Migration Characteristics of Manure-Derived Antibiotic-Resistant Bacteria in Vegetables Under Different Soil Types
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Plant Sample Collection and Processing
2.3. Determination of Total Culturable Endophytic Bacteria and ARB Abundance in Pakchoi
2.4. Pretreatment of TCEB and CREB
2.5. 16S rRNA High-Throughput Sequencing
2.6. Data Analysis
3. Results
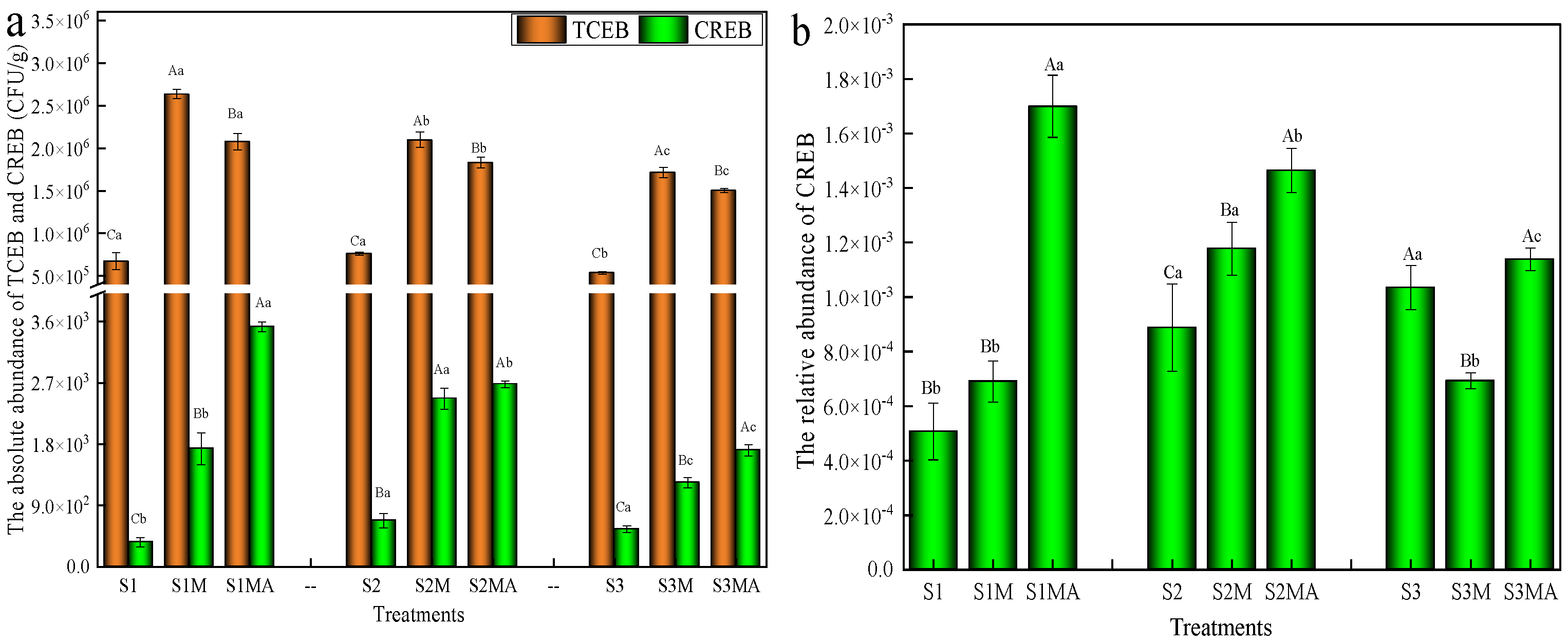
3.1. Changes in the Abundance of Chlortetracycline-Resistant Bacteria in Pakchoi
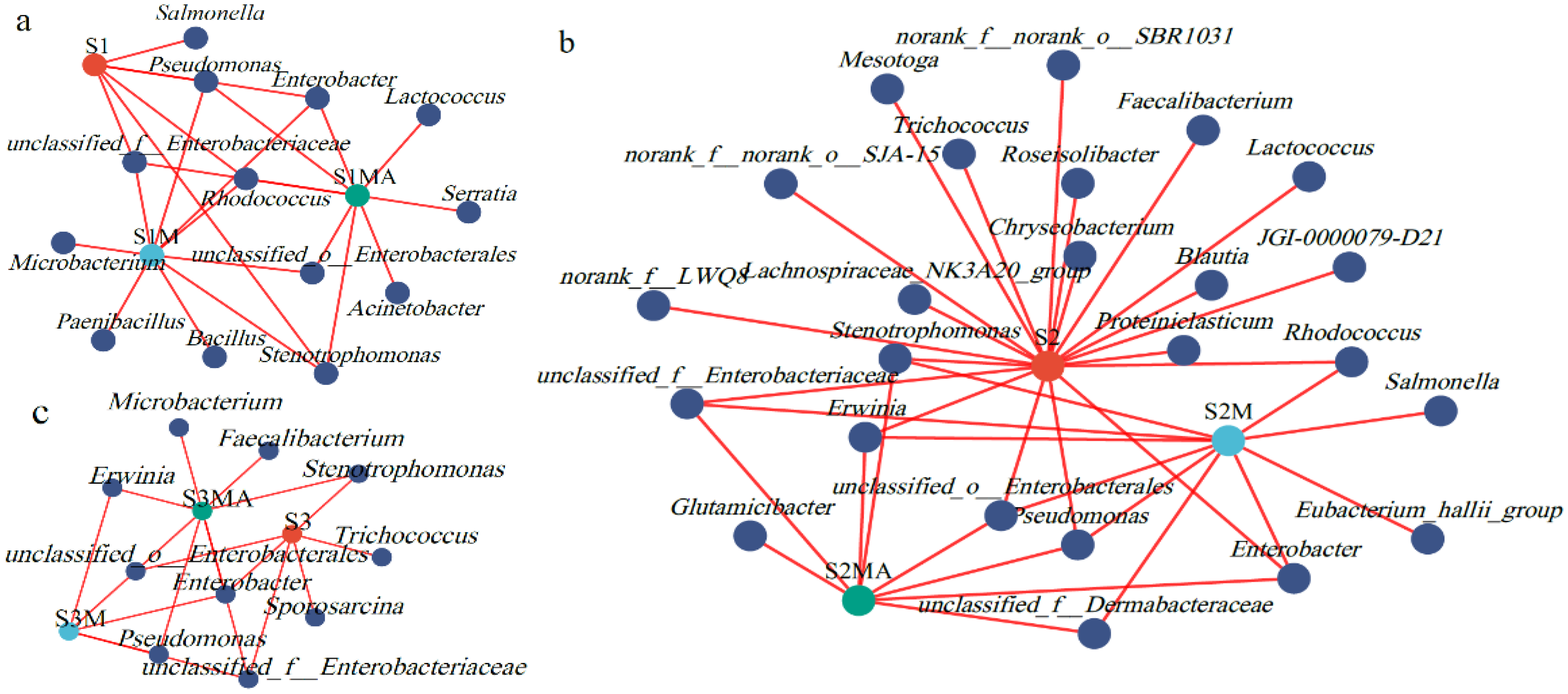
3.2. Changes in the Community Structure of Chlortetracycline-Resistant Endophytic Bacteria
3.3. Analysis of Differential Genera of Chlortetracycline-Resistant Bacteria Under Different Treatments
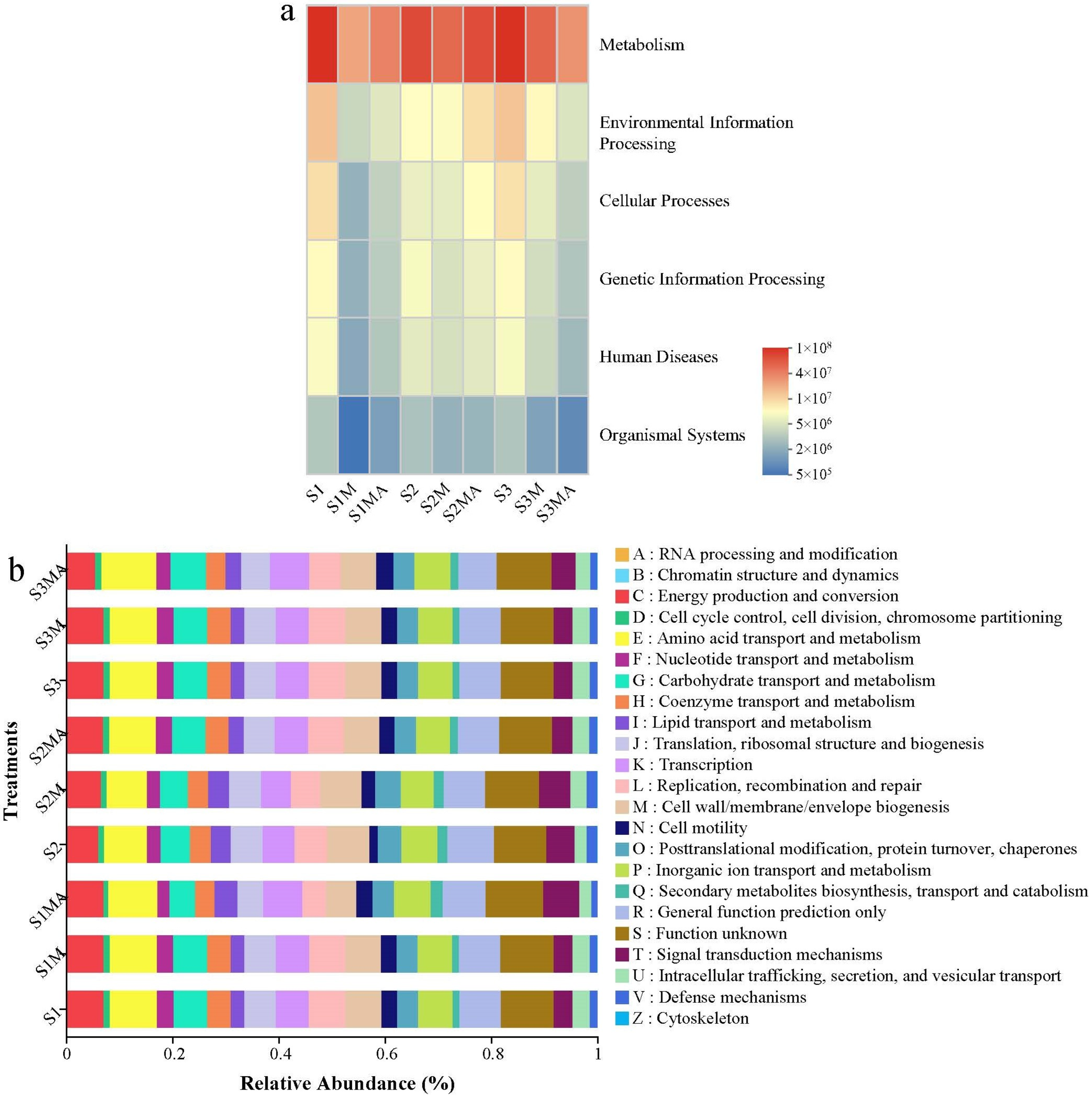
3.4. The Functional Prediction of PICRUSt
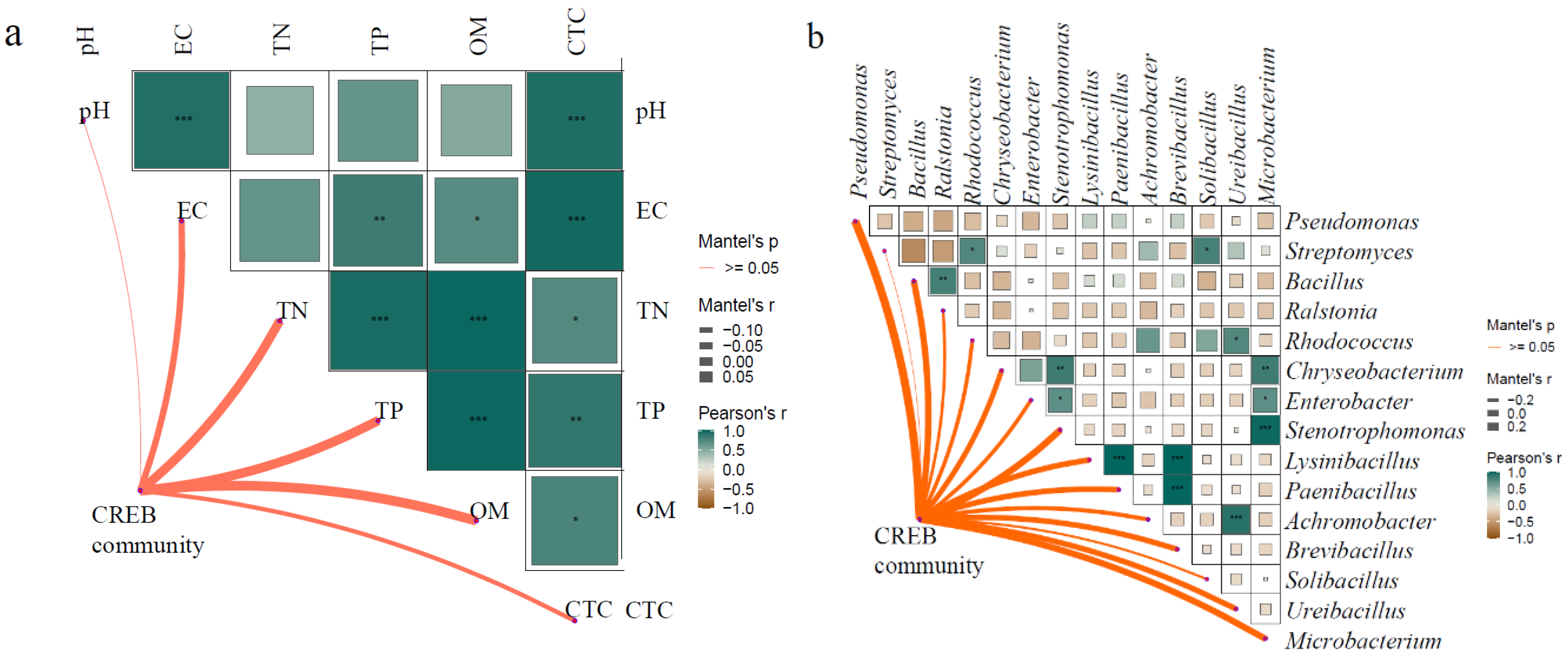
3.5. Analysis of the Impact Factors of CREB
4. Discussion
4.1. Influence of Manure and CTC on CREB Abundance in Pakchoi
4.2. The Influence of Manure and CTC on CREB Community Structure
4.3. The Influence of Manure and CTC on the Functional Prediction of PICRUSt
4.4. The Influence of Soil Physical and Chemical Properties and CRB on CREB of Pakchoi
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suganya, K.; Vasanthrao, R.; Chattopadhyay, I. Impact of Environment on Transmission of Antibiotic-Resistant Superbugs in Humans and Strategies to Lower Dissemination of Antibiotic Resistance. Folia Microbiol. 2023, 68, 657–675. [Google Scholar] [CrossRef]
- Yang, Q.L.; Gao, Y.; Ke, J.; Show, P.L.; Ge, Y.H.; Liu, Y.H.; Guo, R.X.; Chen, J.Q. Antibiotics: An Overview on the Environmental Occurrence, Toxicity, Degradation, and Removal Methods. Bioengineered 2021, 12, 7376–7416. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of Antibiotic Resistance in China and Its Environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Ejue, W.S.; Olayanju, A.; Dunsin, O.; Aboyeji, C.M.; Aremu, C.; Adegbite, K.; Akinpelu, O. Different Organic Manure Sources and NPK Fertilizer on Soil Chemical Properties, Growth, Yield and Quality of Okra. Sci. Rep. 2020, 10, 16083. [Google Scholar] [CrossRef]
