.; Sun, X.; Wang, Y. Improving squalene production by enhancing the NADPH/NADP(+) ratio, modifying the isoprenoid-feeding module and blocking the menaquinone pathway in Escherichia coli. Biotechnol. Biofuels 2019, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Du, M.-M.; Zhang, G.-G.; Zhu, Z.-T.; Zhao, Y.-Q.; Gao, B.; Tao, X.-Y.; Wang, F.-Q.; Wei, D.-Z. Boosting the epoxidation of squalene to produce triterpenoids in Saccharomyces cerevisiae. Biotechnol. Biofuels Bioprod. 2023, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Che, J.; Liu, X.; Wang, Z.; Qi, Q.; Hou, J. Secretory and metabolic engineering of squalene in Yarrowia lipolytica. Bioresour. Technol. 2025, 421, 132171. [Google Scholar] [CrossRef]
- Ma, Y.; Shang, Y.; Stephanopoulos, G. Engineering peroxisomal biosynthetic pathways for maximization of triterpene production in Yarrowia lipolytica. Proc. Natl. Acad. Sci. USA 2024, 121, e2314798121. [Google Scholar] [CrossRef]
- Ning, Y.; Liu, M.; Ru, Z.; Zeng, W.; Liu, S.; Zhou, J. Efficient synthesis of squalene by cytoplasmic-peroxisomal engineering and regulating lipid metabolism in Yarrowia lipolytica. Bioresour. Technol. 2024, 395, 130379. [Google Scholar] [CrossRef]
- Zhu, Z.-T.; Du, M.-M.; Gao, B.; Tao, X.-Y.; Zhao, M.; Ren, Y.-H.; Wang, F.-Q.; Wei, D.-Z. Metabolic compartmentalization in yeast mitochondria: Burden and solution for squalene overproduction. Metab. Eng. 2021, 68, 232–245. [Google Scholar] [CrossRef]
- Donald, K.A.; Hampton, R.Y.; Fritz, I.B. Effects of overproduction of the catalytic domain of 3-hydroxy-3-methylglutaryl coenzyme A reductase on squalene synthesis in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1997, 63, 3341–3344. [Google Scholar] [CrossRef]
- Kwak, S.; Yun, E.J.; Lane, S.; Oh, E.J.; Kim, K.H.; Jin, Y.S. Redirection of the Glycolytic Flux Enhances Isoprenoid Production in Saccharomyces cerevisiae. Biotechnol. J. 2020, 15, e1900173. [Google Scholar] [CrossRef]
- Mantzouridou, F.; Tsimidou, M.Z. Observations on squalene accumulation in Saccharomyces cerevisiae due to the manipulation of HMG2 and ERG6. FEMS Yeast Res. 2010, 10, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Paramasivan, K.; Abdulla, A.; Gupta, N.; Mutturi, S. In silico target-based strain engineering of Saccharomyces cerevisiae for terpene precursor improvement. Integr. Biol. 2022, 14, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, T.E.; Salazar, M.J.; Weng, L.L.; Palsson, B.O.; Feist, A.M. The emergence of adaptive laboratory evolution as an efficient tool for biological discovery and industrial biotechnology. Metab. Eng. 2019, 56, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, H.M.; Kozak, B.U.; Pronk, J.T.; Van Maris, A.J.A. Engineering cytosolic acetyl-coenzyme A supply in Saccharomyces cerevisiae: Pathway stoichiometry, free-energy conservation and redox-cofactor balancing. Metab. Eng. 2016, 36, 99–115. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, M.; Chen, H.; Lu, X.; Tian, Y.; Hu, P.; Zhao, Q.; Li, P.; Li, C.; Ji, X.; et al. Metabolic engineering of Yarrowia lipolytica for high-level production of squalene. Bioresour. Technol. 2024, 394, 130233. [Google Scholar] [CrossRef]
- Paramasivan, K.; Mutturi, S. Regeneration of NADPH coupled with HMG-CoA reductase activity increases squalene synthesis in Saccharomyces cerevisiae. J. Agric. Food. Chem. 2017, 65, 8162–8170. [Google Scholar] [CrossRef]
