Abstract
Poultry production has become the fastest-growing sector in global meat supply. However, the intensification of poultry farming has increased the risk of zoonotic transmission of bacterial pathogens such as Salmonella spp., Campylobacter spp., Escherichia coli, Clostridium perfringens, and Listeria monocytogenes. These bacterial agents pose major public health concerns, contributing to millions of human infections annually and substantial economic losses. Historically, antibiotic growth promoters (AGPs) were widely used to mitigate disease burden and improve poultry productivity. Yet, the global shift away from AGPs due to concerns over antimicrobial resistance has spurred interest in antimicrobial alternatives. Among these, probiotics have been explored as a promising preharvest intervention. This review investigates major bacterial foodborne pathogens associated with poultry and evaluates the practical implementation of probiotic-based strategies in modern poultry production systems, with the goal of reducing pathogen load and enhancing overall food safety.
1. Introduction
Poultry meat plays a vital role in the global food supply as a major source of animal protein. Its production has expanded dramatically in recent decades, from about 15.1 million tonnes in 1970 to roughly 103 million tonnes in 2024/25, making it the fastest-growing meat sector worldwide [1]. In 2024, the US produced 9.33 billion broiler chickens, yielding 61.1 billion pounds of live weight with a total production value of around $70.2 billion [2]. This rapid growth underlines poultry’s economic and nutritional importance. At the same time, intensive poultry production also heightens the risk of zoonotic transmission, as poultry flocks and products can harbor zoonotic pathogens [3,4].
Several bacterial agents, such as Campylobacter spp., Salmonella spp., Escherichia coli, Clostridium perfringens, and Listeria monocytogenes, are frequently associated with poultry and pose food safety risks. Bacterial-induced foodborne illnesses have a significant health and economic burden. According to the U.S. Department of Agriculture (USDA) Economic Research Service (ERS), 15 major pathogens are responsible for 95% of reported foodborne illnesses, resulting in a total economic burden of $15.6 billion. Campylobacter jejuni is cited as the most common bacterial cause of human gastroenteritis globally [5], where poultry products contribute to a staggering 70% of its transmission [6]. Similarly, Salmonella is responsible for ~94 million human gastroenteritis cases and ~155,000 deaths each year [7], making it one of the most important foodborne pathogens in poultry. Non-typhoidal Salmonella serotypes (e.g., S. Enteritidis, S. Typhimurium) persist widely in poultry and eggs. Contaminated table eggs are a leading source of S. Enteritidis, with 70% of human infections attributed to this serovar [8]. L. monocytogenes is less common in poultry, but it causes invasive listeriosis with roughly a 20% case-fatality rate. Sporadic listeriosis cases have been traced to contaminated poultry, being isolated at multiple points in the poultry production environment [9]. Virulent strains of C. perfringens cause necrotic enteritis (NE) in chickens, an intestinal disease that can cause poultry mortality up to ~30%, costing the global poultry industry over $6 billion annually [10]. While C. perfringens strains in poultry differ from classic food poisoning strains, the heat-resistant spores can survive cooking and increase carcass contamination. Finally, pathogenic E. coli strains, particularly avian-pathogenic E. coli (APEC), cause colibacillosis in birds, inflicting heavy production losses. In the US alone, APEC infections are estimated to cause roughly $40 million per year in losses from condemned birds [11]. From a food-safety standpoint, improper handling or undercooking allows these strains to cause urinary tract infections in humans.
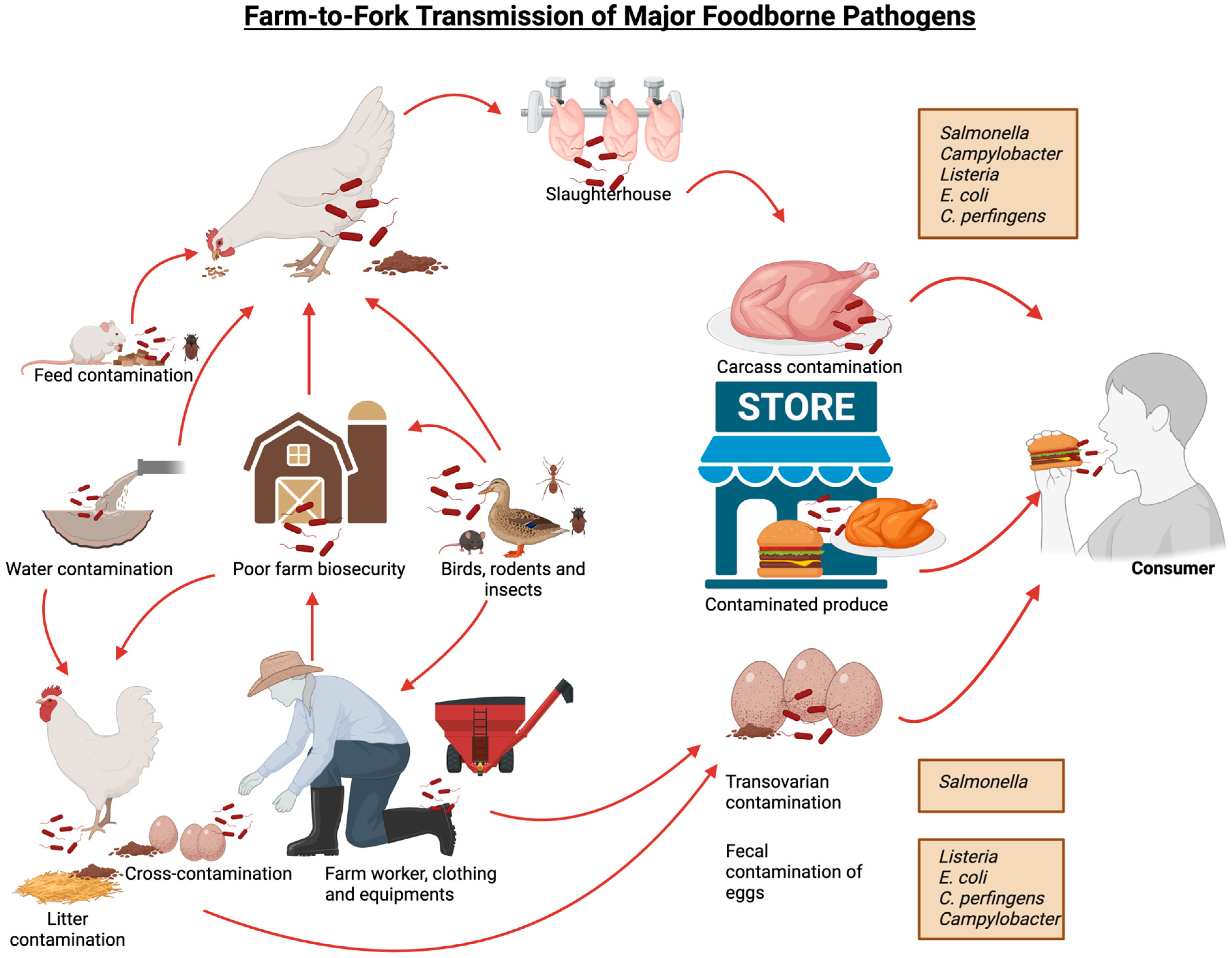
The combined impacts of these pathogens create a dual burden. For poultry producers, disease outbreaks lead to direct losses: high mortality, reduced growth or egg production, and costs for treatment, culling, or sanitation. For example, E. coli and Salmonella outbreaks often force whole flock culling or product recalls. One recent report in 2023 estimates that in the US, foodborne illnesses alone cost about $75 billion annually in economic loss, in large part due to bacterial pathogens. Among these major pathogens, Salmonella alone accounts for $17 billion, Campylobacter for $11 billion, Listeria for $4 billion, diarrheagenic E. coli for $3.7 million and C. perfringens for $343 million in associated costs [12,13]. The impact of these infections is widespread, with an estimated 49 million cases occurring annually in the US, affecting approximately 15% of the population. Reports indicate that foodborne pathogens caused approximately 350 million cases of illness globally in 2010, with food animals serving as asymptomatic carriers of these bacteria. Among the 86 known transmissible diseases from animals to humans, 20 are bacterial in origin [14]. Figure 1 illustrates the different pathways of transmission for these foodborne pathogens.
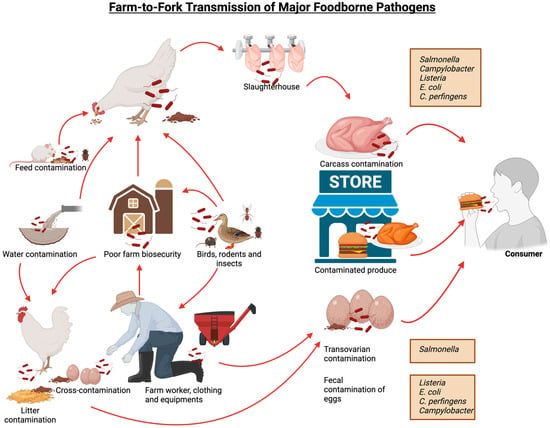
Figure 1.
Farm-to-fork transmission pathways of different bacterial foodborne pathogens.
Historically, antibiotic growth promoters (AGPs) were used to mitigate disease burden, owing to their benefits in reducing subclinical infections and reducing mortality rates, as well as enhancing feed efficiency and growth, in production facilities [15]. Although antibiotics serve important therapeutic purposes, their prolonged sub-therapeutic use (low doses administered over extended periods) is associated with public health concerns, such as antibiotic resistance, environmental antibiotic pollution and antibiotic residues in animal-derived products [16]. The US, EU, Canada, and Mexico, among several leading countries, have placed bans or strict limitations on subtherapeutic use of antibiotics as growth promoters [17].
Thus, alternative preharvest interventions are urgently sought [3,18,19,20,21,22,23]. Among these, probiotic feed additives have attracted attention. Probiotics are defined as “live, beneficial microbes that confer health benefits to the host.” [24]. Probiotics work by multiple complementary mechanisms to inhibit pathogens in the gut and improve gut health. Probiotic bacteria colonize the gut and occupy niches or adhesion sites, outcompeting pathogens for nutrients and space [18,19,20,25,26]. Many probiotics (especially lactic acid bacteria) secrete organic acids (lactic, acetic, etc.) and bacteriocins that directly inhibit pathogens [24,27]. Additionally, they produce useful metabolites, enzymes and induce mucin and defensin secretion, improve intestinal integrity, helping prime both innate and adaptive immunity against enteric pathogens [28,29].
Probiotic species belonging to Lactobacillus, Streptococcus, Bacillus, Bifidobacterium, Enterococcus, Aspergillus, Candida, and Saccharomyces have been proven to exert beneficial effects on broiler production [29]. In the US, probiotics are generally marketed as GRAS (Generally Recognized As Safe) feed additives or animal health products [30]. The selection of probiotics for use in poultry relies on a set of criteria to ensure both biological efficacy and industrial feasibility. Probiotic strains are often inhabitants of the gut microbiota, but they cannot be called ‘probiotics’ until they are well-characterized and demonstrate health benefits both in vivo and in vitro [24]. Selection criteria involve rigorous testing, such as evaluation of acid and bile salt tolerance, antimicrobial activity, adhesion capacity to intestinal epithelial cells, and viability during storage and processing [27,31,32,33,34,35]. Moreover, practical factors like appropriate dosing (≥106–109 CFU per bird), production stage of the bird and compatibility with other feed additives such as prebiotics should be taken into consideration. In this review, we highlight the significant burden posed by major bacterial foodborne pathogens in poultry, namely Salmonella, Campylobacter, E. coli, L. monocytogenes, and C. perfringens and underscore probiotic control strategies to enhance poultry health and food safety.
2. Major Food-Borne Pathogens in Poultry and Probiotic Mitigation Strategies
2.1. Salmonella
Salmonellosis is a foodborne disease that primarily causes diarrhea in humans [5]. Globally, non-typhoidal salmonellosis (NTS) has been reported to cause about 93 million cases of gastroenteritis and 155,000 deaths annually [34]. The Centers for Disease Control and Prevention (CDC) has estimated that it is responsible for 1.35 million infections and 420 deaths annually in the United States [35]. Estimates from the WHO Foodborne Disease Burden Epidemiology Reference Group (FERG, 2007–2015) show that NTS and invasive non-typhoidal salmonellosis (iNTS) are responsible for 4.38 million and 3.9 million Disability-adjusted life years (DALYs), respectively [36], a metric commonly used to measure the impact of a disease or illness on population health. Recently, the Global Burden of Disease (GBD) reported that iNTS caused 594,000 infections, leading to 79,000 deaths and 6.11 million DALYs worldwide [37]. This overlays an annual economic burden of $3.31 billion, with disease severity in humans varying by strain, health status and age.
Poultry meat and eggs are the primary carriers of Salmonella [5], and they cause an annual loss of $64 to $114 million to the US poultry industry. Between 1998 and 2008, poultry was linked to 17.9% of foodborne illnesses in the U.S., with S. Enteritidis and S. Typhimurium responsible for 17% and 34% of poultry-related infections, respectively [38].
2.1.1. Salmonella Virulence, Pathogenicity and Mode of Transmission
Salmonella is a gram-negative, oxidase-negative, non-spore-forming, facultative anaerobic bacterium belonging to the Enterobacteriaceae family [35]. Its cell wall is composed of lipids, lipopolysaccharides, proteins and lipoproteins [39]. The bacterial surface possesses a polysaccharide somatic (O) antigen, composed of short oligosaccharides [40]. Salmonella is classified based on roughly 60 somatic antigens. Additionally, it possesses proteinaceous and heat-labile flagellar (H) antigens. Based on agglutination reactions of O and H antigens, approximately 2643 serotypes of the pathogen were recognized by 2000, a number that has risen to nearly 3000 by 2017 [41,42].
The genus Salmonella is divided into two species, S. enterica and S. bongori [41,43]. Among them, S. enterica is of primary clinical and veterinary importance, and is divided into six subspecies: S. enterica subsp. enterica (I), S. enterica subsp. salamae (II), S. enterica subsp. arizonae (IIIa), S. enterica subsp. diarizonae (IIIb), S. enterica subsp. houtenae (IV), and S. enterica subsp. indica [41,44]. These species are further divided into serovars, which differ in host adaptation, pathogenicity and epidemiological distribution. Salmonella serovars are classified as typhoidal and non-typhoidal based on the type of disease they cause. Typhoidal serovars such as S. Typhi and S. Paratyphi, are strictly adapted to human hosts and are responsible for causing enteric fever, commonly known as typhoid fever, through the fecal-oral route. Contrastingly, non-typhoidal Salmonella is zoonotic and can infect many animals, including birds, reptiles, dogs, cats, rodents, sheep, poultry, and pigs [45]. The common non-typhoidal serovars reported in human and poultry infections include S. Enteritidis, S. Typhimurium, S. Heidelberg, S. Kentucky, and S. Gallinarum [46]. Among these, S. Enteritidis is the most common serovar linked to human salmonellosis outbreaks and is strongly associated with vertical transmission in hens, contaminating eggs and egg products [47]. 70% of S. Enteritidis infections are caused by grade A table eggs. S. Typhimurium has a broader host range, infecting many warm-blooded animals besides poultry, and is commonly found in poultry meat and slaughter environments [8]. Additionally, S. Heidelberg is frequently isolated from all poultry production systems in North America and increasingly associated with multidrug resistance and human salmonellosis. S. Kentucky is present in dairy cattle and broiler chickens [48]. S. Gallinarum is host-restricted to birds, causing fowl typhoid and pullorum disease, which resulted in severe losses to the poultry industry in the US; however, it was entirely eradicated by the 1960s due to the continual efforts of the National Poultry Improvement Plan (NPIP) [47,49].
Contaminated broiler chicken meat is a major source of Salmonella infection in humans. Hassanein et al. (2011) tested 75 samples of beef and chicken and observed that 20% of minced frozen beef, 36% of frozen chicken leg and 52% of the frozen chicken fillets were contaminated with prominent Salmonella serovars like S. Enteritidis and S. Kentucky [50]. Table 1 summarizes the classification of Salmonella serovars.

Table 1.
Based on their host specificity, Salmonella serovars are classified into the following three groups.
Salmonella can be transmitted to poultry via two routes: horizontal and vertical transmission, with horizontal being the most common. In the horizontal transmission, Salmonella spreads between birds through the fecal-oral route, skin wounds, blood, mating, and contaminated equipment [55]. Vectors, including cockroaches, poultry mites (Dermanyssus gallinae), litter beetle (Alphitobius diaperinus), lizards and rodents, are sources of Salmonella infection in poultry [56,57,58]. Several wild birds like passerine birds also serve as asymptomatic carriers of Salmonella, transmitting Salmonella serotypes to poultry during migration, seasonal movements and feeding [59,60,61]. On the other hand, vertical or transovarial transmission occurs when bacteria from infected hens are transferred to their hatched chicks. Salmonella invades reproductive organs, reaching the yolk, vitelline membrane and albumen before eggshell formation and laying [62]. The digestive tract of chickens is recognized as a primary multiplication site for Salmonella, from where the organism is excreted through feces, leading to widespread environmental contamination [63].
These infected chickens and their products, including contaminated meat and eggs, are the primary source of Salmonella infection in humans. Factors like serotype, infective dose of the pathogen and immune status of the host determine the severity of disease in humans. Usually, children below five years of age and immunosuppressed individuals are more vulnerable to infection [64]. Upon infection with pathogenic strains of Salmonella, the host phagocytic cells, mainly macrophages, engulf the bacterial cells, where they get encapsulated in the vacuole. While recognizing bacterial components by host cells typically triggers lysosomal fusion and enzymatic degradation to eliminate invading microbes, Salmonella exhibits resistance to this process by injecting effector proteins into the vacuole through the Type III secretion system, consequently altering the compartment structure. This altered vacuole structure prevents lysosomal fusion and allows the bacteria to survive and multiply within phagocytic cells, causing gastroenteritis [65]. NTS infections can also cause cholecystitis, pancreatitis and appendicitis [39]. Some serotypes like S. Dublin and S. Cholearsuis can enter the bloodstream and cause bacteremia, a condition associated with high fever and, in severe cases, can lead to septic shock and other complications, such as urinary tract infections, pneumonia, endocarditis and meningitis [7,66]. Classification of Salmonella pathogenicity islands (SPIs), the regions on the Salmonella plasmid encoding several virulence factors, and the virulence factors are given in Table 2 and Table 3, respectively.

Table 2.
SPIs: Regions on the Salmonella plasmid encoding several virulence factors.

Table 3.
Other virulence factors involved in Salmonella infection.
2.1.2. Probiotic Efficacy Against Salmonellosis in Poultry
Several preharvest and post-harvest interventions have been adopted to combat Salmonella infections in poultry. Preharvest interventions include management at the farm level, use of feed additives and biosecurity measures. Biosecurity measures include site cleanliness, immunization, boot sanitization, hand hygiene, better rodent, fly and red mite control, and disinfection between the flocks. Antibiotic incorporation is another pre-harvest strategy that has been used in poultry since the 1940s due to its rapid and effective action in controlling enteric pathogens and enhancing the growth performance of the host [81]. Although antibiotics like penicillin, tetracycline, enrofloxacin, ciprofloxacin, azithromycin, ceftriaxone, chloramphenicol, neomycin, polymyxin, nitrofurazolidone, amoxicillin, and chloramphenicol have been used to treat salmonellosis [35,82,83], non-antibiotic-based approaches like probiotics, prebiotics, synbiotics, postbiotics and phytobiotics offer low toxicity, high safety, efficient metabolism, and no environmental contamination. The bioactive derivatives of these compounds, such as unsaturated fatty acids, proteins, polysaccharides, and alkaloids, can be metabolized by the host and its gut microbiota, thereby reducing overall metabolic load on the liver and kidneys. These natural antimicrobial alternatives also disturb bacterial sensing mechanisms by altering biofilm permeability, which affects bacterial survival and growth in host cells. Additionally, they promote the secretion of antibacterial compounds such as inhibitory peptides, defensins, lysozyme and colloidal mucin by the host immune cells, as well as activating the absorption of nutrients in the intestine and balancing the synthesis of intestinal hormones [84,85,86]. Owing to these benefits, there is an increasing interest in the utilization of feed-based additives like probiotics against Salmonella infections in poultry [87,88,89].
Probiotic bacteria belonging to the genera Bifidobacterium, Lactobacillus, Bacillus, Pediococcus and Enterococcus are used to control salmonellosis in poultry [89]. Their demonstrated efficiency lies in their ability to restore gut microbiota and increase the accumulation of short-chain fatty acids (acetate, butyrate and propionate) in Salmonella-infected chickens. Moreover, probiotics can improve the effectiveness of Salmonella vaccines used in poultry, which intensifies the defensive effects of the body’s immune system [90].