- Youssef, M.A.; Farag, M.I.H. Co-Application of Organic Manure and Bio-Fertilizer to Improve Soil Fertility and Production of Quinoa and Proceeding Jew’s Mallow Crops. J. Soil Sci. Plant Nutr. 2021, 21, 2472–2488. [Google Scholar] [CrossRef]
- Bondarczuk, K.; Markowicz, A.; Piotrowska-Seget, Z. The Urgent Need for Risk Assessment on the Antibiotic Resistance Spread via Sewage Sludge Land Application. Environ. Int. 2016, 87, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.L.; An, X.L.; Zheng, B.X.; Ma, Y.B.; Su, J.Q. Long-Term Organic Fertilization Increased Antibiotic Resistome in Phyllosphere of Maize. Sci. Total Environ. 2018, 645, 1230–1237. [Google Scholar] [CrossRef]
- Tien, Y.C.; Li, B.; Zhang, T.; Scott, A.; Murray, R.; Sabourin, L.; Marti, R.; Topp, E. Impact of Dairy Manure Pre-Application Treatment on Manure Composition, Soil Dynamics of Antibiotic Resistance Genes, and Abundance of Antibiotic-Resistance Genes on Vegetables at Harvest. Sci. Total Environ. 2017, 581–582, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Garbisu, C.; Alkorta, I. A Case for the Importance of Following Antibiotic Resistant Bacteria Throughout the Soil Food Web. BioEssays 2023, 45, 2300153. [Google Scholar] [CrossRef]
- Liu, Y.T.; Zheng, L.; Cai, Q.J.; Xu, Y.B.; Xie, Z.F.; Liu, J.Y.; Ning, X. Simultaneous Reduction of Antibiotics and Antibiotic Resistance Genes in Pig Manure Using a Composting Process with a Novel Microbial Agent. Ecotoxicol. Environ. Saf. 2021, 208, 111724. [Google Scholar] [CrossRef]
- Adebayonle, A.; Chisom, L.O.; Tomisin, O.; Oladiti, O.O.; Babatunde, O. Impact of Soil Supplemented with Pig Manure on the Abundance of Antibiotic Resistant Bacteria and Their Associated Genes. J. Antibiot. 2023, 76, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Song, T.T.; Sardar, M.F.; Wang, X.R.; Li, B.X.; Zhang, Z.Y.; Wu, D.M.; Zhu, C.X.; Li, H.N. Distribution of Antibiotic Resistant Bacteria in Different Soil Types Following Manure Application. Soil Ecol. Lett. 2024, 6, 230210. [Google Scholar] [CrossRef]
