Engineering and Application of Biosensors for Aromatic Compounds Production in Escherichia coli
Abstract
1. Introduction
2. Biosynthetic Pathway of Aromatic Compounds in E. coli
3. Construction and Application of Different Biosensors for Aromatic Compounds Production
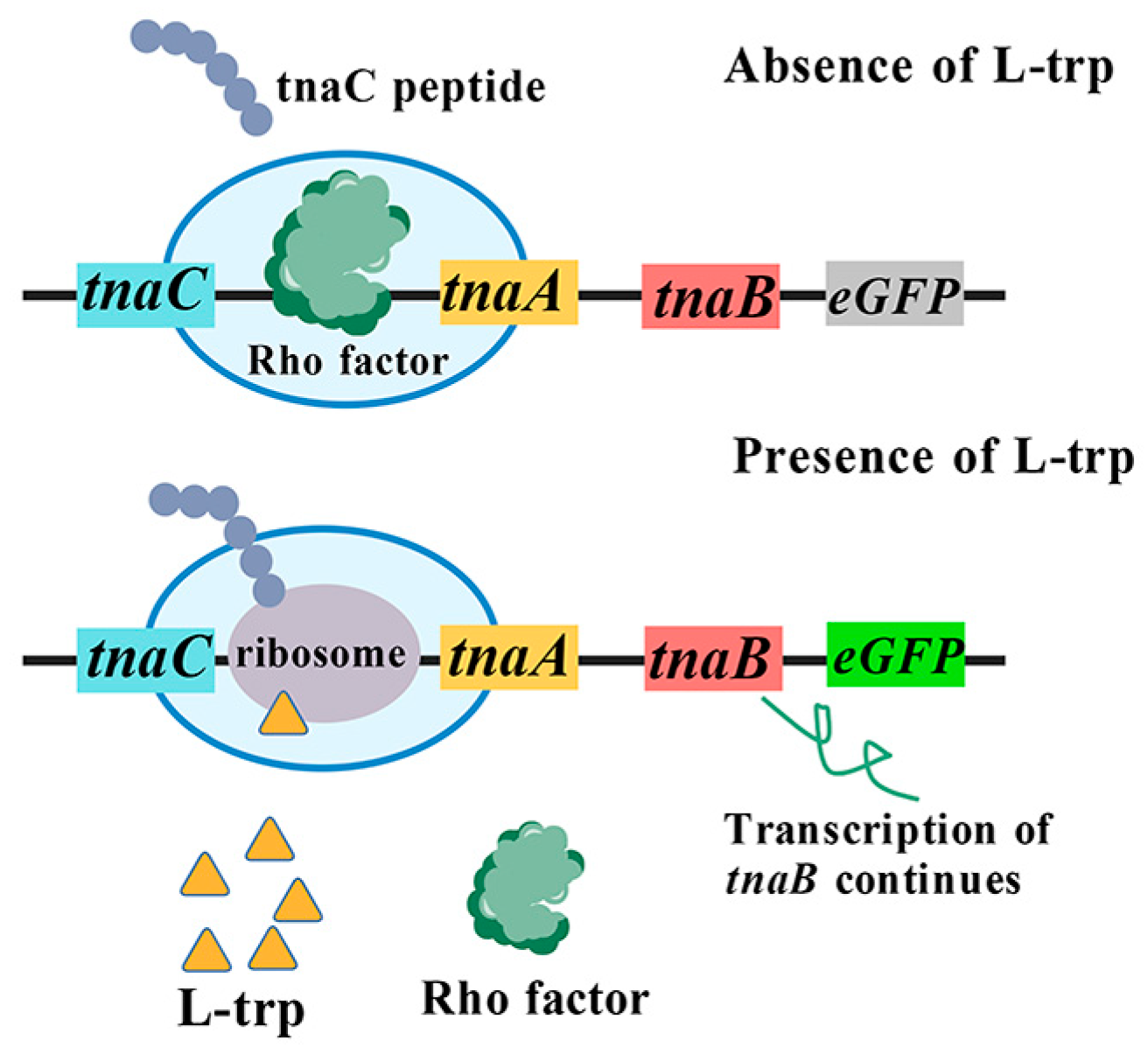
3.1. TnaC-Based L-Tryptophan Biosensor
3.2. Aptamer-Based L-Tryptophan Biosensor
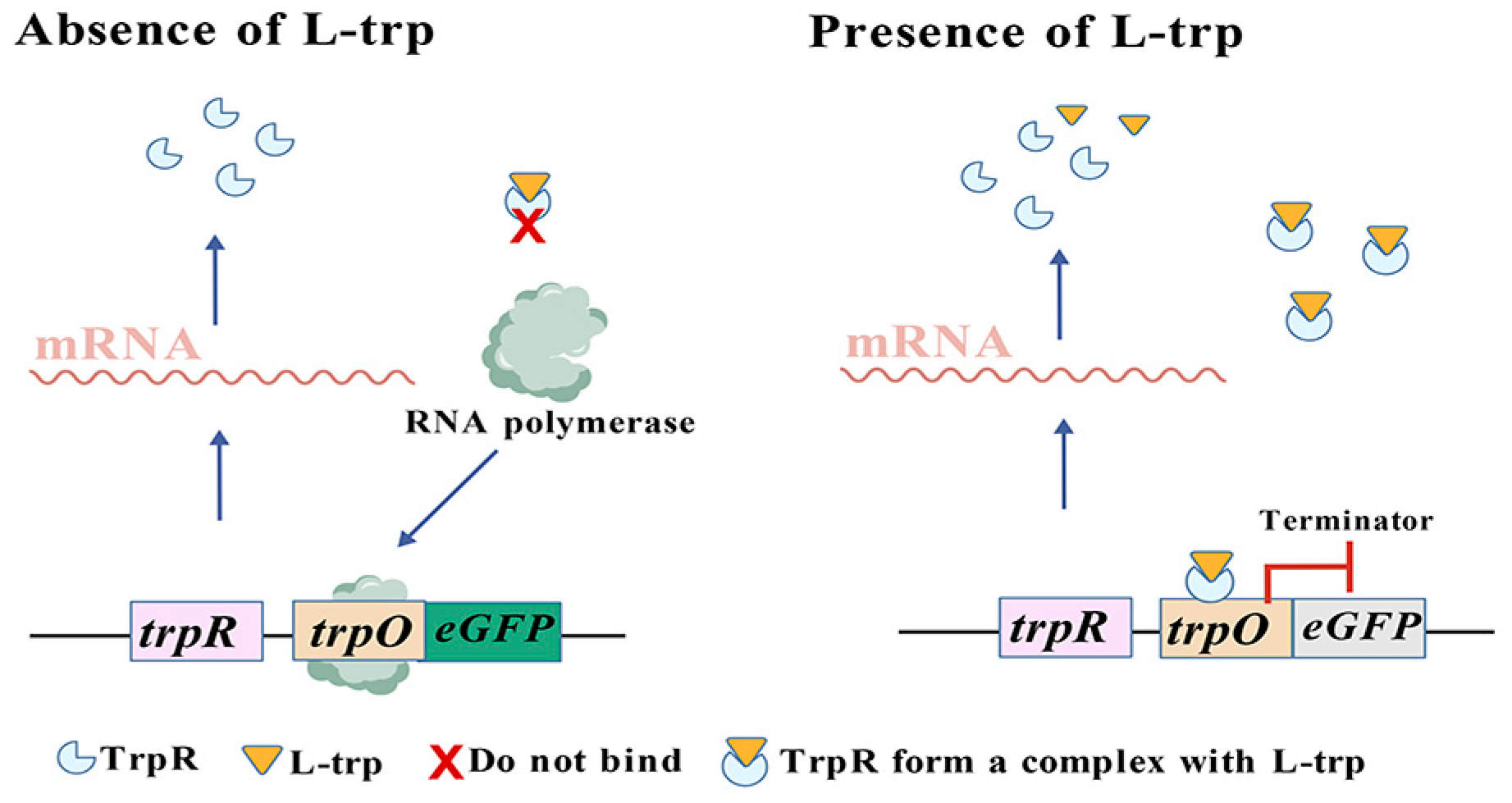
3.3. TF-Based Tryptophan Biosensors
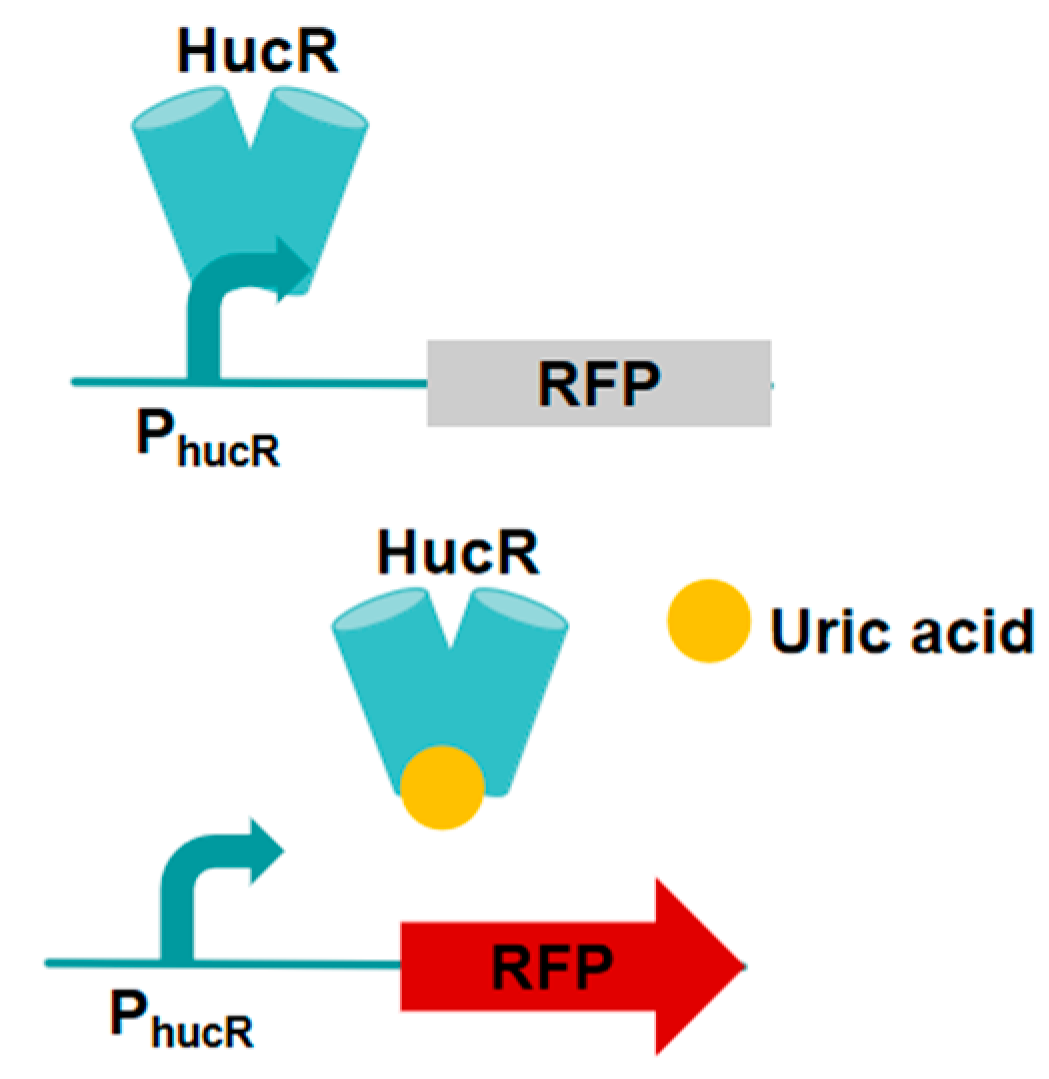
3.4. HucR-V7/PhucR-Based Vanillin Biosensor
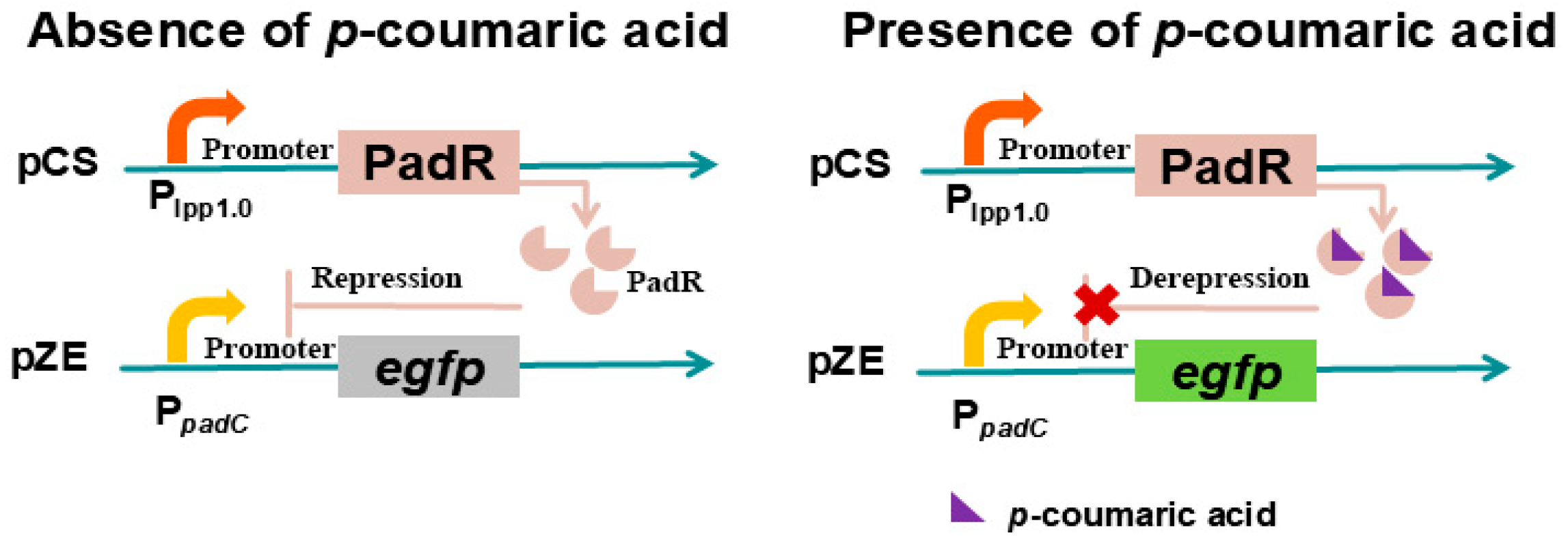
3.5. PadR/PpadC-Based p-Coumaric Acid Biosensor
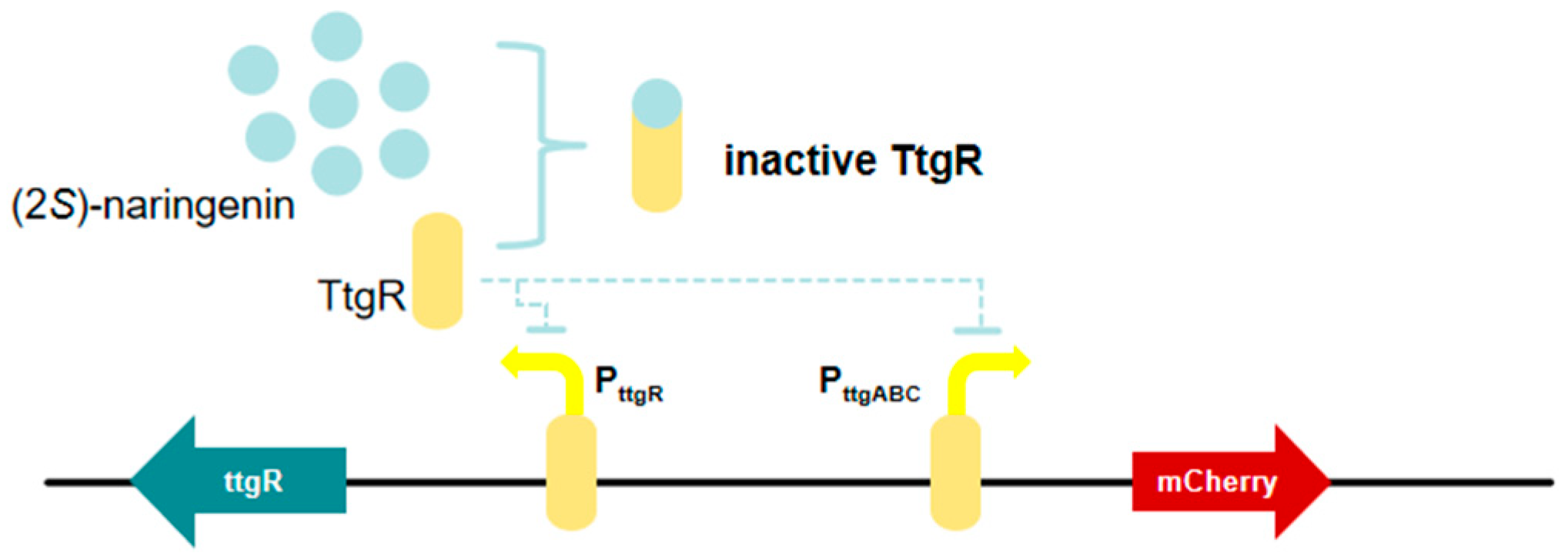
3.6. TtgR-Based (2S)-Naringenin Biosensor
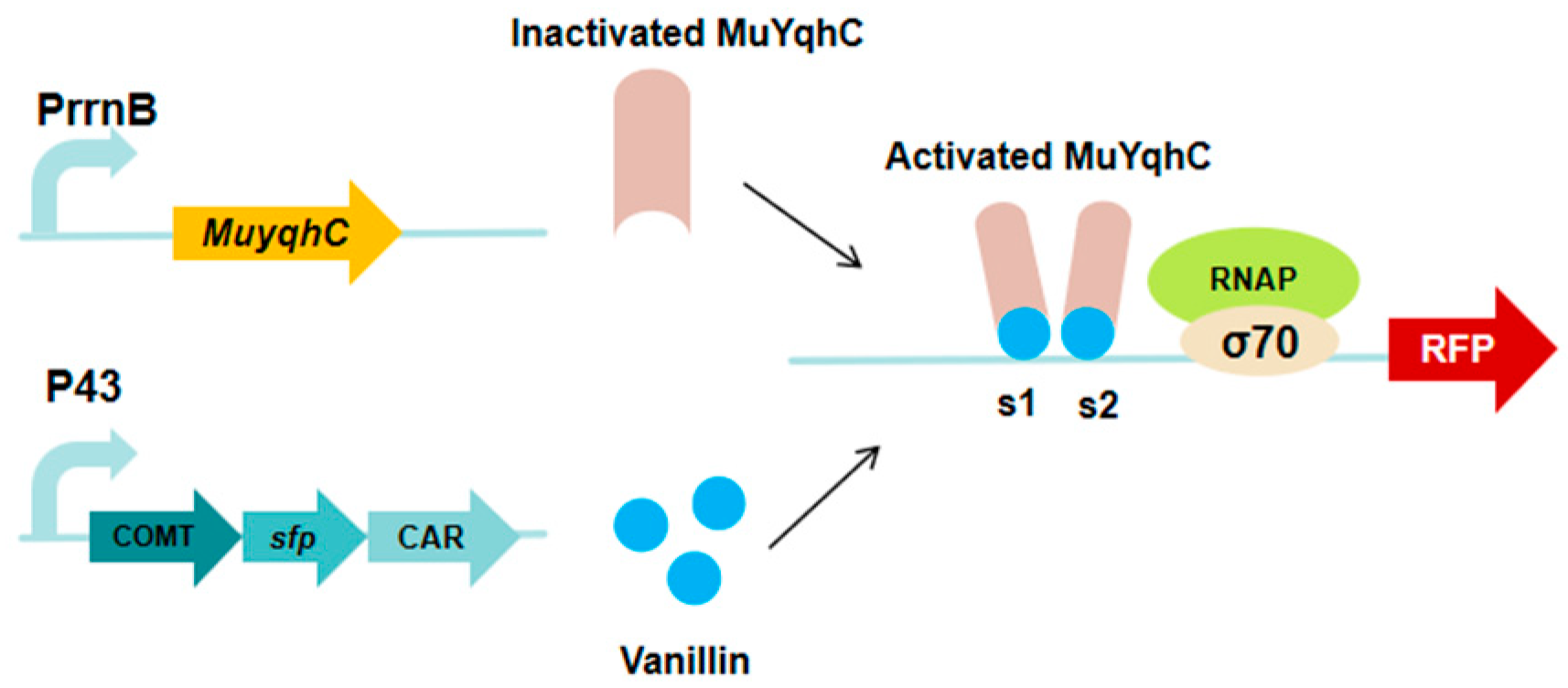
3.7. MuYqhC-Based Vanillin Biosensor
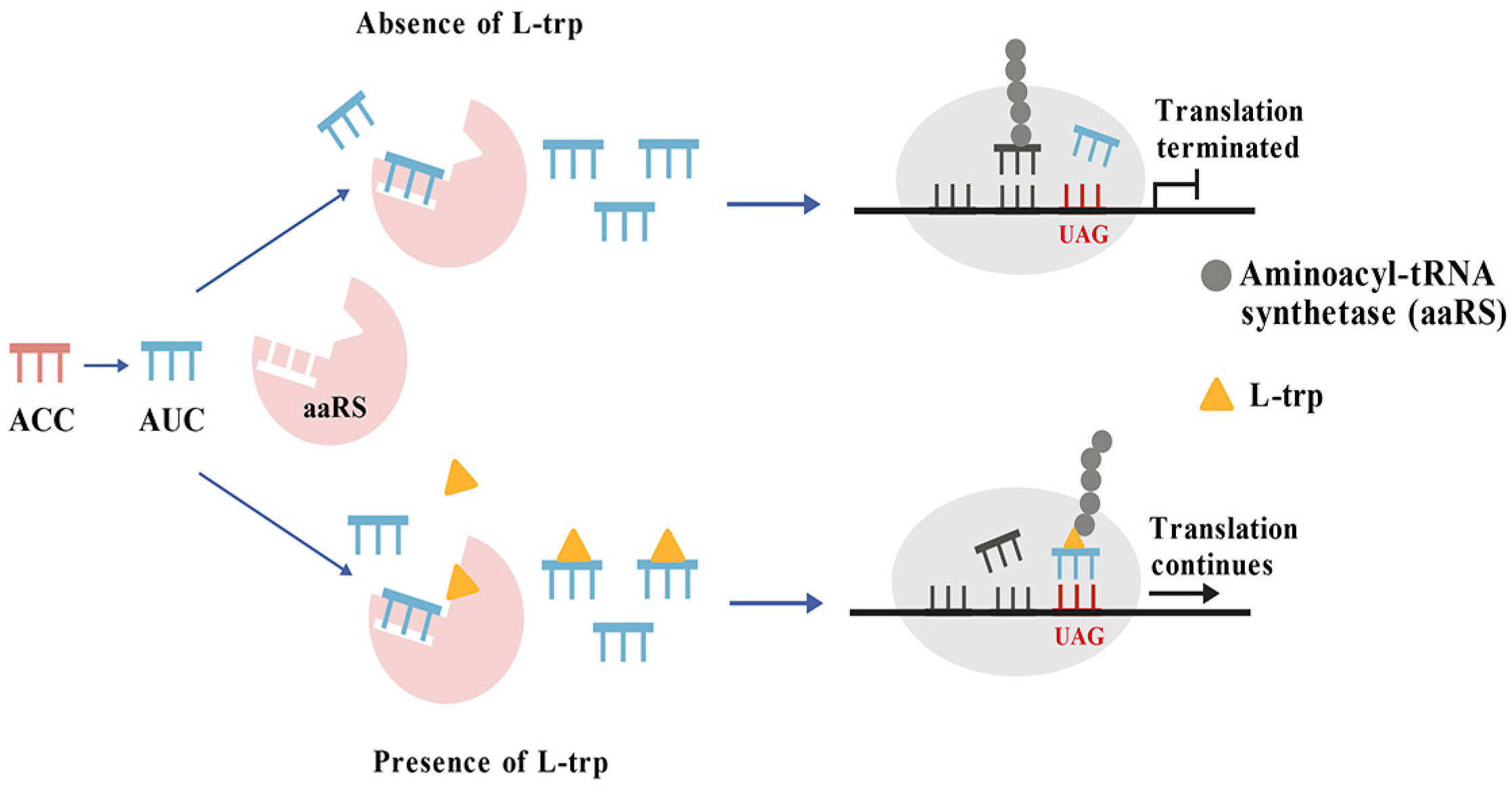
3.8. Protein Translation Elements Based Amino Acids Biosensor
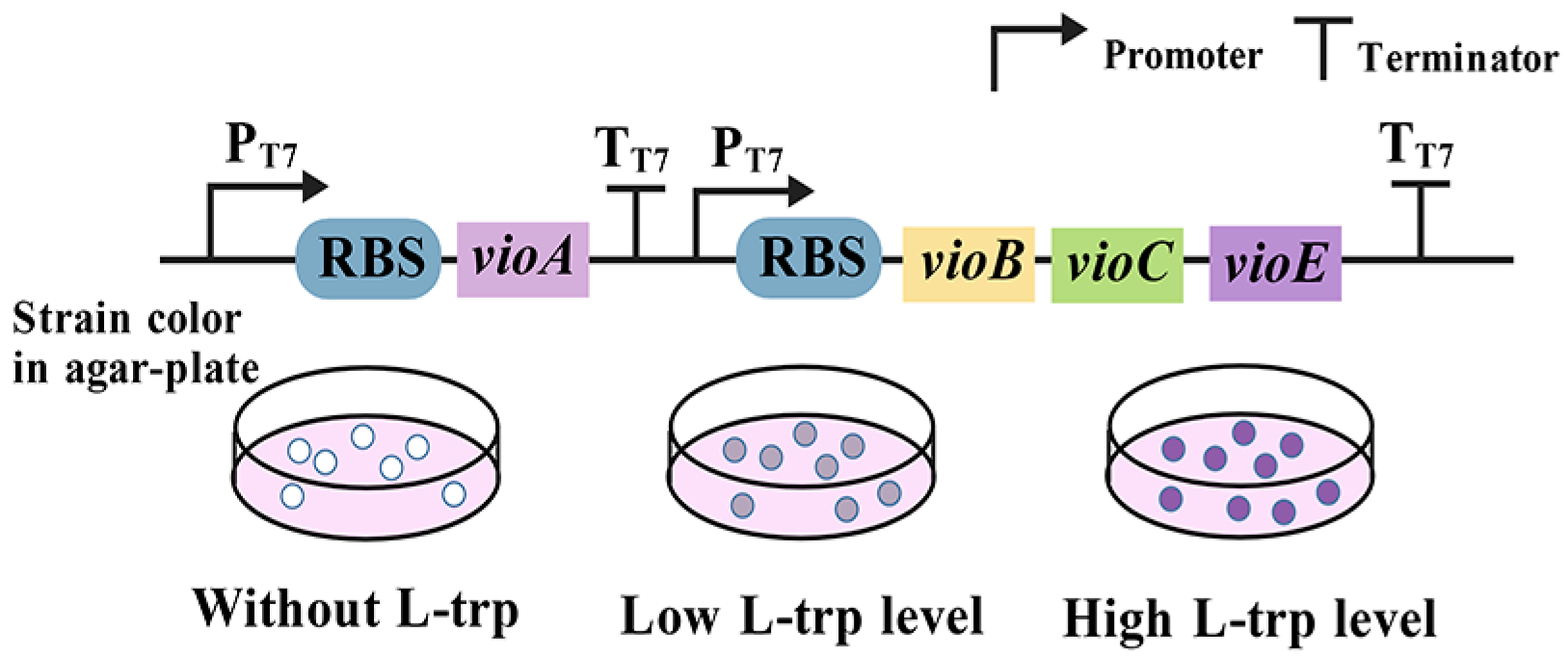
3.9. Enzyme-Coupled Tryptophan Biosensors
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ko, Y.S.; Kim, J.W.; Lee, J.A.; Han, T.; Kim, G.B.; Park, J.E.; Lee, S.Y. Tools and strategies of systems metabolic engineering for the development of microbial cell factories for chemical production. Chem. Soc. Rev. 2020, 49, 4615–4636. [Google Scholar] [CrossRef]
