Major Foodborne Bacterial Pathogens in Poultry: Implications for Human Health and the Poultry Industry and Probiotic Mitigation Strategies
Abstract
1. Introduction
2. Major Food-Borne Pathogens in Poultry and Probiotic Mitigation Strategies
2.1. Salmonella
2.1.1. Salmonella Virulence, Pathogenicity and Mode of Transmission
2.1.2. Probiotic Efficacy Against Salmonellosis in Poultry
2.2. Campylobacter
2.2.1. Campylobacter Virulence, Pathogenicity and Mode of Transmission
2.2.2. Probiotic Efficacy Against Campylobacteriosis in Poultry
| Breed | Strain of Probiotics | Delivery | Campylobacter Strain/Dose | Effect on Campylobacter Colonization | References |
|---|---|---|---|---|---|
| Broiler | Single strain | ||||
| E. faecium NCIMB 11508 | First-day post-hatch and day 28 orally | Naturally infected | There is no reduction in the relative abundance of Campylobacter | [191] | |
| Calsporin® (B. subtilis C-3102) | Day 1–42 in feed | Fecal contamination during processing | 0.2 log10 reduction on chicken carcasses | [185] | |
| Bacillus spp. (10 isolates individually tested) | Per os and intracloacally at one day old | C. jejuni cocktail of 4 strains (2.5 × 106 CFU) | Intracloacally: 1–3 log10 Orally: 1 log10 for only one isolate | [186] | |
| L. salivarius SMXD51 | Given orally on day one and then every two to three days for 35 days | C. jejuni C97ANSES640 (1 × 104 CFU) | 0.8 log10 at 14 days and 2.81 log10 at day 35. | [178] | |
| L. plantarum PA18A | Orally, on days 1 and 4 | C. jejuni strain 12/2 (1 × 104 CFU) | 1 log10 reduction | [181] | |
| E. faecalis MB 5259 | Day 1–21 orally | C. jejuni MB 4185 (KC 40) (2 × 104 CFU) | 0.4 log10 in only one of the groups received 104 CFU E. faecalis No reduction in the chickens received 108 CFU E. faecali | [122] | |
| E. coli Nissle 1917 (free and chitosan micro-encapsulated) | Daily or three times per week supplementation in drinking water at weeks 4 and 5 of age | Cocktail of six C. jejuni strains/orally/(1 × 105 CFU) | Up to 2.6 log10 at the end of the experiment at | [196] | |
| B. amyloliquefaciens-loaded nanoparticles (BNPs) | Per os from 1–35 post-hatch with three different doses of BNPs: I (2.5 × 105 CFU/g), BNPs II (5 × 105 CFU/g), and BNPs III 7.5× (CFU/g) of feed | Crop gavage with pandrug-resistant (PDR) and multi-virulent field C. jejuni 108 CFU/mL at 30 days old | BNPs III inclusion showed significant fecal and cecal reduction at 7 days post-infection (3.86 log10, 3.94 log10, respectively) | [188] | |
| B. subtilis PS-216 spores | 2.5 × 106 CFU/mL in drinking water 1–20 d | 8 d, all of the broilers were inoculated with 4 × 106 CFU C. jejuni 11,168 by oral gavage | 1.2 log10 CFU/g feces in the C. jejuni counts | [187] | |
| L. plantarum 256 | (107 CFU/mL) in drinking water for 6 and 9 weeks L. plantarum strain 256 during baling, providing an inoculum concentration of 108 CFU per gram of fresh matter | 106 CFU/mL of the C. jejuni strain 65 at day 22 for the (6 weeks exp) and at 29 days for the (9 weeks exp) | No significant reduction at the end of the experiments at 42 and 63 days. | [197] | |
| Mixed strains | |||||
| K-bacteria + competitive exclusion Broilact® | Day 1–38 in drinking water | C. jejuni T23/42 (1.3 × 104 CFU) | Up to 2 log10 | [140] | |
| Citrobacter diversus 22 + K. pneumonia 23 + E. coli 25 + mannose | Days 1 and 3 | C. jejuni orally (108 CFU) | Up to 70% reduction | [198] | |
| Avian Pac Soluble (L. acidophilus + Streptococcus faecium) | Day 1–3 in drinking water | C. jejuni C101 (2.7 × 104 CFU) | Two-thirds reduction in C. jejuni shedding | [199] | |
| PrimaLac (L. acidophilus + L. casei + B. thermophilus + E. faecium) | Day 1–42 in feed | Naturally infected | 12% reduction of C. jejuni presence | [193] | |
| B. longum PCB 133 | Day 1–15 intraesophageally | Naturally infected | 1 log10 reduction | [200] | |
| Microencapsulated B. longum PCB133 + oligosaccharides | Day 1–14 in feed | Naturally infected | Up to 1.4 log10 | [201] | |
| PoultryStar sol® (E. faecium + P. acidilactici + B. animalis + L. salivarius + L. reuteri) | Day 1–15 in drinking water | C. jejuni 3015/2010 (104 CFU) | 6 log10 | [184] | |
| L. acidophilus NCFM or L. crispatus JCM5810 or L.s gallinarum ATCC or L. helveticus CNRZ32 | Day 1 and 4 orally | C. jejuni F38011 (108 CFU) | ~2 log10 reduction | [202] | |
| L. gasseri SBT2055 LG2055 WTCM, Dapf1 and Dapf2 mutant strains | Day 2–14 orally Dapf1: No reduction | C. jejuni 81–176 (106 CFU) | WTCM and Dapf2: Up to 270-fold reduction | [182] | |
| Bacillus spp.+ L. salivarius subsp. salivarius + L. salivarius subsp. salicinius | Day 1 orally | C. jejuni cocktail of 4 strains (2.5 × 106 CFU) | 1–2 log10 in only one of the three trials | [203] | |
| L. paracasei J.R + L. rhamnosus 15b + L. lactis Y + L. lactis FOa | Day 1–42 in drinking water | Naturally infected | Up to 5 log10 | [204] | |
| Calsporin® (B. subtilis C-3102) Ecobiol® (B. amyloliquefaciens CECT 5940) | Day 1 and 42 in feed | C. jejuni C97ANSES640 (104 CFU) | Calsporin®: 0.25 log10 reduction on day 14 and 1.7 log10 on day 42 Ecobiol®: 1.12 log10 on day 35 and 1.2 log10 on day 42 | [189] | |
| B. subtilis DSM 17299 or S. cerevisiae boulardii | Day 21–42 in feed | C. jejuni ST45 (104 CFU) | B. subtilis: No reduction S. cerevisiae: Up to 0.3 log10 reduction | [190] | |
| Lavipan (multispecies probiotic): L. lactis IBB 500, Carnobacterium divergens S-1, L. casei OCK 0915, L0915, L. plantarum OCK 0862, and S. cerevisiae OCK 0141 | Day 1–37 in feed | Naturally infected | <1 log10 | [122] | |
| Layers | Citrobacter diversus, K. pneumoniae, and E. coli | Reduced C. jejuni load in ceca | [198] | ||
| Cecal culture | Reduced C. jejuni load in ceca | [205] | |||
| E. faecium EM41 | Orally in drinking water, 109 CFU/mL were received for 21 days | Natural infection | 0.8 log10 reduction at 21 days of starting administration and 0.25 log10 reduction at day 35 (2 weeks after cessation of the additive) | [192] | |
| Enterocin EM41 (Ent EM41) | Enterocin (Ent) EM41 (40 μL/animal/day, 25,600 AU/mL). | Natural infection | 1.95 log10 reduction at 21 days of starting administration and 0.75 log10 reduction on day 35 (2 weeks after cessation of the additive) | [192] | |
| Duck | L. salivarius | Orally in feed 2 × 108 CFU/g for 79 days | Natural infection | No reduction | [179] |
| Turkeys | Bacteriocin of P. polymyxa and L. salivarius | Three successive days on 10–12 post-hatch | Orally, 106 CFU of a mixture of 3 Campylobacter coli isolates. | 4 log10 reduction in Campylobacter concentrations | [180] |
2.3. Clostridium Perfingens
2.3.1. C. perfringens Virulence, Pathogenicity and Mode of Transmission
2.3.2. Probiotic Efficacy Against C. perfringens
| Probiotic Strain(s) | Administration | Concentration | Main Outcomes | References |
|---|---|---|---|---|
| B. amyloliquefaciens | Feed additive | 106 CFU/g feed | 0.8 log10 reduction in cecal C. perfringens counts. Improved FCR and BWG | [218] |
| B. coagulans | Feed additive | 4 × 109 CFU/kg feed | 0.84, 1.46, and 1.79-log10 reductions in C. perfringens cecal counts at days 28, 35, and 42, respectively. Decreased lesion scores and reduced crypt depths in the small intestine | [217] |
| B. subtilis | Feed additive | 2 × 108 CFU/g feed | 0.98 log10 reduction in cecal C. perfringens counts. Improved FCR and BWG | [216] |
| B. subtilis | In vitro | 108 CFU/mL | 6 log10 C. perfringens reduction alone. Efficacy declined when combined with other Bacillus strains | [221] |
| E. faecium, B. animalis, and L. salivarius mix | Feed additive | 2 × 108 CFU/g feed | 0.88 log10 C. perfringens count reduction. Enhanced BWG and FCR | [216] |
| B. subtilis and B. licheniformis mix | Feed additive | 2.5 × 1012 CFU/kg feed | 2.45 log10 C. perfringens reduction. Reduced mortality and lesion scores | [219] |
| E. faecium, B. animalis, P. acidilactici and L. reuteri mix | Water additive | 1 × 109 CFU/mL | 2.91 log10 C. perfringens reduction. Reduced lesion scores and mortalities. | [220] |
2.4. Escherichia coli
2.4.1. E. coli Virulence, Pathogenicity and Mode of Transmission
2.4.2. Probiotic Efficacy Against E. coli
2.5. Listeria Monocytogenes
2.5.1. Listeria Virulence, Pathogenicity and Mode of Transmission
2.5.2. Probiotic Efficacy Against Listeriosis
3. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- United States Department of Agriculture. Poultry—Production and Value; United States Department of Agriculture: Washington, DC, USA, 2025. [Google Scholar]
- United States Department of Agriculture, Foreign Agricultural Service. Production—Chicken Meat. USDA FAS: Washington, DC, USA. Available online: https://www.fas.usda.gov/data/production/commodity/0115000 (accessed on 21 August 2025).
- Kulkarni, R.R.; Taha-Abdelaziz, K.; Shojadoost, B.; Astill, J.; Sharif, S. Gastrointestinal Diseases of Poultry: Causes and Nutritional Strategies for Prevention and Control. In Improving Gut Health in Poultry; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 205–236. [Google Scholar]
- Taha-Abdelaziz, K.; Singh, M.; Sharif, S.; Sharma, S.; Kulkarni, R.R.; Alizadeh, M.; Yitbarek, A.; Helmy, Y.A. Intervention Strategies to Control Campylobacter at Different Stages of the Food Chain. Microorganisms 2023, 11, 113. [Google Scholar] [CrossRef]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef]
- Galán-Relaño, Á.; Valero Díaz, A.; Huerta Lorenzo, B.; Gómez-Gascón, L.; Mena Rodríguez, M.Á.; Carrasco Jiménez, E.; Pérez Rodríguez, F.; Astorga Márquez, R.J. Salmonella and Salmonellosis: An Update on Public Health Implications and Control Strategies. Animals 2023, 13, 3666. [Google Scholar] [CrossRef]
- St Louis, M.E.; Morse, D.L.; Potter, M.E.; DeMelfi, T.M.; Guzewich, J.J.; Tauxe, R.V.; Blake, P.A. The Emergence of Grade A Eggs as a Major Source of Salmonella Enteritidis Infections. New Implications for the Control of Salmonellosis. JAMA 1988, 259, 2103–2107. [Google Scholar] [CrossRef]
- Rothrock, M.J.; Davis, M.L.; Locatelli, A.; Bodie, A.; McIntosh, T.G.; Donaldson, J.R.; Ricke, S.C. Listeria Occurrence in Poultry Flocks: Detection and Potential Implications. Front. Vet. Sci. 2017, 4, 125. [Google Scholar] [CrossRef]
- Jesudhasan, P.R.; Bhatia, S.S.; Sivakumar, K.K.; Praveen, C.; Genovese, K.J.; He, H.L.; Droleskey, R.; McReynolds, J.L.; Byrd, J.A.; Swaggerty, C.L.; et al. Controlling the Colonization of Clostridium Perfringens in Broiler Chickens by an Electron-Beam-Killed Vaccine. Animals 2021, 11, 671. [Google Scholar] [CrossRef]
- Joseph, J.; Zhang, L.; Adhikari, P.; Evans, J.D.; Ramachandran, R. Avian Pathogenic Escherichia coli (APEC) in Broiler Breeders: An Overview. Pathogens 2023, 12, 1280. [Google Scholar] [CrossRef]
- Hoffmann, S.; White, A.E.; McQueen, R.B.; Ahn, J.-W.; Gunn-Sandell, L.B.; Scallan Walter, E.J. Economic Burden of Foodborne Illnesses Acquired in the United States. Foodborne Pathog. Dis. 2025, 22, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Ba, X.; Jin, Y.; Ning, X.; Gao, Y.; Li, W.; Li, Y.; Wang, Y.; Zhou, J. Clostridium Perfringens in the Intestine: Innocent Bystander or Serious Threat? Microorganisms 2024, 12, 1610. [Google Scholar] [CrossRef] [PubMed]
- Grace, D.; Gilbert, J.; Randolph, T.; Kang’ethe, E. The Multiple Burdens of Zoonotic Disease and an Ecohealth Approach to Their Assessment. Trop. Anim. Health Prod. 2012, 44, 67–73. [Google Scholar] [CrossRef]
- Shamshirgaran, M.A.; Golchin, M. A Comprehensive Review of Experimental Models and Induction Protocols for Avian Necrotic Enteritis over the Past 2 Decades. Front. Vet. Sci. 2024, 11, 1429637. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Taha-Abdelaziz, K.; Hawwas, H.A.E.-H.; Ghosh, S.; AlKafaas, S.S.; Moawad, M.M.M.; Saied, E.M.; Kassem, I.I.; Mawad, A.M.M. Antimicrobial Resistance and Recent Alternatives to Antibiotics for the Control of Bacterial Pathogens with an Emphasis on Foodborne Pathogens. Antibiotics 2023, 12, 274. [Google Scholar] [CrossRef]
- Lima, É.; Oliveira, M.B.; Freitas, A. Antibiotics in Intensive Egg Production: Food Safety Tools to Ensure Regulatory Compliance. Food Chem. Adv. 2023, 3, 100548. [Google Scholar] [CrossRef]