Over recent years, Bacillus probiotics have been recognized as effective control agents for salmonellosis in poultry [28,91,92,93]. Spore formers are highly resistant to harsh and dry physical and chemical conditions and bile concentrations and can withstand the intestinal temperatures of the chickens [94]. B. subtilis is one of the most used Bacillus species in the animal feed industry [95]. It is an effective growth promoter and modulator for the gut microbiota population and the innate immune response of broiler chickens [96,97]. B. subtilis produces certain antimicrobials and favors the growth of gut-beneficial microorganisms that reduce invasive pathogens by CE [96]. A previous study showed that the dietary supplementation of B. subtilis QST-713 in Salmonella Gallinarum-challenged chickens improves their growth performance by modulating gut mucosal microarchitecture, including villus height. The treated birds also had less disease severity, a lower mortality rate and improved weight gain [98]. In a study conducted by Knap et al. (2011), the inclusion of B. subtilis (DSM17299) resulted in a 3-log reduction in cecal S. Heidelberg counts in birds, with a transient decrease in fecal shedding of the pathogen [99]. Moreover, B. subtilis can improve systemic immune responses by producing α and β-defensins, supporting host innate immunity [100], promoting immunoglobulin synthesis and improving chicken intestinal microarchitecture. Likewise, Sikandar and colleagues observed an increased bursa follicular area (165.757 μm2), delayed B-cell apoptosis, increased thickness of the thymus cortex and medulla and higher antibody titers against Newcastle disease virus (NDV) in S. Gallinarum-infected chickens receiving B. subtilis-inoculated diet [101]. Contrastingly, birds fed a regular diet had a bursa follicular area of about 59.124 μm2, decreased thymus medulla thickness and reduced germinal area of the spleen. B. methylotrophicus, another novel Bacillus species, possesses probiotic characteristics; therefore, it is used as a feed supplement in poultry as well as livestock production. For instance, in a study, the feed supplementation of either B. subtilis RX7 or B. methylotrophicus C14 at 0.1% of the diet (~1 × 109 CFU/g) resulted in a significant reduction of Salmonella counts in the excreta in S. Gallinarum-infected (1 mL containing 108 CFU/mL) Hy-Line Brown laying hens [102]. The authors also reported an increase in the beneficial intestinal microbiota, such as Lactobacillus, of the treated hens. Hosseindoust and colleagues also observed an improved eggshell strength and thickness, increased intestinal populations of Lactobacillus spp., and a reduction in cecal and fecal shedding in S. Gallinarum-infected Hy-line laying hens receiving a B. subtilis (strain B2A) supplemented diet [103].
Bacillus licheniformis can inhibit enteric pathogens in poultry by secreting antimicrobial surfactant molecules, serine protease, and other enzymes [104]. As a probiotic, B. licheniformis has been shown to improve bird performance, stimulate immunoglobulin production and enhance mucosal barrier function [27], and it can effectively interfere with colonization by pathogenic microbes, including Salmonella species. A recent study found that dietary inclusion of B. licheniformis and B. subtilis to broilers challenged with S. Enteritidis resulted in 0.73, 1.59 and 1.32 log decreases in cecal Salmonella CFU/g at 5, 12 and 21 days, respectively, post-infection [105]. Similar results were obtained in a later study conducted by [106], where feeding the chickens infected with S. Enteritidis a diet supplemented with a mixture of three strains of bacterial probiotic led to a 1.08 log10 reduction in cecal Salmonella content as compared to the control.
Bacillus coagulans is also commonly used as a probiotic, producing bacteriocin-like substances (coagulin) against various pathogens, including Salmonella. It can also enhance intestinal barrier integrity and gut morphology [107,108]. Zhen et al. (2018) studied the effect of B. coagulans in broiler chickens challenged with S. Enteritidis [109]. They observed that its supplementation increased alkaline phosphatase activity, villous height and the number of goblet cells in the jejunum [109]. Moreover, the birds fed a supplemented diet had 0.24, 0.41, and 0.24 log10 less Salmonella counts after 7, 17 and 31 days of infection compared to birds receiving a regular diet.
Bacillus cereus has also been observed to stimulate immune responses, typically macrophages, by enhancing phagocytosis, nitric oxide production and secretion of pro-inflammatory cytokines [110]. It also improves the digestion and absorption of food, which eventually results in improved FCR and broiler weight, as studied by Vilà et al. (2009) [111]. The results of this study indicated that the addition of Toyocerin (containing 109 viable spores/g of B. cereus var. toyoi NCIMB 40112/CNCM I-1012 per gram) in the feed decreased cecal Salmonella load by 100% (42 days post-infection) and improved average daily weight gain (ADG) by 3.4 g, broiler weight (BW) by 141 g and feed conversion ratio (FCR) by 0.060kg/kg in S. Enteritidis-infected broilers.
Bacillus amyloliquefaciens also possesses antimicrobial activity as it can secrete several enzymes, such as barnase, cellulase, protease, amylase and xylanase [112]. For example, Poudel et al. (2025) observed a respective 0.40 and 0.60 log10 reduction in cecal Salmonella content (after 7- and 17-days post-infection) when the birds inoculated with S. Enteritidis were fed a diet containing a mixture of three strains of dried B. subtilis and two strains B. amyloliquefaciens in different concentrations (1 × 106 CFU/kg diet (PRO1) and 2 × 106 CFU/kg diet (PRO2) [113].
Pediococcus pentosaceus GT001 is another promising probiotic strain. When it was included in the diet of S. Typhimurium-infected chickens, it decreased mortality by 6.7% in treated chickens compared to the birds fed with a regular diet, where this decrease was only 3.3%. Beyond this, it also improved growth performance, immune function and intestinal morphology in broilers and brought a 1.2 log reduction in Salmonella count at 14 days post-infection as compared to untreated birds [114]. Khochamit et al. (2020) also conducted a study to examine the synergistic effect of bacteriocin-producing strains of B. subtilis KKU213 and P. pentosaceus NP6 in improving the growth, microbial community and health in broilers [115]. The authors observed that feeding a probiotic-inoculated diet to broilers decreased mortality; however, no Salmonella was detected in the treated birds (on day 18 post-treatment) compared to the control group, where Salmonella was 20%.
Dairy propionibacteria are a group of bacterial species involved in dairy technology and offer in vivo benefits [116]. Their probiotic properties lie in the fact that they can survive and maintain metabolic activity in the gastrointestinal tract. They produce bifidogenic molecules such as 1,4-dihydroxy-2-naphtoic acid (DHNA) and 2-amino-3-carboxy-1,4-naphthoquinone (ACNQ) that promote the growth of intestinal bifidobacteria and inhibit the growth of toxin-producing Bacteroides spp. and Clostridium difficile [117]. It was reported that P. freudenreichii, when administered to S. Heidelberg GT2011-infected turkeys, reduced the pathogen levels in cecal content by about 1 to 2 log CFU/g. Although the effect lasted only two days, supplementation with probiotics increased lactobacilli and Ruminococcaceae after 2 days. It increased the genera Lactococcus, Erysipelatoclostridium, Leuconostoc, and Butyricicoccus after 7 days [118]. It was later reported that in treatments of turkeys with P. freudenreichii B3523, the dissemination of S. Heidelberg in the liver and spleen was reduced from 20% to 60% depending on the age of animals and treatment time [118]. A recent study also observed that P. freudenreichii B3523 significantly reduced Salmonella dissemination in the liver and spleen of turkeys challenged with drug-resistant field turkey isolates of S. Agona, S. Saintpaul, and S. Reading. When combined with a Salmonella vaccine, it eliminated the pathogen from these organs. This synergistic effect of the vaccine and probiotic was found to be more effective than the vaccine alone or when used in combination with Ligilactobacillus salivarius in combating multidrug-resistant Salmonella strains in turkeys [119].
Among Lactic Acid Bacteria (LAB), L. plantarum has the largest genome and is commonly used in fermented foods [120]. A previous study observed that administering a mixture of L. plantarum and B. subtilis decreased the mortality rate of S. Typhimurium-infected chickens and increased body weight gain and feed conversion ratio. When used individually or in combination, it resulted in the complete elimination of Salmonella in the liver, spleen and heart of the chickens at 28 days post-infection [121]. Similarly, other LAB strains have also been used as probiotics in poultry feed. In a study conducted by Smialek et al. (2018), the incorporation of probiotic Lavipan (consisting of four strains of LAB, i.e., Lactococcus lactis IBB 500, Carnobacterium divergens S-1, L. casei ŁOCK 0915, L. plantarum ŁOCK 0862 and a yeast Saccharomyces cerevisiae LOCK 0141 resulted in a 98.84% reduction in cecal Salmonella content at 42 days of age as compared to 58.29% reduction by untreated birds [122]. In a later study, Adhikari et al. (2019) evaluated the impact of in-feed supplementation of L. plantarum (0.05% and 0.10% (w/w) on fecal shedding, cecal and internal organ colonization of S. Enteritidis-infected (2.8 × 108 CFU/mL) White Leghorn laying hens [123]. The authors reported no significant reduction in cecal S. Enteritidis colonization 7 dpi since the mean counts in all challenged groups were around 3.7 log10 CFU/g, but a downregulation of IFN-γ and upregulation of IL-6 and IL-10 were observed in the group receiving a higher dose of probiotic (0.10% (w/w)).
L. acidophilus is another widely used LAB that can tolerate low intestinal pH, competitively excludes enteric pathogens by competing with them for nutrients and attachment sites and modulates immune mechanisms. Similarly, L. salivarius has been known for its immune-modulatory and anti-inflammatory properties when used as a probiotic. Bielecka et al. (2010) compared the effects of the incorporation of different strains of LAB, including L. acidophilus strain BS, L. salivarius strain AWH, L. helveticus strain b9, Bifidobacterium longum strain KNA1 and Bacillus longum in S. Enteritidis-infected chickens [124]. The authors found that these probiotic strains promoted superoxide anion production, stimulated leukocyte multiplication, and increased lysozyme and γ-globulin levels in chickens.
In an earlier study, Khaksefidi and Rahimi (2005) also studied the impact of a multi-strain probiotic diet supplementation consisting of L. acidophilus, L. casei, Bifidobacterium bifidum, Aspergillus oryzae, Streptococcus faecium and Torulopsis spp. on chicken health. They observed that feeding chickens with a probiotic-inoculated diet increased their weight and decreased the mortality rate by 3.2% compared to the control group [125]. Moreover, only 40% of carcass meat from treated chickens tested positive for Salmonella compared to 100% in the untreated group.
Furthermore, yeasts are another probiotic candidate known for their antimicrobial properties. Among all species, S. cerevisiae is one of the most frequently used strains to inhibit enteric pathogens. In an earlier study conducted by Mountzouris et al. (2015), it was observed that the Salmonella load on breast skin and cloacae of Salmonella enterica birds fed S. cerevisiae var. boulardii CNCM I-1079 was approximately 28.6% and 33.3% less, respectively, compared to birds fed a standard diet [126]. Similarly, in a later study, it was reported that supplementing probiotics containing B. amyloliquefaciens, B. licheniformis, and B. pumilus (454 g/ton) and yeast culture (1133 g/ton) to the feed of S. Enteritidis-infected Hy-Line Brown pullets resulted in 0.79 log10 and 0.86 log10 reduction in cecal Salmonella content, respectively, as compared to the control groups [127]. However, in a study conducted by El-Hamd and Hans (2016), the use of a probiotic blend comprising L. plantarum, L. acidophilus, and S. cerevisiae in the drinking water of S. Enteritidis-infected broilers didn’t reduce cecal Salmonella colonization, as the treated birds still harbored the pathogen [128].
Table 4 highlight the promising role of both bacterial and yeast-based probiotics in reducing Salmonella colonization and improving growth performance in poultry, supporting their integration as single-strain or multi-strain probiotics into pre-harvest food safety strategies.

Table 4.
Probiotics’ effectiveness against Salmonella serotypes in broilers.
2.2. Campylobacter
The risk of transmission of foodborne illnesses, such as C. jejuni, to humans through contaminated poultry meat and its products is considered a significant challenge for sustainable poultry production. Thermophilic Campylobacter species, mainly C. jejuni and C. coli, are commonly found in wild birds and domestic fowl (e.g., chickens, turkeys, ducks, and geese) [63,130]. The detection rate of Campylobacter might reach 100% in broiler flocks at slaughterhouses [63]. Despite the high colonization rate (up to 109 CFU/g of cecal contents), Campylobacter infections generally cause little or no clinical disease in chickens [131]. However, the transmission of this pathogen through contaminated poultry products to humans is estimated to cause 1.5 million foodborne infections annually in the US [132]. Though Campylobacter is a self-limiting disease in humans, infection in immunocompromised individuals could lead to severe health complications, such as reactive arthritis, inflammatory bowel disease, and Guillain-Barré syndrome [133] with a hospitalization rate of 10% [134] and 0.2% deaths [135]. The health care costs incurred by this disease in humans were initially estimated at two billion dollars in the US [136]. However, a recent study revealed a substantially increased burden of 11 billion dollars annually [137]. Humans are primarily infected through undercooked poultry meat (50–80%), contaminated food and water or direct contact with infected animals [138].
With the lack of Campylobacter vaccines, other strategies, including feed additives, are being explored to control this pathogen in poultry. Due to their natural ability to inhibit pathogens and modulate host immunity, probiotics have emerged as a promising solution [139,140,141].
2.2.1. Campylobacter Virulence, Pathogenicity and Mode of Transmission
Upon ingestion of contaminated feed and water, Campylobacter colonizes the avian gastrointestinal tract within 24 h, with a small infectious dose of 35 CFUs that can successfully colonize chickens’ guts [142]. Flagellum is a key structure that facilitates C. jejuni motility, adherence and invasion [143]. With its spiral shape, bipolar flagella enable C. jejuni to move in a corkscrew motion and facilitate its penetration of the mucin viscous layer [144]. Chemotaxis is a crucial process that guides C. jejuni to favorable sites for colonization. C. jejuni exhibits chemotactic responses to a variety of attractants, including intestinal mucins, amino acids, carbohydrates, and organic acid salts [145]. To identify these chemical signals, the bacterium uses methyl-accepting chemotaxis proteins (MCPs), including the colonization protein B (DocB) determinant and transducer-like protein 1 (Tlp1). The signal transduction between MCPs and the flagellar motor is mediated by the chemotaxis-regulating protein Y (CheY) [146]. A physical barrier comprising a mucus layer that lines the intestinal epithelium with secretory IgA and antimicrobial peptides, allowing commensal microbes to thrive but preventing excessive interaction [147]. The activity of flaA is downregulated with the viscosity of this layer. To overcome this, C. jejuni modifies its flagella and successfully penetrates the mucus layer [148]. C. jejuni adhesion to epithelial cells necessitates several factors, such as intact flagella, Campylobacter adhesion to fibronectin (CadF), and lipooligosaccharide (LOS). Following adhesion, the flagella apparatus increases the secretion of Campylobacter invasion antigen (Cia) [143]. Simultaneously, LOS enables C. jejuni to evade the host immune response in humans and facilitate its invasion [149].
Despite chickens asymptomatically harboring a high load of C. jejuni in their gut, upon ingesting a small dose of 500–900 CFUs in contaminated poultry products, humans experience severe diarrheal illness. The underlying mechanisms behind this controversy of the pathogenicity between human and avian hosts are not entirely understood. Initial evidence underscores mucus composition, acidity and function as a key point in these diverse outcomes [150]. Byrne et al. (2007) conducted an in vitro study that showed the ability of C. jejuni to adhere and invade epithelial cells [151]. The same group further explored the effect of chicken and human crude mucus on C. jejuni internalization. Chicken mucus was found to reduce the binding and internalization of C. jejuni into human epithelial cells, whereas human mucus enhanced both processes. Unique O-linked glycan structures seen in chickens’ mucins directly disrupt the adherence of C. jejuni. With lesser inhibitory effects shown from small intestinal and cecal mucins, purified chicken mucins from the large intestine diminish bacterial binding to human colonic epithelial cells (HCT-8) by 60–70% [152]. While the MUC2 backbones are similarly structured in human mucins [153], the absence of specific glycans leaves the mucus vulnerable to C. jejuni invasion [147]. Additionally, pathogen surface proteins are effectively impeded by the extensive sialylation and fucosylation patterns observed in chicken mucins [152]. It is hypothesized that the divergent body temperature of chickens (42 °C), compared with human temperature (37 °C), crucially influences the pathogenicity of C. jejuni in both hosts [150]. Human temperature facilitates the expression of virulence factors, such as the zinc exporter CzcD, to counteract the host defense mechanism [154]. However, Zhang and his team revealed that the upregulation of the CadF gene, which controls cell adhesion, is upregulated by C. jejuni at 37 °C and 42 °C, thus demonstrating that C. jejuni can adhere to both human and chicken intestinal cells [155]. Nevertheless, these gene expression variations do not fully explain the distinct inflammatory pathogenesis of C. jejuni in humans and its near-symbiotic colonization in chicken hosts. It is well-documented that host-specific immune response could justify the paradox in the C. jejuni virulence in humans vs. chickens. Most C. jejuni isolates encode for cytolethal distending toxin (CDT). However, immune cells produce neutralizing antibodies against this toxin, implicating its role in human pathogenesis [156]. Additionally, CDT-negative C. jejuni mutants successfully colonize the gut of chickens as wild-type strains, confirming the previous outcome [157]. At the same time, chickens may experience induced proinflammatory cytokines post-C. jejuni inoculation without further heterophil infiltration, robust recruitment of neutrophils and macrophages initiated by TLR-4-mediated recognition of LOS was observed in humans [158]. Another key mechanism by which Campylobacter evades the human immune system is the structural mimicry of C. jejuni LOS and nerve gangliosides, which can ultimately trigger Guillain-Barré syndrome; however, this immune evasion is not observed in chickens. Overall, in chicken infections, it seems that C. jejuni resides in the mucus layer without exposure to intestinal epithelial cells with a state of immunological tolerance, while in humans, it invades the intestinal layer and provokes acute disease. Collectively, it could be concluded that host-specific mucus composition, body temperature, gene expression profile, and immune responses lie behind the contrast of pathogenicity reported in human vs. chicken hosts. Virulence factors associated with C. jejuni pathogenicity are summarized in Table 5.

Table 5.
Key virulence factors associated with Campylobacter jejuni.
2.2.2. Probiotic Efficacy Against Campylobacteriosis in Poultry
Several pre-harvest mitigation strategies against Campylobacter have been investigated in poultry, including biosecurity, bacteriophage therapy, vaccination, bacteriocins, prebiotics, and probiotics [139,141,167]. The limited abilities of the investigated vaccines to elicit cross-protective immunity against various strains of Campylobacter lie in the antigenic variability among these strains and the unsuccessful identification of the antigenic protein/s that provide complete and cross-protection against these strains. These factors remain the primary reasons why effective and reliable immune interventions are still elusive [139,168]. As such, continued investigations are still required to improve our comprehension of the complexity of C. jejuni-host interaction and develop more resilient and broadly applicable interventions to curb C. jejuni infection in chickens. There is no evidence of Campylobacter developing resistance to probiotics, making probiotics a viable long-term strategy for mitigating this infection. However, candidate isolates should be thoroughly screened to ensure their non-pathogenic nature and effectiveness.
Numerous laboratory tests have been developed to investigate the appropriate probiotic candidate (Table 6). The laboratory screening of probiotic isolates is crucial to assess their ability to resist the extreme acidity conditions in chickens’ gut, bile salts, and enzymatic activities and, more importantly, to detect their ability to reduce the growth, invasion, and adhesion of targeted pathogens, such as Campylobacter. The well diffusion assay is mainly applied to evaluate the antagonistic effect of the probiotics’ cell-free supernatants. The presence of inhibitory metabolites. such as organic acids and bacteriocins, which are associated with producing a clearance zone on the agar. On the other hand, agar spot and co-culture suspension are primarily employed to test a live probiotic culture. Despite the beneficial outcomes of testing live probiotic culture using the co-culture suspension and agar spot, it ignores the complexity of the interactions occurring within the host. Consequently, epithelial cells are a more effective screening approach to measure the probiotics’ antagonistic effect against pathogenic bacteria such as Campylobacter. Both candidate probiotics and pathogenic bacteria are cultured with the intestinal monolayer, and subsequently, the adhesion and invasion indices are calculated. A primary concern when using probiotics is the risk of the transfer of antibiotic-resistant genes. Hence, an antibiotic sensitivity assay is widely used to confirm the absence of antibiotic-resistance-associated genes among candidate probiotics.

Table 6.
In vitro screening of probiotics with inhibitory activities against Campylobacter jejuni.
Several researchers have investigated the effectiveness of probiotics against Campylobacter infection in chickens. However, a significant variation in the results was recorded. Several factors contribute to this observed inconsistency, including strain-specific effects, variations in bird age and type, probiotic dosage and combinations, administration routes, dosage, and duration of treatment. Moreover, management and environmental factors, such as housing type and dietary program, may influence outcomes. Understanding these variables is essential for optimizing probiotic use to control Campylobacter in poultry, improve food safety and reduce the risk of human transmission.
LAB group includes several genera, such as Leuconostoc, Lactococcus, Enterococcus, Pediococcus, Streptococcus, Lactobacillus and Bifidobacterium. While in vitro assays offer valuable insight into the probiotic potential of Lactobacillus species and their antagonistic effects against Campylobacter, their efficacy has not been replicated in vivo models. For instance, a recent study showed that although L. sakei L14 demonstrated strong anti-Campylobacter activity in vitro, it failed to reduce C. jejuni counts when administered orally to broiler chickens [177]. In broiler chickens, oral administration of L. salivarius SMXD51 on day one of age, followed by repeated doses every 2–3 days, resulted in a reduction of C. jejuni counts by 0.8 log10 and 2.8 log10 on day 35, respectively [178]. In contrast, dietary supplementation of the same species to mule ducks for 79 days failed to reduce Campylobacter’s burden [179]. When microencapsulated bacteriocins, a byproduct of L. salivarius, were added to the feed, they reduced C. jejuni counts by 4 log units after three consecutive days of administration in turkeys [180]. In another study comparing the effects of various Lactobacillus species, oral administration of L. plantarum PA18A on days 1 and 4 resulted in a 1 log10 reduction in C. jejuni load. However, no reduction was observed in the groups that received L. salivarius, L. crispatus, or L. reuteri [181]. In another study, oral daily administration of L. gasseri SBT2055 for 14 days significantly reduced C. jejuni colonization by up to 2.5 log10 [182]. Similarly, Abdelaziz et al. (2019) showed that L. salivarius, L. johnsonii, L. crispatus, and L. gasseri variably suppressed C. jejuni and downregulated flaA/flaB/flhA, ciaB, and luxS while enhancing chicken macrophage phagocytosis and activation, indicating potent anti-Campylobacter and immunomodulatory potential [183].