- Wang, F.H.; Qiao, M.; Chen, Z.; Su, J.Q.; Zhu, Y.G. Antibiotic Resistance Genes in Manure-Amended Soil and Vegetables at Harvest. J. Hazard. Mater. 2015, 299, 215–221. [Google Scholar] [CrossRef]
- Chen, Q.L.; Hu, H.W.; Zhu, D.; Ding, J.; Yan, Z.Z.; He, J.Z.; Zhu, Y.G. Host Identity Determines Plant Associated Resistomes. Environ. Pollut. 2020, 258, 113709. [Google Scholar] [CrossRef]
- Agler, M.T.; Ruhe, J.; Kroll, S.; Morhenn, C.; Kim, S.T.; Weigel, D.; Kemen, E.M. Microbial Hub Taxa Link Host and Abiotic Factors to Plant Microbiome Variation. PLoS Biol. 2016, 14, e1002352. [Google Scholar] [CrossRef]
- Guo, Y.; Qiu, T.; Gao, M.; Sun, Y.; Cheng, S.; Gao, H.; Wang, X. Diversity and Abundance of Antibiotic Resistance Genes in Rhizosphere Soil and Endophytes of Leafy Vegetables: Focusing on the Effect of the Vegetable Species. J. Hazard. Mater. 2021, 415, 125595. [Google Scholar] [CrossRef]
- Bezanson, G.S.; MacInnis, R.; Potter, G.; Hughes, T. Presence and Potential for Horizontal Transfer of Antibiotic Resistance in Oxidase-Positive Bacteria Populating Raw Salad Vegetables. Int. J. Food Microbiol. 2008, 127, 37–42. [Google Scholar] [CrossRef]
- Zhuang, M.; Achmon, Y.; Cao, Y.; Liang, X.; Chen, L.; Wang, H.; Siame, B.A.; Leung, K.Y. Distribution of Antibiotic Resistance Genes in the Environment. Environ. Pollut. 2021, 285, 117402. [Google Scholar] [CrossRef]
- Xu, H.; Chen, Z.Y.; Huang, R.Y.; Cui, Y.X.; Li, Q.; Zhao, Y.H.; Wang, X.L.; Mao, D.Q.; Luo, Y.; Ren, H.Q. Antibiotic Resistance Gene-Carrying Plasmid Spreads into the Plant Endophytic Bacteria Using Soil Bacteria as Carriers. Environ. Sci. Technol. 2021, 55, 10462–10470. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.W.; Hsiao, W.C.; Chang, B.V. Biodegradation of Sulfonamide Antibiotics in Sludge. Chemosphere 2016, 150, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, F.; Matamoros, V.; Bayona, J.M.; Berendonk, T.U.; Elsinga, G.; Hornstra, L.M.; Piña, B. Antibiotic Resistance Gene Distribution in Agricultural Fields and Crops. A Soil-to-Food Analysis. Environ. Res. 2019, 177, 108608. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Qiu, T.; Sun, Y.; Song, Y.; Wang, X.; Gao, M. Diversity of Tetracycline- and Erythromycin-Resistant Bacteria in Aerosols and Manures from Four Types of Animal Farms in China. Environ. Sci. Pollut. Res. 2019, 26, 24213–24222. [Google Scholar] [CrossRef] [PubMed]
- Zarraonaindia, I.; Owens, S.M.; Weisenhorn, P.; West, K.; Hampton-Marcell, J.; Lax, S.; Bokulich, N.A.; Mills, D.A.; Martin, G.; Taghavi, S.; et al. The Soil Microbiome Influences Grapevine-Associated Microbiota. MBio 2015, 6, e02527-14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Gu, J.; Wang, X.; Li, Y.; Liu, J.; Lu, C.; Qiu, L. Response of Antibiotic Resistance Genes Abundance by Graphene Oxide during the Anaerobic Digestion of Swine Manure with Copper Pollution. Sci. Total Environ. 2019, 654, 292–299. [Google Scholar] [CrossRef]
- Wei, H.; Ding, S.; Qiao, Z.; Su, Y.; Xie, B. Insights into Factors Driving the Transmission of Antibiotic Resistance from Sludge Compost-Amended Soil to Vegetables under Cadmium Stress. Sci. Total Environ. 2020, 729, 138990. [Google Scholar] [CrossRef]
- Liu, D.L.; Zeleke, K.T.; Wang, B.; Macadam, I.; Scott, F.; Martin, R.J. Crop Residue Incorporation Can Mitigate Negative Climate Change Impacts on Crop Yield and Improve Water Use Efficiency in a Semiarid Environment. Eur. J. Agron. 2017, 85, 51–68. [Google Scholar] [CrossRef]
- Song, T.; Li, H.; Li, B.; Yang, J.; Sardar, M.F.; Yan, M.; Li, L.; Tian, Y.; Xue, S.; Zhu, C. Distribution of Antibiotic-Resistant Bacteria in Aerobic Composting of Swine Manure with Different Antibiotics. Environ. Sci. Eur. 2021, 33, 114. [Google Scholar] [CrossRef]