- Kulkarni, R.R.; Gaghan, C.; Gorrell, K.; Sharif, S.; Taha-Abdelaziz, K. Probiotics as Alternatives to Antibiotics for the Prevention and Control of Necrotic Enteritis in Chickens. Pathogens 2022, 11, 692. [Google Scholar] [CrossRef] [PubMed]
- Taha-Abdelaziz, K.; Hodgins, D.C.; Lammers, A.; Alkie, T.N.; Sharif, S. Effects of Early Feeding and Dietary Interventions on Development of Lymphoid Organs and Immune Competence in Neonatal Chickens: A Review. Vet. Immunol. Immunopathol. 2018, 201, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kulkarni, R.R.; Sharif, S.; Hassan, H.; Alizadeh, M.; Pratt, S.; Abdelaziz, K. In Ovo Feeding of Probiotic Lactobacilli Differentially Alters Expression of Genes Involved in the Development and Immunological Maturation of Bursa of Fabricius in Pre-Hatched Chicks. Poult. Sci. 2024, 103, 103237. [Google Scholar] [CrossRef]
- Alkie, T.N.; Yitbarek, A.; Hodgins, D.C.; Kulkarni, R.R.; Taha-Abdelaziz, K.; Sharif, S. Development of Innate Immunity in Chicken Embryos and Newly Hatched Chicks: A Disease Control Perspective. Avian Pathol. 2019, 48, 288–310. [Google Scholar] [CrossRef]
- Alizadeh, M.; Shojadoost, B.; Astill, J.; Taha-Abdelaziz, K.; Karimi, S.H.; Bavananthasivam, J.; Kulkarni, R.R.; Sharif, S. Effects of in Ovo Inoculation of Multi-Strain Lactobacilli on Cytokine Gene Expression and Antibody-Mediated Immune Responses in Chickens. Front. Vet. Sci. 2020, 7, 105. [Google Scholar] [CrossRef]
- Alizadeh, M.; Astill, J.; Alqazlan, N.; Shojadoost, B.; Taha-Abdelaziz, K.; Bavananthasivam, J.; Doost, J.S.; Sedeghiisfahani, N.; Sharif, S. In Ovo Co-Administration of Vitamins (A and D) and Probiotic Lactobacilli Modulates Immune Responses in Broiler Chickens. Poult. Sci. 2022, 101, 101717. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Alizadeh, M.; Bavananthasivam, J.; Shojadoost, B.; Astill, J.; Taha-Abdelaziz, K.; Alqazlan, N.; Boodhoo, N.; Shoja Doost, J.; Sharif, S. In Ovo and Oral Administration of Probiotic Lactobacilli Modulate Cell- and Antibody-Mediated Immune Responses in Newly Hatched Chicks. Front. Immunol. 2021, 12, 664387. [Google Scholar] [CrossRef] [PubMed]
- Shojadoost, B.; Alizadeh, M.; Boodhoo, N.; Astill, J.; Karimi, S.H.; Shoja Doost, J.; Taha-Abdelaziz, K.; Kulkarni, R.; Sharif, S. Effects of Treatment with Lactobacilli on Necrotic Enteritis in Broiler Chickens. Probiotics Antimicrob. Proteins 2022, 14, 1110–1129. [Google Scholar] [CrossRef]
- Hardy, H.; Harris, J.; Lyon, E.; Beal, J.; Foey, A. Probiotics, Prebiotics and Immunomodulation of Gut Mucosal Defences: Homeostasis and Immunopathology. Nutrients 2013, 5, 1869–1912. [Google Scholar] [CrossRef]
- Sleator, R.D.; Hill, C. New Frontiers in Probiotic Research. Lett. Appl. Microbiol. 2007, 46, 143–147. [Google Scholar] [CrossRef]
- Kabir, S.M.L. The Role of Probiotics in the Poultry Industry. Int. J. Mol. Sci. 2009, 10, 3531–3546. [Google Scholar] [CrossRef]
- Maurer, J.J.; Cheng, Y.; Pedroso, A.; Thompson, K.K.; Akter, S.; Kwan, T.; Morota, G.; Kinstler, S.; Porwollik, S.; McClelland, M.; et al. Peeling Back the Many Layers of Competitive Exclusion. Front. Microbiol. 2024, 15, 1342887. [Google Scholar] [CrossRef]
- Sanders, M.E.; Akkermans, L.M.A.; Haller, D.; Hammerman, C.; Heimbach, J.T.; Hörmannsperger, G.; Huys, G. Safety Assessment of Probiotics for Human Use. Gut Microbes 2010, 1, 164–185. [Google Scholar] [CrossRef]
- Patterson, J.; Burkholder, K. Application of Prebiotics and Probiotics in Poultry Production. Poult. Sci. 2003, 82, 627–631. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: Ottawa, ON, Canada, 2002. [Google Scholar]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M. The Global Burden of Nontyphoidal Salmonella Gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Shaji, S.; Selvaraj, R.K.; Shanmugasundaram, R. Salmonella Infection in Poultry: A Review on the Pathogen and Control Strategies. Microorganisms 2023, 11, 2814. [Google Scholar] [CrossRef]
- World Health Organisation. Salmonella (Non-Typhoidal). Available online: https://www.who.int/news-room/fact-sheets/detail/salmonella-(non-typhoidal) (accessed on 21 August 2025).
- Institute for Health Metrics and Evaluation (IHME). Global Burden of Disease (GBD). Available online: https://www.healthdata.org/research-analysis/gbd (accessed on 21 August 2025).
- Painter, J.A.; Hoekstra, R.M.; Ayers, T.; Tauxe, R.V.; Braden, C.R.; Angulo, F.J.; Griffin, P.M. Attribution of Foodborne Illnesses, Hospitalizations, and Deaths to Food Commodities by Using Outbreak Data, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Acheson, D.; Hohmann, E.L. Nontyphoidal Salmonellosis. Clin. Infect. Dis. 2001, 32, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Reeves, M.W.; Evins, G.M.; Heiba, A.A.; Plikaytis, B.D.; Farmer, J.J. Clonal Nature of Salmonella Typhi and Its Genetic Relatedness to Other Salmonellae as Shown by Multilocus Enzyme Electrophoresis, and Proposal of Salmonella Bongori Comb. Nov. J. Clin. Microbiol. 1989, 27, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Popoff, M.Y.; Le Minor, L.E. Salmonella. In Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2015; p. 1. [Google Scholar]
- El-Sharkawy, H.; Tahoun, A.; El-Gohary, A.E.-G.A.; El-Abasy, M.; El-Khayat, F.; Gillespie, T.; Kitade, Y.; Hafez, H.M.; Neubauer, H.; El-Adawy, H. Epidemiological, Molecular Characterization and Antibiotic Resistance of Salmonella enterica Serovars Isolated from Chicken Farms in Egypt. Gut Pathog. 2017, 9, 8. [Google Scholar] [CrossRef]
- Dione, M.M.; Ikumapayi, U.N.; Saha, D.; Mohammed, N.I.; Geerts, S.; Ieven, M.; Adegbola, R.A.; Antonio, M. Clonal Differences between Non-Typhoidal Salmonella (NTS) Recovered from Children and Animals Living in Close Contact in The Gambia. PLoS Negl. Trop. Dis. 2011, 5, e1148. [Google Scholar] [CrossRef]
- Brenner, F.W.; Villar, R.G.; Angulo, F.J.; Tauxe, R.; Swaminathan, B. Salmonella Nomenclature. J. Clin. Microbiol. 2000, 38, 2465–2467. [Google Scholar] [CrossRef]
- Center for Disease Control Salmonella Infection. Available online: https://www.cdc.gov/salmonella/index.html (accessed on 21 August 2025).
- Singh, V. Salmonella Serovars and Their Host Specificity. J. Vet. Sci. Anim. Husb. 2013, 1, 301. [Google Scholar] [CrossRef]
- Foley, S.L.; Johnson, T.J.; Ricke, S.C.; Nayak, R.; Danzeisen, J. Salmonella Pathogenicity and Host Adaptation in Chicken-Associated Serovars. Microbiol. Mol. Biol. Rev. 2013, 77, 582–607. [Google Scholar] [CrossRef]
- Deblais, L.; Lorentz, B.; Scaria, J.; Nagaraja, K.V.; Nisar, M.; Lauer, D.; Voss, S.; Rajashekara, G. Comparative Genomic Studies of Salmonella Heidelberg Isolated From Chicken- and Turkey-Associated Farm Environmental Samples. Front. Microbiol. 2018, 9, 1841. [Google Scholar] [CrossRef]
- Foley, S.L.; Lynne, A.M.; Nayak, R. Salmonella Challenges: Prevalence in Swine and Poultry and Potential Pathogenicity of Such Isolates1,2. J. Anim. Sci. 2008, 86, E149–E162. [Google Scholar] [CrossRef]
- Hassanein, R.; Ali, S.; ElMalek, A.; Moemen, M.; Elsayh, A. Detection and Identification of Salmonella Species in Minced Beef and Chicken Meats by Using Multiplex PCR in Assiut City. Vet. World 2011, 4, 5–11. [Google Scholar] [CrossRef]
- Barrow, P.A.; Freitas Neto, O.C. Pullorum disease and fowl typhoid—new thoughts on old diseases: A review. Avian Pathol. 2011, 40, 1–13. [Google Scholar] [CrossRef]
- Baumler, A.; Fang, F.C. Host Specificity of Bacterial Pathogens. Cold Spring Harb. Perspect. Med. 2013, 3, a010041. [Google Scholar] [CrossRef] [PubMed]
- Uzzau, S.; Leori, G.S.; Petruzzi, V.; Watson, P.R.; Schianchi, G.; Bacciu, D.; Mazzarello, V.; Wallis, T.S.; Rubino, S. Salmonella enterica Serovar-Host Specificity Does Not Correlate with the Magnitude of Intestinal Invasion in Sheep. Infect. Immun. 2001, 69, 3092–3099. [Google Scholar] [CrossRef]
- Ellermeier, J.R.; Slauch, J.M. Adaptation to the Host Environment: Regulation of the SPI1 Type III Secretion System in Salmonella enterica Serovar Typhimurium. Curr. Opin. Microbiol. 2007, 10, 24–29. [Google Scholar] [CrossRef]
- Tariq, S.; Samad, A.; Hamza, M.; Ahmer, A.; Muazzam, A.; Ahmad, S.; Amhabj, A.M.A. Salmonella in Poultry; An Overview. Int. J. Multidiscip. Sci. Arts 2022, 1, 80–84. [Google Scholar] [CrossRef]
- Kopanic, R.J.; Sheldon, B.W.; Wright, C.G. Cockroaches As Vectors of Salmonella: Laboratory and Field Trials. J. Food Prot. 1994, 57, 125–131. [Google Scholar] [CrossRef]
- Sparagano, O. Control of Poultry Mites: Where Do We Stand? Exp. Appl. Acarol. 2009, 48, 1–2. [Google Scholar] [CrossRef]
- Leffer, A.M.; Kuttel, J.; Martins, L.M.; Pedroso, A.C.; Astolfi-Ferreira, C.S.; Ferreira, F.; Ferreira, A.J.P. Vectorial Competence of Larvae and Adults of Alphitobius diaperinus in the Transmission of Salmonella Enteritidis in Poultry. Vector-Borne Zoonotic Dis. 2010, 10, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.A.; Shopland, S.; Wigley, P.; Bradon, H.; Leatherbarrow, A.H.; Williams, N.J.; Bennett, M.; de Pinna, E.; Lawson, B.; Cunningham, A.A.; et al. Characterisation of Salmonella enterica Serotype Typhimurium Isolates from Wild Birds in Northern England from 2005–2006. BMC Vet Res. 2008, 4, 4. [Google Scholar] [CrossRef]
- Alley, M.; Connolly, J.; Fenwick, S.; Mackereth, G.; Leyland, M.; Rogers, L.; Haycock, M.; Nicol, C.; Reed, C. An Epidemic of Salmonellosis Caused by Salmonella Typhimurium DT160 in Wild Birds and Humans in New Zealand. N. Z. Vet. J. 2002, 50, 170–176. [Google Scholar] [CrossRef]
- Sousa, E.; Werther, K.; Berchieri Júnior, A. Assessment of Newcastle and Infectious Bronchitis Pathogens, and Salmonella Spp. in Wild Birds Captured near Poultry Facilities. Arq. Bras. Med. Vet. Zootec. 2010, 62, 219–223. [Google Scholar] [CrossRef]
- De Reu, K.; Grijspeerdt, K.; Messens, W.; Heyndrickx, M.; Uyttendaele, M.; Debevere, J.; Herman, L. Eggshell Factors Influencing Eggshell Penetration and Whole Egg Contamination by Different Bacteria, Including Salmonella Enteritidis. Int. J. Food Microbiol. 2006, 112, 253–260. [Google Scholar] [CrossRef]
- Sahin, O.; Morishita, T.Y.; Zhang, Q. Campylobacter Colonization in Poultry: Sources of Infection and Modes of Transmission. Anim. Health Res. Rev. 2002, 3, 95–105. [Google Scholar] [CrossRef]
- Jones, T.F.; Ingram, L.A.; Fullerton, K.E.; Marcus, R.; Anderson, B.J.; McCarthy, P.V.; Vugia, D.; Shiferaw, B.; Haubert, N.; Wedel, S.; et al. A Case-Control Study of the Epidemiology of Sporadic Salmonella Infection in Infants. Pediatrics 2006, 118, 2380–2387. [Google Scholar] [CrossRef] [PubMed]
- Eng, S.-K.; Pusparajah, P.; Ab Mutalib, N.-S.; Ser, H.-L.; Chan, K.-G.; Lee, L.-H. Salmonella: A Review on Pathogenesis, Epidemiology and Antibiotic Resistance. Front. Life Sci. 2015, 8, 284–293. [Google Scholar] [CrossRef]
- Woods, D.F.; Reen, F.J.; Gilroy, D.; Buckley, J.; Frye, J.G.; Boyd, E.F. Rapid Multiplex PCR and Real-Time TaqMan PCR Assays for Detection of Salmonella enterica and the Highly Virulent Serovars Choleraesuis and Paratyphi C. J. Clin. Microbiol. 2008, 46, 4018–4022. [Google Scholar] [CrossRef]
- McShan, A.C.; Kaur, K.; Chatterjee, S.; Knight, K.M.; De Guzman, R.N. NMR Identification of the Binding Surfaces Involved in the Salmonella and Shigella Type III Secretion Tip-Translocon Protein-Protein Interactions. Proteins Struct. Funct. Bioinform. 2016, 84, 1097–1107. [Google Scholar] [CrossRef]
- Kyrova, K.; Stepanova, H.; Rychlik, I.; Faldyna, M.; Volf, J. SPI-1 Encoded Genes of Salmonella Typhimurium Influence Differential Polarization of Porcine Alveolar Macrophages in Vitro. BMC Vet. Res. 2012, 8, 115. [Google Scholar] [CrossRef]
- Chaudhuri, D.; Roy Chowdhury, A.; Biswas, B.; Chakravortty, D. Salmonella Typhimurium Infection Leads to Colonization of the Mouse Brain and Is Not Completely Cured With Antibiotics. Front. Microbiol. 2018, 9, 1632. [Google Scholar] [CrossRef] [PubMed]
- Uchiya, K.; Barbieri, M.A.; Funato, K.; Shah, A.H.; Stahl, P.D.; Groisman, E.A. A Salmonella Virulence Protein That Inhibits Cellular Trafficking. EMBO J. 1999, 18, 3924–3933. [Google Scholar] [CrossRef]