- Tokuhiro, K.; Muramatsu, M.; Ohto, C.; Kawaguchi, T.; Obata, S.; Muramoto, N.; Hirai, M.; Takahashi, H.; Kondo, A.; Sakuradani, E.; et al. Overproduction of geranylgeraniol by metabolically engineered Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2009, 75, 5536–5543. [Google Scholar] [CrossRef]
- Kwak, S.; Kim, S.R.; Xu, H.; Zhang, G.C.; Lane, S.; Kim, H.; Jin, Y.S. Enhanced isoprenoid production from xylose by engineered Saccharomyces cerevisiae. Biotechnol. Bioeng. 2017, 114, 2581–2591. [Google Scholar] [CrossRef]
- Yang, H.; Tong, J.; Lee, C.W.; Ha, S.; Eom, S.H.; Im, Y.J. Structural mechanism of ergosterol regulation by fungal sterol transcription factor Upc2. Nat. Commun. 2015, 6, 6129. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, Y.; Huang, L.; Zhang, X. Production of miltiradiene by metabolically engineered Saccharomyces cerevisiae. Biotechnol. Bioeng. 2012, 109, 2845–2853. [Google Scholar] [CrossRef]
- Dong, H.; Chen, S.; Zhu, J.; Gao, K.; Zha, W.; Lin, P.; Zi, J. Enhance production of diterpenoids in yeast by overexpression of the fused enzyme of ERG20 and its mutant mERG20. J. Biotechnol. 2020, 307, 29–34. [Google Scholar] [CrossRef]
- Ma, B.-X.; Ke, X.; Tang, X.-L.; Zheng, R.-C.; Zheng, Y.-G. Rate-limiting steps in the Saccharomyces cerevisiae ergosterol pathway: Towards improved ergosta-5,7-dien-3β-ol accumulation by metabolic engineering. World J. Microbiol. Biotechnol. 2018, 34, 55. [Google Scholar] [CrossRef]
- Han, J.Y.; Seo, S.H.; Song, J.M.; Lee, H.; Choi, E.-S. High-level recombinant production of squalene using selected Saccharomyces cerevisiae strains. J. Ind. Microbiol. Biotechnol. 2018, 45, 239–251. [Google Scholar] [CrossRef]
- Song, Y.; Guan, Z.; van Merkerk, R.; Pramastya, H.; Abdallah, I.I.; Setroikromo, R.; Quax, W.J. Production of squalene in Bacillus subtilis by squalene synthase screening and metabolic engineering. J. Agric. Food. Chem. 2020, 68, 4447–4455. [Google Scholar] [CrossRef]
- Ma, Y.; Zu, Y.; Huang, S.; Stephanopoulos, G. Engineering a universal and efficient platform for terpenoid synthesis in yeast. Proc. Natl. Acad. Sci. USA 2023, 120, e2207680120. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liang, H.; Gao, R.; Qin, L.; Xu, P.; Huang, M.; Zong, M.-H.; Cao, Y.; Lou, W.-Y. Yeast metabolism adaptation for efficient terpenoids synthesis via isopentenol utilization. Nat. Commun. 2024, 15, 9844. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; An, T.; Fu, D.; Duan, S.; Jin, H.-L.; Wang, H.-B. Optimization of central carbon metabolism by Warburg effect of human cancer cell improves triterpenes biosynthesis in yeast. Adv. Biotechnol. 2023, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-E.; Jang, I.-S.; Son, S.-H.; Ko, Y.-J.; Cho, B.-K.; Kim, S.C.; Lee, J.Y. Tailoring the Saccharomyces cerevisiae endoplasmic reticulum for functional assembly of terpene synthesis pathway. Metab. Eng. 2019, 56, 50–59. [Google Scholar] [CrossRef]
- Liu, G.-S.; Li, T.; Zhou, W.; Jiang, M.; Tao, X.-Y.; Liu, M.; Zhao, M.; Ren, Y.-H.; Gao, B.; Wang, F.-Q.; et al. The yeast peroxisome: A dynamic storage depot and subcellular factory for squalene overproduction. Metab. Eng. 2020, 57, 151–161. [Google Scholar] [CrossRef]
- Rasool, A.; Ahmed, M.S.; Li, C. Overproduction of squalene synergistically downregulates ethanol production in Saccharomyces cerevisiae. Chem. Eng. Sci. 2016, 152, 370–380. [Google Scholar] [CrossRef]
- Henry, K.W.; Nickels, J.T.; Edlind, T.D. ROX1 and ERG regulation in Saccharomyces cerevisiae: Implications for antifungal susceptibility. Eukaryot. Cell 2002, 1, 1041–1044. [Google Scholar] [CrossRef] [PubMed]
