Cross-Kingdom Enzymatic Strategies for Deoxynivalenol Detoxification: Computational Analysis of Structural Mechanisms and Evolutionary Adaptations
Abstract
1. Introduction
2. Materials and Methods
2.1. Sequence Alignment and Phylogenetic Analysis
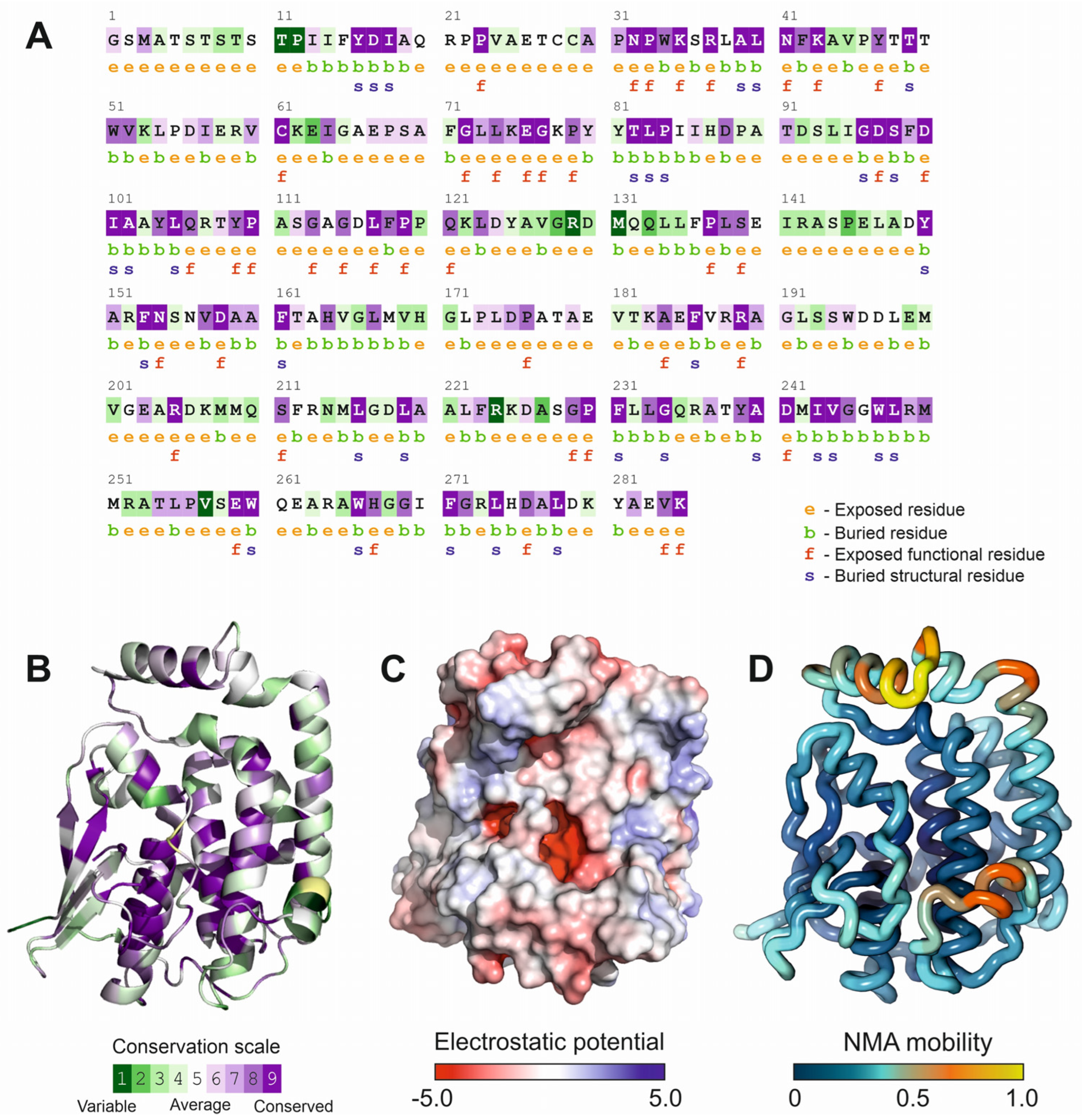
2.2. Residue Functional Conservation and Coevolution Analysis
2.3. Normal Mode Analysis
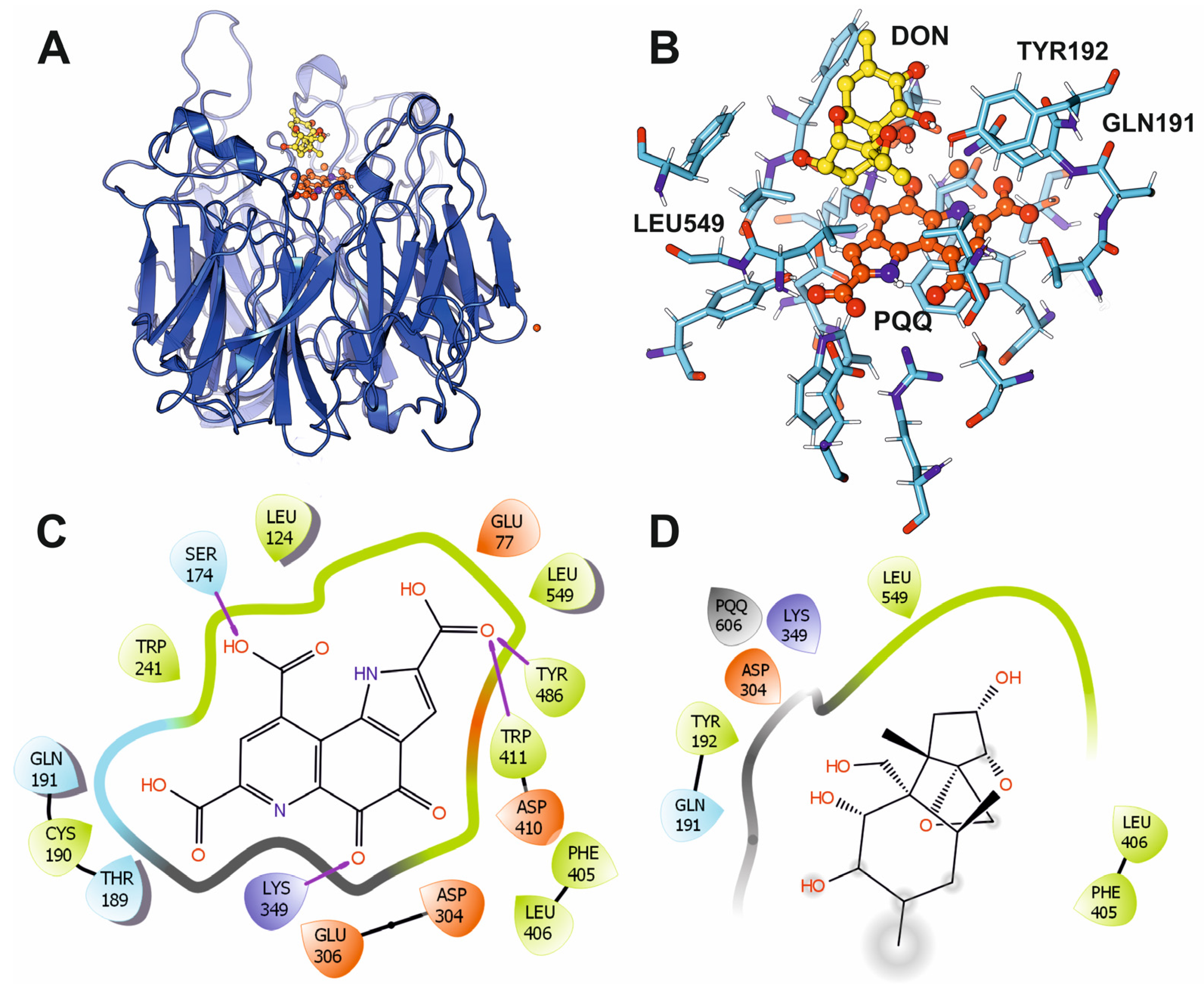
2.4. Molecular Docking
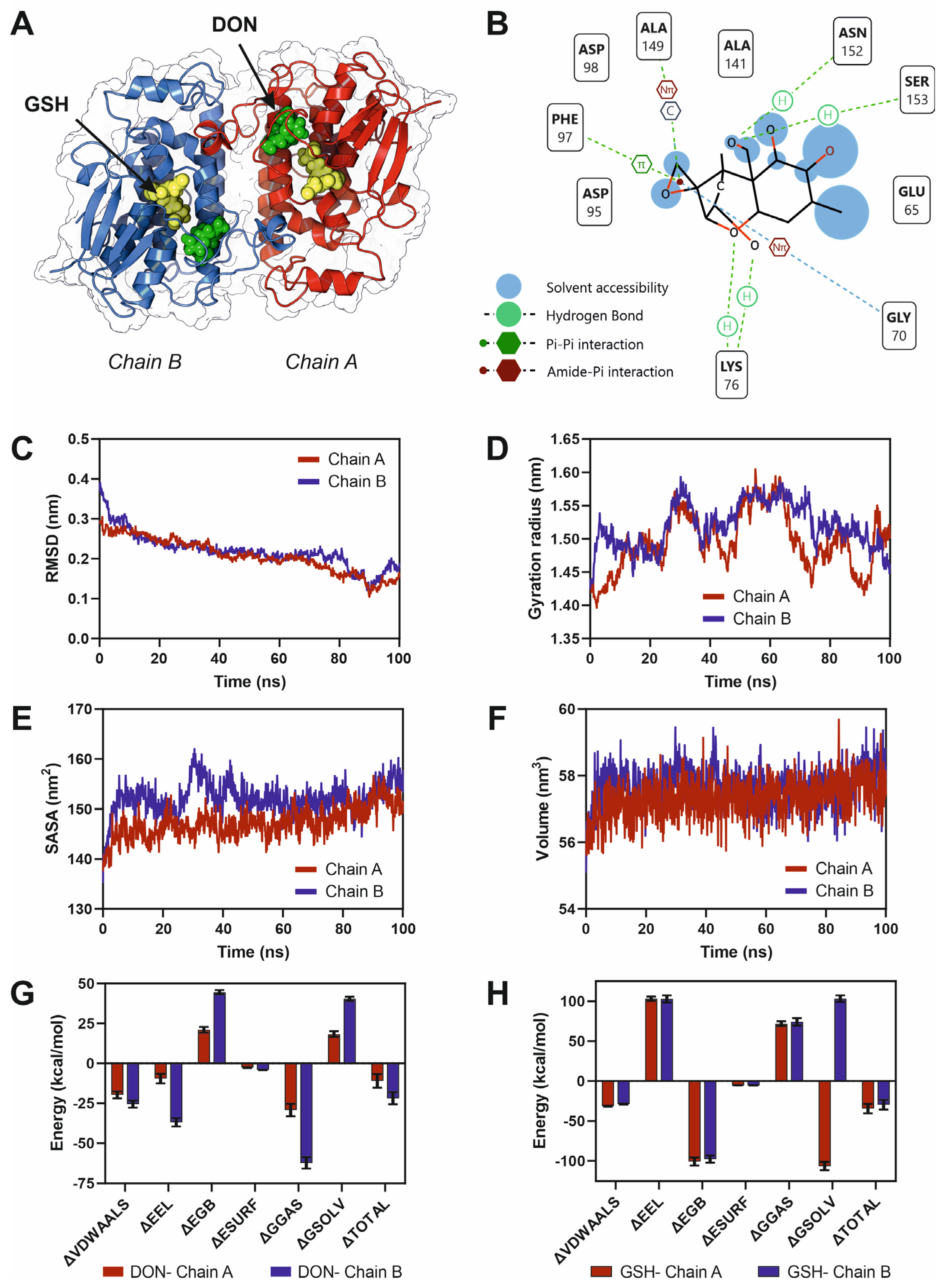
2.5. Molecular Dynamics Simulations and Free Energy Calculations
2.6. Allosteric Signaling and Mutational Analysis
2.7. Structure Analysis and Representation
3. Results
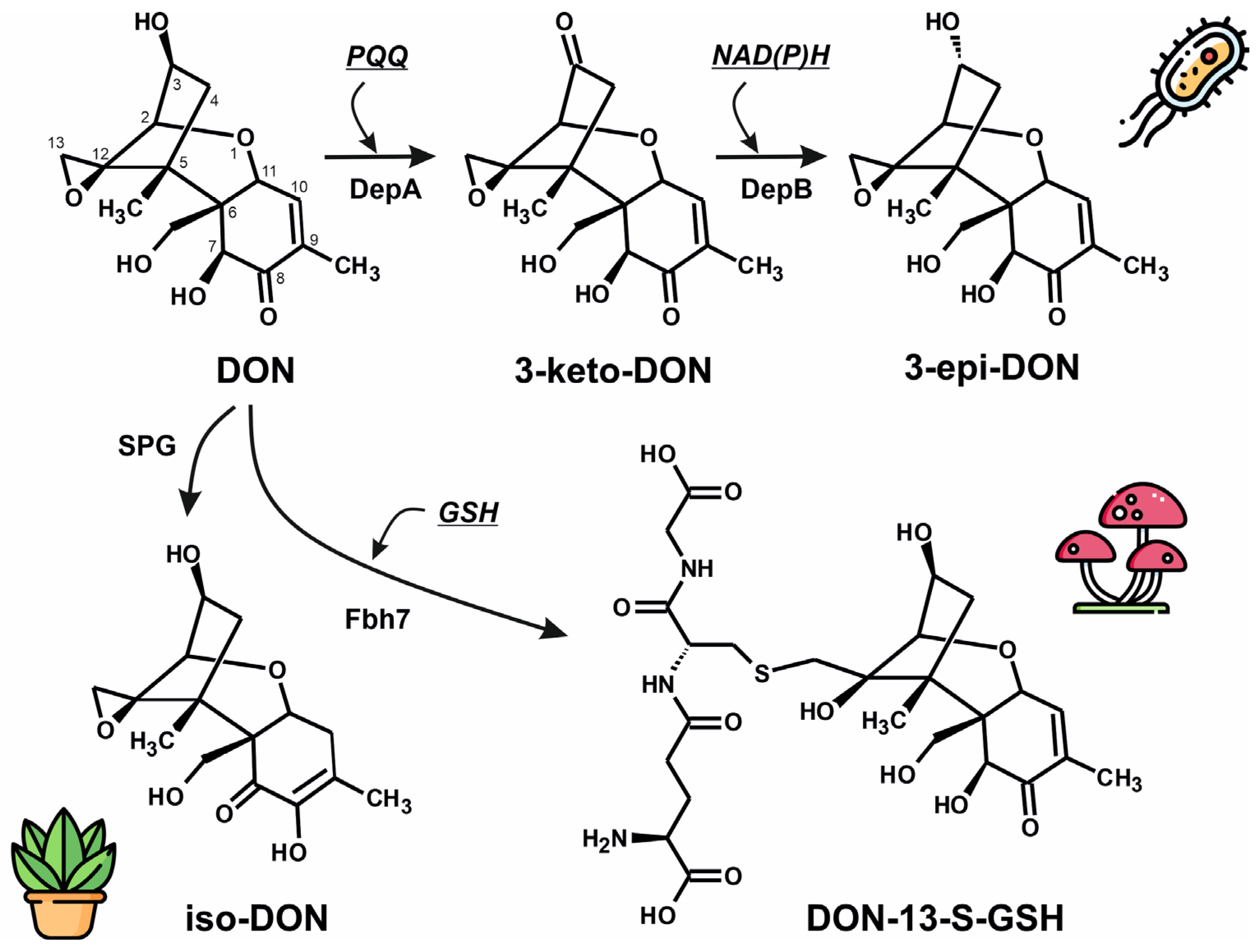
3.1. Fungal Glutathione S-Transferase, Fhb7
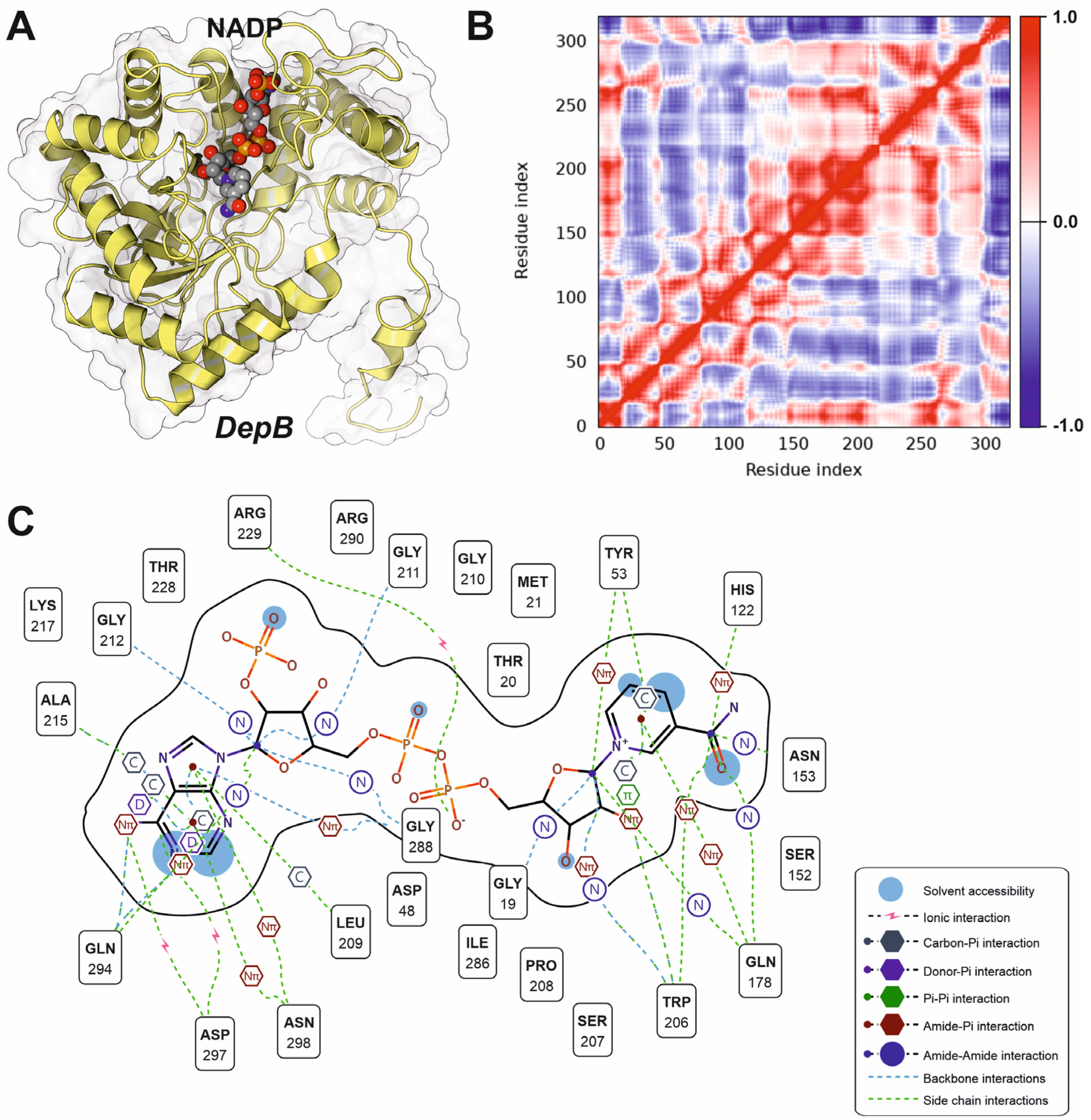
3.2. Bacterial Deoxynivalenol-3-Epimerase System: DepA and DepB
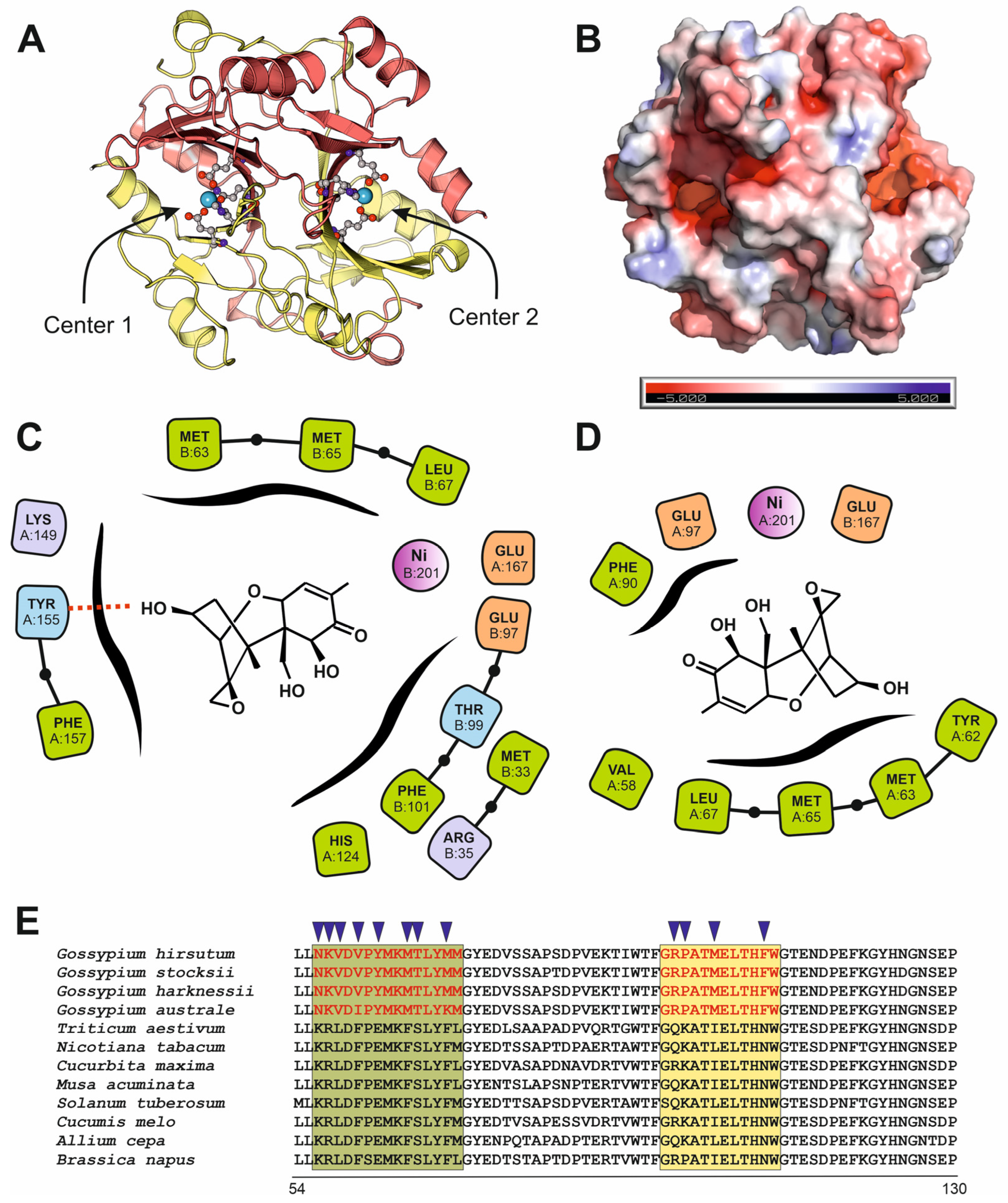
3.3. Plant Deoxynivalenol Glyoxalase, SPG Glyoxalase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, H.; Ji, J.; Wang, J.S.; Sun, X. Deoxynivalenol: Masked forms, fate during food processing, and potential biological remedies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 895–926. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Peng, Z.; Chen, L.; Nussler, A.K.; Liu, L.; Yang, W. Deoxynivalenol, gut microbiota and immunotoxicity: A potential approach? Food Chem. Toxicol. 2018, 112, 342–354. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Hohn, T.M.; McCormick, S.P. Trichothecene biosynthesis in Fusarium species: Chemistry, genetics, and significance. Microbiol. Rev. 1993, 57, 595–604. [Google Scholar] [CrossRef]
- Oldenburg, E.; Schittenhelm, S. Effect of plant water deficit on the deoxynivalenol concentration in Fusarium-infected maize kernels. Mycotoxin Res. 2012, 28, 229–236. [Google Scholar] [CrossRef]
- Mishra, S.; Dixit, S.; Dwivedi, P.D.; Pandey, H.P.; Das, M. Influence of temperature and pH on the degradation of deoxynivalenol (DON) in aqueous medium: Comparative cytotoxicity of DON and degraded product. Food Addit. Contam. Part A 2014, 31, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Wolf-Hall, C.E.; Hanna, M.A.; Bullerman, L.B. Stability of deoxynivalenol in heat-treated foods. J. Food Prot. 1999, 62, 962–964. [Google Scholar] [CrossRef] [PubMed]