- Yang, Q.; Ren, S.; Niu, T.; Guo, Y.; Qi, S.; Han, X.; Liu, D.; Pan, F. Distribution of Antibiotic-Resistant Bacteria in Chicken Manure and Manure-Fertilized Vegetables. Environ. Sci. Pollut. Res. 2014, 21, 1231–1241. [Google Scholar] [CrossRef]
- Griffin, M.O.; Fricovsky, E.; Ceballos, G.; Villarreal, F. Tetracyclines: A Pleitropic Family of Compounds with Promising Therapeutic Properties. Review of the Literature. Am. J. Physiol. Cell Physiol. 2010, 299, 539–548. [Google Scholar] [CrossRef]
- Boehme, S.; Werner, G.; Klare, I.; Reissbrodt, R.; Witte, W. Occurrence of Antibiotic-Resistant Enterobacteria in Agricultural Foodstuffs. Mol. Nutr. Food Res. 2004, 48, 522–531. [Google Scholar] [CrossRef]
- Ullah, H.; Hassan, S.H.A.; Yang, Q.; Salama, E.S.; Liu, P.; Li, X.K. Dynamic Interaction of Antibiotic Resistance between Plant Microbiome and Organic Fertilizers: Sources, Dissemination, and Health Risks. World J. Microbiol. Biotechnol. 2025, 41, 4. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, H.; Guo, Y.; Tian, T. Influence of Chicken Manure Fertilization on Antibiotic-Resistant Bacteria in Soil and the Endophytic Bacteria of Pakchoi. Int. J. Environ. Res. Public Health 2016, 13, 662. [Google Scholar] [CrossRef]
- Doughari, H.J.; Ndakidemi, P.A.; Human, I.S.; Benade, S. The Ecology, Biology and Pathogenesis of Acinetobacter spp.: An Overview. Microbes Environ. 2011, 26, 101–112. [Google Scholar] [CrossRef]
- Hong, C.K.; Kim, J.; Kim, G.Y. Characteristics of Quinolone Resistance in Multidrug-Resistant Acinetobacter baumannii Strains Isolated from General Hospitals. Jundishapur J. Microbiol. 2021, 14, e115015. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, Y.; Chen, G.; Gui, X.; Chen, T.; Zhan, X. Characterization of Organic Matter Degradation during Composting of Manure-Straw Mixtures Spiked with Tetracyclines. Bioresour. Technol. 2011, 102, 7329–7334. [Google Scholar] [CrossRef]