- Hirasawa, T.; Satoh, Y.; Koma, D. Production of aromatic amino acids and their derivatives by Escherichia coli and Corynebacterium glutamicum. World J. Microbiol. Biotechnol. 2025, 41, 65. [Google Scholar] [CrossRef]
- Hu, C.; Yang, J.; Guo, W.; Pan, H.; Guo, D. De Novo Biosynthesis of 4-Vinylanisole in Engineered Escherichia coli. J. Agric. Food Chem. 2024, 72, 4334–4338. [Google Scholar] [CrossRef]
- Maffei, M.E. 5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology. Int. J. Mol. Sci. 2020, 22, 181. [Google Scholar] [CrossRef] [PubMed]
- Dickey, R.M.; Forti, A.M.; Kunjapur, A.M. Advances in engineering microbial biosynthesis of aromatic compounds and related compounds. Bioresour. Bioprocess. 2021, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Dhande, Y.K.; Xiong, M.; Zhang, K. Production of C5 carboxylic acids in engineered Escherichia coli. Process Biochem. 2012, 47, 1965–1971. [Google Scholar] [CrossRef]
- Kang, N.K.; Baek, K.; Koh, H.G.; Atkinson, C.A.; Ort, D.R.; Jin, Y.S. Microalgal metabolic engineering strategies for the production of fuels and chemicals. Bioresour. Technol. 2022, 345, 126529. [Google Scholar] [CrossRef]
- Volk, M.J.; Tran, V.G.; Tan, S.I.; Mishra, S.; Fatma, Z.; Boob, A.; Li, H.; Xue, P.; Martin, T.A.; Zhao, H. Metabolic Engineering: Methodologies and Applications. Chem. Rev. 2023, 123, 5521–5570. [Google Scholar] [CrossRef]