- Ollinger, T.L.; Vu, B.; Murante, D.; Parker, J.E.; Simonicova, L.; Doorley, L.; Stamnes, M.A.; Kelly, S.L.; Rogers, P.D.; Moye-Rowley, W.S.; et al. Loss-of-function ROX1 mutations suppress the fluconazole susceptibility of upc2AΔ mutation in Candida glabrata, implicating additional positive regulators of ergosterol biosynthesis. mSphere 2021, 6, e0083021. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Plan, M.R.; Chrysanthopoulos, P.; Hodson, M.P.; Nielsen, L.K.; Vickers, C.E. A squalene synthase protein degradation method for improved sesquiterpene production in Saccharomyces cerevisiae. Metab. Eng. 2017, 39, 209–219. [Google Scholar] [CrossRef]
- Kamimura, N.; Hidaka, M.; Masaki, H.; Uozumi, T. Construction of squalene-accumulating Saccharomyces cerevisiae mutants by gene disruption through homologous recombination. Appl. Microbiol. Biotechnol. 1994, 42, 353–357. [Google Scholar] [CrossRef]
- Zhou, C.; Li, M.; Lu, S.; Cheng, Y.; Guo, X.; He, X.; Wang, Z.; He, X.P. Engineering of cis-element in Saccharomyces cerevisiae for efficient accumulation of value-added compound squalene via downregulation of the downstream metabolic flux. J. Agric. Food. Chem. 2021, 69, 12474–12484. [Google Scholar] [CrossRef]
- Manzoor, R.; Ahmed, M.; Riaz, N.; Kiani, B.H.; Kaleem, U.; Rashid, Y.; Nawaz, A.; Awan, M.U.F.; Khan, H.; Imtiaz, U.; et al. Self-redirection of metabolic flux toward squalene and ethanol pathways by engineered yeast. Metabolites 2020, 10, 56. [Google Scholar] [CrossRef]
- Jordá, T.; Martínez-Martín, A.; Martínez-Pastor, M.T.; Puig, S. Modulation of yeast Erg1 expression and terbinafine susceptibility by iron bioavailability. Microb. Biotechnol. 2022, 15, 2705–2716. [Google Scholar] [CrossRef]
- Moreira dos Santos, M.; Raghevendran, V.; Kötter, P.; Olsson, L.; Nielsen, J. Manipulation of malic enzyme in Saccharomyces cerevisiae for increasing NADPH production capacity aerobically in different cellular compartments. Metab. Eng. 2004, 6, 352–363. [Google Scholar] [CrossRef]
- Song, W.; Yan, S.; Li, Y.; Feng, S.; Zhang, J.J.; Li, J.R. Functional characterization of squalene epoxidase and NADPH-cytochrome P450 reductase in Dioscorea zingiberensis. Biochem. Biophys. Res. Commun. 2019, 509, 822–827. [Google Scholar] [CrossRef]
- Zhao, X.; Shi, F.; Zhan, W. Overexpression of ZWF1 and POS5 improves carotenoid biosynthesis in recombinant Saccharomyces cerevisiae. Lett. Appl. Microbiol. 2015, 61, 354–360. [Google Scholar] [CrossRef]
- Lu, Q.; McAlister-Henn, L. Peroxisomal localization and function of NADP+ -specific isocitrate dehydrogenases in yeast. Arch. Biochem. Biophys. 2010, 493, 125–134. [Google Scholar] [CrossRef]
- Darkes, M.J.; Scott, L.J.; Goa, K.L. Terbinafine: A review of its use in onychomycosis in adults. Am. J. Clin. Dermatol. 2003, 4, 39–65. [Google Scholar] [CrossRef]
- Ryder, N.S. Terbinafine: Mode of action and properties of the squalene epoxidase inhibition. Br. J. Dermatol. 1992, 126 (Suppl. S39), 2–7. [Google Scholar] [CrossRef]
- Son, S.-H.; Kim, J.-E.; Oh, S.S.; Lee, J.Y. Engineering cell wall integrity enables enhanced squalene production in yeast. J. Agric. Food. Chem. 2020, 68, 4922–4929. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Wei, W.; Xia, L.; Gao, S.; Zeng, W.; Liu, S.; Zhou, J. Regulation of ethanol assimilation for efficient accumulation of squalene in Saccharomyces cerevisiae. J. Agric. Food. Chem. 2023, 71, 6389–6397. [Google Scholar] [CrossRef] [PubMed]