- Kombade, S.; Kaur, N. Pathogenicity Island in Salmonella. In Salmonella spp.—A Global Challenge; IntechOpen: Bristol, UK, 2021. [Google Scholar]
- Dorsey, C.W.; Laarakker, M.C.; Humphries, A.D.; Weening, E.H.; Bäumler, A.J. Salmonella enterica Serotype Typhimurium MisL Is an Intestinal Colonization Factor That Binds Fibronectin. Mol. Microbiol. 2005, 57, 196–211. [Google Scholar] [CrossRef]
- Gerlach, R.G.; Cláudio, N.; Rohde, M.; Jäckel, D.; Wagner, C.; Hensel, M. Cooperation of Salmonella Pathogenicity Islands 1 and 4 Is Required to Breach Epithelial Barriers. Cell Microbiol. 2008, 10, 2364–2376. [Google Scholar] [CrossRef]
- Kiss, T.; Morgan, E.; Nagy, G. Contribution of SPI-4 Genes to the Virulence of Salmonella enterica. FEMS Microbiol. Lett. 2007, 275, 153–159. [Google Scholar] [CrossRef]
- Wood, M.W.; Jones, M.A.; Watson, P.R.; Hedges, S.; Wallis, T.S.; Galyov, E.E. Identification of a Pathogenicity Island Required for Salmonella Enteropathogenicity. Mol. Microbiol. 1998, 29, 883–891. [Google Scholar] [CrossRef]
- Knodler, L.A.; Celli, J.; Hardt, W.; Vallance, B.A.; Yip, C.; Finlay, B.B. Salmonella Effectors within a Single Pathogenicity Island Are Differentially Expressed and Translocated by Separate Type III Secretion Systems. Mol. Microbiol. 2002, 43, 1089–1103. [Google Scholar] [CrossRef] [PubMed]
- Sana, T.G.; Flaugnatti, N.; Lugo, K.A.; Lam, L.H.; Jacobson, A.; Baylot, V.; Durand, E.; Journet, L.; Cascales, E.; Monack, D.M. Salmonella Typhimurium Utilizes a T6SS-Mediated Antibacterial Weapon to Establish in the Host Gut. Proc. Natl. Acad. Sci. USA 2016, 113, E5044–E5051. [Google Scholar] [CrossRef]
- Sabbagh, S.C.; Forest, C.G.; Lepage, C.; Leclerc, J.-M.; Daigle, F. So Similar, yet so Different: Uncovering Distinctive Features in the Genomes of Salmonella enterica Serovars Typhimurium and Typhi. FEMS Microbiol. Lett. 2010, 305, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Asten, A.J.A.M.; Dijk, J.E. Distribution of ÂĨŖClassicâĨŗ; Virulence Factors among Salmonella Spp. FEMS Immunol. Med. Microbiol. 2005, 44, 251–259. [Google Scholar] [CrossRef]
- Ahsan, C.R.; Shamma, F.; Ahsan, N.; Islam, M.J. Environmental Factors Regulate the HlyE Gene Expression in Both S. Typhi and E. coli in a Similar Way to Display Haemolytic Activity. Bangladesh Med. Res. Counc. Bull. 2017, 42, 33–38. [Google Scholar] [CrossRef]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Sachan, S.; Karthik, K.; Dhama, K. The Use of Probiotics as Eco-Friendly Alternatives for Antibiotics in Poultry Nutrition. Environ. Sci. Pollut. Res. 2018, 25, 10611–10618. [Google Scholar] [CrossRef]
- Farhat, M.; Khayi, S.; Berrada, J.; Mouahid, M.; Ameur, N.; El-Adawy, H.; Fellahi, S. Salmonella enterica Serovar Gallinarum Biovars Pullorum and Gallinarum in Poultry: Review of Pathogenesis, Antibiotic Resistance, Diagnosis and Control in the Genomic Era. Antibiotics 2023, 13, 23. [Google Scholar] [CrossRef]
- Ruvalcaba-Gómez, J.M.; Villagrán, Z.; Valdez-Alarcón, J.J.; Martínez-Núñez, M.; Gomez-Godínez, L.J.; Ruesga-Gutiérrez, E.; Anaya-Esparza, L.M.; Arteaga-Garibay, R.I.; Villarruel-López, A. Non-Antibiotics Strategies to Control Salmonella Infection in Poultry. Animals 2022, 12, 102. [Google Scholar] [CrossRef]
- Bialkowski, S.; Toschi, A.; Yu, L.; Schlitzkus, L.; Mann, P.; Grilli, E.; Li, Y. Effects of Microencapsulated Blend of Organic Acids and Botanicals on Growth Performance, Intestinal Barrier Function, Inflammatory Cytokines, and Endocannabinoid System Gene Expression in Broiler Chickens. Poult. Sci. 2023, 102, 102460. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Guo, F.; Abbas, W.; Hu, Z.; Liu, L.; Qiao, J.; Bi, R.; Xu, T.; Zhang, K.; Huang, J.; et al. Effects of Microencapsulated Essential Oils and Organic Acids Preparation on Growth Performance, Slaughter Characteristics, Nutrient Digestibility and Intestinal Microenvironment of Broiler Chickens. Poult. Sci. 2024, 103, 103655. [Google Scholar] [CrossRef]
- Spisni, E.; Petrocelli, G.; Imbesi, V.; Spigarelli, R.; Azzinnari, D.; Donati Sarti, M.; Campieri, M.; Valerii, M.C. Antioxidant, Anti-Inflammatory, and Microbial-Modulating Activities of Essential Oils: Implications in Colonic Pathophysiology. Int. J. Mol. Sci. 2020, 21, 4152. [Google Scholar] [CrossRef]
- Shen, J.; Liu, T.; Qian, Y.; Yan, S.; Liu, Z.; Jia, F. Therapeutic Effect of Probiotic-Fermented Herbal Blend as Antibiotic Alternative on Salmonellosis by Multi-Drug Resistant Salmonella Pullorum. Food Biosci. 2024, 57, 103585. [Google Scholar] [CrossRef]
- Kačániová, M.; Čmiková, N.; Vukovic, N.L.; Verešová, A.; Bianchi, A.; Garzoli, S.; Ben Saad, R.; Ben Hsouna, A.; Ban, Z.; Vukic, M.D. Citrus Limon Essential Oil: Chemical Composition and Selected Biological Properties Focusing on the Antimicrobial (In Vitro, In Situ), Antibiofilm, Insecticidal Activity and Preservative Effect against Salmonella enterica Inoculated in Carrot. Plants 2024, 13, 524. [Google Scholar] [CrossRef] [PubMed]
- Groves, P.J.; Williamson, S.L.; Ahaduzzaman, M.; Diamond, M.; Ngo, M.; Han, A.; Sharpe, S.M. Can a Combination of Vaccination, Probiotic and Organic Acid Treatment in Layer Hens Protect against Early Life Exposure to Salmonella Typhimurium and Challenge at Sexual Maturity? Vaccine 2021, 39, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Deng, X.; Deng, Q.; Liu, Z.; Liu, N. Probiotic Lactobacilli Improved Growth Performance and Attenuated Salmonella Typhimurium Infection Via Jak/Stat Signaling in Broilers. Braz. J. Poult. Sci. 2021, 23. [Google Scholar] [CrossRef]
- Hong, H.A.; Duc, L.H.; Cutting, S.M. The Use of Bacterial Spore Formers as Probiotics: Table 1. FEMS Microbiol. Rev. 2005, 29, 813–835. [Google Scholar] [CrossRef]
- Barbosa, T.M.; Serra, C.R.; La Ragione, R.M.; Woodward, M.J.; Henriques, A.O. Screening for Bacillus Isolates in the Broiler Gastrointestinal Tract. Appl. Environ. Microbiol. 2005, 71, 968–978. [Google Scholar] [CrossRef]
- Duc, L.H.; Hong, H.A.; Barbosa, T.M.; Henriques, A.O.; Cutting, S.M. Characterization of Bacillus Probiotics Available for Human Use. Appl. Environ. Microbiol. 2004, 70, 2161–2171. [Google Scholar] [CrossRef]
- Menconi, A.; Morgan, M.J.; Pumford, N.R.; Hargis, B.M.; Tellez, G. Physiological Properties and Salmonella Growth Inhibition of Probiotic Bacillus Strains Isolated from Environmental and Poultry Sources. Int. J. Bacteriol. 2013, 2013, 958408. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, N.; Han, J.; Chang, C.; Hsiao, F.S.; Yu, Y. Optimization of Surfactin Production from Bacillus subtilis in Fermentation and Its Effects on Clostridium perfringens -induced Necrotic Enteritis and Growth Performance in Broilers. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1232–1244. [Google Scholar] [CrossRef]
- Lee, K.-W.; Kim, D.K.; Lillehoj, H.S.; Jang, S.I.; Lee, S.-H. Immune Modulation by Bacillus Subtilis-Based Direct-Fed Microbials in Commercial Broiler Chickens. Anim. Feed. Sci. Technol. 2015, 200, 76–85. [Google Scholar] [CrossRef]
- Tarradas, J.; Tous, N.; Esteve-Garcia, E.; Brufau, J. The Control of Intestinal Inflammation: A Major Objective in the Research of Probiotic Strains as Alternatives to Antibiotic Growth Promoters in Poultry. Microorganisms 2020, 8, 148. [Google Scholar] [CrossRef]
- Sikandar, A.; Zaneb, H.; Nasir, A.; Adil, M.; Ali, H.M.; Muhammad, N.; Rehman, T.; Rehman, A.; Rehman, H.F. Effects of Bacillus Subtilis on Performance, Immune System and Gut in Salmonella-Challenged Broilers. S. Afr. J. Anim. Sci. 2020, 50, 654–662. [Google Scholar] [CrossRef]
- Knap, I.; Kehlet, A.B.; Bennedsen, M.; Mathis, G.F.; Hofacre, C.L.; Lumpkins, B.S.; Jensen, M.M.; Raun, M.; Lay, A. Bacillus Subtilis (DSM17299) Significantly Reduces Salmonella in Broilers. Poult. Sci. 2011, 90, 1690–1694. [Google Scholar] [CrossRef]
- Guo, M.; Wu, F.; Hao, G.; Qi, Q.; Li, R.; Li, N.; Wei, L.; Chai, T. Bacillus Subtilis Improves Immunity and Disease Resistance in Rabbits. Front. Immunol. 2017, 8, 354. [Google Scholar] [CrossRef]
- Sikandar, A.; Zaneb, H.; Nasir, A.; Rehman, A.; Kashif, M.; Shah, M.; Luqman, Z.; Din, S.; Iqbal, M.; Khan, I.; et al. Effect of Bacillus Subtilis on the Microarchitectural Development of the Immune System in Salmonella-Challenged Broiler Chickens. Vet. Med. 2022, 67, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, S.D.; Hossiendoust, A.; Kim, I.H. Probiotics in Salmonella-Challenged Hy-Line Brown Layers. Poult. Sci. 2016, 95, 1894–1897. [Google Scholar] [CrossRef] [PubMed]
- Hosseindoust, A.; Mohammadi, M.; Yao, Z.P.; Jung, M.; Kim, I.H. Dietary Bacillus subtilis B2A Strain in Laying Hens Challenged with Salmonella gallinarum: Effects on Egg Production, Egg Quality, Blood Haptoglobin and Targeted Intestinal Salmonella Shedding. J. Appl. Anim. Res. 2018, 46, 512–517. [Google Scholar] [CrossRef]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the Use of Bacillus Species for Industrial Production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef]
- Shanmugasundaram, R.; Applegate, T.J.; Selvaraj, R.K. Effect of Bacillus Subtilis and Bacillus Licheniformis Probiotic Supplementation on Cecal Salmonella Load in Broilers Challenged with Salmonella. J. Appl. Poult. Res. 2020, 29, 808–816. [Google Scholar] [CrossRef]
- Connor Padgett, J.; Thomas Price, P.; Allen Byrd, J.; Anthony Bailey, C. Salmonella Enteritidis Control in Mature Laying Hens Through Dry Fed Parietal Yeast Fraction or Bacillus Blend Probiotic. Int. J. Anim. Sci. Technol. 2021, 5, 1. [Google Scholar] [CrossRef]
- Dolin, B.J. Effects of a Propietary Bacillus Coagulans Preparation on Symptoms of Diarrhea-Predominant Irritable Bowel Syndrome. Methods Find. Exp. Clin. Pharmacol. 2009, 31, 655. [Google Scholar] [CrossRef]
- Fitzpatrick, L.R. Probiotics for the Treatment of Clostridium difficile Associated Disease. World J. Gastrointest. Pathophysiol. 2013, 4, 47. [Google Scholar] [CrossRef]
- Zhen, W.; Shao, Y.; Gong, X.; Wu, Y.; Geng, Y.; Wang, Z.; Guo, Y. Effect of Dietary Bacillus Coagulans Supplementation on Growth Performance and Immune Responses of Broiler Chickens Challenged by Salmonella Enteritidis. Poult. Sci. 2018, 97, 2654–2666. [Google Scholar] [CrossRef]
- Higgins, J.P.; Higgins, S.E.; Vicente, J.L.; Wolfenden, A.D.; Tellez, G.; Hargis, B.M. Temporal Effects of Lactic Acid Bacteria Probiotic Culture on Salmonella in Neonatal Broilers. Poult. Sci. 2007, 86, 1662–1666. [Google Scholar] [CrossRef]
- Vilà, B.; Fontgibell, A.; Badiola, I.; Esteve-Garcia, E.; Jiménez, G.; Castillo, M.; Brufau, J. Reduction of Salmonella enterica Var. Enteritidis Colonization and Invasion by Bacillus Cereus Var. Toyoi Inclusion in Poultry Feeds. Poult. Sci. 2009, 88, 975–979. [Google Scholar] [CrossRef]
- Lei, X.; Piao, X.; Ru, Y.; Zhang, H.; Péron, A.; Zhang, H. Effect of Bacillus amyloliquefaciens-Based Direct-Fed Microbial on Performance, Nutrient Utilization, Intestinal Morphology and Cecal Microflora in Broiler Chickens. Asian-Australas. J. Anim. Sci. 2014, 28, 239–246. [Google Scholar] [CrossRef]
- Poudel, I.; Calvert, A.; Kiess, A.S.; Zhang, L.; Adhikari, P.A. Effect of a Bacillus-Based Probiotic on Fecal Shedding and Cecal Colonization of Salmonella Enteritidis in Laying Hens. J. Appl. Poult. Res. 2025, 34, 100532. [Google Scholar] [CrossRef]
- Bumbie, G.Z.; Abormegah, L.; Asiedu, P.; Oduro-Owusu, A.D.; Koranteng, A.A.-A.; Ansah, K.O.; Lamptey, V.K.; Chen, C.; Mohamed, T.M.; Tang, Z. Influence of Pediococcus Pentosaceus GT001 on Performance, Meat Quality, Immune Function, Antioxidant and Cecum Microbial in Broiler Chickens Challenged by Salmonella Typhimurium. Animals 2024, 14, 1676. [Google Scholar] [CrossRef] [PubMed]
- Khochamit, N.; Siripornadulsil, S.; Sukon, P.; Siripornadulsil, W. Bacillus Subtilis and Lactic Acid Bacteria Improve the Growth Performance and Blood Parameters and Reduce Salmonella Infection in Broilers. Vet. World 2020, 13, 2663–2672. [Google Scholar] [CrossRef] [PubMed]