- Ferreira, J.A.G.; Penner, J.C.; Moss, R.B.; Haagensen, J.A.J.; Clemons, K.V.; Spormann, A.M.; Nazik, H.; Cohen, K.; Banaei, N.; Carolino, E.; et al. Inhibition of Aspergillus fumigatus and Its Biofilm by Pseudomonas aeruginosa Is Dependent on the Source, Phenotype and Growth Conditions of the Bacterium. PLoS ONE 2015, 10, e0134692. [Google Scholar] [CrossRef]
- Tang, Q.H.; Sui, Q.W.; Wei, Y.S.; Shen, P.H.; Zhang, J.Y. Swine-Manure Composts Induce the Enrichment of Antibiotic-Resistant Bacteria but Not Antibiotic Resistance Genes in Soils. J. Environ. Manag. 2023, 345, 118707. [Google Scholar] [CrossRef]
- Reiter, B.; Pfeifer, U.; Schwab, H.; Sessitsch, A. Response of Endophytic Bacterial Communities in Potato Plants to Infection with Erwinia carotovora subsp. atroseptica. Appl. Environ. Microbiol. 2002, 68, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ben, W.; Zhang, Y.; Yang, M.; Qiang, Z. Effects of Thermophilic Composting on Oxytetracycline, Sulfamethazine, and Their Corresponding Resistance Genes in Swine Manure. Environ. Sci. Process Impacts 2015, 17, 1654–1660. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.B.; Baloda, S.; Boye, M.; Aarestrup, F.M. Antimicrobial Resistance among Pseudomonas spp. and the Bacillus cereus Group Isolated from Danish Agricultural Soil. Environ. Int. 2001, 26, 581–587. [Google Scholar] [CrossRef]
- Peng, H.L.; Xu, T.Y.; Wang, L.X.; Yu, J.Q.; Chen, X.; Cheng, X.; Li, H.G.; Huang, L.; Wei, L.; Wei, S.J. Effect of Streptomyces JD211 Application on Soil Physicochemical Properties and N2O Emission Characteristics of Rice Rhizosphere. Sci. Total Environ. 2024, 906, 167673. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Venkatesh, S.; Ke, K.; Barratt, M.J.; Gordon, J.I. A Human Gut Faecalibacterium prausnitzii Fatty Acid Amide Hydrolase. Science 2024, 386, 6828. [Google Scholar] [CrossRef]
- Ibekwe, A.D.; Bhattacharjee, A.S.; Phan, D.; Ashworth, D.; Schmidt, M.P.; Murinda, S.E.; Obayiuwana, A.; Murry, M.A.; Schwartz, G.; Lundquist, T.; et al. Potential Reservoirs of Antimicrobial Resistance in Livestock Waste and Treated Wastewater That Can Be Disseminated to Agricultural Land. Sci. Total Environ. 2023, 872, 162194. [Google Scholar] [CrossRef]