- Liu, D.; Evans, T.; Zhang, F. Applications and advances of metabolite biosensors for metabolic engineering. Metab. Eng. 2015, 31, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Zhang, J.; Jiang, T.; Zou, Y.; Gong, X.; Yan, Y. Biosensor-enabled pathway optimization in metabolic engineering. Curr. Opin. Biotechnol. 2022, 75, 102696. [Google Scholar] [CrossRef]
- Mitchler, M.M.; Garcia, J.M.; Montero, N.E.; Williams, G.J. Transcription factor-based biosensors: A molecular-guided approach for natural product engineering. Curr. Opin. Biotechnol. 2021, 69, 172–181. [Google Scholar] [CrossRef]
- Machado, D.; Costa, R.S.; Ferreira, E.C.; Rocha, I.; Tidor, B. Exploring the gap between dynamic and constraint-based models of metabolism. Metab. Eng. 2012, 14, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Boada, Y.; Vignoni, A.; Picó, J.; Carbonell, P. Extended Metabolic Biosensor Design for Dynamic Pathway Regulation of Cell Factories. Iscience 2020, 23, 101305. [Google Scholar] [CrossRef] [PubMed]
- Farmer, W.R.; Liao, J.C. Improving lycopene production in Escherichia coli by engineering metabolic control. Nat. Biotechnol. 2000, 18, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Z.; Prather, K.L. Dynamic pathway regulation: Recent advances and methods of construction. Curr. Opin. Chem. Biol. 2017, 41, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Xu, X.; Jin, K.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Genetically encoded biosensors for microbial synthetic biology: From conceptual frameworks to practical applications. Biotechnol. Adv. 2023, 62, 108077. [Google Scholar] [CrossRef]
- Fang, M.; Wang, T.; Zhang, C.; Bai, J.; Zheng, X.; Zhao, X.; Lou, C.; Xing, X.H. Intermediate-sensor assisted push-pull strategy and its application in heterologous deoxyviolacein production in Escherichia coli. Metab. Eng. 2016, 33, 41–51. [Google Scholar] [CrossRef]
- Gwon, D.A.; Seok, J.Y.; Jung, G.Y.; Lee, J.W. Biosensor-Assisted Adaptive Laboratory Evolution for Violacein Production. Int. J. Mol. Sci. 2021, 22, 6594. [Google Scholar] [CrossRef]
- Yang, J.; Seo, S.W.; Jang, S.; Shin, S.I.; Lim, C.H.; Roh, T.Y.; Jung, G.Y. Synthetic RNA devices to expedite the evolution of metabolite-producing microbes. Nat. Commun. 2013, 4, 1413. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, H.; Ding, D.; Dong, H.; Wang, Q.; Zhang, D. Establishment of a Biosensor-based High-Throughput Screening Platform for Tryptophan Overproduction. ACS Synth. Biol. 2021, 10, 1373–1383. [Google Scholar] [CrossRef]
