Immunogenicity and Breakthrough Outcomes of mRNA Booster Strategies Among Healthcare Workers During the BA.1/BA.2 Omicron Surge
Abstract
1. Introduction
2. Materials and Methods
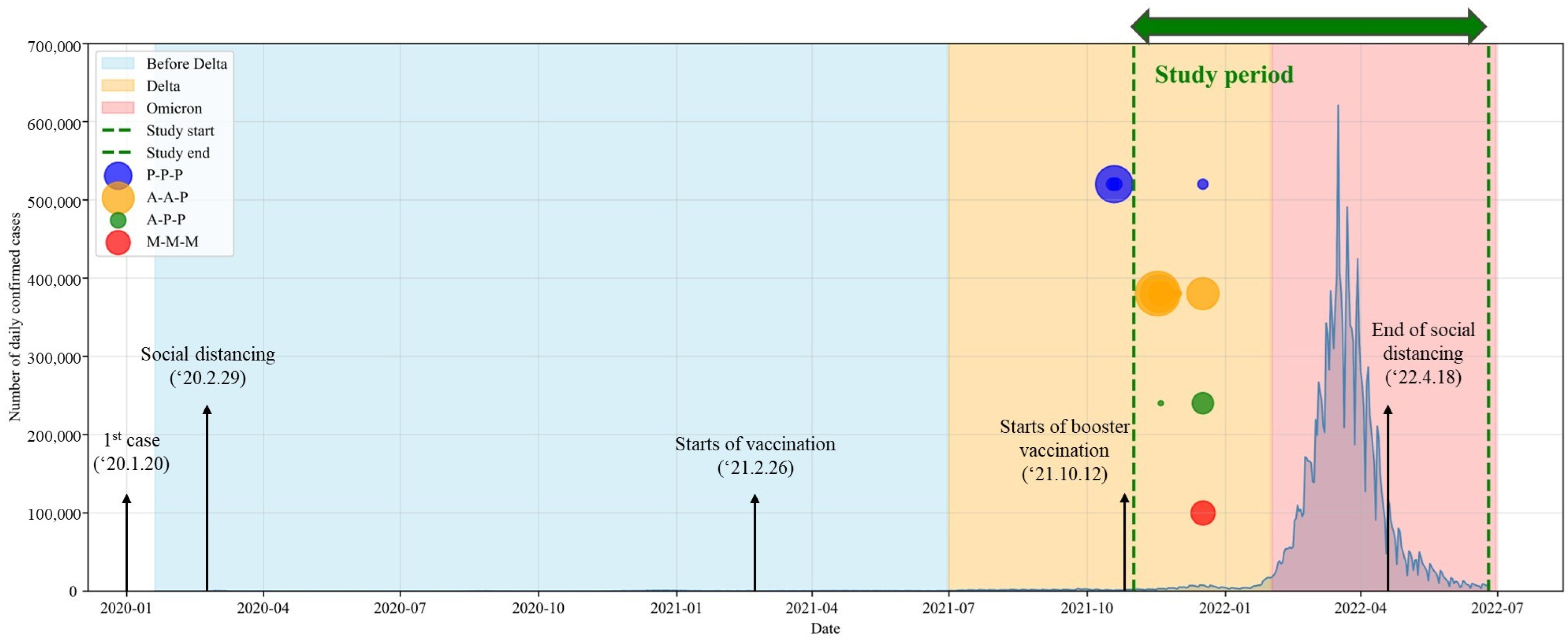