- Olavo, A.A.; Ribeiro, P.R.; Rufini, M.; Cruvinel, I.A.; Casagrande, D.R.; Moreira, F.M.S. Microsymbionts of Forage Peanut under Different Soil and Climate Conditions Belong to a Specific Group of Bradyrhizobium Strains. Appl. Soil Ecol. 2019, 143, 201–212. [Google Scholar] [CrossRef]
- Mehrotra, T.; Konar, D.; Pragasam, A.K.; Kumar, S.; Jana, P.; Babele, P.; Das, B. Antimicrobial Resistance Heterogeneity among Multidrug-Resistant Gram-Negative Pathogens: Phenotypic, Genotypic, and Proteomic Analysis. Proc. Natl. Acad. Sci. USA 2023, 120, 465120. [Google Scholar] [CrossRef]
- Poli, N.; Keel, C.J.; Garrido-Sanz, D. Expanding the Pseudomonas Diversity of the Wheat Rhizosphere: Four Novel Species Antagonizing Fungal Phytopathogens and with Plant-Beneficial Properties. Front. Microbiol. 2024, 15, 1440341. [Google Scholar] [CrossRef]
- Wang, X.L.; Shu, S.; Wang, Y.; Zeng, X.L. Degradation of Sulfathiazole by Heat-Activated Persulfate: Kinetics, Degradation Pathways, and Toxicity Assessment. J. Environ. Eng. 2025, 151, 04025019. [Google Scholar] [CrossRef]
- Wang, Y.X.; Liu, X.H.; Huang, C.D.; Han, W.P.; Gu, P.C.; Jing, R.X.; Yang, Q. Antibiotic Resistance Genes and Virulence Factors in the Plastisphere in Wastewater Treatment Plant Effluent: Health Risk Quantification and Driving Mechanism Interpretation. Water Res. 2025, 271, 122896. [Google Scholar] [CrossRef]
- Cruz-Loya, M.; Kang, T.M.; Lozano, N.A.; Watanabe, R.; Tekin, E.; Damoiseaux, R.; Savage, V.M.; Yeh, P.J. Stressor Interaction Networks Suggest Antibiotic Resistance Co-Opted from Stress Responses to Temperature. ISME J. 2019, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Keet, J.H.; Ellis, A.G.; Hui, C.; Novoa, A.; Roux, J.J.L. Impacts of Invasive Australian Acacias on Soil Bacterial Community Composition, Microbial Enzymatic Activities, and Nutrient Availability in Fynbos Soils. Microb. Ecol. 2021, 82, 704–721. [Google Scholar] [CrossRef] [PubMed]