- Tang, M.; Pan, X.; Yang, T.; You, J.; Zhu, R.; Yang, T.; Zhang, X.; Xu, M.; Rao, Z. Multidimensional engineering of Escherichia coli for efficient synthesis of L-tryptophan. Bioresour. Technol. 2023, 386, 129475. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, R.; Wang, J.; Yan, Y. Engineering of a TrpR-Based Biosensor for Altered Dynamic Range and Ligand Preference. ACS Synth. Biol. 2022, 11, 2175–2183. [Google Scholar] [CrossRef]
- Zhang, J.; Petersen, S.D.; Radivojevic, T.; Ramirez, A.; Pérez-Manríquez, A.; Abeliuk, E.; Sánchez, B.J.; Costello, Z.; Chen, Y.; Fero, M.J.; et al. Combining mechanistic and machine learning models for predictive engineering and optimization of tryptophan metabolism. Nat. Commun. 2020, 11, 4880. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Wu, J.; Mu, S.; Wu, Z.; Jin, J.M.; Tang, S.Y. Dynamic control of toxic natural product biosynthesis by an artificial regulatory circuit. Metab. Eng. 2020, 57, 239–246. [Google Scholar] [CrossRef]
- Jiang, T.; Li, C.; Yan, Y. Optimization of a p-Coumaric Acid Biosensor System for Versatile Dynamic Performance. ACS Synth. Biol. 2021, 10, 132–144. [Google Scholar] [CrossRef]