2.1. Study Design
2.2. Demographics and Serum Samples
2.3. Immunogenicity Assessment
2.4. Breakthrough Infection Rate
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
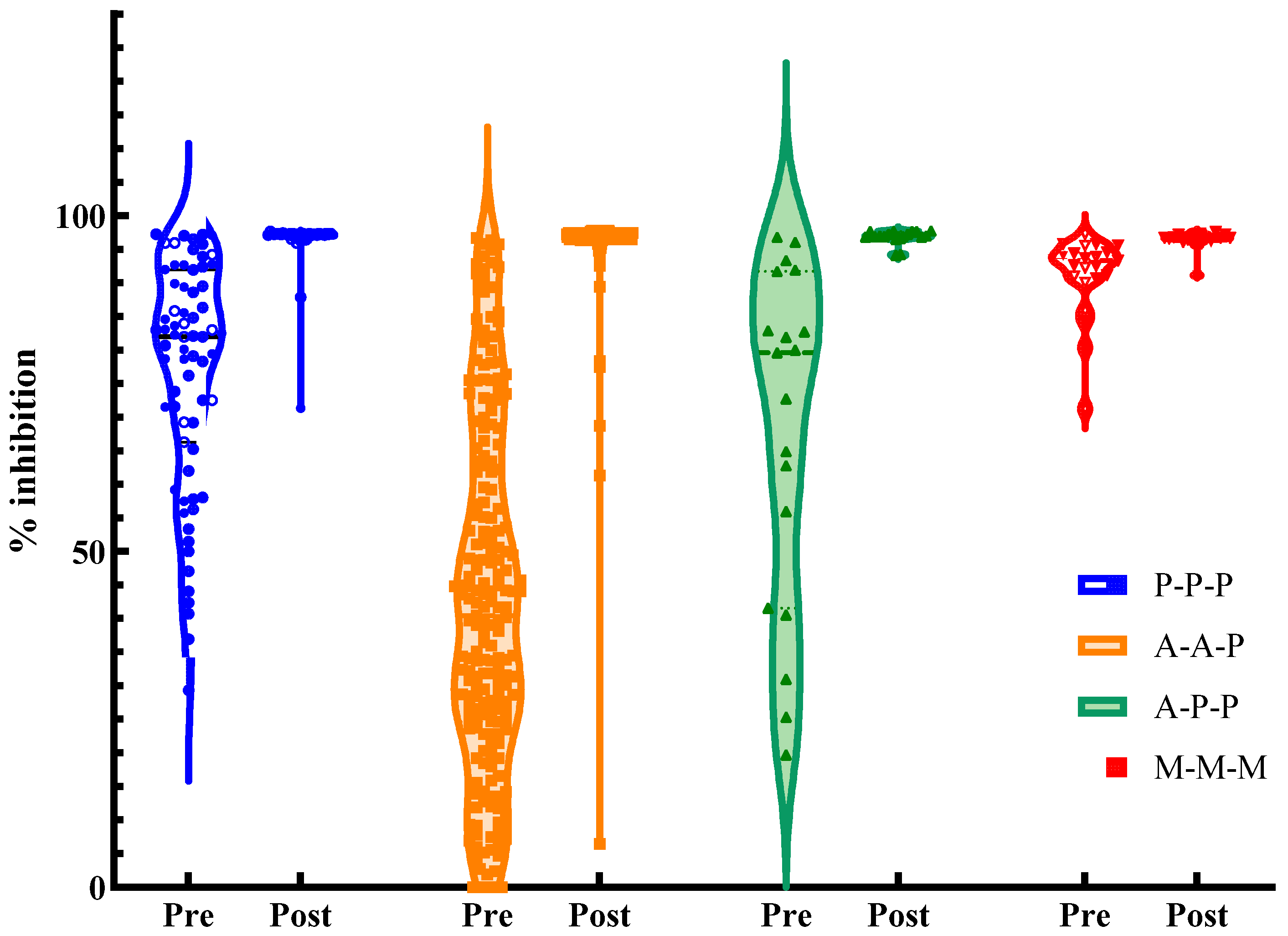
3.2. Immunogenicity After Booster Vaccination