Due to the variability in outcomes with single-species probiotics, some studies demonstrated that incorporating multiple species may provide a more effective approach. For example, administering a mixture of five probiotic species comprising L. salivarius, L. reuteri, E. faecium, Pediococcus acidilactici, and Bifidobacterium animalis significantly reduced C. jejuni count by 6 log10 compared with the control group [184].
The inclusion of Bacillus subtilis C-3102 into the diet for broilers continuously for 42 days improved their body weight and decreased Campylobacter on the processed carcass by 0.2 log10 [185]. In two trials using Cobb 500 broilers, Aguiar and his group tested a mixture of 10 isolates of Bacillus spp. through oral and intracloacal delivery to day-old chicks, and their results revealed that Bacillus delivered orally reduced C. jejuni count by 1 log10, while those delivered intacloacally reduced the C. jejuni load by 1–3 log10 [186]. The inclusion of 2.5 × 106 CFU/mL of B. subtilis PS-216 spores in drinking water from day 1 post-hatch until day 20 was also found to reduce C. jejuni by 1.2 log10 CFU/g [187]. Ismail and colleagues investigated whether the encapsulation of B. amyloliquefaciens into nanoparticles (BNPs) would boost its efficacy against C. jejuni in chickens, and their results revealed that supplementation of BNPs at a concentration of 7.5 × 105 CFU/g to the feed from 1–35 days post-hatch resulted in significant fecal and cecal reduction at 7 days post-infection by 3.8 log10 and 3.9 log10, respectively [188]. In another study, including two B. subtilis C-3102 into the feed from day 1 until day 42 reduced C. jejuni colonization by 0.25 log10 on day 14 and 1.7 log10 on day 42 of age, while B. amyloliquefaciens CECT5940 reduced it by 1.12 log10 on day 14 and 1.2 log10 on day 42 [189]. In contrast to these findings, the inclusion of B. subtilis DSM17299 in the feed from day 21 until day 42 failed to significantly reduce the C. jejuni count in broiler chickens [190].
Enterococcus faecium is a gram-positive, anaerobic bacterium that beneficially colonizes the chicken gut and produces bacteriocin metabolites. A study conducted by Netherwood et al. (1999) showed that oral administration of E. faecium NCIMB 11508 strain at one and 28 days of age failed to reduce the relative abundance of C. jejuni in naturally infected chicks [191]. In another study, adding 109 CFU/mL of E. faecium EM4 to drinking water for 21 days resulted in a 0.8 log10 reduction at 21 days post-administration and a 0.25 log10 reduction on day 35 (2 weeks after cessation of the probiotic) [192]. Dietary supplementation of E. faecium with L. acidophilus, L. casei, and B. thermophilus until 42 days post-hatch resulted in a reduction in C. jejuni abundance by 12% in naturally infected chickens [193]. Probiotic applications against C. jejuni in poultry are summarized in Table 7.
A non-pathogenic isolate of the E. coli family, E. coli Nissle (EcN), was isolated and characterized by Alferd Nissle in 1917 from human feces. Owing to its absence of virulence factors typically present in other pathogenic E. coli strains, such as invasiveness, enterotoxin or cytotoxin production [194,195], combined with its immune-modulatory properties, ability to enhance intestinal barrier function, and production of antimicrobial microcin peptides, EcN 1917 has been used as a probiotic to combat pathogenic bacteria, including Salmonella, L. monocytogenes, Shigella and Campylobacter [194,195]. In a recently conducted study by Helmy et al. (2022), chickens orally treated with free EcN in drinking water for two weeks starting at 3 weeks of age reduced C. jejuni cecal load by 2.0 log10 at week 5 of age [196]. The same group explored different administration routes, timing, and nanoparticle encapsulation of EcN, but observed no significant enhancement, as both encapsulated (9.8 × 108 CFU/bird) and free EcN (1 × 109 CFU/bird) reduced cecal C. jejuni by 2.0 and 2.5 log10, respectively, after oral administration three times weekly during weeks 4 and 5 of age [196].

Table 7.
Probiotics’ effectiveness against Campylobacter jejuni in poultry.
Table 7.
Probiotics’ effectiveness against Campylobacter jejuni in poultry.
| Breed | Strain of Probiotics | Delivery | Campylobacter Strain/Dose | Effect on Campylobacter Colonization | References |
|---|---|---|---|---|---|
| Broiler | Single strain | ||||
| E. faecium NCIMB 11508 | First-day post-hatch and day 28 orally | Naturally infected | There is no reduction in the relative abundance of Campylobacter | [191] | |
| Calsporin® (B. subtilis C-3102) | Day 1–42 in feed | Fecal contamination during processing | 0.2 log10 reduction on chicken carcasses | [185] | |
| Bacillus spp. (10 isolates individually tested) | Per os and intracloacally at one day old | C. jejuni cocktail of 4 strains (2.5 × 106 CFU) | Intracloacally: 1–3 log10 Orally: 1 log10 for only one isolate | [186] | |
| L. salivarius SMXD51 | Given orally on day one and then every two to three days for 35 days | C. jejuni C97ANSES640 (1 × 104 CFU) | 0.8 log10 at 14 days and 2.81 log10 at day 35. | [178] | |
| L. plantarum PA18A | Orally, on days 1 and 4 | C. jejuni strain 12/2 (1 × 104 CFU) | 1 log10 reduction | [181] | |
| E. faecalis MB 5259 | Day 1–21 orally | C. jejuni MB 4185 (KC 40) (2 × 104 CFU) | 0.4 log10 in only one of the groups received 104 CFU E. faecalis No reduction in the chickens received 108 CFU E. faecali | [122] | |
| E. coli Nissle 1917 (free and chitosan micro-encapsulated) | Daily or three times per week supplementation in drinking water at weeks 4 and 5 of age | Cocktail of six C. jejuni strains/orally/(1 × 105 CFU) | Up to 2.6 log10 at the end of the experiment at | [196] | |
| B. amyloliquefaciens-loaded nanoparticles (BNPs) | Per os from 1–35 post-hatch with three different doses of BNPs: I (2.5 × 105 CFU/g), BNPs II (5 × 105 CFU/g), and BNPs III 7.5× (CFU/g) of feed | Crop gavage with pandrug-resistant (PDR) and multi-virulent field C. jejuni 108 CFU/mL at 30 days old | BNPs III inclusion showed significant fecal and cecal reduction at 7 days post-infection (3.86 log10, 3.94 log10, respectively) | [188] | |
| B. subtilis PS-216 spores | 2.5 × 106 CFU/mL in drinking water 1–20 d | 8 d, all of the broilers were inoculated with 4 × 106 CFU C. jejuni 11,168 by oral gavage | 1.2 log10 CFU/g feces in the C. jejuni counts | [187] | |
| L. plantarum 256 | (107 CFU/mL) in drinking water for 6 and 9 weeks L. plantarum strain 256 during baling, providing an inoculum concentration of 108 CFU per gram of fresh matter | 106 CFU/mL of the C. jejuni strain 65 at day 22 for the (6 weeks exp) and at 29 days for the (9 weeks exp) | No significant reduction at the end of the experiments at 42 and 63 days. | [197] | |
| Mixed strains | |||||
| K-bacteria + competitive exclusion Broilact® | Day 1–38 in drinking water | C. jejuni T23/42 (1.3 × 104 CFU) | Up to 2 log10 | [140] | |
| Citrobacter diversus 22 + K. pneumonia 23 + E. coli 25 + mannose | Days 1 and 3 | C. jejuni orally (108 CFU) | Up to 70% reduction | [198] | |
| Avian Pac Soluble (L. acidophilus + Streptococcus faecium) | Day 1–3 in drinking water | C. jejuni C101 (2.7 × 104 CFU) | Two-thirds reduction in C. jejuni shedding | [199] | |
| PrimaLac (L. acidophilus + L. casei + B. thermophilus + E. faecium) | Day 1–42 in feed | Naturally infected | 12% reduction of C. jejuni presence | [193] | |
| B. longum PCB 133 | Day 1–15 intraesophageally | Naturally infected | 1 log10 reduction | [200] | |
| Microencapsulated B. longum PCB133 + oligosaccharides | Day 1–14 in feed | Naturally infected | Up to 1.4 log10 | [201] | |
| PoultryStar sol® (E. faecium + P. acidilactici + B. animalis + L. salivarius + L. reuteri) | Day 1–15 in drinking water | C. jejuni 3015/2010 (104 CFU) | 6 log10 | [184] | |
| L. acidophilus NCFM or L. crispatus JCM5810 or L.s gallinarum ATCC or L. helveticus CNRZ32 | Day 1 and 4 orally | C. jejuni F38011 (108 CFU) | ~2 log10 reduction | [202] | |
| L. gasseri SBT2055 LG2055 WTCM, Dapf1 and Dapf2 mutant strains | Day 2–14 orally Dapf1: No reduction | C. jejuni 81–176 (106 CFU) | WTCM and Dapf2: Up to 270-fold reduction | [182] | |
| Bacillus spp.+ L. salivarius subsp. salivarius + L. salivarius subsp. salicinius | Day 1 orally | C. jejuni cocktail of 4 strains (2.5 × 106 CFU) | 1–2 log10 in only one of the three trials | [203] | |
| L. paracasei J.R + L. rhamnosus 15b + L. lactis Y + L. lactis FOa | Day 1–42 in drinking water | Naturally infected | Up to 5 log10 | [204] | |
| Calsporin® (B. subtilis C-3102) Ecobiol® (B. amyloliquefaciens CECT 5940) | Day 1 and 42 in feed | C. jejuni C97ANSES640 (104 CFU) | Calsporin®: 0.25 log10 reduction on day 14 and 1.7 log10 on day 42 Ecobiol®: 1.12 log10 on day 35 and 1.2 log10 on day 42 | [189] | |
| B. subtilis DSM 17299 or S. cerevisiae boulardii | Day 21–42 in feed | C. jejuni ST45 (104 CFU) | B. subtilis: No reduction S. cerevisiae: Up to 0.3 log10 reduction | [190] | |
| Lavipan (multispecies probiotic): L. lactis IBB 500, Carnobacterium divergens S-1, L. casei OCK 0915, L0915, L. plantarum OCK 0862, and S. cerevisiae OCK 0141 | Day 1–37 in feed | Naturally infected | <1 log10 | [122] | |
| Layers | Citrobacter diversus, K. pneumoniae, and E. coli | Reduced C. jejuni load in ceca | [198] | ||
| Cecal culture | Reduced C. jejuni load in ceca | [205] | |||
| E. faecium EM41 | Orally in drinking water, 109 CFU/mL were received for 21 days | Natural infection | 0.8 log10 reduction at 21 days of starting administration and 0.25 log10 reduction at day 35 (2 weeks after cessation of the additive) | [192] | |
| Enterocin EM41 (Ent EM41) | Enterocin (Ent) EM41 (40 μL/animal/day, 25,600 AU/mL). | Natural infection | 1.95 log10 reduction at 21 days of starting administration and 0.75 log10 reduction on day 35 (2 weeks after cessation of the additive) | [192] | |
| Duck | L. salivarius | Orally in feed 2 × 108 CFU/g for 79 days | Natural infection | No reduction | [179] |
| Turkeys | Bacteriocin of P. polymyxa and L. salivarius | Three successive days on 10–12 post-hatch | Orally, 106 CFU of a mixture of 3 Campylobacter coli isolates. | 4 log10 reduction in Campylobacter concentrations | [180] |
2.3. Clostridium Perfingens
C. perfringens is a Gram-positive, spore-forming, toxin-producing anaerobic bacterium that inhibits the poultry gut [206]. As a foodborne pathogen, C. perfringens has been associated with food poisoning, gas gangrene, and diarrhea in humans and NE in poultry [207]. Epidemiologically, C. perfringens carrying the enterotoxin gene (cpe) is ranked as the second leading cause of foodborne bacterial illness in the United States, accounting for roughly one million incident cases annually and $400 million USD in annual economic losses. Although most infections are self-limiting, they can necessitate hospitalization in vulnerable populations, incurring high healthcare costs.
2.3.1. C. perfringens Virulence, Pathogenicity and Mode of Transmission
Pathogenic strains of C. perfringens can produce over 20 toxins, with six primary toxins being alpha, beta, epsilon, iota, enterotoxin, and necrotic B-like (Net B) toxin. These six toxins are encoded by the genes cpa/plc, cpb, etx, iap/iab/itx, cpe, and netB, respectively. The composition of these specific genes determines the classification of different C. perfringens strains, categorized as toxinotypes, depicted in Table 8. Rood et al. (2018) expanded the scheme to include toxinotypes F and G to compensate for the increasing complexity and diagnostic limitations of the outdated framework [208].

Table 8.
Classification of C. perfringens toxinotypes relevant to poultry and humans.
C. perfringens produces a diverse range of toxins integral to its pathogenicity in humans and animals. Among these, C. perfringens’ CPE, associated with the type F strain, is one of the most clinically relevant toxins in human foodborne infections. CPE binds to claudin receptors on intestinal epithelial cells, disrupting tight junctions and inducing pore formation [209]. This pore formation increases intestinal permeability, fluid accumulation, and epithelial necrosis, leading to symptoms like diarrhea and abdominal cramps commonly seen in foodborne outbreaks.
In poultry, C. perfringens’ type G strain produces NetB toxin, the primary toxin responsible for NE in chickens. NetB forms heptameric pores in the membranes of intestinal epithelial cells, leading to cellular disruption, necrosis, and mucosal damage. It shares structural homology with other pore-forming toxins like beta toxin and Staphylococcus aureus α-hemolysin. Although its receptor has not been definitively identified, its cation-selective pore formation is integral to its pathogenicity in poultry [210]. There is also some suggestion that carriage of tpeL in some netB+ C. perfringens strains may contribute to enhanced virulence in chickens [211,212,213]. NE creates economic losses exceeding $6 billion USD annually for poultry producers due to performance reductions, higher feed conversion ratios, and treatment costs [214]. NE can present acute high mortality rates of 10–40% among two to six-week-old broilers or sub-clinically affect broilers, compromising feed conversion and growth rates [215].
The concern surrounding C. perfringens stems from the zoonotic potential of specific bacterial strains. For example, a strain carrying the netB gene may induce NE in poultry under conditions, such as contaminated meat products and subsequent human ingestion. Once consumed, the bacterium may express the cpe gene, enabling foodborne illness in humans [206]. The toxin-producing capability of C. perfringens imposes a significant threat to human and poultry health in the US, contributing to one million foodborne illness cases in addition to substantial economic losses within the poultry industry [15].
2.3.2. Probiotic Efficacy Against C. perfringens
A review by Kulkarni et al. (2022) highlighted that probiotics, across genera such as Lactobacillus, Enterococcus, Bacillus, Bacteroides, and some yeasts, consistently reduced C. perfringens colonization and NE–related pathology in chickens, while enhancing bird performance [18]. Multiple studies evaluated the effectiveness of mono-strain probiotics in reducing C. perfringens burden in broiler chickens, depicted in Table 9. Granstad et al. (2020) investigated three individual strains: B. subtilis (PB6), B. subtilis (no. 671265), and L. farciminis. B. subtilis synthesizes bacteriocins such as mersacidin and sublancin that disrupt bacterial cell membranes and inhibit cell wall biosynthesis to suppress C. perfringens proliferation [216]. Among these strains in comparison to the control group, B. subtilis strain PB6 led to the highest C. perfringens log10 reduction of 0.98, whereas its other strain only exhibited a 0.19 log reduction, and L. farciminis gave a 0.63 log10 reduction [216]. However, even with such variability among log reduction counts, each probiotic strain reduced FCR and increased the BW of the broilers. Similarly, Gharib-Naseri et al. (2021) and Wu et al. (2018) utilized Bacillus-based probiotics, B. amyloliquefaciens and B. coagulans, respectively, to investigate their roles in gut microbiota [217,218]. It was found that B. amyloliquefaciens exhibited 0.8 log10 reduction of cecal C. perfringens, whereas B. coagulans showed log reductions of 0.84, 1.46, and 1.79 at days 28, 35, and 42 of age, respectively. B. coagulans enhanced mucosal immunity by the production of secretory immunoglobulin A and the reduction of systemic immunoglobulin G levels, while also upregulating antimicrobial peptides, such as folicidin-2 and lysozyme, to suppress C. perfringens. Focusing on the outcomes of Wu et al. (2018), the levels of C. perfringens reductions and growth performance varied, where significant increases in BW and improved FCR were found from day 15 to 21 when supplemented with dietary B. coagulans [217]. Overall, single-strain probiotics can improve broiler health and performance, but can express variability in C. perfringens reductions, depending on the probiotic strain used [217].
Multi-strain probiotic formulations demonstrated greater outcomes through synergistic mechanisms throughout diverse bacterial species. Granstad et al. (2020) created a multi-strain probiotic feed additive using E. faecium, B. animalis, and L. salivarius to determine its impact on broilers [216]. The multi-strain probiotic showed a 0.88 log10 reduction in cecal C. perfringens with improvements in BW and FCR compared to the control birds [216]. Abd El-Ghany et al. (2022) also developed a multi-strain probiotic comprised of B. subtilis and B. licheniformis, administered through the feed, and observed a 2.45 log10 reduction in cecal C. perfringens with reductions in C. perfringens-related mortality rates and lower FCR values [219]. These strains utilized in tandem contribute to the inhibition of C. perfringens growth and toxin production by enhancing mucosal immune responses. Compared with earlier studies, McReynolds et al. (2009) designed a multi-strain probiotic as a water additive rather than a feed additive [220]. The probiotic blend colonized the gut and acidified the environment while producing bacteriocins such as reuterin and hydrogen peroxide to deter C. perfringens establishment, thus resulting in a 2.91 log10 reduction of C. perfringens in the cecal content of broiler chickens [220]. In addition, this probiotic mix showed reductions in intestinal lesions and mortalities compared to the positive controls. The multi-strain probiotic combinations provide many benefits for gut microbiota stability, nutrient absorption, and immune support. However, the strain-specific nature of probiotic efficacy requires precise selections where not all mixes guarantee a significant outcome. Buiatte et al. (2023) performed an in vitro study on a Bacillus-based probiotic mix comprising B. subtilis, B. licheniformis, B. coagulans, and B. pumilus [221]. Inter-strain antagonism was noted when the probiotic mix had a 0.28 log10 reduction in cecal C. perfringens, whereas B. subtilis alone induced a 6 log10 reduction [221]. This difference in bacterial counts suggests that competitive interactions or bacteriocin inhibitors may compromise the overall effectiveness of a multi-strain probiotic formulation.

Table 9.
Probiotics’ effectiveness against C. perfringens in broilers.
Table 9.
Probiotics’ effectiveness against C. perfringens in broilers.
| Probiotic Strain(s) | Administration | Concentration | Main Outcomes | References |
|---|---|---|---|---|
| B. amyloliquefaciens | Feed additive | 106 CFU/g feed | 0.8 log10 reduction in cecal C. perfringens counts. Improved FCR and BWG | [218] |
| B. coagulans | Feed additive | 4 × 109 CFU/kg feed | 0.84, 1.46, and 1.79-log10 reductions in C. perfringens cecal counts at days 28, 35, and 42, respectively. Decreased lesion scores and reduced crypt depths in the small intestine | [217] |
| B. subtilis | Feed additive | 2 × 108 CFU/g feed | 0.98 log10 reduction in cecal C. perfringens counts. Improved FCR and BWG | [216] |
| B. subtilis | In vitro | 108 CFU/mL | 6 log10 C. perfringens reduction alone. Efficacy declined when combined with other Bacillus strains | [221] |
| E. faecium, B. animalis, and L. salivarius mix | Feed additive | 2 × 108 CFU/g feed | 0.88 log10 C. perfringens count reduction. Enhanced BWG and FCR | [216] |
| B. subtilis and B. licheniformis mix | Feed additive | 2.5 × 1012 CFU/kg feed | 2.45 log10 C. perfringens reduction. Reduced mortality and lesion scores | [219] |
| E. faecium, B. animalis, P. acidilactici and L. reuteri mix | Water additive | 1 × 109 CFU/mL | 2.91 log10 C. perfringens reduction. Reduced lesion scores and mortalities. | [220] |
Overall, current findings emphasize the need for precision strain selection and optimized dosing strategies. The route and consistency of administration, viability of spores during feed processing, and age-specific gut microbiota dynamics in broilers are all critical factors determining probiotic success. While individual strains like B. subtilis offer impressive monotherapeutic potential, the growing body of evidence supports the synergistic use of tailored multi-strain or synbiotic formulations, especially in antibiotic-free production systems.
Safety evaluations still need to precede widespread synbiotic and multi-strain probiotics applications. Concerns regarding horizontal gene transfer of antibiotic resistance markers from probiotic strains remain valid; therefore, future applications should adopt genomic screening and resistance profiling practices to ensure regulatory compliance and long-term safety for all parties involved.
2.4. Escherichia coli
E. coli is a gram-negative bacterium commonly present in controlled levels within the digestive tracts of chickens. While many strains of E. coli are nonpathogenic, pathogenic strains such as E. coli O157, K88, and O78 can cause severe illness in broilers [222,223,224,225]. APEC, particularly strain O78, is the primary cause of colibacillosis in broilers, which is reported to cause a mortality rate of 6.56% in infected chickens, with clinical signs of inflammation, yolk sac infections, coligranulomas, swollen head syndrome, avian cellulitis, and enteritis [226]. It has also been estimated that about 30% of broilers are sub-clinically infected with E. coli at a given time [227]. Additionally, the presence of pathogenic strains of E. coli poses a zoonotic threat as they can spread through the consumption of contaminated poultry meat and have also been detected on the shells and in the contents of eggs [228,229].
The economic costs of E. coli both in the US and globally are associated with both the poultry industry and the healthcare industry. Monetary losses in the poultry industry are often due to containment of the disease and mortality of the birds, as well as decreased weight gain and production inefficiencies [230,231]. Additionally, Shiga-toxin-producing E. coli was one of the top three causes for total food recall by the USDA Food Safety and Inspection Service between 1994 and 2015 [232]. A 2019 estimate found that the O157 strain of E. coli alone has accrued economic losses of about $268.3 million, while other strains cost about $7.7 million [233].