- Sirot, V.; Fremy, J.M.; Leblanc, J.C. Dietary exposure to mycotoxins and health risk assessment in the second French total diet study. Food Chem. Toxicol. 2013, 52, 1–11. [Google Scholar] [CrossRef]
- Stuper-Szablewska, K.; Perkowski, J. Level of contamination with mycobiota and contents of mycotoxins from the group of trichothecenes in grain of wheat, oats, barley, rye and triticale harvested in Poland in 2006–2008. Ann. Agric. Environ. Med. 2017, 24, 49–55. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens-the IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Samet, J.M.; Chiu, W.A.; Cogliano, V.; Jinot, J.; Kriebel, D.; Lunn, R.M.; Beland, F.A.; Bero, L.; Browne, P.; Fritschi, L.; et al. The IARC Monographs: Updated Procedures for Modern and Transparent Evidence Synthesis in Cancer Hazard Identification. J. Natl. Cancer Inst. 2020, 112, 30–37. [Google Scholar] [CrossRef]
- Kouadio, J.H.; Mobio, T.A.; Baudrimont, I.; Moukha, S.; Dano, S.D.; Creppy, E.E. Comparative study of cytotoxicity and oxidative stress induced by deoxynivalenol, zearalenone or fumonisin B1 in human intestinal cell line Caco-2. Toxicology 2005, 213, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Schwaiger, S.; Cervellati, R.; Stuppner, H.; Speroni, E.; Guerra, M.C. In vitro evaluation of the chemoprotective action mechanisms of leontopodic acid against aflatoxin B1 and deoxynivalenol-induced cell damage. J. Appl. Toxicol. 2009, 29, 7–14. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, L.; Geng, C.; Cao, J.; Zhong, L. The role of oxidative stress in deoxynivalenol-induced DNA damage in HepG2 cells. Toxicon 2009, 54, 513–518. [Google Scholar] [CrossRef]
- Yang, W.; Yu, M.; Fu, J.; Bao, W.; Wang, D.; Hao, L.; Yao, P.; Nussler, A.K.; Yan, H.; Liu, L. Deoxynivalenol induced oxidative stress and genotoxicity in human peripheral blood lymphocytes. Food Chem. Toxicol. 2014, 64, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Sun, Y.; Chen, J.; Zhao, Y.; Qiao, H.; Chen, R.; Wen, X.; Deng, Y.; Wen, J. Deoxynivalenol globally affects the selection of 3′ splice sites in human cells by suppressing the splicing factors, U2AF1 and SF1. RNA Biol. 2020, 17, 584–595. [Google Scholar] [CrossRef]