- Aprea, G.; Del Matto, I.; Tucci, P.; Marino, L.; Scattolini, S.; Rossi, F. In Vivo Functional Properties of Dairy Bacteria. Microorganisms 2023, 11, 1787. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Santonicola, S.; Giaccone, V.; Truant, A.; Colavita, G. Dairy Propionibacteria: Probiotic Properties and Their Molecular Bases. Biomolecules 2025, 15, 886. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.V.T.; Vazhakkattu Thomas, J.; Dewi, G.; Brannon, J.; Noll, S.L.; Johnson, T.J.; Cox, R.B.; Kollanoor Johny, A. Propionibacterium Freudenreichii Freudenreichii B3523 Reduces Cecal Colonization and Internal Organ Dissemination of Multidrug-Resistant Salmonella Heidelberg in Finishing Turkeys. J. Appl. Poult. Res. 2021, 30, 100107. [Google Scholar] [CrossRef]
- Manjankattil, S.; Dewi, G.; Peichel, C.; Creek, M.; Bina, P.; Lerohl, K.; Deniz, K.; Akhtar, L.; Porter, R.; Johnson, T.J.; et al. Dairy-Origin Propionibacterium Freudenreichii, Turkey-Origin Lactobacillus Salivarius, and a Salmonella Typhimurium Vaccine Elicit Comparable Colonization Resistance on Drug-Resistant Salmonella Serotypes (S. Reading, S. Agona, and S. Saintpaul) in Growing Turkeys after Oral Challenge. J. Appl. Poult. Res. 2024, 33, 100428. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. Biomed. Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef]
- Nam, T.V.B.; Anh, L.H.; Loc, H.T.; Trang, C.T.H.; Thiet, N.; Lan, L.T.T.; Diep, T.H.; Xuan, N.H.; Ngu, N.T. Effects of Probiotic (Lactobacillus Plantarum and Bacillus Subtilis) Supplementation on Mortality, Growth Performance, and Carcass Characteristics of Native Vietnamese Broilers Challenged with Salmonella Typhimurium. Vet. World 2022, 15, 2302–2308. [Google Scholar] [CrossRef]
- Smialek, M.; Kaczorek, E.; Szczucińska, E.; Burchardt, S.; Kowalczyk, J.; Tykałowski, B.; Koncicki, A. Evaluation of Lactobacillus Spp. and Yeast Based Probiotic (Lavipan) Supplementation for the Reduction of Salmonella Enteritidis after Infection of Broiler Chickens. Pol. J. Vet. Sci. 2019, 22, 5–10. [Google Scholar] [CrossRef]
- Adhikari, P.; Lee, C.H.; Cosby, D.E.; Cox, N.A.; Kim, W.K. Effect of Probiotics on Fecal Excretion, Colonization in Internal Organs and Immune Gene Expression in the Ileum of Laying Hens Challenged with SalmonellaEnteritidis. Poult. Sci. 2019, 98, 1235–1242. [Google Scholar] [CrossRef]
- Bielecka, M.; Smoragiewicz, W.; Siwicki, A.K.; Wójcik, R.; Biedrzycka, E.; Orłowski, A.; Kask, S.; Jankowski, J.; Karska-Wysocki, B.; Ham, D. The Effect of Various Probiotic Strains or Avilamycin Feed Additive on Immune Defense Markers and Acute-Phase Response to Salmonella Infection in Chickens. Probiotics Antimicrob. Proteins 2010, 2, 175–185. [Google Scholar] [CrossRef]
- Khaksefidi, A.; Rahimi, S. Effect of Probiotic Inclusion in the Diet of Broiler Chickens on Performance, Feed Efficiency and Carcass Quality. Asian-Australas. J. Anim. Sci. 2005, 18, 1153–1156. [Google Scholar] [CrossRef]
- Mountzouris, K.C.; Dalaka, E.; Palamidi, I.; Paraskeuas, V.; Demey, V.; Theodoropoulos, G.; Fegeros, K. Evaluation of Yeast Dietary Supplementation in Broilers Challenged or Not with Salmonella on Growth Performance, Cecal Microbiota Composition and Salmonella in Ceca, Cloacae and Carcass Skin. Poult. Sci. 2015, 94, 2445–2455. [Google Scholar] [CrossRef]
- Price, P.T.; Gaydos, T.A.; Berghaus, R.D.; Baxter, V.; Hofacre, C.L.; Sims, M.D. Salmonella Enteritidis Reduction in Layer Ceca with a Bacillus Probiotic. Vet. World 2020, 13, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Shibat El-hamd, D.; Mohamed, H. Effect of Probiotics on Salmonella Enteritidis Infection in Broiler Chickens. Egypt. J. Chem. Environ. Health 2016, 2, 298–314. [Google Scholar] [CrossRef]
- Mountzouris, K.C.; Tsirtsikos, P.; Kalamara, E.; Nitsch, S.; Schatzmayr, G.; Fegeros, K. Evaluation of the Efficacy of a Probiotic Containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus Strains in Promoting Broiler Performance and Modulating Cecal Microflora Composition and Metabolic Activities. Poult. Sci. 2007, 86, 309–317. [Google Scholar] [CrossRef]
- Gölz, G.; Rosner, B.; Hofreuter, D.; Josenhans, C.; Kreienbrock, L.; Löwenstein, A.; Schielke, A.; Stark, K.; Suerbaum, S.; Wieler, L.H.; et al. Relevance of Campylobacter to Public Health—The Need for a One Health Approach. Int. J. Med. Microbiol. 2014, 304, 817–823. [Google Scholar] [CrossRef]
- Wagenaar, J.A.; Van Bergen, M.A.P.; Mueller, M.A.; Wassenaar, T.M.; Carlton, R.M. Phage Therapy Reduces Campylobacter jejuni Colonization in Broilers. Vet. Microbiol. 2005, 109, 275–283. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). FoodNet Fast. Available online: https://wwwn.cdc.gov/foodnetfast/ (accessed on 21 August 2025).
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on Quantification of the Risk Posed by Broiler Meat to Human Campylobacteriosis in the EU. EFSA J. 2010, 8, 1437. [Google Scholar] [CrossRef]
- Bessell, P.R.; Matthews, L.; Smith-Palmer, A.; Rotariu, O.; Strachan, N.J.; Forbes, K.J.; Cowden, J.M.; Reid, S.W.; Innocent, G.T. Geographic Determinants of Reported Human Campylobacter Infections in Scotland. BMC Public Health 2010, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Saint-Cyr, M.J.; Guyard-Nicodème, M.; Messaoudi, S.; Chemaly, M.; Cappelier, J.-M.; Dousset, X.; Haddad, N. Recent Advances in Screening of Anti-Campylobacter Activity in Probiotics for Use in Poultry. Front. Microbiol. 2016, 7, 553. [Google Scholar] [CrossRef]
- Batz, M.B.; Hoffmann, S.; Morris, J.G. Ranking the Disease Burden of 14 Pathogens in Food Sources in the United States Using Attribution Data from Outbreak Investigations and Expert Elicitation. J. Food Prot. 2012, 75, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.; Devleesschauwer, B.; Aspinall, W.; Cooke, R.; Corrigan, T.; Havelaar, A.H.; Angulo, F.J.; Gibb, H.J.; Kirk, M.D.; Lake, R.J.; et al. Attribution of global foodborne disease to specific foods: Findings from a World Health Organization structured expert elicitation. PLoS ONE 2017, 12, e0183641. [Google Scholar] [CrossRef] [PubMed]
- Nolte, T.; Spieß, F.; Jacobs, A.-K.; Kemper, N.; Visscher, C. Assessing Concordance between Campylobacter Prevalence in Broilers and Human Cases before and during the COVID-19 Pandemic in Lower Saxony, Germany, Considering Fresh Chicken Meat Consumption Patterns. Front. Vet. Sci. 2024, 11, 1392677. [Google Scholar] [CrossRef]
- Naguib, M.; Sharma, S.; Schneider, A.; Wehmueller, S.; Abdelaziz, K. Comparative Effectiveness of Various Multi-Antigen Vaccines in Controlling Campylobacter jejuni in Broiler Chickens. Vaccines 2024, 12, 908. [Google Scholar] [CrossRef]
- Aho, M.; Nuotio, L.; Nurmi, E.; Kiiskinen, T. Competitive Exclusion of Campylobacters from Poultry with K-Bacteria and Broilact®. Int. J. Food Microbiol. 1992, 15, 265–275. [Google Scholar] [CrossRef]
- Ty, M.; Taha-Abdelaziz, K.; Demey, V.; Castex, M.; Sharif, S.; Parkinson, J. Performance of Distinct Microbial Based Solutions in a Campylobacter Infection Challenge Model in Poultry. Anim. Microbiome 2022, 4, 2. [Google Scholar] [CrossRef]
- Cawthraw, S.A.; Wassenaar, T.M.; Ayling, R.; Newell, D.G. Increased Colonization Potential of Campylobacter jejuni Strain 81116 after Passage through Chickens and Its Implication on the Rate of Transmission within Flocks. Epidemiol. Infect. 1996, 117, 213–215. [Google Scholar] [CrossRef]
- Dasti, J.I.; Tareen, A.M.; Lugert, R.; Zautner, A.E.; Groß, U. Campylobacter Jejuni: A Brief Overview on Pathogenicity-Associated Factors and Disease-Mediating Mechanisms. Int. J. Med. Microbiol. 2010, 300, 205–211. [Google Scholar] [CrossRef]
- Cohen, E.J.; Nakane, D.; Kabata, Y.; Hendrixson, D.R.; Nishizaka, T.; Beeby, M. Campylobacter jejuni Motility Integrates Specialized Cell Shape, Flagellar Filament, and Motor, to Coordinate Action of Its Opposed Flagella. PLoS Pathog. 2020, 16, e1008620. [Google Scholar] [CrossRef] [PubMed]
- Day, C.J.; King, R.M.; Shewell, L.K.; Tram, G.; Najnin, T.; Hartley-Tassell, L.E.; Wilson, J.C.; Fleetwood, A.D.; Zhulin, I.B.; Korolik, V. A Direct-Sensing Galactose Chemoreceptor Recently Evolved in Invasive Strains of Campylobacter jejuni. Nat. Commun. 2016, 7, 13206. [Google Scholar] [CrossRef] [PubMed]
- Hendrixson, D.R.; DiRita, V.J. Identification of Campylobacter jejuni Genes Involved in Commensal Colonization of the Chick Gastrointestinal Tract. Mol. Microbiol. 2004, 52, 471–484. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Lindén, S.K.; Sutton, P.; Florin, T.H. Mucin Dynamics and Enteric Pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar] [CrossRef]
- Ribardo, D.A.; Johnson, J.J.; Hendrixson, D.R. Viscosity-Dependent Determinants of Campylobacter jejuni Impacting the Velocity of Flagellar Motility. mBio 2024, 15, e0254423. [Google Scholar] [CrossRef]
- Kemper, L.; Hensel, A. Campylobacter Jejuni: Targeting Host Cells, Adhesion, Invasion, and Survival. Appl. Microbiol. Biotechnol. 2023, 107, 2725–2754. [Google Scholar] [CrossRef]
- Al Hakeem, W.G.; Fathima, S.; Shanmugasundaram, R.; Selvaraj, R.K. Campylobacter jejuni in Poultry: Pathogenesis and Control Strategies. Microorganisms 2022, 10, 2134. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.M.; Clyne, M.; Bourke, B. Campylobacter jejuni Adhere to and Invade Chicken Intestinal Epithelial Cells in Vitro. Microbiology 2007, 153, 561–569. [Google Scholar] [CrossRef]
- Alemka, A.; Whelan, S.; Gough, R.; Clyne, M.; Gallagher, M.E.; Carrington, S.D.; Bourke, B. Purified Chicken Intestinal Mucin Attenuates Campylobacter jejuni Pathogenicity in Vitro. J. Med. Microbiol. 2010, 59, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Larsson, J.M.H.; Hansson, G.C. The Two Mucus Layers of Colon Are Organized by the MUC2 Mucin, Whereas the Outer Layer Is a Legislator of Host–Microbial Interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 4659–4665. [Google Scholar] [CrossRef] [PubMed]
- Barnawi, H.; Masri, N.; Hussain, N.; Al-Lawati, B.; Mayasari, E.; Gulbicka, A.; Jervis, A.J.; Huang, M.-H.; Cavet, J.S.; Linton, D. RNA-Based Thermoregulation of a Campylobacter jejuni Zinc Resistance Determinant. PLoS Pathog. 2020, 16, e1009008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-J.; Xiao, D.; Zhao, F.; Gu, Y.-X.; Meng, F.-L.; He, L.-H.; Ma, G.-Y.; Zhang, J.-Z. Comparative Proteomic Analysis of Campylobacter jejuni Cultured at 37C and 42C. Jpn. J. Infect. Dis. 2009, 62, 356–361. [Google Scholar] [CrossRef]
- Biswas, D.; Fernando, U.; Reiman, C.; Willson, P.; Potter, A.; Allan, B. Effect of Cytolethal Distending Toxin of Campylobacter jejuni on Adhesion and Internalization in Cultured Cells and in Colonization of the Chicken Gut. Avian Dis. 2006, 50, 586–593. [Google Scholar] [CrossRef]
- AbuOun, M.; Manning, G.; Cawthraw, S.A.; Ridley, A.; Ahmed, I.H.; Wassenaar, T.M.; Newell, D.G. Cytolethal Distending Toxin (CDT)-Negative Campylobacter jejuni Strains and Anti-CDT Neutralizing Antibodies Are Induced during Human Infection but Not during Colonization in Chickens. Infect. Immun. 2005, 73, 3053–3062. [Google Scholar] [CrossRef]
- de Zoete, M.R.; Keestra, A.M.; Roszczenko, P.; van Putten, J.P.M. Activation of Human and Chicken Toll-Like Receptors by Campylobacter spp. Infect. Immun. 2010, 78, 1229–1238. [Google Scholar] [CrossRef]
- Guerry, P.; Alm, R.A.; Power, M.E.; Logan, S.M.; Trust, T.J. Role of Two Flagellin Genes in Campylobacter Motility. J. Bacteriol. 1991, 173, 4757–4764. [Google Scholar] [CrossRef]
- Nachamkin, I.; Yang, X.H.; Stern, N.J. Role of Campylobacter jejuni Flagella as Colonization Factors for Three-Day-Old Chicks: Analysis with Flagellar Mutants. Appl. Environ. Microbiol. 1993, 59, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Morokka, T.; Umeda, A.; Amako, K. Motility as an Intestinal Colonization Factor for Campylobacter jejuni. Microbiology 1985, 131, 1973–1980. [Google Scholar] [CrossRef]
- Konkel, M.E.; Klena, J.D.; Rivera-Amill, V.; Monteville, M.R.; Biswas, D.; Raphael, B.; Mickelson, J. Secretion of Virulence Proteins from Campylobacter jejuni Is Dependent on a Functional Flagellar Export Apparatus. J. Bacteriol. 2004, 186, 3296–3303. [Google Scholar] [CrossRef] [PubMed]