2.4.1. E. coli Virulence, Pathogenicity and Mode of Transmission
Different strains of E. coli exhibit different virulence factors to cause disease in their hosts, which are primarily mammals and birds. Pathogenic strains of E. coli are identified based on their surface antigen, which is either of the O, K, or H variety. Adherence of the bacteria to the colonization surface is often accomplished through adhesion proteins and outer membrane proteins. They have also been known to create biofilms and resistance to the immune complement system, which can increase pathogenicity [234]. The virulence factors associated with pathogenic E. coli strains are provided in Table 10.

Table 10.
Virulence factors of different pathogenic Escherichia coli strains.
E. coli infection in the poultry was previously controlled through the practice of using antibiotics at subtherapeutic levels in the diet [231]. Antimicrobial resistance in E. coli is a growing concern due to the bacterium’s capacity to act as a reservoir and vector for resistance genes, with studies linking poultry-origin E. coli strains to urinary tract infections in humans [231]. This has also caused many strains to become resistant to ampicillin, tetracycline and gentamicin [226]. Quinolone-resistant E. coli strains have been detected in poultry carcasses and increasingly among human clinical isolates in countries like Spain and Taiwan, emphasizing direct foodborne transmission.
2.4.2. Probiotic Efficacy Against E. coli
Probiotics have been used as a safer alternative for controlling E. coli in broilers. Lactobacillus, a non-spore-forming, gram-positive bacterium naturally found in the gut, is widely used as a probiotic due to its safety [224]. However, its effectiveness against E. coli in broilers has shown variable results. L. plantarum has been found to withstand low pH during in vitro studies and survive many different bile level concentrations between 1–3%, causing inhibition of E. coli O157 growth in broilers [243]. Dietary supplementation of L. plantarum showed an increase in IgA secretion and a reduction in E. coli counts in cecal digesta by 0.69 log10 at day 42 of age [244]. In a subsequent study, chicks supplemented with L. plantarum and challenged with E. coli K88 had an increase in ileal mucosal IgA and a reduction in E. coli in the cecal digesta of 0.3 log10. Additionally, decreased ileal mRNA expressions of IL-2, INF-γ, TNF- α, IL-4 and TLR4 were demonstrated [223]. Contrastingly, another study showed that healthy Ross 308 chicks supplemented with 1 × 106 to 1 × 108 CFUs of L. plantarum/mL of drinking water had no significant reduction in cecal E. coli colonization, and this was attributed to its inability to colonize the mucosal wall of the intestines [245].
The use of a commercial Lactobacillus spp. mixture containing 2 strains of L. acidophilus, 3 strains of L. fermentum, 1 strain of L. crispatus, and 6 strains of L. brevis was studied for probiotic tendencies. It was found that Lactobacilli strains effectively reduced naturally occurring E. coli counts in broiler chickens, with one trial showing a reduction of 1.83 log10 and the other showing complete inhibition [246]. Another similar experiment found that supplementing Lactobacillus significantly reduced E. coli colony counts, with a decrease of 0.57 log10 CFUs/g of excreta [224].
The heat-resistant property of B. subtilis allows it to survive feed preparation processes [247]. When B. subtilis was used as a dietary supplement in Ross 308 chicks challenged with E. coli, birds showed a reduction in E. coli colonization by 1.69 log10 CFUs/g. Another study reported a complete inhibition of E. coli in the liver and spleen 48 h post challenge, and a reduction of approximately 3.2 × 107 CFUs/g in the cecum [248]. E. faecalis is a potential commensal probiotic, but has shown pathogenicity when it gains antibiotic resistance, sensitivity to the acidic environment and toxin release into the body [249,250]. When E. faecalis was supplemented in the drinking water to E. coli O78 challenged broilers, no change was found between bacterial or coliform counts, but a significant increase in serum IgY levels was observed [225]. Dong et al. (2019) showed that broilers challenged with E. coli K88 and then given microencapsulated E. faecalis as a dietary supplement had greater serum IgA levels and a reduction in cecal E. coli colonization by approximately 0.5 log10 CFU/g [250]. Encapsulation may protect the probiotic from changes in the external environment, such as food processing or harsh conditions in the gastrointestinal tract, allowing it to survive longer and have its effect. Huang et al. (2019) have also studied E. faecium as a potential probiotic candidate by supplementing it in the diet and challenging broilers with E. coli O78 [251]. Supplemented birds maintained higher levels of claudin-1 and occludin mRNA expression, along with a reduction in E. coli colonization by approximately 2.0 log10 CFUs/g in the liver [251]. A subsequent study challenged male Cobb broilers with E. coli K88 and showed that supplementation with E. faecium increased levels of TNF-α and serum IgA, and there was a reduction of E. coli colonization in the cecum by 0.27 log10 CFU/g [252].
Another probiotic candidate, Clostridium butyricum, when used as a supplement in broilers that were challenged with E. coli K88, showed increased levels of TNF-ɑ and IL-4 [253]. Subsequently, another study found that broilers orally challenged with E. coli K88 and given C. butyricum had reduced cecal E. coli colony counts of approximately 1 log10 CFUs/g. The birds also had increased serum IgA, IgY, and IgM levels on day 3 post-challenge, and increased IgA and IgM levels on day 14 post-challenge.
S. cerevisiae has been used as a probiotic within the poultry industry in the past. Igbafe et al. (2020) tested the antagonistic effect of S. cerevisiae in vitro as a supplement against E. coli O157, with the results showing its ability to withstand low pH values; however, it showed no antagonistic effects when plated with E. coli [254]. Further confirming this finding, an in vivo study by Bortoluzzi et al. (2018) using healthy male Ross 308 chicks fed a basal diet with SINERGIS, produced by Aleris Nutrition (Aleris USA LLC, NV), a commercially available form of S. cerevisiae, found no significant reduction of E. coli colonization in the ileum or cecum [255].
Candida famata, another species of yeast, was used in drinking water at 108 CFUs/mL, and it was found that there was no significant reduction in cecal E. coli colonization. It is believed that while this species had increased proliferation of many other yeast species within the bird’s intestines, it did not have any effect on cecal E. coli colonization [245].
Similarly, another probiotic candidate, Lacticaseibacillus rhamnosus, has been found to have inhibitory effects by producing peptides against APEC. Guo et al. (2021) studied the efficacy of L. rhamnosus against pathogenic E. coli O78 in Leghorn chickens and found a 1.6 log10 CFU reduction in cecal E. coli colonization, with no colonization in the heart and liver [256]. Another study found that L. rhamnosus can act as a competitive excluder against E. coli and prevent its adhesion to the intestinal epithelia of chickens [257]. These supplemented birds also had an upregulation of serum IgA, IgG, and IgM when measured on day 21 of the experiment, and upregulation of proinflammatory cytokines, IFN-α, IL-1β, and IL-6 in the spleen. E. coli-challenged birds supplemented with L. rhamnosus had a significant reduction of E. coli colonization in the heart, lungs, and kidney by 1.5 log10 CFUs/g, 1.0 log10 CFUs/g, and 0.5 log10 CFUs/g, respectively, on day 3 post-challenge [256].
Bifidobacterium lactis has been used in the food industry, specifically the dairy industry, as a probiotic in milk and yogurt [258,259]. It has been found to inhibit colonization of APEC strains in vitro. Kathayat et al. (2022) studied the efficacy of B. lactis in controlling levels of pathogenic E. coli O78 in Leghorn chickens and found that birds treated with B. lactis had a 0.7 log10 CFU reduction in E. coli colonization in the cecal contents, while 10% of chickens were positive for E. coli in the liver, and 20% of chickens were positive for E. coli in the heart [257]. Table 11 summarizes the effectiveness of probiotics against E. coli serotypes in broiler chickens.

Table 11.
Probiotics’ effectiveness against E. coli serotypes in broiler chickens.
2.5. Listeria Monocytogenes
L. monocytogenes is a Gram-positive, rod-shaped, non-spore-forming, facultative anaerobic psychrophilic bacterium responsible for listeriosis. Although there are 21 species of Listeria, only L. monocytogenes and L. ivanovii have been considered pathogenic, with L. monocytogenes being the most important. The CDC estimates approximately 1600 annual cases in the U.S., with a mortality rate of 17.6% in high-risk groups.
L. monocytogenes poses significant risks to public health due to its ability to thrive in harsh conditions, including refrigeration temperatures, acidic environments (pH 2.0–9.6), and high osmolarity, enabling persistence in food processing plants [268]. The ability of L. monocytogenes to produce biofilms also limits the efficacy of disinfectants and antimicrobial agents. It can survive various processing conditions, including freezing, surface dehydration, and simulated spray chilling. It can also survive in vacuum-packaged chicken and other ready-to-eat (RTE) foods.
It is ubiquitous in nature, found in soil, water, and animal feces, and frequently contaminates raw and RTE foods such as poultry, dairy products, fresh vegetables, seafood, and processed meats. The pathogen is particularly dangerous for vulnerable populations, including pregnant women, neonates, the elderly, and immunocompromised individuals, with mortality rates exceeding 20% in these groups [269,270]. Neonatal infections can occur through maternal chorioamnionitis (early-onset sepsis) or birth canal exposure (late-onset meningitis), while immunocompromised adults are at risk of septicemia, meningitis, and rhombencephalitis [271,272] as it can breach the blood-brain barrier. Furthermore, viral pathogens associated with gastroenteritis may allow for L. monocytogenes translocation.
While sporadic in poultry, it primarily affects young chicks, causing septicemia or localized encephalitis. It often occurs alongside other infections like coccidiosis or salmonellosis, highlighting its opportunistic nature [273]. L. monocytogenes exhibits varying prevalence, with broilers (32%) showing higher contamination rates than laying hens (15.5%), and free-range flocks (37%) more affected than conventional systems (28%) [274]. While clinical disease is rare in adult poultry, young chicks are highly susceptible, suffering septicemia or encephalitis with mortality rates up to 40% [270,275].
2.5.1. Listeria Virulence, Pathogenicity and Mode of Transmission
Whole-genome sequencing (WGS) analysis of 90 L. monocytogenes isolates revealed that clonal complex CC9 was the most prevalent in poultry and livestock meat and shared 80 other resistance genes. Poultry meat isolates had a higher number of tetracycline resistance genes (tetZ, tet41, and tetA), virulence genes, such as inlA (which promotes host cell invasion) and bsh (which helps resist bile toxicity), compared to livestock meat isolates [276].
The bacterium contaminates eggs through soil, poultry feces, or processing equipment, with eggshells showing a 1.8–6% prevalence [269]. Conventional caged systems have lower contamination rates (1.6%) due to controlled environments, but small-scale farms (<3000 hens) often escape regulatory oversight, increasing contamination risks [269]. Processing plants are critical contamination sites, with Listeria persisting in biofilms on equipment like egg washers, conveyors, and floor drains. Humans typically contract listeriosis from contaminated raw poultry meat or unhygienic processing conditions, rather than direct bird contact [277]. L. monocytogenes can colonize broiler chickens, but not as easily as other bacterial foodborne pathogens like Salmonella or C. jejuni. Younger birds are more susceptible to colonization than older birds, and the colonization response is dose-dependent. Surveys show contamination rates ranging from 12% (RTE) to 60% (fresh packaged chicken) [268]. Human listeriosis primarily results from consuming contaminated foods, with infective doses as low as 105 CFUs in high-risk individuals and 107–109 CFU in healthy adults, leading to gastrointestinal symptoms [270,278].
Listeria’s virulence is linked to its capability of adhesion, invasion, and translocation across the gut barrier. Specific genetic lineages (e.g., CC1, CC4) and virulence factors, including internalins (InlA, InlB), listeriolysin O (LLO), and ActA, facilitate host cell invasion, intracellular survival, and cell-to-cell spread. Alternative sigma factor σB, a protein subunit of RNA polymerase (RNAP), aids in the ability of Listeria to survive in harsher conditions. Serotypes 1/2a, 1/2b, and 4b cause over 96% of human cases, with serotype 4b associated with severe outbreaks [270,279]. Major outbreaks, such as the 2016–2017 UK cooked chicken incident (linked to recalled products with 20–340 CFU/g) and the 2008 Canadian deli meat outbreak, highlight its public health and economic impact, with U.S. illness costs estimated at $186 million annually [280]. L. monocytogenes is the most serious zoonotic disease, with high rates of hospitalization (92%) and mortality (17.6%) [281]
Control strategies include stringent cooking protocols (e.g., 90 min at 65 °C) to overcome Listeria’s heat resistance, which exceeds that of Salmonella and Campylobacter [275]. Although no commercial probiotics are currently available to control Listeria infections, several research groups have investigated the potential of probiotic strains as natural bio-preservatives and antimicrobial agents against L. monocytogenes. Deng et al. (2020) showed that dietary supplementation of broilers with an equal blend of L. acidophilus and L. plantarum at doses of 106, 108 and 1010 CFU/kg significantly reduced L. monocytogenes loads in the cecum, liver, spleen, and skin by 0.065–0.9 log10 CFU compared to control groups [176]. In the intestine, it lowered proinflammatory cytokines (IL-1β, IL-6, TNF-α, and IFN-γ) by 25.4–51.1%, upregulated anti-inflammatory genes (IL-10, HIF1A, PTGER2, and PTGS2), and downregulated critical virulence genes responsible for adhesion (Ami, FlaA), invasion (InlA, InlB), and cytotoxicity (HlyA) of L. monocytogenes. Apart from this study, no other experimental or field studies specifically testing probiotics (or synbiotics) against Listeria in poultry were identified.
2.5.2. Probiotic Efficacy Against Listeriosis
A couple of in vitro trials assess LAB as potential poultry probiotics against L. monocytogenes. Reuben et al. (2020) demonstrated potent antimicrobial activity of LAB with inhibition zones of 14–20 mm and high co-aggregation capability (up to 83.6%) [282]. Key probiotic candidates included L. reuteri, P. acidilactici, P. pentosaceus, and E. faecium, which exhibited acid and bile tolerance (surviving pH 2.0 and 0.3% bile salts), phenol resistance (withstanding 0.4% phenol), and strong adhesion to intestinal epithelial cells (3.0–6.0 log10 CFU/mL) and showed anti-Listeria activity consistent with pediocin/reuterin production and organic-acid–mediated inhibition. While these in vitro results are promising for controlling Listeria in poultry, further in vivo studies are needed to validate their efficacy under field conditions.
A study by Abouloifa et al. (2022) demonstrated the effectiveness of L. plantarum S61 in controlling L. monocytogenes in minced poultry meat [283]. The cell-free supernatant of this strain exhibited potent antibacterial activity, reducing Listeria counts from 5 log CFU/g to 2 log10 CFU/g after seven days of refrigerated storage. The antimicrobial effect was attributed to proteinaceous compounds, as treatment with protease enzymes neutralized the activity [283]. It also improved the meat’s physicochemical and sensory qualities, like color stability and delaying spoilage caused by other microbes. Additionally, biofilm-forming L. plantarum Y42 has shown high adhesion capacity to intestinal epithelial cells and effectively inhibited L. monocytogenes adhesion and invasion through CE. Its extracellular polymeric substances enhanced its ability to scavenge pre-formed Listeria biofilms more effectively than planktonic cells, suggesting an advantage in food preservation [284].
Regulatory agencies enforce strict microbiological criteria, requiring <100 CFU/g in non-growth-supporting foods [285]. Despite these measures, L. monocytogenes remains a persistent threat, necessitating improved surveillance, WGS for outbreak tracing, and innovative solutions to mitigate its impact on food safety [280].
3. Conclusions and Future Prospects
Bacterial foodborne pathogens such as Salmonella spp., Campylobacter spp., L. monocytogenes, C. perfringens, and pathogenic E. coli compromise poultry health and productivity and pose public health and economic burdens. This review brings together diverse evidence on the potential of probiotics as preharvest interventions to control these major bacterial foodborne pathogens in poultry. The increasing interest in probiotics for poultry production and research is driven by their ability to modulate gut microbiota, enhance gut immunity, and competitively exclude pathogens, while improving bird performance. Overall, although some studies show that probiotics can reduce intestinal colonization and shedding of these bacterial pathogens, their effects vary widely depending on strain, dose, formulation, bird age and type, administration route, treatment duration, sample size, and study design. For Campylobacter, Lactobacillus supplementation has been shown in some studies to reduce cecal loads by ~1–3 log10, while others report no effect; benefits sometimes require repeated dosing or multi-strain combinations. Similarly, Bacillus and LAB supplements can reduce cecal or fecal Salmonella loads by 0.4–3 log units, but some studies show minimal impact. For C. perfringens, single strains may be effective, though multi-strain mixes can either outperform or underperform due to inter-strain interactions, highlighting a design gap. For Listeria, in vivo poultry data are limited, and the lack of field-relevant trials represents a clear research gap.
A general observation is that many research trials use limited bird numbers and rearing conditions that differ from commercial settings, often lacking factors such as co-infections, litter reuse, diet variability, stocking density, vaccination, or litter microbiota interactions that influence pathogen dynamics. Studies also vary widely in their endpoints, ranging from cecal CFU and fecal shedding to carcass contamination, making comparisons between studies difficult. Formulation is another gap, as bacterial survival through pelleting, storage, and digestion is rarely verified. Most pathogen challenges use a single strain, whereas field flocks face diverse, multidrug-resistant serovars and mixed exposures, leaving cross-strain protection untested. Furthermore, it is also important to emphasize that supplementing poultry with individual probiotic species or a limited cocktail of a few strains may be insufficient to dominate the gut microbiota and confer the intended benefits, potentially allowing pathogenic bacteria to colonize. Safety and quality standards also need strengthening through consistent genomic screening for resistance genes, toxins, potency, and stability. Finally, integration with other interventions such as vaccines and synbiotics shows potential. By integrating findings on strain efficacy, delivery strategies, and pathogen outcomes, this review highlights how probiotics can effectively reduce foodborne risks while promoting bird health and performance.
With recent advancements in the multi-omics tools, microbiome profiling can now be performed at the species level, enabling the identification of numerous beneficial microbes that could not be detected using traditional culture-based methods. Functional characterization of these microbes through metagenomic and metabolomic approaches can facilitate the development of a consortium of well-defined, well-characterized probiotic strains. Such next-generation poultry-specific probiotics could establish a stable and synergistic microbiome in neonatal chicks, thereby enhancing microbial interactions and minimizing colonization by pathogenic bacteria.
Author Contributions
Conceptualization, K.A.; data curation, S.S., S.K., M.N., A.B. and A.S.; writing—original draft preparation, S.S., S.K., M.N., A.B. and A.S.; writing—review and editing, S.S., R.R.K., A.N. and K.A.; supervision, K.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We would like to thank Oluseyi Taiwo, Department of Biological Sciences, Clemson University, for his help in creating the figure in this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- United States Department of Agriculture. Poultry—Production and Value; United States Department of Agriculture: Washington, DC, USA, 2025. [Google Scholar]
- United States Department of Agriculture, Foreign Agricultural Service. Production—Chicken Meat. USDA FAS: Washington, DC, USA. Available online: https://www.fas.usda.gov/data/production/commodity/0115000 (accessed on 21 August 2025).