- Bhat, R.V.; Beedu, S.R.; Ramakrishna, Y.; Munshi, K.L. Outbreak of trichothecene mycotoxicosis associated with consumption of mould-damaged wheat production in Kashmir Valley, India. Lancet 1989, 1, 35–37. [Google Scholar] [CrossRef]
- Ruan, F.; Chen, J.G.; Chen, L.; Lin, X.T.; Zhou, Y.; Zhu, K.J.; Guo, Y.T.; Tan, A.J. Food Poisoning Caused by Deoxynivalenol at a School in Zhuhai, Guangdong, China, in 2019. Foodborne Pathog. Dis. 2020, 17, 429–433. [Google Scholar] [CrossRef]
- Martins, C.; Vidal, A.; De Boevre, M.; De Saeger, S.; Nunes, C.; Torres, D.; Goios, A.; Lopes, C.; Assuncao, R.; Alvito, P. Exposure assessment of Portuguese population to multiple mycotoxins: The human biomonitoring approach. Int. J. Hyg. Environ. Health 2019, 222, 913–925. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Mohan, K.; Karthick Rajan, D.; Pillay, A.A.; Palanisami, T.; Sathishkumar, P.; Conterno, L. Distribution, toxicity, interactive effects, and detection of ochratoxin and deoxynivalenol in food: A review. Food Chem. 2022, 378, 131978. [Google Scholar] [CrossRef]
- Thapa, A.; Horgan, K.A.; White, B.; Walls, D. Deoxynivalenol and Zearalenone-Synergistic or Antagonistic Agri-Food Chain Co-Contaminants? Toxins 2021, 13, 561. [Google Scholar] [CrossRef]
- Chain, E.P.o.C.i.t.F.; Knutsen, H.K.; Alexander, J.; Barregard, L.; Bignami, M.; Bruschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; et al. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, e04718. [Google Scholar] [CrossRef] [PubMed]
- Schmied, A.; Marske, L.; Berger, M.; Kujath, P.; Weber, T.; Kolossa-Gehring, M. Human biomonitoring of deoxynivalenol (DON)—Assessment of the exposure of young German adults from 1996–2021. Int. J. Hyg. Environ. Health 2023, 252, 114198. [Google Scholar] [CrossRef]
- Brera, C.; de Santis, B.; Debegnach, F.; Miano, B.; Moretti, G.; Lanzone, A.; Del Sordo, G.; Buonsenso, D.; Chiaretti, A.; Hardie, L.; et al. Experimental study of deoxynivalenol biomarkers in urine. EFSA Support. Publ. 2015, EN-818, 136. [Google Scholar]
- Yao, Y.; Long, M. The biological detoxification of deoxynivalenol: A review. Food Chem. Toxicol. 2020, 145, 111649. [Google Scholar] [CrossRef]