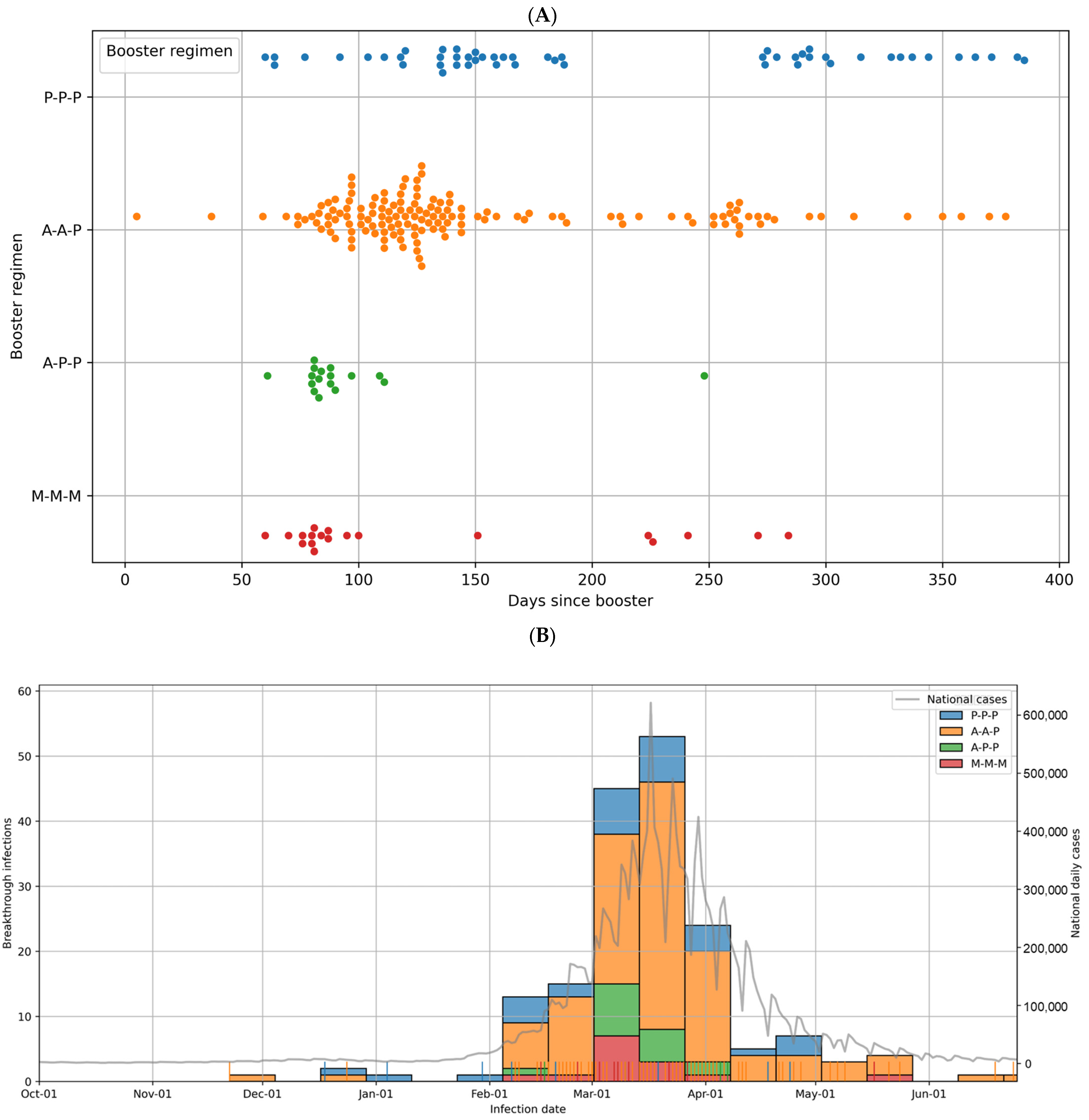
3.3. Breakthrough Infections
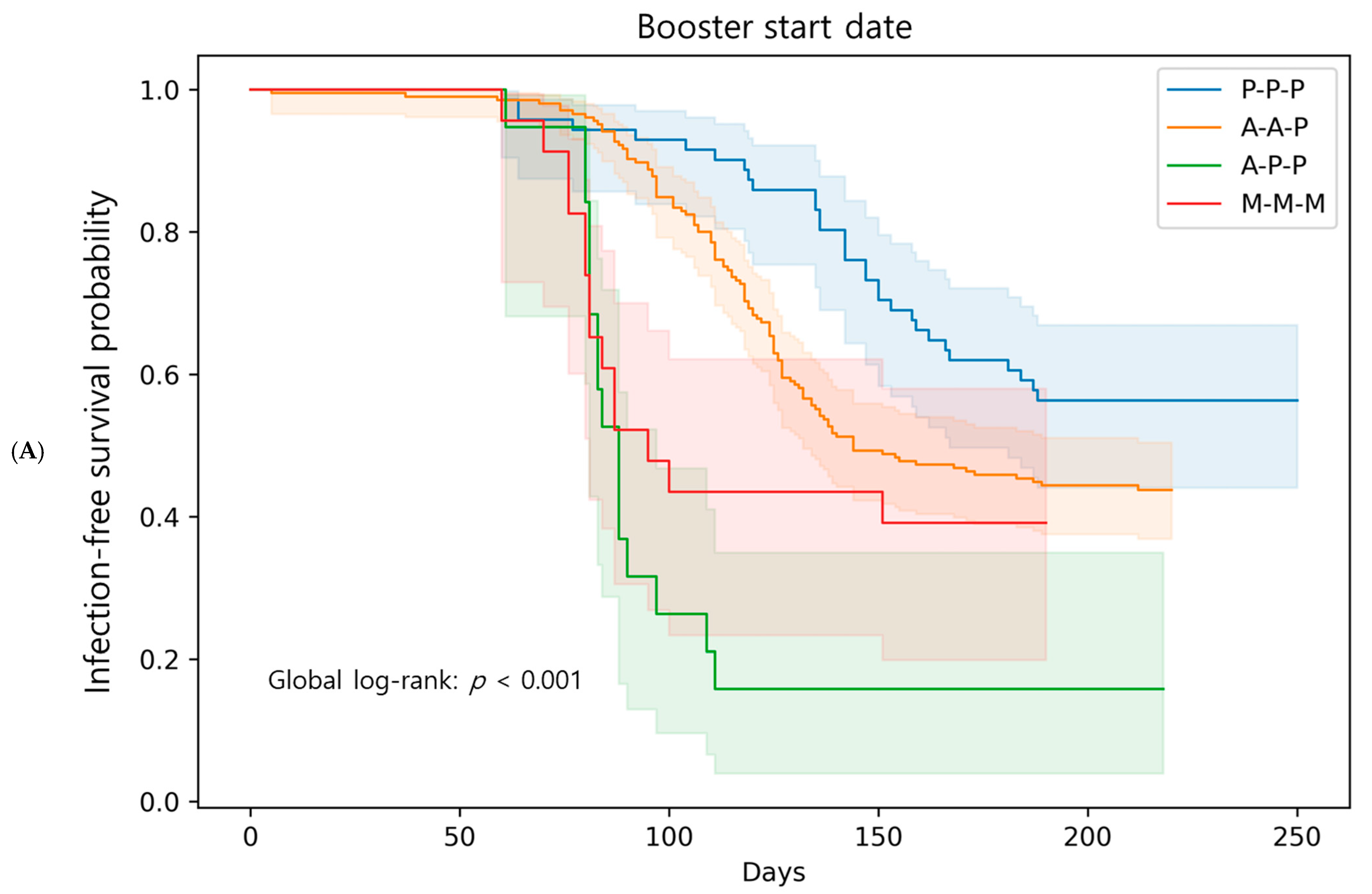
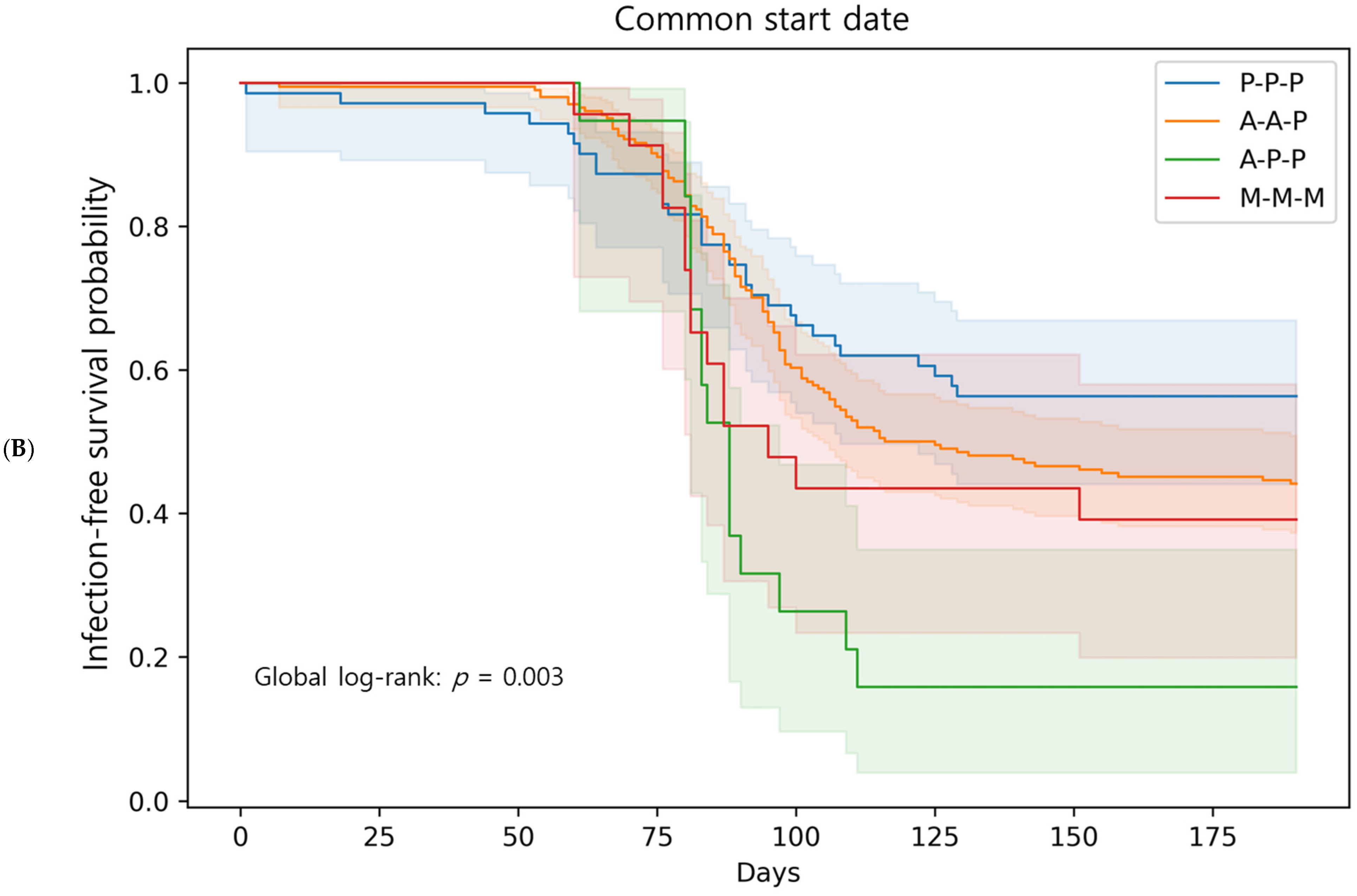
3.4. Breakthrough Infections: Comparison Among Booster Regimens
3.5. Occupation-Based Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COVID-19 | Coronavirus disease 2019 |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus-2 |
| HR | Hazard ratio |
| CI | Confidence interval |
| HCWs | Health care workers |
| IQR | Interquartile range |
| KDCA | Korea Disease Control and Prevention Agency |
References
- World Health Organization Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 23 July 2025).