- Elmi, A.; Nasher, F.; Dorrell, N.; Wren, B.; Gundogdu, O. Revisiting Campylobacter jejuni Virulence and Fitness Factors: Role in Sensing, Adapting, and Competing. Front. Cell Infect. Microbiol. 2021, 10, 607704. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Burr, D.H.; Guerry, P. CheY-mediated Modulation of Campylobacter jejuni Virulence. Mol. Microbiol. 1997, 23, 1021–1031. [Google Scholar] [CrossRef]
- Godschalk, P.C.R.; Heikema, A.P.; Gilbert, M.; Komagamine, T.; Ang, C.W.; Glerum, J.; Brochu, D.; Li, J.; Yuki, N.; Jacobs, B.C.; et al. The Crucial Role of Campylobacter jejuni Genes in Anti-Ganglioside Antibody Induction in Guillain-Barré Syndrome. J. Clin. Investig. 2004, 114, 1659–1665. [Google Scholar] [CrossRef]
- Boehm, M.; Krause-Gruszczynska, M.; Rohde, M.; Tegtmeyer, N.; Takahashi, S.; Oyarzabal, O.A.; Backert, S. Major Host Factors Involved in Epithelial Cell Invasion of Campylobacter Jejuni: Role of Fibronectin, Integrin Beta1, FAK, Tiam-1, and DOCK180 in Activating Rho GTPase Rac1. Front. Cell Infect. Microbiol. 2011, 1, 17. [Google Scholar] [CrossRef]
- Smith, J.L.; Bayles, D.O. The Contribution of Cytolethal Distending Toxin to Bacterial Pathogenesis. Crit. Rev. Microbiol. 2006, 32, 227–248. [Google Scholar] [CrossRef]
- Soro, A.B.; Whyte, P.; Bolton, D.J.; Tiwari, B.K. Strategies and Novel Technologies to Control Campylobacter in the Poultry Chain: A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1353–1377. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.F.; Boris, S.; Barbes, C. Probiotic Properties of Human Lactobacilli Strains to Be Used in the Gastrointestinal Tract. J. Appl. Microbiol. 2003, 94, 449–455. [Google Scholar] [CrossRef]
- Chaveerach, P.; Lipman, L.J.A.; van Knapen, F. Antagonistic Activities of Several Bacteria on in Vitro Growth of 10 Strains of Campylobacter Jejuni/Coli. Int. J. Food Microbiol. 2004, 90, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Wine, E.; Gareau, M.G.; Johnson-Henry, K.; Sherman, P.M. Strain-Specific Probiotic (Lactobacillus helveticus) Inhibition of Campylobacter jejuni invasion of Human Intestinal Epithelial Cells. FEMS Microbiol. Lett. 2009, 300, 146–152. [Google Scholar] [CrossRef]
- Tareb, R.; Bernardeau, M.; Gueguen, M.; Vernoux, J.-P. In Vitro Characterization of Aggregation and Adhesion Properties of Viable and Heat-Killed Forms of Two Probiotic Lactobacillus Strains and Interaction with Foodborne Zoonotic Bacteria, Especially Campylobacter jejuni. J. Med. Microbiol. 2013, 62, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, M.A.; Kurzak, P.; Bauer, J.; Vogel, R.F. Characterization of Lactobacilli towards Their Use as Probiotic Adjuncts in Poultry. J. Appl. Microbiol. 2002, 92, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Taheri, H.R.; Moravej, H.; Tabandeh, F.; Zaghari, M.; Shivazad, M. Screening of Lactic Acid Bacteria toward Their Selection as a Source of Chicken Probiotic. Poult. Sci. 2009, 88, 1586–1593. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Deng, Q.; Shi, H.; Luo, Y.; Zhao, H.; Liu, N. Effect of Dietary Lactobacilli Mixture on Listeria Monocytogenes Infection and Virulence Property in Broilers. Poult. Sci. 2020, 99, 3655–3662. [Google Scholar] [CrossRef] [PubMed]
- Catacutan, J.R.P.; Subejano, M.S.E.P.; Penuliar, G.M. In Vitro and in Vivo Activity of Lactobacillus sakei L14 Strain against Campylobacter jejuni DC3 Strain. J. Vet. Res. 2022, 66, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Saint-Cyr, M.J.; Haddad, N.; Taminiau, B.; Poezevara, T.; Quesne, S.; Amelot, M.; Daube, G.; Chemaly, M.; Dousset, X.; Guyard-Nicodème, M. Use of the Potential Probiotic Strain Lactobacillus Salivarius SMXD51 to Control Campylobacter jejuni in Broilers. Int. J. Food Microbiol. 2017, 247, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Even, M.; Davail, S.; Rey, M.; Tavernier, A.; Houssier, M.; Bernadet, M.D.; Gontier, K.; Pascal, G.; Ricaud, K. Probiotics Strains Modulate Gut Microbiota and Lipid Metabolism in Mule Ducks. Open Microbiol. J. 2018, 12, 71–93. [Google Scholar] [CrossRef]
- Cole, K.; Farnell, M.B.; Donoghue, A.M.; Stern, N.J.; Svetoch, E.A.; Eruslanov, B.N.; Volodina, L.I.; Kovalev, Y.N.; Perelygin, V.V.; Mitsevich, E.V.; et al. Bacteriocins Reduce Campylobacter Colonization and Alter Gut Morphology in Turkey Poults. Poult. Sci. 2006, 85, 1570–1575. [Google Scholar] [CrossRef]
- Kobierecka, P.A.; Wyszyńska, A.K.; Aleksandrzak-Piekarczyk, T.; Kuczkowski, M.; Tuzimek, A.; Piotrowska, W.; Górecki, A.; Adamska, I.; Wieliczko, A.; Bardowski, J.; et al. In Vitro Characteristics of Lactobacillus Spp. Strains Isolated from the Chicken Digestive Tract and Their Role in the Inhibition of Campylobacter Colonization. Microbiologyopen 2017, 6, e00512. [Google Scholar] [CrossRef]
- Nishiyama, K.; Seto, Y.; Yoshioka, K.; Kakuda, T.; Takai, S.; Yamamoto, Y.; Mukai, T. Lactobacillus Gasseri SBT2055 Reduces Infection by and Colonization of Campylobacter jejuni. PLoS ONE 2014, 9, e108827. [Google Scholar] [CrossRef]
- Taha-Abdelaziz, K.; Astill, J.; Kulkarni, R.R.; Read, L.R.; Najarian, A.; Farber, J.M.; Sharif, S. In Vitro Assessment of Immunomodulatory and Anti-Campylobacter Activities of Probiotic Lactobacilli. Sci. Rep. 2019, 9, 17903. [Google Scholar] [CrossRef]
- Ghareeb, K.; Awad, W.A.; Mohnl, M.; Porta, R.; Biarnés, M.; Böhm, J.; Schatzmayr, G. Evaluating the Efficacy of an Avian-Specific Probiotic to Reduce the Colonization of Campylobacter jejuni in Broiler Chickens. Poult. Sci. 2012, 91, 1825–1832. [Google Scholar] [CrossRef]
- Fritts, C.A.; Kersey, J.H.; Motl, M.A.; Kroger, E.C.; Yan, F.; Si, J.; Jiang, Q.; Campos, M.M.; Waldroup, A.L.; Waldroup, P.W. Bacillus Subtilis C-3102 (Calsporin) Improves Live Performance and Microbiological Status of Broiler Chickens. J. Appl. Poult. Res. 2000, 9, 149–155. [Google Scholar] [CrossRef]
- Aguiar, V.F.; Donoghue, A.M.; Arsi, K.; Reyes-Herrera, I.; Metcalf, J.H.; de los Santos, F.S.; Blore, P.J.; Donoghue, D.J. Targeting Motility Properties of Bacteria in the Development of Probiotic Cultures Against Campylobacter jejuni in Broiler Chickens. Foodborne Pathog. Dis. 2013, 10, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Šimunović, K.; Sahin, O.; Erega, A.; Štefanič, P.; Zhang, Q.; Mandic Mulec, I.; Smole Možina, S.; Klančnik, A. Bacillus Subtilis PS-216 Spores Supplemented in Broiler Chicken Drinking Water Reduce Campylobacter jejuni Colonization and Increases Weight Gain. Front. Microbiol. 2022, 13, 910616. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.; Ibrahim, D.; El Sayed, S.; Wahdan, A.; El-Tarabili, R.M.; Rizk El-Ghareeb, W.; Abdullah Alhawas, B.; Alahmad, B.A.-H.Y.; Abdel-Raheem, S.M.; El-Hamid, M.I.A. Prospective Application of Nanoencapsulated Bacillus Amyloliquefaciens on Broiler Chickens’ Performance and Gut Health with Efficacy against Campylobacter jejuni Colonization. Animals 2023, 13, 775. [Google Scholar] [CrossRef]
- Guyard-Nicodème, M.; Keita, A.; Quesne, S.; Amelot, M.; Poezevara, T.; Le Berre, B.; Sánchez, J.; Vesseur, P.; Martín, Á.; Medel, P.; et al. Efficacy of Feed Additives against Campylobacter in Live Broilers during the Entire Rearing Period. Poult. Sci. 2016, 95, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Gracia, M.I.; Millán, C.; Sánchez, J.; Guyard-Nicodème, M.; Mayot, J.; Carre, Y.; Csorbai, A.; Chemaly, M.; Medel, P. Efficacy of Feed Additives against Campylobacter in Live Broilers during the Entire Rearing Period: Part B. Poult. Sci. 2016, 95, 886–892. [Google Scholar] [CrossRef]
- Netherwood, T.; Gilbert, H.J.; Parker, D.S.; O’Donnell, A.G. Probiotics Shown To Change Bacterial Community Structure in the Avian Gastrointestinal Tract. Appl. Environ. Microbiol. 1999, 65, 5134–5138. [Google Scholar] [CrossRef]
- Lauková, A.; Kandričáková, A.; Ščerbová, J.; Szabóová, R. In Vivo Model Experiment Using Laying Hens Treated with Enterococcus Faecium EM41 from Ostrich Faeces and Its Enterocin EM41. Maced. Vet. Rev. 2017, 40. [Google Scholar] [CrossRef]
- Willis, W.L.; Reid, L. Investigating the Effects of Dietary Probiotic Feeding Regimens on Broiler Chicken Production and Campylobacter jejuni Presence. Poult. Sci. 2008, 87, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Sonnenborn, U.; Schulze, J. The Non-Pathogenic Escherichia coli Strain Nissle 1917—Features of a Versatile Probiotic. Microb. Ecol. Health Dis. 2009, 21, 122–158. [Google Scholar] [CrossRef]
- Splichalova, A.; Trebichavsky, I.; Rada, V.; Vlkova, E.; Sonnenborn, U.; Splichal, I. Interference of Bifidobacterium choerinum or Escherichia coli Nissle 1917 with Salmonella Typhimurium in Gnotobiotic Piglets Correlates with Cytokine Patterns in Blood and Intestine. Clin. Exp. Immunol. 2011, 163, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Helmy, Y.A.; Closs, G.; Jung, K.; Kathayat, D.; Vlasova, A.; Rajashekara, G. Effect of Probiotic E. coli Nissle 1917 Supplementation on the Growth Performance, Immune Responses, Intestinal Morphology, and Gut Microbes of Campylobacter jejuni Infected Chickens. Infect. Immun. 2022, 90, e00337-22. [Google Scholar] [CrossRef]
- Valečková, E.; Sun, L.; Wang, H.; Dube, F.; Ivarsson, E.; Kasmaei, K.M.; Ellström, P.; Wall, H. Intestinal Colonization with Campylobacter jejuni Affects Broiler Gut Microbiota Composition but Is Not Inhibited by Daily Intake of Lactiplantibacillus Plantarum. Front. Microbiol. 2023, 14, 1205797. [Google Scholar] [CrossRef] [PubMed]
- Schoeni, J.L.; Wong, A.C. Inhibition of Campylobacter jejuni Colonization in Chicks by Defined Competitive Exclusion Bacteria. Appl. Environ. Microbiol. 1994, 60, 1191–1197. [Google Scholar] [CrossRef]
- Morishita, T.Y.; Aye, P.P.; Harr, B.S.; Cobb, C.W.; Clifford, J.R. Evaluation of an Avian-Specific Probiotic to Reduce the Colonization and Shedding of Campylobacter jejuni in Broilers. Avian Dis. 1997, 41, 850–855. [Google Scholar] [CrossRef]
- Santini, C.; Baffoni, L.; Gaggia, F.; Granata, M.; Gasbarri, R.; Di Gioia, D.; Biavati, B. Characterization of Probiotic Strains: An Application as Feed Additives in Poultry against Campylobacter jejuni. Int. J. Food Microbiol. 2010, 141, S98–S108. [Google Scholar] [CrossRef]
- Baffoni, L.; Gaggìa, F.; Di Gioia, D.; Santini, C.; Mogna, L.; Biavati, B. A Bifidobacterium-Based Synbiotic Product to Reduce the Transmission of C. Jejuni along the Poultry Food Chain. Int. J. Food Microbiol. 2012, 157, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Neal-McKinney, J.M.; Lu, X.; Duong, T.; Larson, C.L.; Call, D.R.; Shah, D.H.; Konkel, M.E. Production of Organic Acids by Probiotic Lactobacilli Can Be Used to Reduce Pathogen Load in Poultry. PLoS ONE 2012, 7, e43928. [Google Scholar] [CrossRef]
- Arsi, K.; Donoghue, A.M.; Woo-Ming, A.; Blore, P.J.; Donoghue, D.J. The Efficacy of Selected Probiotic and Prebiotic Combinations in Reducing Campylobacter Colonization in Broiler Chickens. J. Appl. Poult. Res. 2015, 24, 327–334. [Google Scholar] [CrossRef]
- Cean, A.; Stef, L.; Simiz, E.; Julean, C.; Dumitrescu, G.; Vasile, A.; Pet, E.; Drinceanu, D.; Corcionivoschi, N. Effect of Human Isolated Probiotic Bacteria on Preventing Campylobacter jejuni Colonization of Poultry. Foodborne Pathog. Dis. 2015, 12, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Schoeni, J.L.; Doyle, M.P. Reduction of Campylobacter jejuni Colonization of Chicks by Cecum-Colonizing Bacteria Producing Anti-C. Jejuni Metabolites. Appl. Environ. Microbiol. 1992, 58, 664–670. [Google Scholar] [CrossRef]
- Fu, Y.; Alenezi, T.; Sun, X. Clostridium Perfringens-Induced Necrotic Diseases: An Overview. Immuno 2022, 2, 387–407. [Google Scholar] [CrossRef]
- Grenda, T.; Jarosz, A.; Sapała, M.; Grenda, A.; Patyra, E.; Kwiatek, K. Clostridium Perfringens—Opportunistic Foodborne Pathogen, Its Diversity and Epidemiological Significance. Pathogens 2023, 12, 768. [Google Scholar] [CrossRef]
- Rood, J.I.; Adams, V.; Lacey, J.; Lyras, D.; McClane, B.A.; Melville, S.B.; Moore, R.J.; Popoff, M.R.; Sarker, M.R.; Songer, J.G.; et al. Expansion of the Clostridium Perfringens Toxin-Based Typing Scheme. Anaerobe 2018, 53, 5–10. [Google Scholar] [CrossRef]
- Freedman, J.; Shrestha, A.; McClane, B. Clostridium Perfringens Enterotoxin: Action, Genetics, and Translational Applications. Toxins 2016, 8, 73. [Google Scholar] [CrossRef]