- Kulkarni, R.R.; Taha-Abdelaziz, K.; Shojadoost, B.; Astill, J.; Sharif, S. Gastrointestinal Diseases of Poultry: Causes and Nutritional Strategies for Prevention and Control. In Improving Gut Health in Poultry; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 205–236. [Google Scholar]
- Taha-Abdelaziz, K.; Singh, M.; Sharif, S.; Sharma, S.; Kulkarni, R.R.; Alizadeh, M.; Yitbarek, A.; Helmy, Y.A. Intervention Strategies to Control Campylobacter at Different Stages of the Food Chain. Microorganisms 2023, 11, 113. [Google Scholar] [CrossRef]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef]
- Galán-Relaño, Á.; Valero Díaz, A.; Huerta Lorenzo, B.; Gómez-Gascón, L.; Mena Rodríguez, M.Á.; Carrasco Jiménez, E.; Pérez Rodríguez, F.; Astorga Márquez, R.J. Salmonella and Salmonellosis: An Update on Public Health Implications and Control Strategies. Animals 2023, 13, 3666. [Google Scholar] [CrossRef]
- St Louis, M.E.; Morse, D.L.; Potter, M.E.; DeMelfi, T.M.; Guzewich, J.J.; Tauxe, R.V.; Blake, P.A. The Emergence of Grade A Eggs as a Major Source of Salmonella Enteritidis Infections. New Implications for the Control of Salmonellosis. JAMA 1988, 259, 2103–2107. [Google Scholar] [CrossRef]
- Rothrock, M.J.; Davis, M.L.; Locatelli, A.; Bodie, A.; McIntosh, T.G.; Donaldson, J.R.; Ricke, S.C. Listeria Occurrence in Poultry Flocks: Detection and Potential Implications. Front. Vet. Sci. 2017, 4, 125. [Google Scholar] [CrossRef]
- Jesudhasan, P.R.; Bhatia, S.S.; Sivakumar, K.K.; Praveen, C.; Genovese, K.J.; He, H.L.; Droleskey, R.; McReynolds, J.L.; Byrd, J.A.; Swaggerty, C.L.; et al. Controlling the Colonization of Clostridium Perfringens in Broiler Chickens by an Electron-Beam-Killed Vaccine. Animals 2021, 11, 671. [Google Scholar] [CrossRef]
- Joseph, J.; Zhang, L.; Adhikari, P.; Evans, J.D.; Ramachandran, R. Avian Pathogenic Escherichia coli (APEC) in Broiler Breeders: An Overview. Pathogens 2023, 12, 1280. [Google Scholar] [CrossRef]
- Hoffmann, S.; White, A.E.; McQueen, R.B.; Ahn, J.-W.; Gunn-Sandell, L.B.; Scallan Walter, E.J. Economic Burden of Foodborne Illnesses Acquired in the United States. Foodborne Pathog. Dis. 2025, 22, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Ba, X.; Jin, Y.; Ning, X.; Gao, Y.; Li, W.; Li, Y.; Wang, Y.; Zhou, J. Clostridium Perfringens in the Intestine: Innocent Bystander or Serious Threat? Microorganisms 2024, 12, 1610. [Google Scholar] [CrossRef] [PubMed]
- Grace, D.; Gilbert, J.; Randolph, T.; Kang’ethe, E. The Multiple Burdens of Zoonotic Disease and an Ecohealth Approach to Their Assessment. Trop. Anim. Health Prod. 2012, 44, 67–73. [Google Scholar] [CrossRef]
- Shamshirgaran, M.A.; Golchin, M. A Comprehensive Review of Experimental Models and Induction Protocols for Avian Necrotic Enteritis over the Past 2 Decades. Front. Vet. Sci. 2024, 11, 1429637. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Taha-Abdelaziz, K.; Hawwas, H.A.E.-H.; Ghosh, S.; AlKafaas, S.S.; Moawad, M.M.M.; Saied, E.M.; Kassem, I.I.; Mawad, A.M.M. Antimicrobial Resistance and Recent Alternatives to Antibiotics for the Control of Bacterial Pathogens with an Emphasis on Foodborne Pathogens. Antibiotics 2023, 12, 274. [Google Scholar] [CrossRef]
- Lima, É.; Oliveira, M.B.; Freitas, A. Antibiotics in Intensive Egg Production: Food Safety Tools to Ensure Regulatory Compliance. Food Chem. Adv. 2023, 3, 100548. [Google Scholar] [CrossRef]
- Kulkarni, R.R.; Gaghan, C.; Gorrell, K.; Sharif, S.; Taha-Abdelaziz, K. Probiotics as Alternatives to Antibiotics for the Prevention and Control of Necrotic Enteritis in Chickens. Pathogens 2022, 11, 692. [Google Scholar] [CrossRef] [PubMed]
- Taha-Abdelaziz, K.; Hodgins, D.C.; Lammers, A.; Alkie, T.N.; Sharif, S. Effects of Early Feeding and Dietary Interventions on Development of Lymphoid Organs and Immune Competence in Neonatal Chickens: A Review. Vet. Immunol. Immunopathol. 2018, 201, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kulkarni, R.R.; Sharif, S.; Hassan, H.; Alizadeh, M.; Pratt, S.; Abdelaziz, K. In Ovo Feeding of Probiotic Lactobacilli Differentially Alters Expression of Genes Involved in the Development and Immunological Maturation of Bursa of Fabricius in Pre-Hatched Chicks. Poult. Sci. 2024, 103, 103237. [Google Scholar] [CrossRef]
- Alkie, T.N.; Yitbarek, A.; Hodgins, D.C.; Kulkarni, R.R.; Taha-Abdelaziz, K.; Sharif, S. Development of Innate Immunity in Chicken Embryos and Newly Hatched Chicks: A Disease Control Perspective. Avian Pathol. 2019, 48, 288–310. [Google Scholar] [CrossRef]
- Alizadeh, M.; Shojadoost, B.; Astill, J.; Taha-Abdelaziz, K.; Karimi, S.H.; Bavananthasivam, J.; Kulkarni, R.R.; Sharif, S. Effects of in Ovo Inoculation of Multi-Strain Lactobacilli on Cytokine Gene Expression and Antibody-Mediated Immune Responses in Chickens. Front. Vet. Sci. 2020, 7, 105. [Google Scholar] [CrossRef]
- Alizadeh, M.; Astill, J.; Alqazlan, N.; Shojadoost, B.; Taha-Abdelaziz, K.; Bavananthasivam, J.; Doost, J.S.; Sedeghiisfahani, N.; Sharif, S. In Ovo Co-Administration of Vitamins (A and D) and Probiotic Lactobacilli Modulates Immune Responses in Broiler Chickens. Poult. Sci. 2022, 101, 101717. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Alizadeh, M.; Bavananthasivam, J.; Shojadoost, B.; Astill, J.; Taha-Abdelaziz, K.; Alqazlan, N.; Boodhoo, N.; Shoja Doost, J.; Sharif, S. In Ovo and Oral Administration of Probiotic Lactobacilli Modulate Cell- and Antibody-Mediated Immune Responses in Newly Hatched Chicks. Front. Immunol. 2021, 12, 664387. [Google Scholar] [CrossRef] [PubMed]
- Shojadoost, B.; Alizadeh, M.; Boodhoo, N.; Astill, J.; Karimi, S.H.; Shoja Doost, J.; Taha-Abdelaziz, K.; Kulkarni, R.; Sharif, S. Effects of Treatment with Lactobacilli on Necrotic Enteritis in Broiler Chickens. Probiotics Antimicrob. Proteins 2022, 14, 1110–1129. [Google Scholar] [CrossRef]
- Hardy, H.; Harris, J.; Lyon, E.; Beal, J.; Foey, A. Probiotics, Prebiotics and Immunomodulation of Gut Mucosal Defences: Homeostasis and Immunopathology. Nutrients 2013, 5, 1869–1912. [Google Scholar] [CrossRef]
- Sleator, R.D.; Hill, C. New Frontiers in Probiotic Research. Lett. Appl. Microbiol. 2007, 46, 143–147. [Google Scholar] [CrossRef]
- Kabir, S.M.L. The Role of Probiotics in the Poultry Industry. Int. J. Mol. Sci. 2009, 10, 3531–3546. [Google Scholar] [CrossRef]
- Maurer, J.J.; Cheng, Y.; Pedroso, A.; Thompson, K.K.; Akter, S.; Kwan, T.; Morota, G.; Kinstler, S.; Porwollik, S.; McClelland, M.; et al. Peeling Back the Many Layers of Competitive Exclusion. Front. Microbiol. 2024, 15, 1342887. [Google Scholar] [CrossRef]
- Sanders, M.E.; Akkermans, L.M.A.; Haller, D.; Hammerman, C.; Heimbach, J.T.; Hörmannsperger, G.; Huys, G. Safety Assessment of Probiotics for Human Use. Gut Microbes 2010, 1, 164–185. [Google Scholar] [CrossRef]
- Patterson, J.; Burkholder, K. Application of Prebiotics and Probiotics in Poultry Production. Poult. Sci. 2003, 82, 627–631. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: Ottawa, ON, Canada, 2002. [Google Scholar]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M. The Global Burden of Nontyphoidal Salmonella Gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Shaji, S.; Selvaraj, R.K.; Shanmugasundaram, R. Salmonella Infection in Poultry: A Review on the Pathogen and Control Strategies. Microorganisms 2023, 11, 2814. [Google Scholar] [CrossRef]
- World Health Organisation. Salmonella (Non-Typhoidal). Available online: https://www.who.int/news-room/fact-sheets/detail/salmonella-(non-typhoidal) (accessed on 21 August 2025).
- Institute for Health Metrics and Evaluation (IHME). Global Burden of Disease (GBD). Available online: https://www.healthdata.org/research-analysis/gbd (accessed on 21 August 2025).
- Painter, J.A.; Hoekstra, R.M.; Ayers, T.; Tauxe, R.V.; Braden, C.R.; Angulo, F.J.; Griffin, P.M. Attribution of Foodborne Illnesses, Hospitalizations, and Deaths to Food Commodities by Using Outbreak Data, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Acheson, D.; Hohmann, E.L. Nontyphoidal Salmonellosis. Clin. Infect. Dis. 2001, 32, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Reeves, M.W.; Evins, G.M.; Heiba, A.A.; Plikaytis, B.D.; Farmer, J.J. Clonal Nature of Salmonella Typhi and Its Genetic Relatedness to Other Salmonellae as Shown by Multilocus Enzyme Electrophoresis, and Proposal of Salmonella Bongori Comb. Nov. J. Clin. Microbiol. 1989, 27, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Popoff, M.Y.; Le Minor, L.E. Salmonella. In Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2015; p. 1. [Google Scholar]
- El-Sharkawy, H.; Tahoun, A.; El-Gohary, A.E.-G.A.; El-Abasy, M.; El-Khayat, F.; Gillespie, T.; Kitade, Y.; Hafez, H.M.; Neubauer, H.; El-Adawy, H. Epidemiological, Molecular Characterization and Antibiotic Resistance of Salmonella enterica Serovars Isolated from Chicken Farms in Egypt. Gut Pathog. 2017, 9, 8. [Google Scholar] [CrossRef]
- Dione, M.M.; Ikumapayi, U.N.; Saha, D.; Mohammed, N.I.; Geerts, S.; Ieven, M.; Adegbola, R.A.; Antonio, M. Clonal Differences between Non-Typhoidal Salmonella (NTS) Recovered from Children and Animals Living in Close Contact in The Gambia. PLoS Negl. Trop. Dis. 2011, 5, e1148. [Google Scholar] [CrossRef]
- Brenner, F.W.; Villar, R.G.; Angulo, F.J.; Tauxe, R.; Swaminathan, B. Salmonella Nomenclature. J. Clin. Microbiol. 2000, 38, 2465–2467. [Google Scholar] [CrossRef]
- Center for Disease Control Salmonella Infection. Available online: https://www.cdc.gov/salmonella/index.html (accessed on 21 August 2025).
- Singh, V. Salmonella Serovars and Their Host Specificity. J. Vet. Sci. Anim. Husb. 2013, 1, 301. [Google Scholar] [CrossRef][Green Version]
- Foley, S.L.; Johnson, T.J.; Ricke, S.C.; Nayak, R.; Danzeisen, J. Salmonella Pathogenicity and Host Adaptation in Chicken-Associated Serovars. Microbiol. Mol. Biol. Rev. 2013, 77, 582–607. [Google Scholar] [CrossRef]
- Deblais, L.; Lorentz, B.; Scaria, J.; Nagaraja, K.V.; Nisar, M.; Lauer, D.; Voss, S.; Rajashekara, G. Comparative Genomic Studies of Salmonella Heidelberg Isolated From Chicken- and Turkey-Associated Farm Environmental Samples. Front. Microbiol. 2018, 9, 1841. [Google Scholar] [CrossRef]
- Foley, S.L.; Lynne, A.M.; Nayak, R. Salmonella Challenges: Prevalence in Swine and Poultry and Potential Pathogenicity of Such Isolates1,2. J. Anim. Sci. 2008, 86, E149–E162. [Google Scholar] [CrossRef]
- Hassanein, R.; Ali, S.; ElMalek, A.; Moemen, M.; Elsayh, A. Detection and Identification of Salmonella Species in Minced Beef and Chicken Meats by Using Multiplex PCR in Assiut City. Vet. World 2011, 4, 5–11. [Google Scholar] [CrossRef]
- Barrow, P.A.; Freitas Neto, O.C. Pullorum disease and fowl typhoid—new thoughts on old diseases: A review. Avian Pathol. 2011, 40, 1–13. [Google Scholar] [CrossRef]
- Baumler, A.; Fang, F.C. Host Specificity of Bacterial Pathogens. Cold Spring Harb. Perspect. Med. 2013, 3, a010041. [Google Scholar] [CrossRef] [PubMed]
- Uzzau, S.; Leori, G.S.; Petruzzi, V.; Watson, P.R.; Schianchi, G.; Bacciu, D.; Mazzarello, V.; Wallis, T.S.; Rubino, S. Salmonella enterica Serovar-Host Specificity Does Not Correlate with the Magnitude of Intestinal Invasion in Sheep. Infect. Immun. 2001, 69, 3092–3099. [Google Scholar] [CrossRef]
- Ellermeier, J.R.; Slauch, J.M. Adaptation to the Host Environment: Regulation of the SPI1 Type III Secretion System in Salmonella enterica Serovar Typhimurium. Curr. Opin. Microbiol. 2007, 10, 24–29. [Google Scholar] [CrossRef]
- Tariq, S.; Samad, A.; Hamza, M.; Ahmer, A.; Muazzam, A.; Ahmad, S.; Amhabj, A.M.A. Salmonella in Poultry; An Overview. Int. J. Multidiscip. Sci. Arts 2022, 1, 80–84. [Google Scholar] [CrossRef]
- Kopanic, R.J.; Sheldon, B.W.; Wright, C.G. Cockroaches As Vectors of Salmonella: Laboratory and Field Trials. J. Food Prot. 1994, 57, 125–131. [Google Scholar] [CrossRef]
- Sparagano, O. Control of Poultry Mites: Where Do We Stand? Exp. Appl. Acarol. 2009, 48, 1–2. [Google Scholar] [CrossRef]
- Leffer, A.M.; Kuttel, J.; Martins, L.M.; Pedroso, A.C.; Astolfi-Ferreira, C.S.; Ferreira, F.; Ferreira, A.J.P. Vectorial Competence of Larvae and Adults of Alphitobius diaperinus in the Transmission of Salmonella Enteritidis in Poultry. Vector-Borne Zoonotic Dis. 2010, 10, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.A.; Shopland, S.; Wigley, P.; Bradon, H.; Leatherbarrow, A.H.; Williams, N.J.; Bennett, M.; de Pinna, E.; Lawson, B.; Cunningham, A.A.; et al. Characterisation of Salmonella enterica Serotype Typhimurium Isolates from Wild Birds in Northern England from 2005–2006. BMC Vet Res. 2008, 4, 4. [Google Scholar] [CrossRef]
- Alley, M.; Connolly, J.; Fenwick, S.; Mackereth, G.; Leyland, M.; Rogers, L.; Haycock, M.; Nicol, C.; Reed, C. An Epidemic of Salmonellosis Caused by Salmonella Typhimurium DT160 in Wild Birds and Humans in New Zealand. N. Z. Vet. J. 2002, 50, 170–176. [Google Scholar] [CrossRef]
- Sousa, E.; Werther, K.; Berchieri Júnior, A. Assessment of Newcastle and Infectious Bronchitis Pathogens, and Salmonella Spp. in Wild Birds Captured near Poultry Facilities. Arq. Bras. Med. Vet. Zootec. 2010, 62, 219–223. [Google Scholar] [CrossRef]
- De Reu, K.; Grijspeerdt, K.; Messens, W.; Heyndrickx, M.; Uyttendaele, M.; Debevere, J.; Herman, L. Eggshell Factors Influencing Eggshell Penetration and Whole Egg Contamination by Different Bacteria, Including Salmonella Enteritidis. Int. J. Food Microbiol. 2006, 112, 253–260. [Google Scholar] [CrossRef]
- Sahin, O.; Morishita, T.Y.; Zhang, Q. Campylobacter Colonization in Poultry: Sources of Infection and Modes of Transmission. Anim. Health Res. Rev. 2002, 3, 95–105. [Google Scholar] [CrossRef]
- Jones, T.F.; Ingram, L.A.; Fullerton, K.E.; Marcus, R.; Anderson, B.J.; McCarthy, P.V.; Vugia, D.; Shiferaw, B.; Haubert, N.; Wedel, S.; et al. A Case-Control Study of the Epidemiology of Sporadic Salmonella Infection in Infants. Pediatrics 2006, 118, 2380–2387. [Google Scholar] [CrossRef] [PubMed]
- Eng, S.-K.; Pusparajah, P.; Ab Mutalib, N.-S.; Ser, H.-L.; Chan, K.-G.; Lee, L.-H. Salmonella: A Review on Pathogenesis, Epidemiology and Antibiotic Resistance. Front. Life Sci. 2015, 8, 284–293. [Google Scholar] [CrossRef]
- Woods, D.F.; Reen, F.J.; Gilroy, D.; Buckley, J.; Frye, J.G.; Boyd, E.F. Rapid Multiplex PCR and Real-Time TaqMan PCR Assays for Detection of Salmonella enterica and the Highly Virulent Serovars Choleraesuis and Paratyphi C. J. Clin. Microbiol. 2008, 46, 4018–4022. [Google Scholar] [CrossRef]
- McShan, A.C.; Kaur, K.; Chatterjee, S.; Knight, K.M.; De Guzman, R.N. NMR Identification of the Binding Surfaces Involved in the Salmonella and Shigella Type III Secretion Tip-Translocon Protein-Protein Interactions. Proteins Struct. Funct. Bioinform. 2016, 84, 1097–1107. [Google Scholar] [CrossRef]
- Kyrova, K.; Stepanova, H.; Rychlik, I.; Faldyna, M.; Volf, J. SPI-1 Encoded Genes of Salmonella Typhimurium Influence Differential Polarization of Porcine Alveolar Macrophages in Vitro. BMC Vet. Res. 2012, 8, 115. [Google Scholar] [CrossRef]
- Chaudhuri, D.; Roy Chowdhury, A.; Biswas, B.; Chakravortty, D. Salmonella Typhimurium Infection Leads to Colonization of the Mouse Brain and Is Not Completely Cured With Antibiotics. Front. Microbiol. 2018, 9, 1632. [Google Scholar] [CrossRef] [PubMed]
- Uchiya, K.; Barbieri, M.A.; Funato, K.; Shah, A.H.; Stahl, P.D.; Groisman, E.A. A Salmonella Virulence Protein That Inhibits Cellular Trafficking. EMBO J. 1999, 18, 3924–3933. [Google Scholar] [CrossRef]
- Kombade, S.; Kaur, N. Pathogenicity Island in Salmonella. In Salmonella spp.—A Global Challenge; IntechOpen: Bristol, UK, 2021. [Google Scholar]
- Dorsey, C.W.; Laarakker, M.C.; Humphries, A.D.; Weening, E.H.; Bäumler, A.J. Salmonella enterica Serotype Typhimurium MisL Is an Intestinal Colonization Factor That Binds Fibronectin. Mol. Microbiol. 2005, 57, 196–211. [Google Scholar] [CrossRef]
- Gerlach, R.G.; Cláudio, N.; Rohde, M.; Jäckel, D.; Wagner, C.; Hensel, M. Cooperation of Salmonella Pathogenicity Islands 1 and 4 Is Required to Breach Epithelial Barriers. Cell Microbiol. 2008, 10, 2364–2376. [Google Scholar] [CrossRef]