- Kim, I.H.; No, J.S.; Kim, J.A.; Park, A.K.; Lee, H.; Kim, J.M.; Lee, N.J.; Kim, C.K.; Lee, C.Y.; Woo, S.; et al. Genomic epidemiology of SARS-CoV-2 variants in South Korea between January 2020 and February 2023. Virology 2023, 587, 109869. [Google Scholar] [CrossRef] [PubMed]
- Cameroni, E.; Bowen, J.E.; Rosen, L.E.; Saliba, C.; Zepeda, S.K.; Culap, K.; Pinto, D.; VanBlargan, L.A.; De Marco, A.; di Iulio, J.; et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature 2022, 602, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Pajon, R.; Doria-Rose, N.A.; Shen, X.; Schmidt, S.D.; O’Dell, S.; McDanal, C.; Feng, W.; Tong, J.; Eaton, A.; Maglinao, M.; et al. SARS-CoV-2 Omicron variant neutralization after mRNA-1273 booster vaccination. N. Engl. J. Med. 2022, 386, 1088–1091. [Google Scholar] [CrossRef]
- Regev-Yochay, G.; Gonen, T.; Gilboa, M.; Mandelboim, M.; Indenbaum, V.; Amit, S.; Meltzer, L.; Asraf, K.; Cohen, C.; Fluss, R.; et al. Efficacy of a fourth mRNA vaccine dose against Omicron. N. Engl. J. Med. 2022, 386, 1377–1380. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100, Erratum in Nat. Rev. Immunol. 2021, 21, 129. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.; van der Meer, J.W.; Mhlanga, M.M.; Mulder, W.J.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Jung, J. Preparing for the coronavirus disease (COVID-19) vaccination: Evidence, plans, and implications. J. Korean Med. Sci. 2021, 36, e59. [Google Scholar] [CrossRef]
- Nahm, E.; Song, J.Y.; Noh, J.Y.; Cheong, H.J.; Kim, W.J. COVID-19 vaccination in Korea: Past, present, and the way forward. J. Korean Med. Sci. 2022, 37, e351. [Google Scholar] [CrossRef]
- Borobia, A.M.; Carcas, A.J.; Pérez-Olmeda, M.; Castaño, L.; Bertran, M.J.; García-Pérez, J.; Campins, M.; Portolés, A.; González-Pérez, M.; García Morales, M.T.; et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 2021, 398, 121–130, Erratum in Lancet 2021, 398, 582. [Google Scholar] [CrossRef]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous vs heterologous COVID-19 boosters. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef]
- Asante, M.A.; Michelsen, M.E.; Balakumar, M.M.; Kumburegama, B.; Sharifan, A.; Thomsen, A.R.; Korang, S.K.; Gluud, C.; Menon, S. Heterologous versus homologous COVID-19 booster vaccinations for adults: Systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMC Med. 2024, 22, 263. [Google Scholar] [CrossRef]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, 847–859.e11. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.; Tiu, C.; Hu, Z.; Chen, V.C.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- Hwang, J.; Hwang, S.S.; Kim, H.B.; Lee, J. Risk compensation after COVID-19 vaccination: Evidence from vaccine rollout by exact birth date in South Korea. Health Econ. 2024, 33, 1811–1830. [Google Scholar] [CrossRef]
- Kwon, S.L.; Oh, J. COVID-19 vaccination program in South Korea: A long journey toward a new normal. Health Policy Technol. 2022, 11, 100601. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111, Erratum in Lancet 2021, 397, 98. [Google Scholar] [CrossRef]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021, 398, 2258–2276, Erratum in Lancet 2021, 398, 2246. [Google Scholar] [CrossRef] [PubMed]
- Barros-Martins, J.; Hammerschmidt, S.I.; Cossmann, A.; Odak, I.; Stankov, M.V.; Morillas Ramos, G.; Dopfer-Jablonka, A.; Heidemann, A.; Ritter, C.; Friedrichsen, M.; et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat. Med. 2021, 27, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Klemis, V.; Schub, D.; Mihm, J.; Hielscher, F.; Marx, S.; Abu-Omar, A.; Ziegler, L.; Guckelmus, C.; Urschel, R.; et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021, 27, 1530–1535. [Google Scholar] [CrossRef]
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022, 602, 654–656. [Google Scholar] [CrossRef]
- Nordström, P.; Ballin, M.; Nordström, A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic COVID-19 infection in Sweden: A nationwide cohort study. Lancet Reg. Health Eur. 2021, 11, 100249. [Google Scholar] [CrossRef]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022, 602, 671–675. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). FDA Authorizes Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines for Use as a Booster Dose. 31 August 2022. Available online: https://web.archive.org/web/20230208030813/https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use (accessed on 20 August 2025).
- Chalkias, S.; Harper, C.; Vrbicky, K.; Walsh, S.R.; Essink, B.; Brosz, A.; McGhee, N.; Tomassini, J.E.; Chen, X.; Chang, Y.; et al. A bivalent omicron-containing booster vaccine against COVID-19. N. Engl. J. Med. 2022, 387, 1279–1291. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Takes Action on Updated mRNA COVID-19 Vaccines to Better Protect Against Currently Circulating Variants. Available online: https://www.fda.gov/news-events/press-announcements/fda-takes-action-updated-mrna-covid-19-vaccines-better-protect-against-currently-circulating (accessed on 20 August 2025).