- Mehdizadeh Gohari, I.; Navarro, M.A.; Li, J.; Shrestha, A.; Uzal, F.; McClane, B.A. Pathogenicity and Virulence of Clostridium perfringens. Virulence 2021, 12, 723–753. [Google Scholar] [CrossRef]
- Kulkarni, R.R.; Gaghan, C.; Gorrell, K.; Fletcher, O.J. Mucosal and Systemic Lymphoid Immune Responses against Clostridium Perfringens Strains with Variable Virulence in the Production of Necrotic Enteritis in Broiler Chickens. Avian Pathol. 2023, 52, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Lillehoj, H.S.; Sun, Z.; Lee, Y.; Zhao, H.; Xianyu, Z.; Yan, X.; Wang, Y.; Lin, S.; Liu, L.; et al. Characterization of Virulent NetB+/TpeL+Clostridium Perfringens Strains from Necrotic Enteritis-Affected Broiler Chicken Farms. Avian Dis. 2019, 63, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Coursodon, C.F.; Glock, R.D.; Moore, K.L.; Cooper, K.K.; Songer, J.G. TpeL-Producing Strains of Clostridium Perfringens Type A Are Highly Virulent for Broiler Chicks. Anaerobe 2012, 18, 117–121. [Google Scholar] [CrossRef]
- Camargo, A.; Ramírez, J.D.; Kiu, R.; Hall, L.J.; Muñoz, M. Unveiling the Pathogenic Mechanisms of Clostridium perfringens Toxins and Virulence Factors. Emerg. Microbes Infect. 2024, 13, 2341968. [Google Scholar] [CrossRef]
- Muneeb, M.; Khan, E.; Ahmad, S.; Suleman, S. Potential of Probiotics against Necrotic Enteritis in Commercial Broilers. In Complementary and Alternative Medicine: Prebiotics and Probiotics; Unique Scientific Publishers: Faisalabad, Pakistan, 2024; pp. 274–283. [Google Scholar] [CrossRef]
- Granstad, S.; Kristoffersen, A.B.; Benestad, S.L.; Sjurseth, S.K.; David, B.; Sørensen, L.; Fjermedal, A.; Edvardsen, D.H.; Sanson, G.; Løvland, A.; et al. Effect of Feed Additives as Alternatives to In-Feed Antimicrobials on Production Performance and Intestinal Clostridium Perfringens Counts in Broiler Chickens. Animals 2020, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shao, Y.; Song, B.; Zhen, W.; Wang, Z.; Guo, Y.; Shahid, M.S.; Nie, W. Effects of Bacillus Coagulans Supplementation on the Growth Performance and Gut Health of Broiler Chickens with Clostridium Perfringens-Induced Necrotic Enteritis. J. Anim. Sci. Biotechnol. 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Gharib-Naseri, K.; Dorigam, J.C.P.; Doranalli, K.; Morgan, N.; Swick, R.A.; Choct, M.; Wu, S.-B. Bacillus Amyloliquefaciens CECT 5940 Improves Performance and Gut Function in Broilers Fed Different Levels of Protein and/or under Necrotic Enteritis Challenge. Anim. Nutr. 2021, 7, 185–197. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A.; Abdel-Latif, M.A.; Hosny, F.; Alatfeehy, N.M.; Noreldin, A.E.; Quesnell, R.R.; Chapman, R.; Sakai, L.; Elbestawy, A.R. Comparative Efficacy of Postbiotic, Probiotic, and Antibiotic against Necrotic Enteritis in Broiler Chickens. Poult. Sci. 2022, 101, 101988. [Google Scholar] [CrossRef] [PubMed]
- McReynolds, J.; Waneck, C.; Byrd, J.; Genovese, K.; Duke, S.; Nisbet, D. Efficacy of Multistrain Direct-Fed Microbial and Phytogenetic Products in Reducing Necrotic Enteritis in Commercial Broilers. Poult. Sci. 2009, 88, 2075–2080. [Google Scholar] [CrossRef]
- Buiatte, V.; Schultheis, M.; Lorenzoni, A.G. Deconstruction of a Multi-Strain Bacillus-Based Probiotic Used for Poultry: An in Vitro Assessment of Its Individual Components against C. Perfringens. BMC Res. Notes 2023, 16, 117. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, K.; Zhang, A.; Chang, W.; Zheng, A.; Chen, Z.; Cai, H.; Liu, G. Effects of Lactobacillus Acidophilus on the Growth Performance, Immune Response, and Intestinal Barrier Function of Broiler Chickens Challenged with Escherichia coli O157. Poult. Sci. 2021, 100, 101323. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Q.; Jia, H.M.; Zeng, X.F.; Zhu, J.L.; Hou, C.L.; Liu, X.T.; Yang, F.J.; Qiao, S.Y. Prevention of Escherichia coli Infection in Broiler Chickens with Lactobacillus Plantarum B1. Poult. Sci. 2017, 96, 2576–2586. [Google Scholar] [CrossRef]
- Hidayat, M.N.; Malaka, R.; Agustina, L.; Pakiding, W. Effect of Lactobacillus Sp. Probiotics on Intestinal Histology, Escherichia coli in Excreta and Broiler Performance. J. Indones. Trop. Anim. Agric. 2018, 43, 445. [Google Scholar] [CrossRef]
- Tarabees, R.; El-Sayed, M.S.; Shehata, A.A.; Diab, M.S. Effects of the Probiotic Candidate E. Faecalis-1, the Poulvac E. coli Vaccine, and Their Combination on Growth Performance, Caecal Microbial Composition, Immune Response, and Protection against E. coli O78 Challenge in Broiler Chickens. Probiotics Antimicrob. Proteins 2020, 12, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Islam, M.; Samad, M.; Kabir, S. Characterization and antibiogram of Escherichia coli associated with mortality in broilers and ducklings in Bangladesh. Bangladesh J. Vet. Med. 1970, 2, 9–14. [Google Scholar] [CrossRef]
- Johnson, T.J.; Wannemuehler, Y.; Doetkott, C.; Johnson, S.J.; Rosenberger, S.C.; Nolan, L.K. Identification of Minimal Predictors of Avian Pathogenic Escherichia coli Virulence for Use as a Rapid Diagnostic Tool. J. Clin. Microbiol. 2008, 46, 3987–3996. [Google Scholar] [CrossRef]
- Manges, A.R. Escherichia coli and Urinary Tract Infections: The Role of Poultry-Meat. Clin. Microbiol. Infect. 2016, 22, 122–129. [Google Scholar] [CrossRef]
- Vinayananda, C.O.; Fairoze, N.; Madhavaprasad, C.B.; Byregowda, S.M.; Nagaraj, C.S.; Bagalkot, P.; Karabasanavar, N. Studies on Occurrence, Characterisation and Decontamination of Emerging Pathogenic Escherichia coli (STEC, ETEC and EIEC) in Table Eggs. Br. Poult. Sci. 2017, 58, 664–672. [Google Scholar] [CrossRef]
- Liang, W.; Li, H.; Zhou, H.; Wang, M.; Zhao, X.; Sun, X.; Li, C.; Zhang, X. Effects of Taraxacum and Astragalus Extracts Combined with Probiotic Bacillus Subtilis and Lactobacillus on Escherichia coli–Infected Broiler Chickens. Poult. Sci. 2021, 100, 101007. [Google Scholar] [CrossRef]
- Mellata, M. Human and Avian Extraintestinal Pathogenic Escherichia coli: Infections, Zoonotic Risks, and Antibiotic Resistance Trends. Foodborne Pathog. Dis. 2013, 10, 916–932. [Google Scholar] [CrossRef] [PubMed]
- Gorton, A.; Stasiewicz, M.J. Twenty-Two Years of U.S. Meat and Poultry Product Recalls: Implications for Food Safety and Food Waste. J. Food Prot. 2017, 80, 674–684. [Google Scholar] [CrossRef]
- Scharff, R.L. Food Attribution and Economic Cost Estimates for Meat- and Poultry-Related Illnesses. J. Food Prot. 2020, 83, 959–967. [Google Scholar] [CrossRef]
- Mainil, J. Escherichia coli Virulence Factors. Vet. Immunol. Immunopathol. 2013, 152, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Trabulsi, L.R.; Keller, R.; Gomes, T.A.T. Typical and Atypical Enteropathogenic Escherichia coli. Emerg. Infect. Dis. 2002, 8, 508–513. [Google Scholar] [CrossRef]
- Mazariego-Espinosa, K.; Cruz, A.; Ledesma, M.A.; Ochoa, S.A.; Xicohtencatl-Cortes, J. Longus, a Type IV Pilus of Enterotoxigenic Escherichia coli, Is Involved in Adherence to Intestinal Epithelial Cells. J. Bacteriol. 2010, 192, 2791–2800. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Richards, P.; Windhorst, D.; Imre, A.; Bukovinski, A.; Ruggeri, J.; Elazomi, A.; Barrow, P. Generation of an Inactivated Vaccine for Avian Pathogenic Escherichia coli Using Microarrays; a More Rational Approach to Inactivated Vaccine Design. Open Vet. J. 2022, 12, 221. [Google Scholar] [CrossRef]
- Bernier, C.; Gounon, P.; Le Bouguénec, C. Identification of an Aggregative Adhesion Fimbria (AAF) Type III-Encoding Operon in Enteroaggregative Escherichia coli as a Sensitive Probe for Detecting the AAF-Encoding Operon Family. Infect. Immun. 2002, 70, 4302–4311. [Google Scholar] [CrossRef] [PubMed]
- Abe, A.; De Grado, M.; Pfuetzner, R.A.; Sánchez-SanMartín, C.; DeVinney, R.; Puente, J.L.; Strynadka, N.C.J.; Finlay, B.B. Enteropathogenic Escherichia coli Translocated Intimin Receptor, Tir, Requires a Specific Chaperone for Stable Secretion. Mol. Microbiol. 1999, 33, 1162–1175. [Google Scholar] [CrossRef]
- Johura, F.-T.; Parveen, R.; Islam, A.; Sadique, A.; Rahim, M.N.; Monira, S.; Khan, A.R.; Ahsan, S.; Ohnishi, M.; Watanabe, H.; et al. Occurrence of Hybrid Escherichia coli Strains Carrying Shiga Toxin and Heat-Stable Toxin in Livestock of Bangladesh. Front. Public Health 2017, 4, 287. [Google Scholar] [CrossRef]
- Trung, N.V.; Nhung, H.N.; Carrique-Mas, J.J.; Mai, H.H.; Tuyen, H.T.; Campbell, J.; Nhung, N.T.; Van Minh, P.; Wagenaar, J.A.; Mai, N.T.N.; et al. Colonization of Enteroaggregative Escherichia coli and Shiga Toxin-Producing Escherichia coli in Chickens and Humans in Southern Vietnam. BMC Microbiol. 2016, 16, 208. [Google Scholar] [CrossRef]
- Madni, W.; Zahoor, M.; Nawaz, Z.; Khurshid, M. Prevalence and Sequence Analysis of Escherichia coli Harboring Colistin, Gentamicin, Streptomycin, Tetracycline and Quinolones Resistant Genes from Commercial Broilers. Pak. Vet. J. 2025, 45, 390–395. [Google Scholar] [CrossRef]
- Jin, L.Z.; Ho, Y.W.; Abdullah, N.; Jalaludin, S. Acid and bile tolerance of Lactobacillus isolated from chicken intestines and their antimicrobial activities against pathogenic bacteria. Lett. Appl. Microbiol. 1998, 27, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zeng, X.F.; Zhu, J.L.; Wang, S.; Liu, X.T.; Hou, C.L.; Thacker, P.A.; Qiao, S.Y. Effects of Dietary Lactobacillus Plantarum B1 on Growth Performance, Intestinal Microbiota, and Short Chain Fatty Acid Profiles in Broiler Chickens. Poult. Sci. 2016, 95, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Aldeieg, M.; Woodward, M.J.; Juniper, D.T.; Rymer, C. The Effect of Candida Famata and Lactobacillus Plantarum on the Number of Coliforms and the Antibiotic Resistance and Virulence of Escherichia coli in the Gut of Broilers. Animal 2021, 15, 100310. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalaifa, H.; Al-Nasser, A.; Al-Surayee, T.; Al-Kandari, S.; Al-Enzi, N.; Al-Sharrah, T.; Ragheb, G.; Al-Qalaf, S.; Mohammed, A. Effect of Dietary Probiotics and Prebiotics on the Performance of Broiler Chickens. Poult. Sci. 2019, 98, 4465–4479. [Google Scholar] [CrossRef]
- Novak, J.S.; Call, J.; Tomasula, P.; Luchansky, J.B. An Assessment of Pasteurization Treatment of Water, Media, and Milk with Respect to Bacillus Spores. J. Food Prot. 2005, 68, 751–757. [Google Scholar] [CrossRef]
- La Ragione, R. Bacillus Subtilis Spores Competitively Exclude Escherichia coli O78:K80 in Poultry. Vet. Microbiol. 2001, 79, 133–142. [Google Scholar] [CrossRef]
- McBride, S.M.; Fischetti, V.A.; LeBlanc, D.J.; Moellering, R.C.; Gilmore, M.S. Genetic Diversity among Enterococcus Faecalis. PLoS ONE 2007, 2, e582. [Google Scholar] [CrossRef]
- Dong, Z.L.; Wang, Y.W.; Song, D.; Wang, W.W.; Liu, K.B.; Wang, L.; Li, A.K. Effects of Microencapsulated Probiotics and Plant Extract on Antioxidant Ability, Immune Status and Caecal Microflora in Escherichia coli K88-Challenged Broiler Chickens. Food Agric. Immunol. 2019, 30, 1123–1134. [Google Scholar] [CrossRef]
- Huang, L.; Luo, L.; Zhang, Y.; Wang, Z.; Xia, Z. Effects of the Dietary Probiotic, Enterococcus Faecium NCIMB11181, on the Intestinal Barrier and System Immune Status in Escherichia coli O78-Challenged Broiler Chickens. Probiotics Antimicrob. Proteins 2019, 11, 946–956. [Google Scholar] [CrossRef]
- Cao, G.T.; Zeng, X.F.; Chen, A.G.; Zhou, L.; Zhang, L.; Xiao, Y.P.; Yang, C.M. Effects of a Probiotic, Enterococcus Faecium, on Growth Performance, Intestinal Morphology, Immune Response, and Cecal Microflora in Broiler Chickens Challenged with Escherichia coli K88. Poult. Sci. 2013, 92, 2949–2955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Zhan, X.; Zeng, X.; Zhou, L.; Cao, G.; Chen, A.; Yang, C. Effects of Dietary Supplementation of Probiotic, Clostridium Butyricum, on Growth Performance, Immune Response, Intestinal Barrier Function, and Digestive Enzyme Activity in Broiler Chickens Challenged with Escherichia coli K88. J. Anim. Sci. Biotechnol. 2016, 7, 3. [Google Scholar] [CrossRef]
- Igbafe, J.; Kilonzo-Nthenge, A.; Nahashon, S.N.; Mafiz, A.I.; Nzomo, M. Probiotics and Antimicrobial Effect of Lactiplantibacillus Plantarum, Saccharomyces Cerevisiae, and Bifidobacterium Longum against Common Foodborne Pathogens in Poultry. Agriculture 2020, 10, 368. [Google Scholar] [CrossRef]