- Kiss, T.; Morgan, E.; Nagy, G. Contribution of SPI-4 Genes to the Virulence of Salmonella enterica. FEMS Microbiol. Lett. 2007, 275, 153–159. [Google Scholar] [CrossRef]
- Wood, M.W.; Jones, M.A.; Watson, P.R.; Hedges, S.; Wallis, T.S.; Galyov, E.E. Identification of a Pathogenicity Island Required for Salmonella Enteropathogenicity. Mol. Microbiol. 1998, 29, 883–891. [Google Scholar] [CrossRef]
- Knodler, L.A.; Celli, J.; Hardt, W.; Vallance, B.A.; Yip, C.; Finlay, B.B. Salmonella Effectors within a Single Pathogenicity Island Are Differentially Expressed and Translocated by Separate Type III Secretion Systems. Mol. Microbiol. 2002, 43, 1089–1103. [Google Scholar] [CrossRef] [PubMed]
- Sana, T.G.; Flaugnatti, N.; Lugo, K.A.; Lam, L.H.; Jacobson, A.; Baylot, V.; Durand, E.; Journet, L.; Cascales, E.; Monack, D.M. Salmonella Typhimurium Utilizes a T6SS-Mediated Antibacterial Weapon to Establish in the Host Gut. Proc. Natl. Acad. Sci. USA 2016, 113, E5044–E5051. [Google Scholar] [CrossRef]
- Sabbagh, S.C.; Forest, C.G.; Lepage, C.; Leclerc, J.-M.; Daigle, F. So Similar, yet so Different: Uncovering Distinctive Features in the Genomes of Salmonella enterica Serovars Typhimurium and Typhi. FEMS Microbiol. Lett. 2010, 305, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Asten, A.J.A.M.; Dijk, J.E. Distribution of ÂĨŖClassicâĨŗ; Virulence Factors among Salmonella Spp. FEMS Immunol. Med. Microbiol. 2005, 44, 251–259. [Google Scholar] [CrossRef]
- Ahsan, C.R.; Shamma, F.; Ahsan, N.; Islam, M.J. Environmental Factors Regulate the HlyE Gene Expression in Both S. Typhi and E. coli in a Similar Way to Display Haemolytic Activity. Bangladesh Med. Res. Counc. Bull. 2017, 42, 33–38. [Google Scholar] [CrossRef][Green Version]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Sachan, S.; Karthik, K.; Dhama, K. The Use of Probiotics as Eco-Friendly Alternatives for Antibiotics in Poultry Nutrition. Environ. Sci. Pollut. Res. 2018, 25, 10611–10618. [Google Scholar] [CrossRef]
- Farhat, M.; Khayi, S.; Berrada, J.; Mouahid, M.; Ameur, N.; El-Adawy, H.; Fellahi, S. Salmonella enterica Serovar Gallinarum Biovars Pullorum and Gallinarum in Poultry: Review of Pathogenesis, Antibiotic Resistance, Diagnosis and Control in the Genomic Era. Antibiotics 2023, 13, 23. [Google Scholar] [CrossRef]
- Ruvalcaba-Gómez, J.M.; Villagrán, Z.; Valdez-Alarcón, J.J.; Martínez-Núñez, M.; Gomez-Godínez, L.J.; Ruesga-Gutiérrez, E.; Anaya-Esparza, L.M.; Arteaga-Garibay, R.I.; Villarruel-López, A. Non-Antibiotics Strategies to Control Salmonella Infection in Poultry. Animals 2022, 12, 102. [Google Scholar] [CrossRef]
- Bialkowski, S.; Toschi, A.; Yu, L.; Schlitzkus, L.; Mann, P.; Grilli, E.; Li, Y. Effects of Microencapsulated Blend of Organic Acids and Botanicals on Growth Performance, Intestinal Barrier Function, Inflammatory Cytokines, and Endocannabinoid System Gene Expression in Broiler Chickens. Poult. Sci. 2023, 102, 102460. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Guo, F.; Abbas, W.; Hu, Z.; Liu, L.; Qiao, J.; Bi, R.; Xu, T.; Zhang, K.; Huang, J.; et al. Effects of Microencapsulated Essential Oils and Organic Acids Preparation on Growth Performance, Slaughter Characteristics, Nutrient Digestibility and Intestinal Microenvironment of Broiler Chickens. Poult. Sci. 2024, 103, 103655. [Google Scholar] [CrossRef]
- Spisni, E.; Petrocelli, G.; Imbesi, V.; Spigarelli, R.; Azzinnari, D.; Donati Sarti, M.; Campieri, M.; Valerii, M.C. Antioxidant, Anti-Inflammatory, and Microbial-Modulating Activities of Essential Oils: Implications in Colonic Pathophysiology. Int. J. Mol. Sci. 2020, 21, 4152. [Google Scholar] [CrossRef]
- Shen, J.; Liu, T.; Qian, Y.; Yan, S.; Liu, Z.; Jia, F. Therapeutic Effect of Probiotic-Fermented Herbal Blend as Antibiotic Alternative on Salmonellosis by Multi-Drug Resistant Salmonella Pullorum. Food Biosci. 2024, 57, 103585. [Google Scholar] [CrossRef]
- Kačániová, M.; Čmiková, N.; Vukovic, N.L.; Verešová, A.; Bianchi, A.; Garzoli, S.; Ben Saad, R.; Ben Hsouna, A.; Ban, Z.; Vukic, M.D. Citrus Limon Essential Oil: Chemical Composition and Selected Biological Properties Focusing on the Antimicrobial (In Vitro, In Situ), Antibiofilm, Insecticidal Activity and Preservative Effect against Salmonella enterica Inoculated in Carrot. Plants 2024, 13, 524. [Google Scholar] [CrossRef] [PubMed]
- Groves, P.J.; Williamson, S.L.; Ahaduzzaman, M.; Diamond, M.; Ngo, M.; Han, A.; Sharpe, S.M. Can a Combination of Vaccination, Probiotic and Organic Acid Treatment in Layer Hens Protect against Early Life Exposure to Salmonella Typhimurium and Challenge at Sexual Maturity? Vaccine 2021, 39, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Deng, X.; Deng, Q.; Liu, Z.; Liu, N. Probiotic Lactobacilli Improved Growth Performance and Attenuated Salmonella Typhimurium Infection Via Jak/Stat Signaling in Broilers. Braz. J. Poult. Sci. 2021, 23. [Google Scholar] [CrossRef]
- Hong, H.A.; Duc, L.H.; Cutting, S.M. The Use of Bacterial Spore Formers as Probiotics: Table 1. FEMS Microbiol. Rev. 2005, 29, 813–835. [Google Scholar] [CrossRef]
- Barbosa, T.M.; Serra, C.R.; La Ragione, R.M.; Woodward, M.J.; Henriques, A.O. Screening for Bacillus Isolates in the Broiler Gastrointestinal Tract. Appl. Environ. Microbiol. 2005, 71, 968–978. [Google Scholar] [CrossRef]
- Duc, L.H.; Hong, H.A.; Barbosa, T.M.; Henriques, A.O.; Cutting, S.M. Characterization of Bacillus Probiotics Available for Human Use. Appl. Environ. Microbiol. 2004, 70, 2161–2171. [Google Scholar] [CrossRef]
- Menconi, A.; Morgan, M.J.; Pumford, N.R.; Hargis, B.M.; Tellez, G. Physiological Properties and Salmonella Growth Inhibition of Probiotic Bacillus Strains Isolated from Environmental and Poultry Sources. Int. J. Bacteriol. 2013, 2013, 958408. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, N.; Han, J.; Chang, C.; Hsiao, F.S.; Yu, Y. Optimization of Surfactin Production from Bacillus subtilis in Fermentation and Its Effects on Clostridium perfringens -induced Necrotic Enteritis and Growth Performance in Broilers. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1232–1244. [Google Scholar] [CrossRef]
- Lee, K.-W.; Kim, D.K.; Lillehoj, H.S.; Jang, S.I.; Lee, S.-H. Immune Modulation by Bacillus Subtilis-Based Direct-Fed Microbials in Commercial Broiler Chickens. Anim. Feed. Sci. Technol. 2015, 200, 76–85. [Google Scholar] [CrossRef]
- Tarradas, J.; Tous, N.; Esteve-Garcia, E.; Brufau, J. The Control of Intestinal Inflammation: A Major Objective in the Research of Probiotic Strains as Alternatives to Antibiotic Growth Promoters in Poultry. Microorganisms 2020, 8, 148. [Google Scholar] [CrossRef]
- Sikandar, A.; Zaneb, H.; Nasir, A.; Adil, M.; Ali, H.M.; Muhammad, N.; Rehman, T.; Rehman, A.; Rehman, H.F. Effects of Bacillus Subtilis on Performance, Immune System and Gut in Salmonella-Challenged Broilers. S. Afr. J. Anim. Sci. 2020, 50, 654–662. [Google Scholar] [CrossRef]
- Knap, I.; Kehlet, A.B.; Bennedsen, M.; Mathis, G.F.; Hofacre, C.L.; Lumpkins, B.S.; Jensen, M.M.; Raun, M.; Lay, A. Bacillus Subtilis (DSM17299) Significantly Reduces Salmonella in Broilers. Poult. Sci. 2011, 90, 1690–1694. [Google Scholar] [CrossRef]
- Guo, M.; Wu, F.; Hao, G.; Qi, Q.; Li, R.; Li, N.; Wei, L.; Chai, T. Bacillus Subtilis Improves Immunity and Disease Resistance in Rabbits. Front. Immunol. 2017, 8, 354. [Google Scholar] [CrossRef]
- Sikandar, A.; Zaneb, H.; Nasir, A.; Rehman, A.; Kashif, M.; Shah, M.; Luqman, Z.; Din, S.; Iqbal, M.; Khan, I.; et al. Effect of Bacillus Subtilis on the Microarchitectural Development of the Immune System in Salmonella-Challenged Broiler Chickens. Vet. Med. 2022, 67, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, S.D.; Hossiendoust, A.; Kim, I.H. Probiotics in Salmonella-Challenged Hy-Line Brown Layers. Poult. Sci. 2016, 95, 1894–1897. [Google Scholar] [CrossRef] [PubMed]
- Hosseindoust, A.; Mohammadi, M.; Yao, Z.P.; Jung, M.; Kim, I.H. Dietary Bacillus subtilis B2A Strain in Laying Hens Challenged with Salmonella gallinarum: Effects on Egg Production, Egg Quality, Blood Haptoglobin and Targeted Intestinal Salmonella Shedding. J. Appl. Anim. Res. 2018, 46, 512–517. [Google Scholar] [CrossRef]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the Use of Bacillus Species for Industrial Production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef]
- Shanmugasundaram, R.; Applegate, T.J.; Selvaraj, R.K. Effect of Bacillus Subtilis and Bacillus Licheniformis Probiotic Supplementation on Cecal Salmonella Load in Broilers Challenged with Salmonella. J. Appl. Poult. Res. 2020, 29, 808–816. [Google Scholar] [CrossRef]
- Connor Padgett, J.; Thomas Price, P.; Allen Byrd, J.; Anthony Bailey, C. Salmonella Enteritidis Control in Mature Laying Hens Through Dry Fed Parietal Yeast Fraction or Bacillus Blend Probiotic. Int. J. Anim. Sci. Technol. 2021, 5, 1. [Google Scholar] [CrossRef]
- Dolin, B.J. Effects of a Propietary Bacillus Coagulans Preparation on Symptoms of Diarrhea-Predominant Irritable Bowel Syndrome. Methods Find. Exp. Clin. Pharmacol. 2009, 31, 655. [Google Scholar] [CrossRef]
- Fitzpatrick, L.R. Probiotics for the Treatment of Clostridium difficile Associated Disease. World J. Gastrointest. Pathophysiol. 2013, 4, 47. [Google Scholar] [CrossRef]
- Zhen, W.; Shao, Y.; Gong, X.; Wu, Y.; Geng, Y.; Wang, Z.; Guo, Y. Effect of Dietary Bacillus Coagulans Supplementation on Growth Performance and Immune Responses of Broiler Chickens Challenged by Salmonella Enteritidis. Poult. Sci. 2018, 97, 2654–2666. [Google Scholar] [CrossRef]
- Higgins, J.P.; Higgins, S.E.; Vicente, J.L.; Wolfenden, A.D.; Tellez, G.; Hargis, B.M. Temporal Effects of Lactic Acid Bacteria Probiotic Culture on Salmonella in Neonatal Broilers. Poult. Sci. 2007, 86, 1662–1666. [Google Scholar] [CrossRef]
- Vilà, B.; Fontgibell, A.; Badiola, I.; Esteve-Garcia, E.; Jiménez, G.; Castillo, M.; Brufau, J. Reduction of Salmonella enterica Var. Enteritidis Colonization and Invasion by Bacillus Cereus Var. Toyoi Inclusion in Poultry Feeds. Poult. Sci. 2009, 88, 975–979. [Google Scholar] [CrossRef]
- Lei, X.; Piao, X.; Ru, Y.; Zhang, H.; Péron, A.; Zhang, H. Effect of Bacillus amyloliquefaciens-Based Direct-Fed Microbial on Performance, Nutrient Utilization, Intestinal Morphology and Cecal Microflora in Broiler Chickens. Asian-Australas. J. Anim. Sci. 2014, 28, 239–246. [Google Scholar] [CrossRef]
- Poudel, I.; Calvert, A.; Kiess, A.S.; Zhang, L.; Adhikari, P.A. Effect of a Bacillus-Based Probiotic on Fecal Shedding and Cecal Colonization of Salmonella Enteritidis in Laying Hens. J. Appl. Poult. Res. 2025, 34, 100532. [Google Scholar] [CrossRef]
- Bumbie, G.Z.; Abormegah, L.; Asiedu, P.; Oduro-Owusu, A.D.; Koranteng, A.A.-A.; Ansah, K.O.; Lamptey, V.K.; Chen, C.; Mohamed, T.M.; Tang, Z. Influence of Pediococcus Pentosaceus GT001 on Performance, Meat Quality, Immune Function, Antioxidant and Cecum Microbial in Broiler Chickens Challenged by Salmonella Typhimurium. Animals 2024, 14, 1676. [Google Scholar] [CrossRef] [PubMed]
- Khochamit, N.; Siripornadulsil, S.; Sukon, P.; Siripornadulsil, W. Bacillus Subtilis and Lactic Acid Bacteria Improve the Growth Performance and Blood Parameters and Reduce Salmonella Infection in Broilers. Vet. World 2020, 13, 2663–2672. [Google Scholar] [CrossRef] [PubMed]
- Aprea, G.; Del Matto, I.; Tucci, P.; Marino, L.; Scattolini, S.; Rossi, F. In Vivo Functional Properties of Dairy Bacteria. Microorganisms 2023, 11, 1787. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Santonicola, S.; Giaccone, V.; Truant, A.; Colavita, G. Dairy Propionibacteria: Probiotic Properties and Their Molecular Bases. Biomolecules 2025, 15, 886. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.V.T.; Vazhakkattu Thomas, J.; Dewi, G.; Brannon, J.; Noll, S.L.; Johnson, T.J.; Cox, R.B.; Kollanoor Johny, A. Propionibacterium Freudenreichii Freudenreichii B3523 Reduces Cecal Colonization and Internal Organ Dissemination of Multidrug-Resistant Salmonella Heidelberg in Finishing Turkeys. J. Appl. Poult. Res. 2021, 30, 100107. [Google Scholar] [CrossRef]
- Manjankattil, S.; Dewi, G.; Peichel, C.; Creek, M.; Bina, P.; Lerohl, K.; Deniz, K.; Akhtar, L.; Porter, R.; Johnson, T.J.; et al. Dairy-Origin Propionibacterium Freudenreichii, Turkey-Origin Lactobacillus Salivarius, and a Salmonella Typhimurium Vaccine Elicit Comparable Colonization Resistance on Drug-Resistant Salmonella Serotypes (S. Reading, S. Agona, and S. Saintpaul) in Growing Turkeys after Oral Challenge. J. Appl. Poult. Res. 2024, 33, 100428. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. Biomed. Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef]
- Nam, T.V.B.; Anh, L.H.; Loc, H.T.; Trang, C.T.H.; Thiet, N.; Lan, L.T.T.; Diep, T.H.; Xuan, N.H.; Ngu, N.T. Effects of Probiotic (Lactobacillus Plantarum and Bacillus Subtilis) Supplementation on Mortality, Growth Performance, and Carcass Characteristics of Native Vietnamese Broilers Challenged with Salmonella Typhimurium. Vet. World 2022, 15, 2302–2308. [Google Scholar] [CrossRef]
- Smialek, M.; Kaczorek, E.; Szczucińska, E.; Burchardt, S.; Kowalczyk, J.; Tykałowski, B.; Koncicki, A. Evaluation of Lactobacillus Spp. and Yeast Based Probiotic (Lavipan) Supplementation for the Reduction of Salmonella Enteritidis after Infection of Broiler Chickens. Pol. J. Vet. Sci. 2019, 22, 5–10. [Google Scholar] [CrossRef]
- Adhikari, P.; Lee, C.H.; Cosby, D.E.; Cox, N.A.; Kim, W.K. Effect of Probiotics on Fecal Excretion, Colonization in Internal Organs and Immune Gene Expression in the Ileum of Laying Hens Challenged with SalmonellaEnteritidis. Poult. Sci. 2019, 98, 1235–1242. [Google Scholar] [CrossRef]
- Bielecka, M.; Smoragiewicz, W.; Siwicki, A.K.; Wójcik, R.; Biedrzycka, E.; Orłowski, A.; Kask, S.; Jankowski, J.; Karska-Wysocki, B.; Ham, D. The Effect of Various Probiotic Strains or Avilamycin Feed Additive on Immune Defense Markers and Acute-Phase Response to Salmonella Infection in Chickens. Probiotics Antimicrob. Proteins 2010, 2, 175–185. [Google Scholar] [CrossRef]
- Khaksefidi, A.; Rahimi, S. Effect of Probiotic Inclusion in the Diet of Broiler Chickens on Performance, Feed Efficiency and Carcass Quality. Asian-Australas. J. Anim. Sci. 2005, 18, 1153–1156. [Google Scholar] [CrossRef]
- Mountzouris, K.C.; Dalaka, E.; Palamidi, I.; Paraskeuas, V.; Demey, V.; Theodoropoulos, G.; Fegeros, K. Evaluation of Yeast Dietary Supplementation in Broilers Challenged or Not with Salmonella on Growth Performance, Cecal Microbiota Composition and Salmonella in Ceca, Cloacae and Carcass Skin. Poult. Sci. 2015, 94, 2445–2455. [Google Scholar] [CrossRef]
- Price, P.T.; Gaydos, T.A.; Berghaus, R.D.; Baxter, V.; Hofacre, C.L.; Sims, M.D. Salmonella Enteritidis Reduction in Layer Ceca with a Bacillus Probiotic. Vet. World 2020, 13, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Shibat El-hamd, D.; Mohamed, H. Effect of Probiotics on Salmonella Enteritidis Infection in Broiler Chickens. Egypt. J. Chem. Environ. Health 2016, 2, 298–314. [Google Scholar] [CrossRef]
- Mountzouris, K.C.; Tsirtsikos, P.; Kalamara, E.; Nitsch, S.; Schatzmayr, G.; Fegeros, K. Evaluation of the Efficacy of a Probiotic Containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus Strains in Promoting Broiler Performance and Modulating Cecal Microflora Composition and Metabolic Activities. Poult. Sci. 2007, 86, 309–317. [Google Scholar] [CrossRef]
- Gölz, G.; Rosner, B.; Hofreuter, D.; Josenhans, C.; Kreienbrock, L.; Löwenstein, A.; Schielke, A.; Stark, K.; Suerbaum, S.; Wieler, L.H.; et al. Relevance of Campylobacter to Public Health—The Need for a One Health Approach. Int. J. Med. Microbiol. 2014, 304, 817–823. [Google Scholar] [CrossRef]
- Wagenaar, J.A.; Van Bergen, M.A.P.; Mueller, M.A.; Wassenaar, T.M.; Carlton, R.M. Phage Therapy Reduces Campylobacter jejuni Colonization in Broilers. Vet. Microbiol. 2005, 109, 275–283. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). FoodNet Fast. Available online: https://wwwn.cdc.gov/foodnetfast/ (accessed on 21 August 2025).