- Carere, J.; Hassan, Y.I.; Lepp, D.; Zhou, T. The Identification of DepB: An Enzyme Responsible for the Final Detoxification Step in the Deoxynivalenol Epimerization Pathway in Devosia mutans 17-2-E-8. Front. Microbiol. 2018, 9, 1573. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, M.; Tang, Y.; Huang, Z.; Guo, Y.; Ma, Q.; Zhao, L. A NADPH-Dependent Aldo/Keto Reductase Is Responsible for Detoxifying 3-Keto-Deoxynivalenol to 3-epi-Deoxynivalenol in Pelagibacterium halotolerans ANSP101. Foods 2024, 13, 1064. [Google Scholar] [CrossRef]
- Wang, H.; Sun, S.; Ge, W.; Zhao, L.; Hou, B.; Wang, K.; Lyu, Z.; Chen, L.; Xu, S.; Guo, J.; et al. Horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat. Science 2020, 368, eaba5435. [Google Scholar] [CrossRef]
- Hu, Y.; Li, H.; Min, J.; Yu, Y.; Liu, W.; Huang, J.W.; Zhang, L.; Yang, Y.; Dai, L.; Chen, C.C.; et al. Crystal structure and biochemical analysis of the specialized deoxynivalenol-detoxifying glyoxalase SPG from Gossypium hirsutum. Int. J. Biol. Macromol. 2022, 200, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Y.; Noble, W.S.; Keich, U. A BLAST from the past: Revisiting blastp’s E-value. Bioinformatics 2024, 40, btae729. [Google Scholar] [CrossRef]
- Kumar, S.; Nei, M.; Dudley, J.; Tamura, K. MEGA: A biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 2008, 9, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazy, H.; Abadi, S.; Martz, E.; Chay, O.; Mayrose, I.; Pupko, T.; Ben-Tal, N. ConSurf 2016: An improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016, 44, W344–W350. [Google Scholar] [CrossRef]
- Colell, E.A.; Iserte, J.A.; Simonetti, F.L.; Marino-Buslje, C. MISTIC2: Comprehensive server to study coevolution in protein families. Nucleic Acids Res. 2018, 46, W323–W328. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Lopez-Blanco, J.R.; Aliaga, J.I.; Quintana-Orti, E.S.; Chacon, P. iMODS: Internal coordinates normal mode analysis server. Nucleic Acids Res. 2014, 42, W271–W276. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, Y. Protein-Ligand Blind Docking Using CB-Dock2. Methods Mol. Biol. 2024, 2714, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Hutchinson, E.G.; Michie, A.D.; Wallace, A.C.; Jones, M.L.; Thornton, J.M. PDBsum: A Web-based database of summaries and analyses of all PDB structures. Trends Biochem. Sci. 1997, 22, 488–490. [Google Scholar] [CrossRef]