- Bortoluzzi, C.; Barbosa, J.G.M.; Pereira, R.; Fagundes, N.S.; Rafael, J.M.; Menten, J.F.M. Autolyzed Yeast (Saccharomyces Cerevisiae) Supplementation Improves Performance While Modulating the Intestinal Immune-System and Microbiology of Broiler Chickens. Front. Sustain. Food Syst. 2018, 2, 85. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, C.; Zhang, C.; Zhang, X.; Wu, Y. Lacticaseibacillus Rhamnosus Reduces the Pathogenicity of Escherichia coli in Chickens. Front. Microbiol. 2021, 12, 664604. [Google Scholar] [CrossRef]
- Kathayat, D.; Closs, G.; Helmy, Y.A.; Deblais, L.; Srivastava, V.; Rajashekara, G. In Vitro and In Vivo Evaluation of Lacticaseibacillus Rhamnosus GG and Bifidobacterium lactis Bb12 Against Avian Pathogenic Escherichia coli and Identification of Novel Probiotic-Derived Bioactive Peptides. Probiotics Antimicrob. Proteins 2022, 14, 1012–1028. [Google Scholar] [CrossRef]
- Yang, Y.-X.; He, M.; Hu, G.; Wei, J.; Pages, P.; Yang, X.-H.; Bourdu-Naturel, S. Effect of a Fermented Milk Containing Bifidobacterium lactis DN-173010 on Chinese Constipated Women. World J. Gastroenterol. 2008, 14, 6237. [Google Scholar] [CrossRef]
- Ventura, M.; Zink, R. Rapid Identification, Differentiation, and Proposed New Taxonomic Classification of Bifidobacterium lactis. Appl. Environ. Microbiol. 2002, 68, 6429–6434. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhang, Y.; Xiao, K.; Jiang, F.; Wang, H.; Tang, D.; Liu, D.; Liu, B.; Liu, Y. The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome 2018, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Ying, Z.; Xue, M.; Xiaoxian, P.; Xiaorong, L.; Chunyang, L.; Yu, W.; Mingcheng, L.; Binxian, L. A Novel Lactobacillus Bulgaricus Isolate Can Maintain the Intestinal Health, Improve the Growth Performance and Reduce the Colonization of E. coli O157:H7 in Broilers. Br. Poult. Sci. 2022, 63, 621–632. [Google Scholar] [CrossRef]
- Manafi, M.; Khalaji, S.; Hedayati, M.; Pirany, N. Efficacy of Bacillus Subtilis and Bacitracin Methylene Disalicylate on Growth Performance, Digestibility, Blood Metabolites, Immunity, and Intestinal Microbiota after Intramuscular Inoculation with Escherichia coli in Broilers. Poult. Sci. 2017, 96, 1174–1183. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, G.T.; Zeng, X.F.; Zhou, L.; Ferket, P.R.; Xiao, Y.P.; Chen, A.G.; Yang, C.M. Effects of Clostridium Butyricum on Growth Performance, Immune Function, and Cecal Microflora in Broiler Chickens Challenged with Escherichia coli K88. Poult. Sci. 2014, 93, 46–53. [Google Scholar] [CrossRef]
- Younis, H. Trials on the Role of Prebiotics and Probiotics in Colonization and Immune Response of Broiler Chickens Challenged with Escherichia coli K88. Alex. J. Vet. Sci. 2018, 59, 48. [Google Scholar] [CrossRef]
- ul Haq, I.; Hafeez, A.; Khan, R.U. Protective Effect of Nigella Sativa and Saccharomyces Cerevisiae on Zootechnical Characteristics, Fecal Escherichia coli and Hematopoietic Potential in Broiler Infected with Experimental Colibacillosis. Livest. Sci. 2020, 239, 104119. [Google Scholar] [CrossRef]
- Papouskova, A.; Rychlik, I.; Harustiakova, D.; Cizek, A. Research Note: A Mixture of Bacteroides Spp. and Other Probiotic Intestinal Anaerobes Reduces Colonization by Pathogenic E. coli Strain O78:H4-ST117 in Newly Hatched Chickens. Poult. Sci. 2023, 102, 102529. [Google Scholar] [CrossRef] [PubMed]
- Istiqomah, L.; Hayati, S.N.; Damayanti, E.; Julendra, H.; Sakti, A.A.; Untari, T. Performance and Meat Quality of Broilers Infected with Escherichia coli and Administered with Bio Additive, Probiotic, and Antibiotic. Media Peternak. Fak. Peternak. Inst. Pertan. Bogor. 2013, 36, 14–20. [Google Scholar] [CrossRef]
- Farber, J.M.; Peterkin, P.I. Listeria Monocytogenes, a Food-Borne Pathogen. Microbiol. Rev. 1991, 55, 476–511. [Google Scholar] [CrossRef]
- Ricke, S.C.; O’Bryan, C.A.; Rothrock, M.J. Listeria Occurrence in Conventional and Alternative Egg Production Systems. Microorganisms 2023, 11, 2164. [Google Scholar] [CrossRef] [PubMed]
- Quereda, J.J.; Morón-García, A.; Palacios-Gorba, C.; Dessaux, C.; García-del Portillo, F.; Pucciarelli, M.G.; Ortega, A.D. Pathogenicity and Virulence of Listeria monocytogenes: A Trip from Environmental to Medical Microbiology. Virulence 2021, 12, 2509–2545. [Google Scholar] [CrossRef]
- Allerberger, F.; Wagner, M. Listeriosis: A resurgent foodborne infection. Clin. Microbiol. Infect. 2010, 16, 16–23. [Google Scholar] [CrossRef]
- Walter, J. Ecological Role of Lactobacilli in the Gastrointestinal Tract: Implications for Fundamental and Biomedical Research. Appl. Environ. Microbiol. 2008, 74, 4985–4996. [Google Scholar] [CrossRef]
- Dhama, K.; Verma, A.K.; Rajagunala, S.; Kumar, A.; Tiwari, R.; Chakrabort, S.; Kumar, R. Listeria Monocytogenes Infection in Poultry and Its Public Health Importance with Special Reference to Food Borne Zoonoses. Pak. J. Biol. Sci. 2013, 16, 301–308. [Google Scholar] [CrossRef]
- Chemaly, M.; Toquin, M.-T.; Le Nôtre, Y.; Fravalo, P. Prevalence of Listeria Monocytogenes in Poultry Production in France. J. Food Prot. 2008, 71, 1996–2000. [Google Scholar] [CrossRef]
- Shimojima, Y.; Shimojima, H.; Morita, Y. Survival of Campylobacter Jejuni, Salmonella, and Listeria Monocytogenes and Temperature Change in Low-Temperature–Longtime-Cooked Chicken Meat. J. Food Prot. 2022, 85, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Chang, X.; Qin, S.; Song, Y.; Tian, J.; Ma, A. Analysis of 90 Listeria Monocytogenes Contaminated in Poultry and Livestock Meat through Whole-Genome Sequencing. Food Res. Int. 2022, 159, 111641. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- Public Health Agency of Canada. Pathogen Safety Data Sheets: Infectious Substances—Salmonella enterica spp. Available online: https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/salmonella-enterica.html (accessed on 21 August 2025).
- Liu, D.; Lawrence, M.L.; Ainsworth, A.J.; Austin, F.W. Toward an Improved Laboratory Definition of Listeria Monocytogenes Virulence. Int. J. Food Microbiol. 2007, 118, 101–115. [Google Scholar] [CrossRef]
- McLauchlin, J.; Aird, H.; Amar, C.; Barker, C.; Dallman, T.; Elviss, N.; Jørgensen, F.; Willis, C. Listeria Monocytogenes in Cooked Chicken: Detection of an Outbreak in the United Kingdom (2016 to 2017) and Analysis of L. Monocytogenes from Unrelated Monitoring of Foods (2013 to 2017). J. Food Prot. 2020, 83, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Girones, R.; Herman, L.; Koutsoumanis, K.; Nørrung, B.; et al. Listeria Monocytogenes Contamination of Ready-to-eat Foods and the Risk for Human Health in the EU. EFSA J. 2018, 16, e05134. [Google Scholar] [CrossRef]
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Rubayet Ul Alam, A.S.M.; Jahid, I.K. Characterization and Evaluation of Lactic Acid Bacteria from Indigenous Raw Milk for Potential Probiotic Properties. J. Dairy. Sci. 2020, 103, 1223–1237. [Google Scholar] [CrossRef]
- Abouloifa, H.; Hasnaoui, I.; Ben Slima, S.; Rokni, Y.; Gaamouche, S.; Trabelsi, I.; Bellaouchi, R.; Ghabbour, N.; Ben Salah, R.; Jaouadi, B.; et al. Bio-Preservation Effect of Probiotic Lactiplantibacillus plantarum S61 Against Rhodotorula glutinis and Listeria monocytogenes in Poultry Meat. Curr. Microbiol. 2022, 79, 232. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mu, G.; Tuo, Y. Phenotypic Traits and Probiotic Functions of Lactiplantibacillus Plantarum Y42 in Planktonic and Biofilm Forms. Foods 2023, 12, 1516. [Google Scholar] [CrossRef] [PubMed]
- The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e05926. [CrossRef] [PubMed]
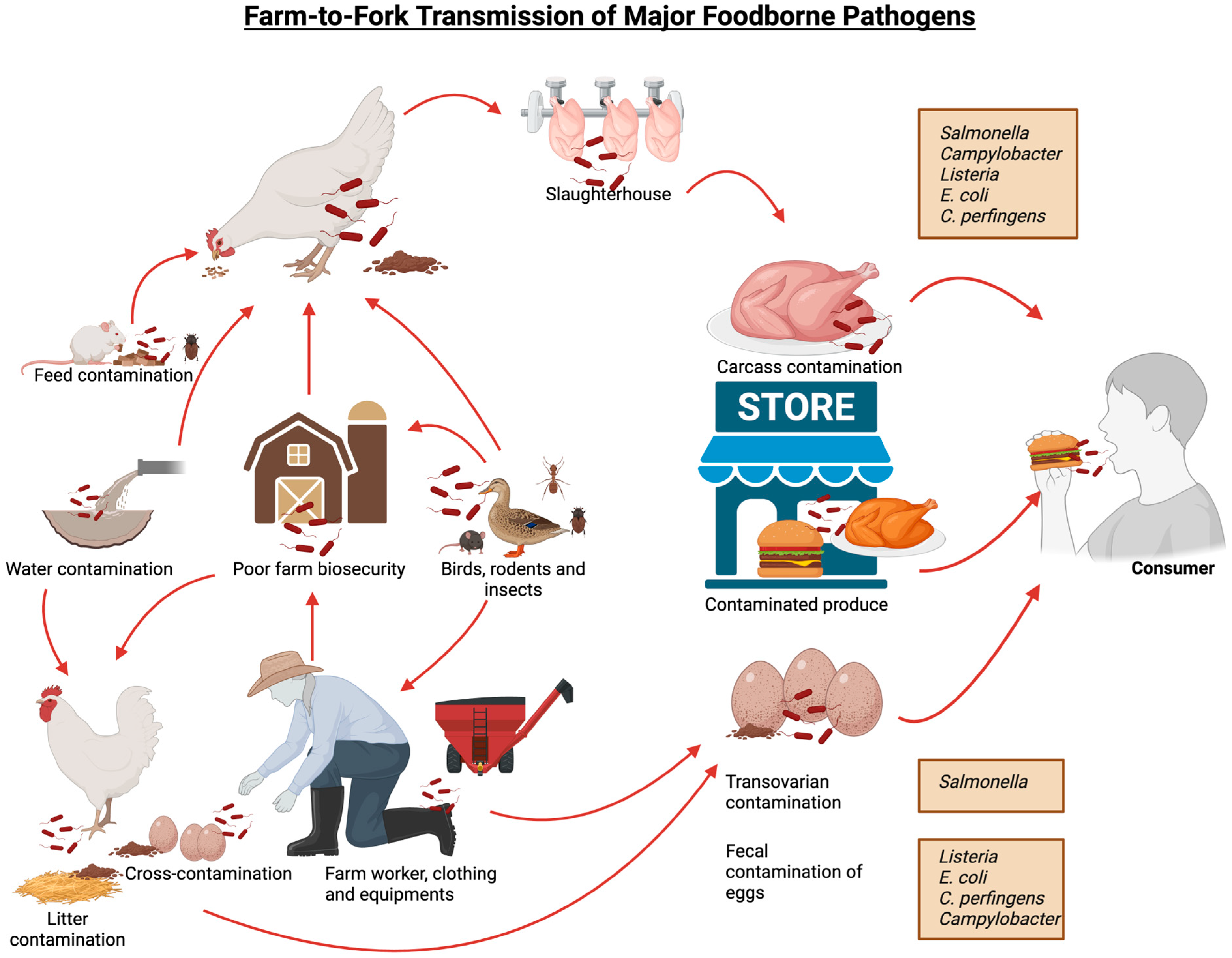
| Group | Host | Serovars | Infections | References |
|---|---|---|---|---|
| Group I (Host restricted) | Humans and higher primates | S. Typhi, S. Paratyphi A, B, C, and S. Sendai | Typhus, diarrhea, septicemia, and abortion in mares. Serovars such as S. Paratyphi A, S. Paratyphi B, S. Paratyphi C, and S. Sendai are the causal agents of typhoid fever. | [51,52] |
| Group II (Host) | Specific animal hosts | S. Dublin in cattle, S. Gallinarum in poultry, S. Abortusequi in horses, S. Abortusovis in sheep, and S. Choleraesuis in pigs | Septicemia, enterocolitis in cattle, fatal systemic infection in swine, bacteremia in humans and mouse. Causes subclinical infection in later hosts, making them a reservoir and carrier of the pathogen. | [51,52,53] |
| Group III (Unrestricted serovars) | Wide host range, including humans, animals, and the environment | S. Typhimurium and S. Enteritidis | Cause relatively less severe enteric diseases as compared to the serovars from Group I and Group II. Lack mechanisms for invading the mature immune system of older hosts, therefore causing more severe diseases in young hosts. Enterocolitis in humans and swine, asymptomatic carriers in poultry and cattle, and septicemia in mouse. | [52,54] |
| SPI Type | Function | References |
|---|---|---|
| SPI-1 | Host cell invasion and macrophage apoptosis induction. Possesses translocons involved in Salmonella contact and invasion and colonization of mammalian epithelial cells. T3SS encoded by SPI-1 functions for suppressing early proinflammatory cytokine expression in macrophages, including that of IL-1β, IL-8, TNF-α, IL-23α, GM-CSF, and IL-18. MHC II downregulation and polarization to the M2 phenotype in macrophages increases the blood-brain barrier penetration along with protein A genes. | [67,68,69] |
| SPI-2 | Proliferation within macrophages and systemic infections. Maintains Salmonella-containing vacuole (SCV) as an intracellular niche for the survival and proliferation of the bacterium. Involved in phagosome tubulation and other alterations by translocating proteins. | [70,71] |
| SPI-3 | Ensure survival in macrophages and growth in Mg-deficient environments. It encodes for the MgtCB (Magnesium transport system) operon, essential for bacterial survival in nutritionally deprived conditions. It also encodes for MisL protein (an anti-transport protein) that is essential for adhesion and long-term intestinal survival of Salmonella. | [71,72] |
| SPI-4 | Possess genes for toxin secretion, apoptosis and intramacrophage survival. Responsible for gastrointestinal inflammation, required for adhesion to epithelial cells as well as membrane ruffle formation. | [73,74] |
| SPI-5 | Possess genes that encode for T3SS effector proteins. Plays a role in enteropathogenicity and encodes for the proteins that are associated with intestinal mucosal fluid secretion as well as inflammatory response. | [75,76] |
| SPI-6 | Responds to external stimuli to transport proteins to host cells. Possesses the saf gene (encodes for fimbriae) and the pagN gene (encodes for invasion protein). Essential for intramacrophage survival and successful establishment of the bacterium in the host’s gut during infection. | [76,77] |
| Virulence Factor | Location | Function | References |
|---|---|---|---|
| Fimbriae (adhesins) | Bacterial cell surface | Adhesion to host cell, biofilm formation, seroconversion, hemagglutination, cellular invasion and macrophage interactions | [78] |
| Flagella | Bacterial cell surface | Display flagellin phase variation, creating phenotypic heterogeneity of the flagellar antigens, which minimizes the host immune response towards the pathogen. | [79] |
| Surface polysaccharides | Bacterial cell surface | Allow persistence of the bacteria in the gut of the hosts. | [47] |
| Type III secretion system | Present on the bacterial cell surface. Encoded on SPI-1, SPI-2 and other SPIs | Modification of host cell biology and successful infection by secreting several effector proteins into the host cell. | [39] |
| hylE protein (product of hylE gene) | Outer membrane of a bacterial cell | Pathogenesis of systemic salmonellosis is utilized in subserovar-level typing. | [80] |
| Salmonella plasmid virulence locus | Located on the Salmonella virulence plasmid. | Multiplication of Salmonella in the reticuloendothelial system. | [80] |