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on Quantification of the Risk Posed by Broiler Meat to Human Campylobacteriosis in the EU. EFSA J. 2010, 8, 1437. [Google Scholar] [CrossRef]
- Bessell, P.R.; Matthews, L.; Smith-Palmer, A.; Rotariu, O.; Strachan, N.J.; Forbes, K.J.; Cowden, J.M.; Reid, S.W.; Innocent, G.T. Geographic Determinants of Reported Human Campylobacter Infections in Scotland. BMC Public Health 2010, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Saint-Cyr, M.J.; Guyard-Nicodème, M.; Messaoudi, S.; Chemaly, M.; Cappelier, J.-M.; Dousset, X.; Haddad, N. Recent Advances in Screening of Anti-Campylobacter Activity in Probiotics for Use in Poultry. Front. Microbiol. 2016, 7, 553. [Google Scholar] [CrossRef]
- Batz, M.B.; Hoffmann, S.; Morris, J.G. Ranking the Disease Burden of 14 Pathogens in Food Sources in the United States Using Attribution Data from Outbreak Investigations and Expert Elicitation. J. Food Prot. 2012, 75, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.; Devleesschauwer, B.; Aspinall, W.; Cooke, R.; Corrigan, T.; Havelaar, A.H.; Angulo, F.J.; Gibb, H.J.; Kirk, M.D.; Lake, R.J.; et al. Attribution of global foodborne disease to specific foods: Findings from a World Health Organization structured expert elicitation. PLoS ONE 2017, 12, e0183641. [Google Scholar] [CrossRef] [PubMed]
- Nolte, T.; Spieß, F.; Jacobs, A.-K.; Kemper, N.; Visscher, C. Assessing Concordance between Campylobacter Prevalence in Broilers and Human Cases before and during the COVID-19 Pandemic in Lower Saxony, Germany, Considering Fresh Chicken Meat Consumption Patterns. Front. Vet. Sci. 2024, 11, 1392677. [Google Scholar] [CrossRef]
- Naguib, M.; Sharma, S.; Schneider, A.; Wehmueller, S.; Abdelaziz, K. Comparative Effectiveness of Various Multi-Antigen Vaccines in Controlling Campylobacter jejuni in Broiler Chickens. Vaccines 2024, 12, 908. [Google Scholar] [CrossRef]
- Aho, M.; Nuotio, L.; Nurmi, E.; Kiiskinen, T. Competitive Exclusion of Campylobacters from Poultry with K-Bacteria and Broilact®. Int. J. Food Microbiol. 1992, 15, 265–275. [Google Scholar] [CrossRef]
- Ty, M.; Taha-Abdelaziz, K.; Demey, V.; Castex, M.; Sharif, S.; Parkinson, J. Performance of Distinct Microbial Based Solutions in a Campylobacter Infection Challenge Model in Poultry. Anim. Microbiome 2022, 4, 2. [Google Scholar] [CrossRef]
- Cawthraw, S.A.; Wassenaar, T.M.; Ayling, R.; Newell, D.G. Increased Colonization Potential of Campylobacter jejuni Strain 81116 after Passage through Chickens and Its Implication on the Rate of Transmission within Flocks. Epidemiol. Infect. 1996, 117, 213–215. [Google Scholar] [CrossRef]
- Dasti, J.I.; Tareen, A.M.; Lugert, R.; Zautner, A.E.; Groß, U. Campylobacter Jejuni: A Brief Overview on Pathogenicity-Associated Factors and Disease-Mediating Mechanisms. Int. J. Med. Microbiol. 2010, 300, 205–211. [Google Scholar] [CrossRef]
- Cohen, E.J.; Nakane, D.; Kabata, Y.; Hendrixson, D.R.; Nishizaka, T.; Beeby, M. Campylobacter jejuni Motility Integrates Specialized Cell Shape, Flagellar Filament, and Motor, to Coordinate Action of Its Opposed Flagella. PLoS Pathog. 2020, 16, e1008620. [Google Scholar] [CrossRef] [PubMed]
- Day, C.J.; King, R.M.; Shewell, L.K.; Tram, G.; Najnin, T.; Hartley-Tassell, L.E.; Wilson, J.C.; Fleetwood, A.D.; Zhulin, I.B.; Korolik, V. A Direct-Sensing Galactose Chemoreceptor Recently Evolved in Invasive Strains of Campylobacter jejuni. Nat. Commun. 2016, 7, 13206. [Google Scholar] [CrossRef] [PubMed]
- Hendrixson, D.R.; DiRita, V.J. Identification of Campylobacter jejuni Genes Involved in Commensal Colonization of the Chick Gastrointestinal Tract. Mol. Microbiol. 2004, 52, 471–484. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Lindén, S.K.; Sutton, P.; Florin, T.H. Mucin Dynamics and Enteric Pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar] [CrossRef]
- Ribardo, D.A.; Johnson, J.J.; Hendrixson, D.R. Viscosity-Dependent Determinants of Campylobacter jejuni Impacting the Velocity of Flagellar Motility. mBio 2024, 15, e0254423. [Google Scholar] [CrossRef]
- Kemper, L.; Hensel, A. Campylobacter Jejuni: Targeting Host Cells, Adhesion, Invasion, and Survival. Appl. Microbiol. Biotechnol. 2023, 107, 2725–2754. [Google Scholar] [CrossRef]
- Al Hakeem, W.G.; Fathima, S.; Shanmugasundaram, R.; Selvaraj, R.K. Campylobacter jejuni in Poultry: Pathogenesis and Control Strategies. Microorganisms 2022, 10, 2134. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.M.; Clyne, M.; Bourke, B. Campylobacter jejuni Adhere to and Invade Chicken Intestinal Epithelial Cells in Vitro. Microbiology 2007, 153, 561–569. [Google Scholar] [CrossRef]
- Alemka, A.; Whelan, S.; Gough, R.; Clyne, M.; Gallagher, M.E.; Carrington, S.D.; Bourke, B. Purified Chicken Intestinal Mucin Attenuates Campylobacter jejuni Pathogenicity in Vitro. J. Med. Microbiol. 2010, 59, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Larsson, J.M.H.; Hansson, G.C. The Two Mucus Layers of Colon Are Organized by the MUC2 Mucin, Whereas the Outer Layer Is a Legislator of Host–Microbial Interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 4659–4665. [Google Scholar] [CrossRef] [PubMed]
- Barnawi, H.; Masri, N.; Hussain, N.; Al-Lawati, B.; Mayasari, E.; Gulbicka, A.; Jervis, A.J.; Huang, M.-H.; Cavet, J.S.; Linton, D. RNA-Based Thermoregulation of a Campylobacter jejuni Zinc Resistance Determinant. PLoS Pathog. 2020, 16, e1009008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-J.; Xiao, D.; Zhao, F.; Gu, Y.-X.; Meng, F.-L.; He, L.-H.; Ma, G.-Y.; Zhang, J.-Z. Comparative Proteomic Analysis of Campylobacter jejuni Cultured at 37C and 42C. Jpn. J. Infect. Dis. 2009, 62, 356–361. [Google Scholar] [CrossRef]
- Biswas, D.; Fernando, U.; Reiman, C.; Willson, P.; Potter, A.; Allan, B. Effect of Cytolethal Distending Toxin of Campylobacter jejuni on Adhesion and Internalization in Cultured Cells and in Colonization of the Chicken Gut. Avian Dis. 2006, 50, 586–593. [Google Scholar] [CrossRef]
- AbuOun, M.; Manning, G.; Cawthraw, S.A.; Ridley, A.; Ahmed, I.H.; Wassenaar, T.M.; Newell, D.G. Cytolethal Distending Toxin (CDT)-Negative Campylobacter jejuni Strains and Anti-CDT Neutralizing Antibodies Are Induced during Human Infection but Not during Colonization in Chickens. Infect. Immun. 2005, 73, 3053–3062. [Google Scholar] [CrossRef]
- de Zoete, M.R.; Keestra, A.M.; Roszczenko, P.; van Putten, J.P.M. Activation of Human and Chicken Toll-Like Receptors by Campylobacter spp. Infect. Immun. 2010, 78, 1229–1238. [Google Scholar] [CrossRef]
- Guerry, P.; Alm, R.A.; Power, M.E.; Logan, S.M.; Trust, T.J. Role of Two Flagellin Genes in Campylobacter Motility. J. Bacteriol. 1991, 173, 4757–4764. [Google Scholar] [CrossRef]
- Nachamkin, I.; Yang, X.H.; Stern, N.J. Role of Campylobacter jejuni Flagella as Colonization Factors for Three-Day-Old Chicks: Analysis with Flagellar Mutants. Appl. Environ. Microbiol. 1993, 59, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Morokka, T.; Umeda, A.; Amako, K. Motility as an Intestinal Colonization Factor for Campylobacter jejuni. Microbiology 1985, 131, 1973–1980. [Google Scholar] [CrossRef]
- Konkel, M.E.; Klena, J.D.; Rivera-Amill, V.; Monteville, M.R.; Biswas, D.; Raphael, B.; Mickelson, J. Secretion of Virulence Proteins from Campylobacter jejuni Is Dependent on a Functional Flagellar Export Apparatus. J. Bacteriol. 2004, 186, 3296–3303. [Google Scholar] [CrossRef] [PubMed]
- Elmi, A.; Nasher, F.; Dorrell, N.; Wren, B.; Gundogdu, O. Revisiting Campylobacter jejuni Virulence and Fitness Factors: Role in Sensing, Adapting, and Competing. Front. Cell Infect. Microbiol. 2021, 10, 607704. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Burr, D.H.; Guerry, P. CheY-mediated Modulation of Campylobacter jejuni Virulence. Mol. Microbiol. 1997, 23, 1021–1031. [Google Scholar] [CrossRef]
- Godschalk, P.C.R.; Heikema, A.P.; Gilbert, M.; Komagamine, T.; Ang, C.W.; Glerum, J.; Brochu, D.; Li, J.; Yuki, N.; Jacobs, B.C.; et al. The Crucial Role of Campylobacter jejuni Genes in Anti-Ganglioside Antibody Induction in Guillain-Barré Syndrome. J. Clin. Investig. 2004, 114, 1659–1665. [Google Scholar] [CrossRef]
- Boehm, M.; Krause-Gruszczynska, M.; Rohde, M.; Tegtmeyer, N.; Takahashi, S.; Oyarzabal, O.A.; Backert, S. Major Host Factors Involved in Epithelial Cell Invasion of Campylobacter Jejuni: Role of Fibronectin, Integrin Beta1, FAK, Tiam-1, and DOCK180 in Activating Rho GTPase Rac1. Front. Cell Infect. Microbiol. 2011, 1, 17. [Google Scholar] [CrossRef]
- Smith, J.L.; Bayles, D.O. The Contribution of Cytolethal Distending Toxin to Bacterial Pathogenesis. Crit. Rev. Microbiol. 2006, 32, 227–248. [Google Scholar] [CrossRef]
- Soro, A.B.; Whyte, P.; Bolton, D.J.; Tiwari, B.K. Strategies and Novel Technologies to Control Campylobacter in the Poultry Chain: A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1353–1377. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.F.; Boris, S.; Barbes, C. Probiotic Properties of Human Lactobacilli Strains to Be Used in the Gastrointestinal Tract. J. Appl. Microbiol. 2003, 94, 449–455. [Google Scholar] [CrossRef]
- Chaveerach, P.; Lipman, L.J.A.; van Knapen, F. Antagonistic Activities of Several Bacteria on in Vitro Growth of 10 Strains of Campylobacter Jejuni/Coli. Int. J. Food Microbiol. 2004, 90, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Wine, E.; Gareau, M.G.; Johnson-Henry, K.; Sherman, P.M. Strain-Specific Probiotic (Lactobacillus helveticus) Inhibition of Campylobacter jejuni invasion of Human Intestinal Epithelial Cells. FEMS Microbiol. Lett. 2009, 300, 146–152. [Google Scholar] [CrossRef]
- Tareb, R.; Bernardeau, M.; Gueguen, M.; Vernoux, J.-P. In Vitro Characterization of Aggregation and Adhesion Properties of Viable and Heat-Killed Forms of Two Probiotic Lactobacillus Strains and Interaction with Foodborne Zoonotic Bacteria, Especially Campylobacter jejuni. J. Med. Microbiol. 2013, 62, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, M.A.; Kurzak, P.; Bauer, J.; Vogel, R.F. Characterization of Lactobacilli towards Their Use as Probiotic Adjuncts in Poultry. J. Appl. Microbiol. 2002, 92, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Taheri, H.R.; Moravej, H.; Tabandeh, F.; Zaghari, M.; Shivazad, M. Screening of Lactic Acid Bacteria toward Their Selection as a Source of Chicken Probiotic. Poult. Sci. 2009, 88, 1586–1593. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Deng, Q.; Shi, H.; Luo, Y.; Zhao, H.; Liu, N. Effect of Dietary Lactobacilli Mixture on Listeria Monocytogenes Infection and Virulence Property in Broilers. Poult. Sci. 2020, 99, 3655–3662. [Google Scholar] [CrossRef] [PubMed]
- Catacutan, J.R.P.; Subejano, M.S.E.P.; Penuliar, G.M. In Vitro and in Vivo Activity of Lactobacillus sakei L14 Strain against Campylobacter jejuni DC3 Strain. J. Vet. Res. 2022, 66, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Saint-Cyr, M.J.; Haddad, N.; Taminiau, B.; Poezevara, T.; Quesne, S.; Amelot, M.; Daube, G.; Chemaly, M.; Dousset, X.; Guyard-Nicodème, M. Use of the Potential Probiotic Strain Lactobacillus Salivarius SMXD51 to Control Campylobacter jejuni in Broilers. Int. J. Food Microbiol. 2017, 247, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Even, M.; Davail, S.; Rey, M.; Tavernier, A.; Houssier, M.; Bernadet, M.D.; Gontier, K.; Pascal, G.; Ricaud, K. Probiotics Strains Modulate Gut Microbiota and Lipid Metabolism in Mule Ducks. Open Microbiol. J. 2018, 12, 71–93. [Google Scholar] [CrossRef]
- Cole, K.; Farnell, M.B.; Donoghue, A.M.; Stern, N.J.; Svetoch, E.A.; Eruslanov, B.N.; Volodina, L.I.; Kovalev, Y.N.; Perelygin, V.V.; Mitsevich, E.V.; et al. Bacteriocins Reduce Campylobacter Colonization and Alter Gut Morphology in Turkey Poults. Poult. Sci. 2006, 85, 1570–1575. [Google Scholar] [CrossRef]
- Kobierecka, P.A.; Wyszyńska, A.K.; Aleksandrzak-Piekarczyk, T.; Kuczkowski, M.; Tuzimek, A.; Piotrowska, W.; Górecki, A.; Adamska, I.; Wieliczko, A.; Bardowski, J.; et al. In Vitro Characteristics of Lactobacillus Spp. Strains Isolated from the Chicken Digestive Tract and Their Role in the Inhibition of Campylobacter Colonization. Microbiologyopen 2017, 6, e00512. [Google Scholar] [CrossRef]
- Nishiyama, K.; Seto, Y.; Yoshioka, K.; Kakuda, T.; Takai, S.; Yamamoto, Y.; Mukai, T. Lactobacillus Gasseri SBT2055 Reduces Infection by and Colonization of Campylobacter jejuni. PLoS ONE 2014, 9, e108827. [Google Scholar] [CrossRef]
- Taha-Abdelaziz, K.; Astill, J.; Kulkarni, R.R.; Read, L.R.; Najarian, A.; Farber, J.M.; Sharif, S. In Vitro Assessment of Immunomodulatory and Anti-Campylobacter Activities of Probiotic Lactobacilli. Sci. Rep. 2019, 9, 17903. [Google Scholar] [CrossRef]
- Ghareeb, K.; Awad, W.A.; Mohnl, M.; Porta, R.; Biarnés, M.; Böhm, J.; Schatzmayr, G. Evaluating the Efficacy of an Avian-Specific Probiotic to Reduce the Colonization of Campylobacter jejuni in Broiler Chickens. Poult. Sci. 2012, 91, 1825–1832. [Google Scholar] [CrossRef]
- Fritts, C.A.; Kersey, J.H.; Motl, M.A.; Kroger, E.C.; Yan, F.; Si, J.; Jiang, Q.; Campos, M.M.; Waldroup, A.L.; Waldroup, P.W. Bacillus Subtilis C-3102 (Calsporin) Improves Live Performance and Microbiological Status of Broiler Chickens. J. Appl. Poult. Res. 2000, 9, 149–155. [Google Scholar] [CrossRef]
- Aguiar, V.F.; Donoghue, A.M.; Arsi, K.; Reyes-Herrera, I.; Metcalf, J.H.; de los Santos, F.S.; Blore, P.J.; Donoghue, D.J. Targeting Motility Properties of Bacteria in the Development of Probiotic Cultures Against Campylobacter jejuni in Broiler Chickens. Foodborne Pathog. Dis. 2013, 10, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Šimunović, K.; Sahin, O.; Erega, A.; Štefanič, P.; Zhang, Q.; Mandic Mulec, I.; Smole Možina, S.; Klančnik, A. Bacillus Subtilis PS-216 Spores Supplemented in Broiler Chicken Drinking Water Reduce Campylobacter jejuni Colonization and Increases Weight Gain. Front. Microbiol. 2022, 13, 910616. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.; Ibrahim, D.; El Sayed, S.; Wahdan, A.; El-Tarabili, R.M.; Rizk El-Ghareeb, W.; Abdullah Alhawas, B.; Alahmad, B.A.-H.Y.; Abdel-Raheem, S.M.; El-Hamid, M.I.A. Prospective Application of Nanoencapsulated Bacillus Amyloliquefaciens on Broiler Chickens’ Performance and Gut Health with Efficacy against Campylobacter jejuni Colonization. Animals 2023, 13, 775. [Google Scholar] [CrossRef]
- Guyard-Nicodème, M.; Keita, A.; Quesne, S.; Amelot, M.; Poezevara, T.; Le Berre, B.; Sánchez, J.; Vesseur, P.; Martín, Á.; Medel, P.; et al. Efficacy of Feed Additives against Campylobacter in Live Broilers during the Entire Rearing Period. Poult. Sci. 2016, 95, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Gracia, M.I.; Millán, C.; Sánchez, J.; Guyard-Nicodème, M.; Mayot, J.; Carre, Y.; Csorbai, A.; Chemaly, M.; Medel, P. Efficacy of Feed Additives against Campylobacter in Live Broilers during the Entire Rearing Period: Part B. Poult. Sci. 2016, 95, 886–892. [Google Scholar] [CrossRef]
- Netherwood, T.; Gilbert, H.J.; Parker, D.S.; O’Donnell, A.G. Probiotics Shown To Change Bacterial Community Structure in the Avian Gastrointestinal Tract. Appl. Environ. Microbiol. 1999, 65, 5134–5138. [Google Scholar] [CrossRef]
- Lauková, A.; Kandričáková, A.; Ščerbová, J.; Szabóová, R. In Vivo Model Experiment Using Laying Hens Treated with Enterococcus Faecium EM41 from Ostrich Faeces and Its Enterocin EM41. Maced. Vet. Rev. 2017, 40. [Google Scholar] [CrossRef]
- Willis, W.L.; Reid, L. Investigating the Effects of Dietary Probiotic Feeding Regimens on Broiler Chicken Production and Campylobacter jejuni Presence. Poult. Sci. 2008, 87, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Sonnenborn, U.; Schulze, J. The Non-Pathogenic Escherichia coli Strain Nissle 1917—Features of a Versatile Probiotic. Microb. Ecol. Health Dis. 2009, 21, 122–158. [Google Scholar] [CrossRef]
- Splichalova, A.; Trebichavsky, I.; Rada, V.; Vlkova, E.; Sonnenborn, U.; Splichal, I. Interference of Bifidobacterium choerinum or Escherichia coli Nissle 1917 with Salmonella Typhimurium in Gnotobiotic Piglets Correlates with Cytokine Patterns in Blood and Intestine. Clin. Exp. Immunol. 2011, 163, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Helmy, Y.A.; Closs, G.; Jung, K.; Kathayat, D.; Vlasova, A.; Rajashekara, G. Effect of Probiotic E. coli Nissle 1917 Supplementation on the Growth Performance, Immune Responses, Intestinal Morphology, and Gut Microbes of Campylobacter jejuni Infected Chickens. Infect. Immun. 2022, 90, e00337-22. [Google Scholar] [CrossRef]
- Valečková, E.; Sun, L.; Wang, H.; Dube, F.; Ivarsson, E.; Kasmaei, K.M.; Ellström, P.; Wall, H. Intestinal Colonization with Campylobacter jejuni Affects Broiler Gut Microbiota Composition but Is Not Inhibited by Daily Intake of Lactiplantibacillus Plantarum. Front. Microbiol. 2023, 14, 1205797. [Google Scholar] [CrossRef] [PubMed]
- Schoeni, J.L.; Wong, A.C. Inhibition of Campylobacter jejuni Colonization in Chicks by Defined Competitive Exclusion Bacteria. Appl. Environ. Microbiol. 1994, 60, 1191–1197. [Google Scholar] [CrossRef]
- Morishita, T.Y.; Aye, P.P.; Harr, B.S.; Cobb, C.W.; Clifford, J.R. Evaluation of an Avian-Specific Probiotic to Reduce the Colonization and Shedding of Campylobacter jejuni in Broilers. Avian Dis. 1997, 41, 850–855. [Google Scholar] [CrossRef]
- Santini, C.; Baffoni, L.; Gaggia, F.; Granata, M.; Gasbarri, R.; Di Gioia, D.; Biavati, B. Characterization of Probiotic Strains: An Application as Feed Additives in Poultry against Campylobacter jejuni. Int. J. Food Microbiol. 2010, 141, S98–S108. [Google Scholar] [CrossRef]
- Baffoni, L.; Gaggìa, F.; Di Gioia, D.; Santini, C.; Mogna, L.; Biavati, B. A Bifidobacterium-Based Synbiotic Product to Reduce the Transmission of C. Jejuni along the Poultry Food Chain. Int. J. Food Microbiol. 2012, 157, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Neal-McKinney, J.M.; Lu, X.; Duong, T.; Larson, C.L.; Call, D.R.; Shah, D.H.; Konkel, M.E. Production of Organic Acids by Probiotic Lactobacilli Can Be Used to Reduce Pathogen Load in Poultry. PLoS ONE 2012, 7, e43928. [Google Scholar] [CrossRef]
- Arsi, K.; Donoghue, A.M.; Woo-Ming, A.; Blore, P.J.; Donoghue, D.J. The Efficacy of Selected Probiotic and Prebiotic Combinations in Reducing Campylobacter Colonization in Broiler Chickens. J. Appl. Poult. Res. 2015, 24, 327–334. [Google Scholar] [CrossRef]
- Cean, A.; Stef, L.; Simiz, E.; Julean, C.; Dumitrescu, G.; Vasile, A.; Pet, E.; Drinceanu, D.; Corcionivoschi, N. Effect of Human Isolated Probiotic Bacteria on Preventing Campylobacter jejuni Colonization of Poultry. Foodborne Pathog. Dis. 2015, 12, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Schoeni, J.L.; Doyle, M.P. Reduction of Campylobacter jejuni Colonization of Chicks by Cecum-Colonizing Bacteria Producing Anti-C. Jejuni Metabolites. Appl. Environ. Microbiol. 1992, 58, 664–670. [Google Scholar] [CrossRef]
- Fu, Y.; Alenezi, T.; Sun, X. Clostridium Perfringens-Induced Necrotic Diseases: An Overview. Immuno 2022, 2, 387–407. [Google Scholar] [CrossRef]
- Grenda, T.; Jarosz, A.; Sapała, M.; Grenda, A.; Patyra, E.; Kwiatek, K. Clostridium Perfringens—Opportunistic Foodborne Pathogen, Its Diversity and Epidemiological Significance. Pathogens 2023, 12, 768. [Google Scholar] [CrossRef]
- Rood, J.I.; Adams, V.; Lacey, J.; Lyras, D.; McClane, B.A.; Melville, S.B.; Moore, R.J.; Popoff, M.R.; Sarker, M.R.; Songer, J.G.; et al. Expansion of the Clostridium Perfringens Toxin-Based Typing Scheme. Anaerobe 2018, 53, 5–10. [Google Scholar] [CrossRef]
- Freedman, J.; Shrestha, A.; McClane, B. Clostridium Perfringens Enterotoxin: Action, Genetics, and Translational Applications. Toxins 2016, 8, 73. [Google Scholar] [CrossRef]