- Mehnaz, K.R.; Keitel, C.; Dijkstra, F.A. Effects of Carbon and Phosphorus Addition on Microbial Respiration, N2O Emission, and Gross Nitrogen Mineralization in a Phosphorus-Limited Grassland Soil. Biol. Fertil. Soils 2018, 54, 481–493. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Hu, H.W.; Chen, Q.L.; Singh, B.K.; Yan, H.; Chen, D.; He, J.Z. Transfer of Antibiotic Resistance from Manure-Amended Soils to Vegetable Microbiomes. Environ. Int. 2019, 130, 104912. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Delgado-Baquerizo, M.; Su, J.Q.; Ding, J.; Li, H.; Gillings, M.R.; Peñuelas, J.; Zhu, Y.G. Deciphering Potential Roles of Earthworms in Mitigation of Antibiotic Resistance in the Soils from Diverse Ecosystems. Environ. Sci. Technol. 2021, 55, 7445–7455. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, T.; Zhu, C.; Teng, H.; Li, B.; Zhong, S.; Qin, Y.; He, J.; Li, H. Migration Characteristics of Manure-Derived Antibiotic-Resistant Bacteria in Vegetables Under Different Soil Types. Microorganisms 2025, 13, 2398. https://doi.org/10.3390/microorganisms13102398
Song T, Zhu C, Teng H, Li B, Zhong S, Qin Y, He J, Li H. Migration Characteristics of Manure-Derived Antibiotic-Resistant Bacteria in Vegetables Under Different Soil Types. Microorganisms. 2025; 13(10):2398. https://doi.org/10.3390/microorganisms13102398
Chicago/Turabian StyleSong, Tingting, Changxiong Zhu, Honghui Teng, Binxu Li, Shuang Zhong, Yan Qin, Jiawei He, and Hongna Li. 2025. "Migration Characteristics of Manure-Derived Antibiotic-Resistant Bacteria in Vegetables Under Different Soil Types" Microorganisms 13, no. 10: 2398. https://doi.org/10.3390/microorganisms13102398
APA StyleSong, T., Zhu, C., Teng, H., Li, B., Zhong, S., Qin, Y., He, J., & Li, H. (2025). Migration Characteristics of Manure-Derived Antibiotic-Resistant Bacteria in Vegetables Under Different Soil Types. Microorganisms, 13(10), 2398. https://doi.org/10.3390/microorganisms13102398