- Tong, Y.; Li, N.; Zhou, S.; Zhang, L.; Xu, S.; Zhou, J. Improvement of Chalcone Synthase Activity and High-Efficiency Fermentative Production of (2S)-Naringenin via In Vivo Biosensor-Guided Directed Evolution. ACS Synth. Biol. 2024, 13, 1454–1466. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Fan, Y.; Huo, Y.X.; Sun, L.; Guo, S. Pathway-Adapted Biosensor for High-Throughput Screening of O-Methyltransferase and its Application in Vanillin Synthesis. ACS Synth. Biol. 2024, 13, 2873–2886. [Google Scholar] [CrossRef]
- Guo, H.; Wang, N.; Ding, T.; Zheng, B.; Guo, L.; Huang, C.; Zhang, W.; Sun, L.; Ma, X.; Huo, Y.X. A tRNA Modification-based strategy for Identifying amino acid Overproducers (AMINO). Metab. Eng. 2023, 78, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, L.; Liu, C.; Liu, X.; Bai, Z.; Yang, Y.; Li, Y. Development of an L-tryptophan biosensor based on the violacein biosynthesis pathway. Biotechnol. Bull. 2023, 39, 80–92. [Google Scholar]
- Mir, R.; Jallu, S.; Singh, T.P. The shikimate pathway: Review of amino acid sequence, function and three-dimensional structures of the enzymes. Crit. Rev. Microbiol. 2015, 41, 172–189. [Google Scholar] [CrossRef]
- Li, M.; Liu, C.; Yang, J.; Nian, R.; Xian, M.; Li, F.; Zhang, H. Common problems associated with the microbial productions of aromatic compounds and corresponding metabolic engineering strategies. Biotechnol. Adv. 2020, 41, 107548. [Google Scholar] [CrossRef]