- Mehdizadeh Gohari, I.; Navarro, M.A.; Li, J.; Shrestha, A.; Uzal, F.; McClane, B.A. Pathogenicity and Virulence of Clostridium perfringens. Virulence 2021, 12, 723–753. [Google Scholar] [CrossRef]
- Kulkarni, R.R.; Gaghan, C.; Gorrell, K.; Fletcher, O.J. Mucosal and Systemic Lymphoid Immune Responses against Clostridium Perfringens Strains with Variable Virulence in the Production of Necrotic Enteritis in Broiler Chickens. Avian Pathol. 2023, 52, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Lillehoj, H.S.; Sun, Z.; Lee, Y.; Zhao, H.; Xianyu, Z.; Yan, X.; Wang, Y.; Lin, S.; Liu, L.; et al. Characterization of Virulent NetB+/TpeL+Clostridium Perfringens Strains from Necrotic Enteritis-Affected Broiler Chicken Farms. Avian Dis. 2019, 63, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Coursodon, C.F.; Glock, R.D.; Moore, K.L.; Cooper, K.K.; Songer, J.G. TpeL-Producing Strains of Clostridium Perfringens Type A Are Highly Virulent for Broiler Chicks. Anaerobe 2012, 18, 117–121. [Google Scholar] [CrossRef]
- Camargo, A.; Ramírez, J.D.; Kiu, R.; Hall, L.J.; Muñoz, M. Unveiling the Pathogenic Mechanisms of Clostridium perfringens Toxins and Virulence Factors. Emerg. Microbes Infect. 2024, 13, 2341968. [Google Scholar] [CrossRef]
- Muneeb, M.; Khan, E.; Ahmad, S.; Suleman, S. Potential of Probiotics against Necrotic Enteritis in Commercial Broilers. In Complementary and Alternative Medicine: Prebiotics and Probiotics; Unique Scientific Publishers: Faisalabad, Pakistan, 2024; pp. 274–283. [Google Scholar] [CrossRef]
- Granstad, S.; Kristoffersen, A.B.; Benestad, S.L.; Sjurseth, S.K.; David, B.; Sørensen, L.; Fjermedal, A.; Edvardsen, D.H.; Sanson, G.; Løvland, A.; et al. Effect of Feed Additives as Alternatives to In-Feed Antimicrobials on Production Performance and Intestinal Clostridium Perfringens Counts in Broiler Chickens. Animals 2020, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shao, Y.; Song, B.; Zhen, W.; Wang, Z.; Guo, Y.; Shahid, M.S.; Nie, W. Effects of Bacillus Coagulans Supplementation on the Growth Performance and Gut Health of Broiler Chickens with Clostridium Perfringens-Induced Necrotic Enteritis. J. Anim. Sci. Biotechnol. 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Gharib-Naseri, K.; Dorigam, J.C.P.; Doranalli, K.; Morgan, N.; Swick, R.A.; Choct, M.; Wu, S.-B. Bacillus Amyloliquefaciens CECT 5940 Improves Performance and Gut Function in Broilers Fed Different Levels of Protein and/or under Necrotic Enteritis Challenge. Anim. Nutr. 2021, 7, 185–197. [Google Scholar] [CrossRef]
- Abd El-Ghany, W.A.; Abdel-Latif, M.A.; Hosny, F.; Alatfeehy, N.M.; Noreldin, A.E.; Quesnell, R.R.; Chapman, R.; Sakai, L.; Elbestawy, A.R. Comparative Efficacy of Postbiotic, Probiotic, and Antibiotic against Necrotic Enteritis in Broiler Chickens. Poult. Sci. 2022, 101, 101988. [Google Scholar] [CrossRef] [PubMed]
- McReynolds, J.; Waneck, C.; Byrd, J.; Genovese, K.; Duke, S.; Nisbet, D. Efficacy of Multistrain Direct-Fed Microbial and Phytogenetic Products in Reducing Necrotic Enteritis in Commercial Broilers. Poult. Sci. 2009, 88, 2075–2080. [Google Scholar] [CrossRef]
- Buiatte, V.; Schultheis, M.; Lorenzoni, A.G. Deconstruction of a Multi-Strain Bacillus-Based Probiotic Used for Poultry: An in Vitro Assessment of Its Individual Components against C. Perfringens. BMC Res. Notes 2023, 16, 117. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, K.; Zhang, A.; Chang, W.; Zheng, A.; Chen, Z.; Cai, H.; Liu, G. Effects of Lactobacillus Acidophilus on the Growth Performance, Immune Response, and Intestinal Barrier Function of Broiler Chickens Challenged with Escherichia coli O157. Poult. Sci. 2021, 100, 101323. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Q.; Jia, H.M.; Zeng, X.F.; Zhu, J.L.; Hou, C.L.; Liu, X.T.; Yang, F.J.; Qiao, S.Y. Prevention of Escherichia coli Infection in Broiler Chickens with Lactobacillus Plantarum B1. Poult. Sci. 2017, 96, 2576–2586. [Google Scholar] [CrossRef]
- Hidayat, M.N.; Malaka, R.; Agustina, L.; Pakiding, W. Effect of Lactobacillus Sp. Probiotics on Intestinal Histology, Escherichia coli in Excreta and Broiler Performance. J. Indones. Trop. Anim. Agric. 2018, 43, 445. [Google Scholar] [CrossRef]
- Tarabees, R.; El-Sayed, M.S.; Shehata, A.A.; Diab, M.S. Effects of the Probiotic Candidate E. Faecalis-1, the Poulvac E. coli Vaccine, and Their Combination on Growth Performance, Caecal Microbial Composition, Immune Response, and Protection against E. coli O78 Challenge in Broiler Chickens. Probiotics Antimicrob. Proteins 2020, 12, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Islam, M.; Samad, M.; Kabir, S. Characterization and antibiogram of Escherichia coli associated with mortality in broilers and ducklings in Bangladesh. Bangladesh J. Vet. Med. 1970, 2, 9–14. [Google Scholar] [CrossRef]
- Johnson, T.J.; Wannemuehler, Y.; Doetkott, C.; Johnson, S.J.; Rosenberger, S.C.; Nolan, L.K. Identification of Minimal Predictors of Avian Pathogenic Escherichia coli Virulence for Use as a Rapid Diagnostic Tool. J. Clin. Microbiol. 2008, 46, 3987–3996. [Google Scholar] [CrossRef]
- Manges, A.R. Escherichia coli and Urinary Tract Infections: The Role of Poultry-Meat. Clin. Microbiol. Infect. 2016, 22, 122–129. [Google Scholar] [CrossRef]
- Vinayananda, C.O.; Fairoze, N.; Madhavaprasad, C.B.; Byregowda, S.M.; Nagaraj, C.S.; Bagalkot, P.; Karabasanavar, N. Studies on Occurrence, Characterisation and Decontamination of Emerging Pathogenic Escherichia coli (STEC, ETEC and EIEC) in Table Eggs. Br. Poult. Sci. 2017, 58, 664–672. [Google Scholar] [CrossRef]
- Liang, W.; Li, H.; Zhou, H.; Wang, M.; Zhao, X.; Sun, X.; Li, C.; Zhang, X. Effects of Taraxacum and Astragalus Extracts Combined with Probiotic Bacillus Subtilis and Lactobacillus on Escherichia coli–Infected Broiler Chickens. Poult. Sci. 2021, 100, 101007. [Google Scholar] [CrossRef]
- Mellata, M. Human and Avian Extraintestinal Pathogenic Escherichia coli: Infections, Zoonotic Risks, and Antibiotic Resistance Trends. Foodborne Pathog. Dis. 2013, 10, 916–932. [Google Scholar] [CrossRef] [PubMed]
- Gorton, A.; Stasiewicz, M.J. Twenty-Two Years of U.S. Meat and Poultry Product Recalls: Implications for Food Safety and Food Waste. J. Food Prot. 2017, 80, 674–684. [Google Scholar] [CrossRef]
- Scharff, R.L. Food Attribution and Economic Cost Estimates for Meat- and Poultry-Related Illnesses. J. Food Prot. 2020, 83, 959–967. [Google Scholar] [CrossRef]
- Mainil, J. Escherichia coli Virulence Factors. Vet. Immunol. Immunopathol. 2013, 152, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Trabulsi, L.R.; Keller, R.; Gomes, T.A.T. Typical and Atypical Enteropathogenic Escherichia coli. Emerg. Infect. Dis. 2002, 8, 508–513. [Google Scholar] [CrossRef]
- Mazariego-Espinosa, K.; Cruz, A.; Ledesma, M.A.; Ochoa, S.A.; Xicohtencatl-Cortes, J. Longus, a Type IV Pilus of Enterotoxigenic Escherichia coli, Is Involved in Adherence to Intestinal Epithelial Cells. J. Bacteriol. 2010, 192, 2791–2800. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Richards, P.; Windhorst, D.; Imre, A.; Bukovinski, A.; Ruggeri, J.; Elazomi, A.; Barrow, P. Generation of an Inactivated Vaccine for Avian Pathogenic Escherichia coli Using Microarrays; a More Rational Approach to Inactivated Vaccine Design. Open Vet. J. 2022, 12, 221. [Google Scholar] [CrossRef]
- Bernier, C.; Gounon, P.; Le Bouguénec, C. Identification of an Aggregative Adhesion Fimbria (AAF) Type III-Encoding Operon in Enteroaggregative Escherichia coli as a Sensitive Probe for Detecting the AAF-Encoding Operon Family. Infect. Immun. 2002, 70, 4302–4311. [Google Scholar] [CrossRef] [PubMed]
- Abe, A.; De Grado, M.; Pfuetzner, R.A.; Sánchez-SanMartín, C.; DeVinney, R.; Puente, J.L.; Strynadka, N.C.J.; Finlay, B.B. Enteropathogenic Escherichia coli Translocated Intimin Receptor, Tir, Requires a Specific Chaperone for Stable Secretion. Mol. Microbiol. 1999, 33, 1162–1175. [Google Scholar] [CrossRef]
- Johura, F.-T.; Parveen, R.; Islam, A.; Sadique, A.; Rahim, M.N.; Monira, S.; Khan, A.R.; Ahsan, S.; Ohnishi, M.; Watanabe, H.; et al. Occurrence of Hybrid Escherichia coli Strains Carrying Shiga Toxin and Heat-Stable Toxin in Livestock of Bangladesh. Front. Public Health 2017, 4, 287. [Google Scholar] [CrossRef][Green Version]
- Trung, N.V.; Nhung, H.N.; Carrique-Mas, J.J.; Mai, H.H.; Tuyen, H.T.; Campbell, J.; Nhung, N.T.; Van Minh, P.; Wagenaar, J.A.; Mai, N.T.N.; et al. Colonization of Enteroaggregative Escherichia coli and Shiga Toxin-Producing Escherichia coli in Chickens and Humans in Southern Vietnam. BMC Microbiol. 2016, 16, 208. [Google Scholar] [CrossRef]
- Madni, W.; Zahoor, M.; Nawaz, Z.; Khurshid, M. Prevalence and Sequence Analysis of Escherichia coli Harboring Colistin, Gentamicin, Streptomycin, Tetracycline and Quinolones Resistant Genes from Commercial Broilers. Pak. Vet. J. 2025, 45, 390–395. [Google Scholar] [CrossRef]
- Jin, L.Z.; Ho, Y.W.; Abdullah, N.; Jalaludin, S. Acid and bile tolerance of Lactobacillus isolated from chicken intestines and their antimicrobial activities against pathogenic bacteria. Lett. Appl. Microbiol. 1998, 27, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zeng, X.F.; Zhu, J.L.; Wang, S.; Liu, X.T.; Hou, C.L.; Thacker, P.A.; Qiao, S.Y. Effects of Dietary Lactobacillus Plantarum B1 on Growth Performance, Intestinal Microbiota, and Short Chain Fatty Acid Profiles in Broiler Chickens. Poult. Sci. 2016, 95, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Aldeieg, M.; Woodward, M.J.; Juniper, D.T.; Rymer, C. The Effect of Candida Famata and Lactobacillus Plantarum on the Number of Coliforms and the Antibiotic Resistance and Virulence of Escherichia coli in the Gut of Broilers. Animal 2021, 15, 100310. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalaifa, H.; Al-Nasser, A.; Al-Surayee, T.; Al-Kandari, S.; Al-Enzi, N.; Al-Sharrah, T.; Ragheb, G.; Al-Qalaf, S.; Mohammed, A. Effect of Dietary Probiotics and Prebiotics on the Performance of Broiler Chickens. Poult. Sci. 2019, 98, 4465–4479. [Google Scholar] [CrossRef]
- Novak, J.S.; Call, J.; Tomasula, P.; Luchansky, J.B. An Assessment of Pasteurization Treatment of Water, Media, and Milk with Respect to Bacillus Spores. J. Food Prot. 2005, 68, 751–757. [Google Scholar] [CrossRef]
- La Ragione, R. Bacillus Subtilis Spores Competitively Exclude Escherichia coli O78:K80 in Poultry. Vet. Microbiol. 2001, 79, 133–142. [Google Scholar] [CrossRef]
- McBride, S.M.; Fischetti, V.A.; LeBlanc, D.J.; Moellering, R.C.; Gilmore, M.S. Genetic Diversity among Enterococcus Faecalis. PLoS ONE 2007, 2, e582. [Google Scholar] [CrossRef]
- Dong, Z.L.; Wang, Y.W.; Song, D.; Wang, W.W.; Liu, K.B.; Wang, L.; Li, A.K. Effects of Microencapsulated Probiotics and Plant Extract on Antioxidant Ability, Immune Status and Caecal Microflora in Escherichia coli K88-Challenged Broiler Chickens. Food Agric. Immunol. 2019, 30, 1123–1134. [Google Scholar] [CrossRef]
- Huang, L.; Luo, L.; Zhang, Y.; Wang, Z.; Xia, Z. Effects of the Dietary Probiotic, Enterococcus Faecium NCIMB11181, on the Intestinal Barrier and System Immune Status in Escherichia coli O78-Challenged Broiler Chickens. Probiotics Antimicrob. Proteins 2019, 11, 946–956. [Google Scholar] [CrossRef]
- Cao, G.T.; Zeng, X.F.; Chen, A.G.; Zhou, L.; Zhang, L.; Xiao, Y.P.; Yang, C.M. Effects of a Probiotic, Enterococcus Faecium, on Growth Performance, Intestinal Morphology, Immune Response, and Cecal Microflora in Broiler Chickens Challenged with Escherichia coli K88. Poult. Sci. 2013, 92, 2949–2955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Zhan, X.; Zeng, X.; Zhou, L.; Cao, G.; Chen, A.; Yang, C. Effects of Dietary Supplementation of Probiotic, Clostridium Butyricum, on Growth Performance, Immune Response, Intestinal Barrier Function, and Digestive Enzyme Activity in Broiler Chickens Challenged with Escherichia coli K88. J. Anim. Sci. Biotechnol. 2016, 7, 3. [Google Scholar] [CrossRef]
- Igbafe, J.; Kilonzo-Nthenge, A.; Nahashon, S.N.; Mafiz, A.I.; Nzomo, M. Probiotics and Antimicrobial Effect of Lactiplantibacillus Plantarum, Saccharomyces Cerevisiae, and Bifidobacterium Longum against Common Foodborne Pathogens in Poultry. Agriculture 2020, 10, 368. [Google Scholar] [CrossRef]
- Bortoluzzi, C.; Barbosa, J.G.M.; Pereira, R.; Fagundes, N.S.; Rafael, J.M.; Menten, J.F.M. Autolyzed Yeast (Saccharomyces Cerevisiae) Supplementation Improves Performance While Modulating the Intestinal Immune-System and Microbiology of Broiler Chickens. Front. Sustain. Food Syst. 2018, 2, 85. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, C.; Zhang, C.; Zhang, X.; Wu, Y. Lacticaseibacillus Rhamnosus Reduces the Pathogenicity of Escherichia coli in Chickens. Front. Microbiol. 2021, 12, 664604. [Google Scholar] [CrossRef]
- Kathayat, D.; Closs, G.; Helmy, Y.A.; Deblais, L.; Srivastava, V.; Rajashekara, G. In Vitro and In Vivo Evaluation of Lacticaseibacillus Rhamnosus GG and Bifidobacterium lactis Bb12 Against Avian Pathogenic Escherichia coli and Identification of Novel Probiotic-Derived Bioactive Peptides. Probiotics Antimicrob. Proteins 2022, 14, 1012–1028. [Google Scholar] [CrossRef]
- Yang, Y.-X.; He, M.; Hu, G.; Wei, J.; Pages, P.; Yang, X.-H.; Bourdu-Naturel, S. Effect of a Fermented Milk Containing Bifidobacterium lactis DN-173010 on Chinese Constipated Women. World J. Gastroenterol. 2008, 14, 6237. [Google Scholar] [CrossRef]
- Ventura, M.; Zink, R. Rapid Identification, Differentiation, and Proposed New Taxonomic Classification of Bifidobacterium lactis. Appl. Environ. Microbiol. 2002, 68, 6429–6434. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, P.; Zhang, Y.; Xiao, K.; Jiang, F.; Wang, H.; Tang, D.; Liu, D.; Liu, B.; Liu, Y. The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome 2018, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Ying, Z.; Xue, M.; Xiaoxian, P.; Xiaorong, L.; Chunyang, L.; Yu, W.; Mingcheng, L.; Binxian, L. A Novel Lactobacillus Bulgaricus Isolate Can Maintain the Intestinal Health, Improve the Growth Performance and Reduce the Colonization of E. coli O157:H7 in Broilers. Br. Poult. Sci. 2022, 63, 621–632. [Google Scholar] [CrossRef]
- Manafi, M.; Khalaji, S.; Hedayati, M.; Pirany, N. Efficacy of Bacillus Subtilis and Bacitracin Methylene Disalicylate on Growth Performance, Digestibility, Blood Metabolites, Immunity, and Intestinal Microbiota after Intramuscular Inoculation with Escherichia coli in Broilers. Poult. Sci. 2017, 96, 1174–1183. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, G.T.; Zeng, X.F.; Zhou, L.; Ferket, P.R.; Xiao, Y.P.; Chen, A.G.; Yang, C.M. Effects of Clostridium Butyricum on Growth Performance, Immune Function, and Cecal Microflora in Broiler Chickens Challenged with Escherichia coli K88. Poult. Sci. 2014, 93, 46–53. [Google Scholar] [CrossRef]
- Younis, H. Trials on the Role of Prebiotics and Probiotics in Colonization and Immune Response of Broiler Chickens Challenged with Escherichia coli K88. Alex. J. Vet. Sci. 2018, 59, 48. [Google Scholar] [CrossRef]
- ul Haq, I.; Hafeez, A.; Khan, R.U. Protective Effect of Nigella Sativa and Saccharomyces Cerevisiae on Zootechnical Characteristics, Fecal Escherichia coli and Hematopoietic Potential in Broiler Infected with Experimental Colibacillosis. Livest. Sci. 2020, 239, 104119. [Google Scholar] [CrossRef]
- Papouskova, A.; Rychlik, I.; Harustiakova, D.; Cizek, A. Research Note: A Mixture of Bacteroides Spp. and Other Probiotic Intestinal Anaerobes Reduces Colonization by Pathogenic E. coli Strain O78:H4-ST117 in Newly Hatched Chickens. Poult. Sci. 2023, 102, 102529. [Google Scholar] [CrossRef] [PubMed]
- Istiqomah, L.; Hayati, S.N.; Damayanti, E.; Julendra, H.; Sakti, A.A.; Untari, T. Performance and Meat Quality of Broilers Infected with Escherichia coli and Administered with Bio Additive, Probiotic, and Antibiotic. Media Peternak. Fak. Peternak. Inst. Pertan. Bogor. 2013, 36, 14–20. [Google Scholar] [CrossRef]
- Farber, J.M.; Peterkin, P.I. Listeria Monocytogenes, a Food-Borne Pathogen. Microbiol. Rev. 1991, 55, 476–511. [Google Scholar] [CrossRef]
- Ricke, S.C.; O’Bryan, C.A.; Rothrock, M.J. Listeria Occurrence in Conventional and Alternative Egg Production Systems. Microorganisms 2023, 11, 2164. [Google Scholar] [CrossRef] [PubMed]
- Quereda, J.J.; Morón-García, A.; Palacios-Gorba, C.; Dessaux, C.; García-del Portillo, F.; Pucciarelli, M.G.; Ortega, A.D. Pathogenicity and Virulence of Listeria monocytogenes: A Trip from Environmental to Medical Microbiology. Virulence 2021, 12, 2509–2545. [Google Scholar] [CrossRef]
- Allerberger, F.; Wagner, M. Listeriosis: A resurgent foodborne infection. Clin. Microbiol. Infect. 2010, 16, 16–23. [Google Scholar] [CrossRef]
- Walter, J. Ecological Role of Lactobacilli in the Gastrointestinal Tract: Implications for Fundamental and Biomedical Research. Appl. Environ. Microbiol. 2008, 74, 4985–4996. [Google Scholar] [CrossRef]
- Dhama, K.; Verma, A.K.; Rajagunala, S.; Kumar, A.; Tiwari, R.; Chakrabort, S.; Kumar, R. Listeria Monocytogenes Infection in Poultry and Its Public Health Importance with Special Reference to Food Borne Zoonoses. Pak. J. Biol. Sci. 2013, 16, 301–308. [Google Scholar] [CrossRef]
- Chemaly, M.; Toquin, M.-T.; Le Nôtre, Y.; Fravalo, P. Prevalence of Listeria Monocytogenes in Poultry Production in France. J. Food Prot. 2008, 71, 1996–2000. [Google Scholar] [CrossRef]
- Shimojima, Y.; Shimojima, H.; Morita, Y. Survival of Campylobacter Jejuni, Salmonella, and Listeria Monocytogenes and Temperature Change in Low-Temperature–Longtime-Cooked Chicken Meat. J. Food Prot. 2022, 85, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Chang, X.; Qin, S.; Song, Y.; Tian, J.; Ma, A. Analysis of 90 Listeria Monocytogenes Contaminated in Poultry and Livestock Meat through Whole-Genome Sequencing. Food Res. Int. 2022, 159, 111641. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- Public Health Agency of Canada. Pathogen Safety Data Sheets: Infectious Substances—Salmonella enterica spp. Available online: https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/salmonella-enterica.html (accessed on 21 August 2025).
- Liu, D.; Lawrence, M.L.; Ainsworth, A.J.; Austin, F.W. Toward an Improved Laboratory Definition of Listeria Monocytogenes Virulence. Int. J. Food Microbiol. 2007, 118, 101–115. [Google Scholar] [CrossRef]
- McLauchlin, J.; Aird, H.; Amar, C.; Barker, C.; Dallman, T.; Elviss, N.; Jørgensen, F.; Willis, C. Listeria Monocytogenes in Cooked Chicken: Detection of an Outbreak in the United Kingdom (2016 to 2017) and Analysis of L. Monocytogenes from Unrelated Monitoring of Foods (2013 to 2017). J. Food Prot. 2020, 83, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Girones, R.; Herman, L.; Koutsoumanis, K.; Nørrung, B.; et al. Listeria Monocytogenes Contamination of Ready-to-eat Foods and the Risk for Human Health in the EU. EFSA J. 2018, 16, e05134. [Google Scholar] [CrossRef]
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Rubayet Ul Alam, A.S.M.; Jahid, I.K. Characterization and Evaluation of Lactic Acid Bacteria from Indigenous Raw Milk for Potential Probiotic Properties. J. Dairy. Sci. 2020, 103, 1223–1237. [Google Scholar] [CrossRef]
- Abouloifa, H.; Hasnaoui, I.; Ben Slima, S.; Rokni, Y.; Gaamouche, S.; Trabelsi, I.; Bellaouchi, R.; Ghabbour, N.; Ben Salah, R.; Jaouadi, B.; et al. Bio-Preservation Effect of Probiotic Lactiplantibacillus plantarum S61 Against Rhodotorula glutinis and Listeria monocytogenes in Poultry Meat. Curr. Microbiol. 2022, 79, 232. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mu, G.; Tuo, Y. Phenotypic Traits and Probiotic Functions of Lactiplantibacillus Plantarum Y42 in Planktonic and Biofilm Forms. Foods 2023, 12, 1516. [Google Scholar] [CrossRef] [PubMed]
- The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e05926. [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).