- Xue, L.C.; Rodrigues, J.P.; Kastritis, P.L.; Bonvin, A.M.; Vangone, A. PRODIGY: A web server for predicting the binding affinity of protein-protein complexes. Bioinformatics 2016, 32, 3676–3678. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; de Groot, B.L.; Grubmuller, H.; MacKerell, A.D., Jr. CHARMM36m: An improved force field for folded and intrinsically disordered proteins. Nat. Methods 2017, 14, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Tresanco, M.S.; Valdes-Tresanco, M.E.; Valiente, P.A.; Moreno, E. gmx_MMPBSA: A New Tool to Perform End-State Free Energy Calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef]
- Guarnera, E.; Tan, Z.W.; Zheng, Z.; Berezovsky, I.N. AlloSigMA: Allosteric signaling and mutation analysis server. Bioinformatics 2017, 33, 3996–3998. [Google Scholar] [CrossRef]
- Lill, M.A.; Danielson, M.L. Computer-aided drug design platform using PyMOL. J. Comput. Aided Mol. Des. 2011, 25, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, G.; Armenia, I.; Molla, G. The Protein Imager: A full-featured online molecular viewer interface with server-side HQ-rendering capabilities. Bioinformatics 2020, 36, 2909–2911. [Google Scholar] [CrossRef]
- Luo, K.; Guo, J.; He, D.; Li, G.; Ouellet, T. Deoxynivalenol accumulation and detoxification in cereals and its potential role in wheat-Fusarium graminearum interactions. aBIOTECH 2023, 4, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Jurrus, E.; Engel, D.; Star, K.; Monson, K.; Brandi, J.; Felberg, L.E.; Brookes, D.H.; Wilson, L.; Chen, J.; Liles, K.; et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 2018, 27, 112–128. [Google Scholar] [CrossRef]
- Zhu, Y.; Chan, E.T.S.; Abraham, N.; Li, X.Z.; Wang, W.; Mats, L.; Zhu, H.; Carere, J.; Zhou, T. Unveiling the Broad Substrate Specificity of Deoxynivalenol Oxidation Enzyme DepA and Its Role in Detoxifying Trichothecene Mycotoxins. Toxins 2024, 16, 136. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, R.; Li, Y.; Lu, Z.; Bie, X.; Zhao, H.; Lu, F.; Chen, M. Structure-Function Analysis of a Quinone-Dependent Dehydrogenase Capable of Deoxynivalenol Detoxification. J. Agric. Food Chem. 2022, 70, 6764–6774. [Google Scholar] [CrossRef]
- Guo, C.; Wen, J.; Sun, Y.; Liang, G.; Wang, Z.; Pan, L.; Huang, J.; Liao, Y.; Wang, Z.; Chen, Q.; et al. Pyrroloquinoline quinone production defines the ability of Devosia species to degrade deoxynivalenol. Food Funct. 2024, 15, 6134–6146. [Google Scholar] [CrossRef]