- Son, S.-H.; Kim, J.-E.; Park, G.; Ko, Y.-J.; Sung, B.H.; Seo, J.; Oh, S.S.; Lee, J.Y. Metabolite trafficking enables membrane-impermeable-terpene secretion by yeast. Nat. Commun. 2022, 13, 2605. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Jin, K.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. In Silico prediction and mining of exporters for secretory bioproduction of terpenoids in Saccharomyces cerevisiae. ACS Synth. Biol. 2023, 12, 863–876. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, C.; Zhao, F.; Li, D.; Lu, W. Biosynthesis of ursolic acid and oleanolic acid in Saccharomyces cerevisiae. AlChE J. 2018, 64, 3794–3802. [Google Scholar] [CrossRef]
- Jin, K.; Shi, X.; Liu, J.; Yu, W.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Combinatorial metabolic engineering enables the efficient production of ursolic acid and oleanolic acid in Saccharomyces cerevisiae. Bioresour. Technol. 2023, 374, 128819. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Li, J.; Zang, G.; Yu, Y.; Jin, X.; He, Y.; Feng, M.; Na, X.; Wang, Y.; Li, C. Engineering Saccharomyces cerevisiae for high-efficient production of ursolic acid via cofactor engineering and acetyl-CoA optimization. Biochem. Eng. J. 2024, 203, 109189. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, X.; Li, W.; Qiao, J.; Zhao, G.-R. Modular metabolic engineering of Saccharomyces cerevisiae for enhanced production of ursolic acid. J. Agric. Food. Chem. 2025, 73, 3580–3590. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, Y.; Wei, P.; Gao, X.; Li, M.; Zhang, C.; Zhou, Z.; Lu, W. Metabolic engineering of Yarrowia lipolytica for heterologous oleanolic acid production. Chem. Eng. Sci. 2020, 218, 115529. [Google Scholar] [CrossRef]
- Tang, M.; Xu, X.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Combinatorial metabolic engineering for improving betulinic acid biosynthesis in Saccharomyces cerevisiae. ACS Synth. Biol. 2024, 13, 1798–1808. [Google Scholar] [CrossRef]
- Tang, S.; Ji, W.; Zhao, Y.; Zhang, J.; Wei, D.; Wang, F.-Q. De novo biosynthesis of betulinic acid in engineered Saccharomyces cerevisiae. Bioorg. Chem. 2024, 152, 107737. [Google Scholar] [CrossRef]
- Wang, P.; Wei, W.; Ye, W.; Li, X.; Zhao, W.; Yang, C.; Li, C.; Yan, X.; Zhou, Z. Synthesizing ginsenoside Rh2 in Saccharomyces cerevisiae cell factory at high-efficiency. Cell Discov. 2019, 5, 5. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yang, G.Y.; Chen, X.; Liu, Q.; Zhang, X.; Deng, Z.; Feng, Y. Biosynthesis of plant-derived ginsenoside Rh2 in yeast via repurposing a key promiscuous microbial enzyme. Metab. Eng. 2017, 42, 25–32. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Zhao, X.; Zhang, Z.; He, S.; Bian, X.; Wang, H.; Zhang, C.; Lu, W. Efficient production of ginsenoside Rh2 from xylose by remodeling metabolism in Saccharomyces cerevisiae. Chem. Eng. J. 2024, 494, 153120. [Google Scholar] [CrossRef]
- Liu, X.-B.; Liu, M.; Tao, X.-Y.; Zhang, Z.-X.; Wang, F.-Q.; Wei, D.-Z. Metabolic engineering of Pichia pastoris for the production of dammarenediol-II. J. Biotechnol. 2015, 216, 47–55. [Google Scholar] [CrossRef]