- Winokur, P.; Gayed, J.; Fitz-Patrick, D.; Thomas, S.J.; Diya, O.; Lockhart, S.; Xu, X.; Zhang, Y.; Bangad, V.; Schwartz, H.I.; et al. Bivalent Omicron BA.1-adapted BNT162b2 booster in adults older than 55 years. N. Engl. J. Med. 2023, 388, 214–227. [Google Scholar] [CrossRef]
- Link-Gelles, R.; Ciesla, A.A.; Fleming-Dutra, K.E.; Smith, Z.R.; Britton, A.; Wiegand, R.E.; Miller, J.D.; Accorsi, E.K.; Schrag, S.J.; Verani, J.R.; et al. Effectiveness of bivalent mRNA vaccines in preventing symptomatic SARS-CoV-2 infection—Increasing community access to testing program, United States, September-November 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1526–1530. [Google Scholar] [CrossRef]
- Wang, Q.; Bowen, A.; Valdez, R.; Gherasim, C.; Gordon, A.; Liu, L.; Ho, D.D. Antibody responses to Omicron BA.4/BA.5 bivalent booster. N. Engl. J. Med. 2023, 388, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Kurhade, C.; Patel, S.; Kitchin, N.; Tompkins, K.; Cutler, M.; Cooper, D.; Yang, Q.; Cai, H.; Muik, A.; et al. Neutralization of BA.4-BA.5, BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 with the bivalent vaccine. N. Engl. J. Med. 2023, 388, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Azzi, L.; Dalla Gasperina, D.; Veronesi, G.; Shallak, M.; Ietto, G.; Iovino, D.; Baj, A.; Gianfagna, F.; Maurino, V.; Focosi, D.; et al. Mucosal immune response in BNT162b2 COVID-19 vaccine recipients. EBioMedicine 2022, 75, 103750. [Google Scholar] [CrossRef] [PubMed]
- Sood, N.; Lam, C.N.; Kawaguchi, E.; Pernet, O.; Kovacs, A.; Unger, J.B.; Hu, H. Association between levels of receptor binding domain antibodies of SARS-CoV-2, receipt of booster, and risk of breakthrough infections: LA pandemic surveillance cohort study. Sci. Rep. 2023, 13, 47261. [Google Scholar] [CrossRef]
- Yamamoto, S.; Matsuda, K.; Maeda, K.; Horii, K.; Okudera, K.; Oshiro, Y.; Inamura, N.; Takeuchi, J.S.; Konishi, M.; Ozeki, M.; et al. Neutralizing antibodies following three doses of BNT162b2 vaccine, breakthrough infection, and symptoms during the Omicron predominant wave. Int. J. Infect. Dis. 2023, 128, 347–354. [Google Scholar] [CrossRef]
- Lingas, G.; Planas, D.; Péré, H.; Porrot, F.; Guivel-Benhassine, F.; Staropoli, I.; Duffy, D.; Chapuis, N.; Gobeaux, C.; Veyer, D.; et al. Neutralizing antibody levels as a correlate of protection against SARS-CoV-2 infection: A modeling analysis. Clin. Pharmacol. Ther. 2024, 115, 86–94. [Google Scholar] [CrossRef]
- Montefiori, D.C. Enhanced immunity after Ad26.COV-2.S vaccine breakthrough infection. Cell Rep. Med. 2022, 3, 100579. [Google Scholar] [CrossRef]
- Seow, J.; Shalim, Z.A.; Graham, C.; Kimuda, S.; Pillai, A.; Lechmere, T.; Kurshan, A.; Khimji, A.M.; Snell, L.B.; Nebbia, G.; et al. Broad and potent neutralizing mAbs are elicited in vaccinated individuals following Delta/BA.1 breakthrough infection. mBio 2023, 14, e0120623. [Google Scholar] [CrossRef]