- Herrmann, K.M.; Weaver, L.M. The Shikimate Pathway. Annu. Rev. Plant Biol. 1999, 50, 473–503. [Google Scholar] [CrossRef] [PubMed]
- Priya, V.K.; Sarkar, S.; Sinha, S. Evolution of tryptophan biosynthetic pathway in microbial genomes: A comparative genetic study. Syst. Synth. Biol. 2014, 8, 59–72. [Google Scholar] [CrossRef]
- Tzin, V.; Galili, G.; Aharoni, A. The shikimate pathway and aromatic amino Acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Yang, F.; Kang, J.; Wang, Q.; Qi, Q. One-step of tryptophan attenuator inactivation and promoter swapping to improve the production of L-tryptophan in Escherichia coli. Microb. Cell Factories 2012, 11, 30. [Google Scholar] [CrossRef]
- Ding, D.; Bai, D.; Li, J.; Mao, Z.; Zhu, Y.; Liu, P.; Lin, J.; Ma, H.; Zhang, D. Analyzing the genetic characteristics of a tryptophan-overproducing Escherichia coli. Bioprocess. Biosyst. Eng. 2021, 44, 1685–1697. [Google Scholar] [CrossRef]
- Patrick, J.S.; Lagu, A.L. A method for the quantitation of tryptophan in Escherichia coli fermentation broth by isocratic high-performance liquid chromatography with ultraviolet detection. Anal. Biochem. 1991, 199, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Delhaye, S.; Landry, J. High-performance liquid chromatography and ultraviolet spectrophotometry for quantitation of tryptophan in barytic hydrolysates. Anal. Biochem. 1986, 159, 175–178. [Google Scholar] [CrossRef]