- He, W.J.; Shi, M.M.; Yang, P.; Huang, T.; Zhao, Y.; Wu, A.B.; Dong, W.B.; Li, H.P.; Zhang, J.B.; Liao, Y.C. A quinone-dependent dehydrogenase and two NADPH-dependent aldo/keto reductases detoxify deoxynivalenol in wheat via epimerization in a Devosia strain. Food Chem. 2020, 321, 126703. [Google Scholar] [CrossRef]
- Deswal, R.; Chakaravarty, T.N.; Sopory, S.K. The glyoxalase system in higher plants: Regulation in growth and differentiation. Biochem. Soc. Trans. 1993, 21, 527–530. [Google Scholar] [CrossRef]
- Huang, J.Q.; Fang, X.; Tian, X.; Chen, P.; Lin, J.L.; Guo, X.X.; Li, J.X.; Fan, Z.; Song, W.M.; Chen, F.Y.; et al. Aromatization of natural products by a specialized detoxification enzyme. Nat. Chem. Biol. 2020, 16, 250–256. [Google Scholar] [CrossRef]
- Gullner, G.; Komives, T.; Kiraly, L.; Schroder, P. Glutathione S-Transferase Enzymes in Plant-Pathogen Interactions. Front. Plant Sci. 2018, 9, 1836. [Google Scholar] [CrossRef]
- Mimma, A.A.; Akter, T.; Haque, M.A.; Bhuiyan, M.A.B.; Chowdhury, M.Z.H.; Sultana, S.; Islam, S.M.N. Effect of Metarhizium anisopliae (MetA1) on growth enhancement and antioxidative defense mechanism against Rhizoctonia root rot in okra. Heliyon 2023, 9, e18978. [Google Scholar] [CrossRef]
- Goral, T.; Wisniewska, H.; Ochodzki, P.; Walentyn-Gorall, D.; Grzeszczak, I.; Beletr, J.; Banaszak, Z.; Pojmaj, M.; Kurleto, D.; Konieczny, M.; et al. Performance of winter triticale lines under high disease (Fusarium head blight) pressure. Commun. Agric. Appl. Biol. Sci. 2014, 79, 122–127. [Google Scholar]
- Mehrabi, P.; Di Pietrantonio, C.; Kim, T.H.; Sljoka, A.; Taverner, K.; Ing, C.; Kruglyak, N.; Pomes, R.; Pai, E.F.; Prosser, R.S. Substrate-Based Allosteric Regulation of a Homodimeric Enzyme. J. Am. Chem. Soc. 2019, 141, 11540–11556. [Google Scholar] [CrossRef]
- Hegazy, U.M.; Musdal, Y.; Mannervik, B. Hidden allostery in human glutathione transferase p1-1 unveiled by unnatural amino acid substitutions and inhibition studies. J. Mol. Biol. 2013, 425, 1509–1514. [Google Scholar] [CrossRef]