- Perera, R.A.; Ko, R.; Tsang, O.T.Y.; Hui, D.S.C.; Kwan, M.Y.W.; Brackman, C.J.; To, E.M.W.; Yen, H.-L.; Leung, K.; Cheng, S.M.S.; et al. Evaluation of a SARS-CoV-2 Surrogate Virus Neutralization Test for Detection of Antibody in Human, Canine, Cat, and Hamster Sera. J. Clin. Microbiol. 2021, 27, 1136–1139. [Google Scholar] [CrossRef]
| Characteristics | Total (n = 318) | P-P-P (n = 71) | A-A-P (n = 205) | A-P-P (n = 19) | M-M-M (n = 23) | p-Value |
|---|---|---|---|---|---|---|
| Age, years, median ± IQR | 32 (27–44) | 28 (26–37) | 35 (27–49) | 41 (34–44) | 25 (23–31) | 0.000 |
| Sex: Female, n (%) | 238 (74.8) | 42 (59.2) | 159 (77.6) | 15 (78.9) | 22 (95.7) | 0.001 |
| Occupation: Clinical, n (%) | 234 (73.6) | 59 (83.1) | 152 (74.1) | 5 (26.3) | 18 (78.3) | 0.000 |
| Pre-booster infection, n (%) | 7 (2.2) | 1 (1.4) | 3 (1.5) | 2 (10.5) | 1 (4.3) | 0.068 |
| Follow-up duration, days, median (IQR) | 154 (110–220) | 249 (147–277) | 144 (113–220) | 84 (81–94) | 95 (80–190) | 0.000 |
| Outcome | P-P-P (n = 71) | A-A-P (n = 205) | A-P-P (n = 19) | M-M-M (n = 23) | p-Value |
|---|---|---|---|---|---|
| Before booster | |||||
| Inhibition (%), median (IQR) | 82 (68–91) | 40 (25–62) | 80 (49–87) | 93 (91–94) | 0.000 |
| Seropositive rate n (%) | 70 (98.6) | 132 (64.4) | 17 (89.5) | 23 (100.0) | 0.000 |
| 1-month post-booster | |||||
| Inhibition %, median (IQR) | 97 (97–97) | 97 (97–97) | 97 (97–97) | 97 (97–97) | 0.002 |
| Δ Absolute change (V2–V1), median (IQR) | 15 (5–30) | 55 (34–71) | 18 (10–47) | 3 (2–6) | 0.000 |
| Seropositive rate, n (%) | 71 (100.0) | 204 (99.5) | 19 (100.0) | 23 (100.0) | 0.907 |
| High responder, n (%) | 69 (97.2) | 199 (97.1) | 19 (100.0) | 23 (100.0) | 0.741 |
| Breakthrough infection no event (%) | 31 (43.7) | 115 (56.1) | 16 (84.2) | 14 (60.9) | 0.0137 |
| Inhibition % (median) at post-booster, by infection status | 97.2 vs. 97.2 | 96.9 vs. 97.0 | 97.0 vs. 96.9 | 96.7 vs. 96.6 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.M.; An, J.N.; Kwon, J.H.; Kim, S.G.; Kim, H.W. Immunogenicity and Breakthrough Outcomes of mRNA Booster Strategies Among Healthcare Workers During the BA.1/BA.2 Omicron Surge. Microorganisms 2025, 13, 2362. https://doi.org/10.3390/microorganisms13102362
Moon SM, An JN, Kwon JH, Kim SG, Kim HW. Immunogenicity and Breakthrough Outcomes of mRNA Booster Strategies Among Healthcare Workers During the BA.1/BA.2 Omicron Surge. Microorganisms. 2025; 13(10):2362. https://doi.org/10.3390/microorganisms13102362
Chicago/Turabian StyleMoon, Song Mi, Jung Nam An, Jae Hyun Kwon, Sung Gyun Kim, and Han Wool Kim. 2025. "Immunogenicity and Breakthrough Outcomes of mRNA Booster Strategies Among Healthcare Workers During the BA.1/BA.2 Omicron Surge" Microorganisms 13, no. 10: 2362. https://doi.org/10.3390/microorganisms13102362
APA StyleMoon, S. M., An, J. N., Kwon, J. H., Kim, S. G., & Kim, H. W. (2025). Immunogenicity and Breakthrough Outcomes of mRNA Booster Strategies Among Healthcare Workers During the BA.1/BA.2 Omicron Surge. Microorganisms, 13(10), 2362. https://doi.org/10.3390/microorganisms13102362