| Probiotic Strain(s) | Administration | Salmonella Strain | Effect on Salmonella Colonization | References |
|---|---|---|---|---|
| B. subtilis QST-713 | In feed | S. Gallinarum | Reduction in Salmonella content was not quantified. | [98] |
| B. subtilis DSM17299 | In feed | S. Heidelberg | 3 log10 reduction in cecal Salmonella content. | [99] |
| B. subtilis | In feed | S. Gallinarum | Reduction in Salmonella content was not quantified. | [101] |
| B. subtilis RX7 or B. methylotrophicus C14 | In feed | S. Gallinarum | Reduction in Salmonella content was not quantified | [102] |
| B. subtilis B2A | In feed | S. Gallinarum | Reduction in Salmonella content was not quantified | [103] |
| Three-strain Bacillus probiotic | In feed | S. Enteritidis | 1.08 log10 CFU/g reduction in cecal Salmonella content. | [106] |
| B. licheniformis, B. subtilis | In feed | S. Enteritidis | 0.73, 1.59 and 1.32 log10 reduction at 5-, 12- and 21-days post-infection. | [15] |
| B. coagulans | In feed | S. Enteritidis | 0.24, 0.41, and 0.24 log10 less Salmonella counts after 7-, 17-, and 31-days post-infection in treated birds than untreated birds. | [109] |
| B. coagulans | In feed | S. Enteritidis | 0.40 and 0.60 log10 reduction in cecal Salmonella content, 0.80 and 0.75 log10 reduction in fecal Salmonella content | [113] |
| B. subtilis KKU213, P. pentosaceus NP6 | In feed | S. Typhimurium | Complete elimination of Salmonella content was observed in treated birds (on day 18 post-treatment) | [115] |
| P. freudenreichii B3523 | In drinking water | S. Heidelberg | 1–2 log10 CFU/g reduction in cecal Salmonella content | [118] |
| P. freudenreichii B3523 | In drinking water | S. Reading, S. Agona and S. Saintpaul | 1.4–2.0 log10 CFU/g reduction in cecal Salmonella content | [119] |
| Toyocerin containing 1010 viable spores of B. cereus var. toyoi NCIMB 40112/CNCM I-1012 per gram | In feed | S. Enteritidis | 100% reduction in cecal Salmonella content | [111] |
| L. plantarum and B. subtilis | In feed | S. Typhimurium | complete elimination of Salmonella in the liver, spleen and heart of the chickens 28 days post-infection | [121] |
| Lavipan (Lactobacillus spp. + yeast) | In feed | S. Enteritidis | Approx. 98.84% reduction in cecal Salmonella content compared to control (58.29%) on 42 days of life. | [122] |
| L. plantarum | In feed | S. Enteritidis | No reduction in Salmonella content | [123] |
| L. acidophilus, L. casei, B. bifidum, Aspergillus oryzae, Streptococcus faecium and Torulopsis spp. | In feed | Salmonella was isolated from carcasses. | 40% carcass meat tested positive for Salmonella, as compared to 100% prevalence in carcass meat from untreated birds | [125] |
| S. cerevisiae var. boulardii | In feed | S. Enteritidis | Led to a 28.6% and 33.3% reduction in Salmonella content on breast skin and cloacae. | [129] |
| B. amyloliquefaciens, B. licheniformis, and B. pumilus, yeast culture, and yeast cell wall | In feed | S. Enteritidis | Led to a 0.79 log10 MPN/g reduction in the probiotic group as compared to the control, and a 0.86 log10 MPN/g reduction in the yeast culture-treated group as compared to the control. | [127] |
| L. plantarum, L. acidophilus and S. cerevisiae | In drinking water | S. Enteritidis | No reduction in cecal Salmonella content was observed | [128] |
| Structure | Function | Initial Role | References |
|---|---|---|---|
| Flagella | Motility | The absence of either FlaA or FlaB leads to changes in the filament construction and the inability to move | [159] |
| Binding and adhesion | Non-motile mutants exhibit less adherence | [143] | |
| Invasion | Lack of flagellins leads to the absence of infectivity in chicks | [160] | |
| Colonization | Lack of flagellar apparatus leads to insufficient colonization of the mouse intestines | [161] | |
| Secretory system | Aflagellated mutants fail to secrete invasion antigens as a Cia | [162] | |
| Immune evasion | Modified flagella evade recognition by toll-like receptor 5 (TLR5) | [163] | |
| CheY | Chemotaxis | Absence of chemotaxis protein CheY leads to loss of invasiveness and motility | [164] |
| LOS | Immune evasion | Mimicry with nerve ganglioside initiates Guillain-Barré syndrome | [143] |
| Evade antibody recognition and modulate immune response | [165] | ||
| CadF | Binding | Fibronectin-binding outer membrane protein | [162] |
| Cia | Invasion | Inhibition of Cia secretion significantly reduces the severity of C. jejuni in vivo | [166] |
| CDT | Toxins | CDT, particularly CdtB, causes apoptosis and cell cycle arrest | [167] |
| Effectors | Test | Mechanism | Aim | References |
|---|---|---|---|---|
| Probiotics’ metabolites (cell-free supernatant) |
| Organic acids Bacteriocins | Testing their direct effect on C. jejuni or indirectly through modulating host immunity | [169] |
| Live culture of probiotics |
| Auto-aggregation Co-aggregation Organic acids production Bacteriocins production | Testing probiotic candidates’ ability to:
| [135,170,171,172] |
| Probiotics stability |
| Resisting lower acids Resisting bile salts | Testing probiotic candidates’ ability to tolerate harsh gut conditions such as lower pH and bile salts. | [173,174] |
| Probiotics safety concern | Antibiotic sensitivity test | Lack of antibiotic resistance genes | Testing the absence of antibiotic-resistance genes | [175,176] |
| Toxinotype | α-Toxin | β-Toxin | ε-Toxin | ι-Toxin | Enterotoxin | NetB | Associated Diseases | References |
|---|---|---|---|---|---|---|---|---|
| Genes | cpa/plc | cpb | etx | Iap/ibp/itx | cpe | netB | ||
| A | + | - | - | - | - | - | Gas gangrene, food poisoning, NE in fowls | [209] |
| C | + | + | - | - | +/- | - | Necrotizing enteritis in humans | [208] |
| F | + | - | - | - | + | - | Food poisoning and abdominal cramps | [208] |
| G | + | - | - | - | - | + | Avian NE | [208] |
| Virulence Factor | Role in Pathogenesis | References |
|---|---|---|
| Bundle-Forming Pili (BFP) | Helps bacteria adhere to each other to form a colony and facilitates the initial interaction between bacteria and host epithelial cells. Allows for the bacteria to attach in a localized manner. | [235] |
| Longus Pillus | Promotes intestinal colonization by aiding bacterial adherence and enabling mobility through twitching motility. | [236] |
| Curli fimbriae | Formation of biofilms on organic and non-organic surfaces in E. coli O157:H7. Facilitate the attachment of E. coli to host epithelial and endothelial cells, thereby enhancing bacterial dissemination and persistence in host tissues and the bloodstream. | [237] |
| Aggregative Adhesion Fimbriae (AAF) | Enteroaggregative E. coli exhibit agglutination and adhere to components of the extracellular matrix | [238] |
| Translocated intimin receptor (Tir) protein | Binds to intimin on the host cells to enable attachment and effacing lesions characteristic of enterohaemorrhagic and enteropathogenic strains of E. coli | [239] |
| Injectisome protein | Connects the pathogenic bacteria to the eukaryotic host cells, and allows proteins to enter enterocytes | [239] |
| STa Oligopeptide Toxin and A1B5 Heat-Labile Enterotoxin | Induces disruption of electrolyte and water homeostasis by modulating ion transport mechanisms, leading to watery diarrhea | [240] |
| Vero toxin or Shiga toxin | Interferes with protein synthesis by targeting ribosomal function to cause blood vessel and tissue damage within the intestinal and renal systems | [241] |
| HlyA toxin | Contributes to tissue invasion by lysing red blood cells and host cells, promoting bacterial dissemination, thus damaging host tissues. | [242] |
| Type of Probiotic | Administration | Probiotic Dose | E. coli Challenge Strain | Effect on E. coli Colonization | References |
|---|---|---|---|---|---|
| L. plantarum B1 | In feed | 2 × 1012 CFUs/kg added to basal diet | E. coli K88 | Decreased E. coli colonization in cecal digesta by 0.3 log10 | [260] |
| L. bulgaricus | Oral gavage | 3 × 109 CFU/mL | E. coli O157:H7 | Decreased colonization in the cecum | [261] |
| Bacillus subtilis (Calsporin®) | In feed | 0.1% B. subtilis in diet | E. coli strain PTCC-1399 | Reduction in E. coli colonization by 1.7 log10 CFUs/g | [262] |
| B. subtilis PY79 | In feed | 2 × 109 CFU/kg of diet | E. coli O78:K80 | Decreased E. coli colonization by 3.2 × 107 CFUs/g | [248] |
| E. faecalis-1 | Oral gavage daily for three days | 1 × 108 CFU | E. coli O78 | No significant change | [225] |
| Microencapsulated E. faecalis | In feed | 1 × 1010 CFU/kg of diet | E. coli K88 | Reduction in E. coli colonization by 0.5 log10 CFUs/g | [250] |
| E. faecium NCIMB11181 | In feed | 5.1 × 1010 CFU/kg of diet | E. coli O78 | E. coli reduction of approximately 2 log10 CFUs/g in liver | [251] |
| E. faecium HJEF005 | In feed | 109 CFUs/kg of feed | E. coli K88 | E. coli reduction of approximately 0.27 log10 CFUs/g in the cecum | [252] |
| C. butyricum HJCB998 | In feed | 2 × 107 CFUs/kg of feed | E. coli K88 | Decreased cecal E. coli colonization by approximately 1 log10 CFUs/g | [263] |
| Probac Plus (L. acidophilus, L. plantarum, L. bervis, L. bifidobacterial, and S. cerevisae) | In feed for 1–7 days | 0.5 g/kg | E. coli K88 | Decreased colonization by approximately 5.4 × 106 CFUs | [264] |
| S. cerevisiae | In feed | Not specified | E. coli strain isolated from infected birds | Reduced E. coli fecal load by 2.4 × 108 CFUs | [265] |
| Lacticaseibacillus rhamnosus GG | Oral inoculation | 108 CFUs/bird | E. coli O78 | Reduction of E. coli colonization by approximately 1.6 log10 CFUs in the cecum, and inhibition of colonization in the heart and liver | [257] |
| Lacticaseibacillus rhamnosus GG ATCC 53103 | In feed | 106 CFUs/g of feed | E. coli isolated from clinically infected ducks | Reduction of E. coli colonization by approximately 1.5 log10 CFUs/g in the heart, 1 log10 CFUs/g in the lungs, and 0.5 log10 CFUs/g in the kidneys | [256] |
| B. lactis Bb12 | Oral inoculation | 108 CFUs/bird | E. coli O78 | Reduction of E. coli colonization by approximately 0.6 log10 in cecal contents | [257] |
| Mixed strain (Bacteroides caecicola, Bacteroides plebeius, Megasphaera stantonii, Megamonas hypermegale, Megamonas funiformis, Phascolarctobacterium faecium, and Sutterella massiliensis) | Oral inoculation | 0.1 mL of gut anaerobes | E. coli O78:H4 ST117 | Inhibition of pathogenic E. coli growth in experimental chickens (with one exception) | [266] |
| L. acidophilus, B. subtilis, Bacillus megaterium, L. bulgaricus, Candida pintolopesii, and S. cerevisiae, Aspergillus oryzae, and Streptococcus thermophilus | Drinking water | 1012 CFUs/g | APEC | Noted increase in mortality, potentially due to disease and decreased immunity | [267] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Kaur, S.; Naguib, M.; Bragg, A.; Schneider, A.; Kulkarni, R.R.; Nazmi, A.; Abdelaziz, K. Major Foodborne Bacterial Pathogens in Poultry: Implications for Human Health and the Poultry Industry and Probiotic Mitigation Strategies. Microorganisms 2025, 13, 2363. https://doi.org/10.3390/microorganisms13102363
Sharma S, Kaur S, Naguib M, Bragg A, Schneider A, Kulkarni RR, Nazmi A, Abdelaziz K. Major Foodborne Bacterial Pathogens in Poultry: Implications for Human Health and the Poultry Industry and Probiotic Mitigation Strategies. Microorganisms. 2025; 13(10):2363. https://doi.org/10.3390/microorganisms13102363
Chicago/Turabian StyleSharma, Shreeya, Sukhman Kaur, Mostafa Naguib, Ari Bragg, Abigail Schneider, Raveendra R. Kulkarni, Ali Nazmi, and Khaled Abdelaziz. 2025. "Major Foodborne Bacterial Pathogens in Poultry: Implications for Human Health and the Poultry Industry and Probiotic Mitigation Strategies" Microorganisms 13, no. 10: 2363. https://doi.org/10.3390/microorganisms13102363
APA StyleSharma, S., Kaur, S., Naguib, M., Bragg, A., Schneider, A., Kulkarni, R. R., Nazmi, A., & Abdelaziz, K. (2025). Major Foodborne Bacterial Pathogens in Poultry: Implications for Human Health and the Poultry Industry and Probiotic Mitigation Strategies. Microorganisms, 13(10), 2363. https://doi.org/10.3390/microorganisms13102363