- Byun, J.Y.; Nguyen, T.T.; Cho, B.K.; Park, S.H.; Kim, S.C. Rap1 overexpression boosts triterpenoid saponin production in yeast by enhancing precursor supply and heterologous gene expression. Microb. Cell Fact. 2025, 24, 47. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.; Song, Y.; Xu, X.; Liu, Y.; Li, J.; Du, G.; Liu, L.; Li, Y.; Lv, X. De novo biosynthesis of mogroside V by multiplexed engineered yeasts. Metab. Eng. 2025, 88, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhang, Y.; Wei, W.; Zhao, X.; Xu, S.; Gao, S.; Zhou, J. Overproduction of cucurbitadienol through modular metabolic engineering and fermentation optimization in Saccharomyces cerevisiae. J. Agric. Food. Chem. 2025, 73, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-J.; Xiao, W.-H.; Wang, Y.; Yao, M.-D.; Zeng, B.-X.; Liu, H.; Zhao, G.-R.; Yuan, Y.-J. Metabolic engineering of Saccharomyces cerevisiae for 7-dehydrocholesterol overproduction. Biotechnol. Biofuels 2018, 11, 192. [Google Scholar] [CrossRef]
- Guo, X.J.; Yao, M.D.; Xiao, W.H.; Wang, Y.; Zhao, G.R.; Yuan, Y.J. Compartmentalized reconstitution of post-squalene pathway for 7-dehydrocholesterol overproduction in Saccharomyces cerevisiae. Front. Microbiol. 2021, 12, 663973. [Google Scholar] [CrossRef]
- Qu, L.; Xiu, X.; Sun, G.; Zhang, C.; Yang, H.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Engineered yeast for efficient de novo synthesis of 7-dehydrocholesterol. Biotechnol. Bioeng. 2022, 119, 1278–1289. [Google Scholar] [CrossRef]
- Wei, W.; Gao, S.; Yi, Q.; Liu, A.; Yu, S.; Zhou, J. Reengineering of 7-dehydrocholesterol biosynthesis in Saccharomyces cerevisiae using combined pathway and organelle strategies. Front. Microbiol. 2022, 13, 978074. [Google Scholar] [CrossRef]
- Xiu, X.; Sun, Y.; Wu, Y.; Jin, K.; Qu, L.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Modular remodeling of sterol metabolism for overproduction of 7-dehydrocholesterol in engineered yeast. Bioresour. Technol. 2022, 360, 127572. [Google Scholar] [CrossRef]
- Ye, Z.; Xu, X.; Wu, Y.; Liu, Y.; Li, J.; Du, G.; Liu, L.; Lv, X. Efficient 7-Dehydrocholesterol production by multiple metabolic engineering of diploid Saccharomyces cerevisiae. J. Agric. Food Chem. 2024, 72, 25186–25196. [Google Scholar] [CrossRef]
- Bi, K.; Wang, W.; Tang, D.; Shi, Z.; Tian, S.; Huang, L.; Lian, J.; Xu, Z. Engineering sub-organelles of a diploid Saccharomyces cerevisiae to enhance the production of 7-dehydrocholesterol. Metab. Eng. 2024, 84, 169–179. [Google Scholar] [CrossRef]
- Xiu, X.; Xu, X.; Wu, Y.; Liu, Y.; Li, J.; Du, G.; Chen, J.; Lv, X.; Liu, L. Hyperproduction of 7-dehydrocholesterol by rewiring the post-squalene module in lipid droplets of Saccharomyces cerevisiae. Metab. Eng. 2024, 86, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Li, D.; Pan, Y.; Lv, B.; Gao, J.; Zuo, Y.; Huang, L.; Lian, J. Establishing Komagataella phaffii as a cell factory for efficient production of cholesterol sulfate. ACS Sustainable Chem. Eng. 2025, 13, 174–186. [Google Scholar] [CrossRef]
- Winegar, P.H.; Hudson, G.A.; Dell, L.B.; Astolfi, M.C.T.; Reed, J.; Payet, R.D.; Ombredane, H.C.J.; Iavarone, A.T.; Chen, Y.; Gin, J.W.; et al. Verazine biosynthesis from simple sugars in engineered Saccharomyces cerevisiae. Metab. Eng. 2024, 85, 145–158. [Google Scholar] [CrossRef]
- Cheng, X.; Pang, Y.; Ban, Y.; Cui, S.; Shu, T.; Lv, B.; Li, C. Application of multiple strategies to enhance oleanolic acid biosynthesis by engineered Saccharomyces cerevisiae. Bioresour. Technol. 2024, 401, 130716. [Google Scholar] [CrossRef]
- Dale, M.P.; Moses, T.; Johnston, E.J.; Rosser, S.J. A systematic comparison of triterpenoid biosynthetic enzymes for the production of oleanolic acid in Saccharomyces cerevisiae. PLoS ONE 2020, 15, e0231980. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, B.B.; Liu, Y.; Shi, M.Y.; Xiao, D.G.; Huang, L.Q.; Dai, Z.B.; Zhang, X.L. Optimization of synthetic pathway and fermentation process of yeast cell factories for production of oleanoic acid. Zhongguo Zhong Yao Za Zhi 2014, 39, 2640–2645. [Google Scholar]