- Gong, H.; Jiao, Y.; Hu, W.W.; Pua, E.C. Expression of glutathione-S-transferase and its role in plant growth and development in vivo and shoot morphogenesis in vitro. Plant Mol. Biol. 2005, 57, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Nianiou-Obeidat, I.; Madesis, P.; Kissoudis, C.; Voulgari, G.; Chronopoulou, E.; Tsaftaris, A.; Labrou, N.E. Plant glutathione transferase-mediated stress tolerance: Functions and biotechnological applications. Plant Cell Rep. 2017, 36, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Sirota, F.L.; Maurer-Stroh, S.; Li, Z.; Eisenhaber, F.; Eisenhaber, B. Functional Classification of Super-Large Families of Enzymes Based on Substrate Binding Pocket Residues for Biocatalysis and Enzyme Engineering Applications. Front. Bioeng. Biotechnol. 2021, 9, 701120. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, Y.; Ji, F.; Xu, L.; Yu, M.; Shi, J.; Xu, J. Biodegradation of deoxynivalenol and its derivatives by Devosia insulae A16. Food Chem. 2019, 276, 436–442. [Google Scholar] [CrossRef]
- He, J.W.; Bondy, G.S.; Zhou, T.; Caldwell, D.; Boland, G.J.; Scott, P.M. Toxicology of 3-epi-deoxynivalenol, a deoxynivalenol-transformation product by Devosia mutans 17-2-E-8. Food Chem. Toxicol. 2015, 84, 250–259. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Man, H.; Lee, Y.W.; Shi, J.; Xu, J. Metabolomics-guided analysis reveals a two-step epimerization of deoxynivalenol catalyzed by the bacterial consortium IFSN-C1. Appl. Microbiol. Biotechnol. 2020, 104, 6045–6056. [Google Scholar] [CrossRef]
- Sato, I.; Ito, M.; Ishizaka, M.; Ikunaga, Y.; Sato, Y.; Yoshida, S.; Koitabashi, M.; Tsushima, S. Thirteen novel deoxynivalenol-degrading bacteria are classified within two genera with distinct degradation mechanisms. FEMS Microbiol. Lett. 2012, 327, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J. Glyoxalase I--structure, function and a critical role in the enzymatic defence against glycation. Biochem. Soc. Trans. 2003, 31, 1343–1348. [Google Scholar] [CrossRef]
- Turra, G.L.; Agostini, R.B.; Fauguel, C.M.; Presello, D.A.; Andreo, C.S.; Gonzalez, J.M.; Campos-Bermudez, V.A. Structure of the novel monomeric glyoxalase I from Zea mays. Acta Crystallogr. D Biol. Crystallogr. 2015, 71, 2009–2020. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, K.K.; Gupta, B.K.; Kaur, C.; Joshi, R.; Pareek, A.; Sopory, S.K.; Singla-Pareek, S.L. Methylglyoxal-glyoxalase system as a possible selection module for raising marker-safe plants in rice. Physiol. Mol. Biol. Plants 2021, 27, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- Zambounis, A.; Ganopoulos, I.; Kalivas, A.; Tsaftaris, A.; Madesis, P. Identification and evidence of positive selection upon resistance gene analogs in cotton (Gossypium hirsutum L.). Physiol. Mol. Biol. Plants 2016, 22, 415–421. [Google Scholar] [CrossRef]
- Ryan, A.; Wang, C.J.; Laurieri, N.; Westwood, I.; Sim, E. Reaction mechanism of azoreductases suggests convergent evolution with quinone oxidoreductases. Protein Cell 2010, 1, 780–790. [Google Scholar] [CrossRef]
- Luo, F.; Zhou, Q.; Chen, F.; Liu, X.; Chiu, T.Y.; Zhu, G.Y.; Huang, A.C. Divergent multifunctional P450s-empowered biosynthesis of bioactive tripterifordin and cryptic atiserenoids in Aconitum implies convergent evolution. Nat. Commun. 2025, 16, 5857. [Google Scholar] [CrossRef]
- Richards, T.A.; Soanes, D.M.; Jones, M.D.; Vasieva, O.; Leonard, G.; Paszkiewicz, K.; Foster, P.G.; Hall, N.; Talbot, N.J. Horizontal gene transfer facilitated the evolution of plant parasitic mechanisms in the oomycetes. Proc. Natl. Acad. Sci. USA 2011, 108, 15258–15263. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enguita, F.J.; Leitão, A.L. Cross-Kingdom Enzymatic Strategies for Deoxynivalenol Detoxification: Computational Analysis of Structural Mechanisms and Evolutionary Adaptations. Microorganisms 2025, 13, 2384. https://doi.org/10.3390/microorganisms13102384
Enguita FJ, Leitão AL. Cross-Kingdom Enzymatic Strategies for Deoxynivalenol Detoxification: Computational Analysis of Structural Mechanisms and Evolutionary Adaptations. Microorganisms. 2025; 13(10):2384. https://doi.org/10.3390/microorganisms13102384
Chicago/Turabian StyleEnguita, Francisco J., and Ana Lúcia Leitão. 2025. "Cross-Kingdom Enzymatic Strategies for Deoxynivalenol Detoxification: Computational Analysis of Structural Mechanisms and Evolutionary Adaptations" Microorganisms 13, no. 10: 2384. https://doi.org/10.3390/microorganisms13102384
APA StyleEnguita, F. J., & Leitão, A. L. (2025). Cross-Kingdom Enzymatic Strategies for Deoxynivalenol Detoxification: Computational Analysis of Structural Mechanisms and Evolutionary Adaptations. Microorganisms, 13(10), 2384. https://doi.org/10.3390/microorganisms13102384