- Zhao, Y.; Fan, J.; Wang, C.; Feng, X.; Li, C. Enhancing oleanolic acid production in engineered Saccharomyces cerevisiae. Bioresour. Technol. 2018, 257, 339–343. [Google Scholar] [CrossRef]
- Chu, L.L.; Huy, N.Q.; Tung, N.H. Microorganisms for ginsenosides biosynthesis: Recent progress, challenges, and perspectives. Molecules 2023, 28, 1437. [Google Scholar] [CrossRef]
- Li, M.; Ma, M.; Wu, Z.; Liang, X.; Zheng, Q.; Li, D.; An, T.; Wang, G. Advances in the biosynthesis and metabolic engineering of rare ginsenosides. Appl. Microbiol. Biotechnol. 2023, 107, 3391–3404. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Diao, M.; Peng, L.; Huang, S.; Xie, N. Characterization of a group of UDP-Glycosyltransferases involved in the biosynthesis of triterpenoid saponins of Panax notoginseng. ACS Synth. Biol. 2022, 11, 770–779. [Google Scholar] [CrossRef]
- Su, W.; Xiao, W.-H.; Wang, Y.; Liu, D.; Zhou, X.; Yuan, Y.-J. Alleviating redox imbalance enhances 7-dehydrocholesterol production in engineered Saccharomyces cerevisiae. PLoS ONE 2015, 10, e0130840. [Google Scholar] [CrossRef] [PubMed]
- Piet, M.; Paduch, R. Ursolic and oleanolic acids in combination therapy inhibit migration of colon cancer cells through down-regulation of the uPA/uPAR-dependent MMPs pathway. Chem. Biol. Interact. 2022, 368, 110202. [Google Scholar] [CrossRef] [PubMed]
- Vilkickyte, G.; Petrikaite, V.; Marksa, M.; Ivanauskas, L.; Jakstas, V.; Raudone, L. Fractionation and characterization of triterpenoids from vaccinium vitis-idaea l. Cuticular Waxes and Their Potential as Anticancer Agents. Antioxidants 2023, 12, 465. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, H.; Fan, D.; Deng, J. The anticancer activity and mechanisms of ginsenosides: An updated review. eFood 2020, 1, 226–241. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, J.; Huang, J.; Cen, S. Research progress on anti-aging effects and mechanisms of the new ginsenoside Compound K. J. Dermatol. Sci. Cosmet. Technol. 2025, 2, 100072. [Google Scholar] [CrossRef]
- Feng, S.; Li, T.; Wei, X.; Zheng, Y.; Zhang, Y.; Li, G.; Zhao, Y. The antioxidant and anti-fatigue effects of rare ginsenosides and γ-aminobutyric acid in fermented ginseng and germinated brown rice puree. Int. J. Mol. Sci. 2024, 25, 10359. [Google Scholar] [CrossRef]
- Irfan, M.; Kwak, Y.-S.; Han, C.-K.; Hyun, S.H.; Rhee, M.H. Adaptogenic effects of Panax ginseng on modulation of cardiovascular functions. J. Ginseng Res. 2020, 44, 538–543. [Google Scholar] [CrossRef]
- Son, S.-H.; Kim, J.-E.; Moon, S.Y.; Jang, I.-S.; Yu, B.J.; Lee, J.Y. Metabolic recycling of storage lipids promotes squalene biosynthesis in yeast. Biotechnol. Biofuels Bioprod. 2022, 15, 108. [Google Scholar] [CrossRef]
- Xu, M.; Yang, N.; Pan, J.; Hua, Q.; Li, C.-X.; Xu, J.-H. Remodeling the homologous recombination mechanism of Yarrowia lipolytica for high-level biosynthesis of squalene. J. Agric. Food. Chem. 2024, 72, 9984–9993. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Jian, X.-X.; Lv, Y.-B.; Nian, K.-Q.; Gao, Q.; Chen, J.; Wei, L.-J.; Hua, Q. Enhanced squalene biosynthesis in Yarrowia lipolytica based on metabolically engineered acetyl-CoA metabolism. J. Biotechnol. 2018, 281, 106–114. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).