- Gruen, L.C.; Rivett, D.E. Specificity of Spies and Chambers reagent for colorimetric estimation of tryptophan. Anal. Biochem. 1971, 44, 519–522. [Google Scholar] [CrossRef]
- Duan, X.; Chen, Z.; Tang, S.; Ge, M.; Wei, H.; Guan, Y.; Zhao, G. A Strategy Employing a TF-Splinting Duplex Nanoswitch to Achieve Single-Step, Enzyme-Free, Signal-On Detection of l-Tryptophan. ACS Sens. 2020, 5, 837–844. [Google Scholar] [CrossRef]
- Jang, S.; Jung, G.Y. Systematic optimization of L-tryptophan riboswitches for efficient monitoring of the metabolite in Escherichia coli. Biotechnol. Bioeng. 2018, 115, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Chiba, S. Arrest peptides: Cis-acting modulators of translation. Annu. Rev. Biochem. 2013, 82, 171–202. [Google Scholar] [CrossRef]
- Majerfeld, I.; Yarus, M. A diminutive and specific RNA binding site for L-tryptophan. Nucleic Acids Res. 2005, 33, 5482–5493. [Google Scholar] [CrossRef]
- Deeley, M.C.; Yanofsky, C. Nucleotide sequence of the structural gene for tryptophanase of Escherichia coli K-12. J. Bacteriol. 1981, 147, 787–796. [Google Scholar] [CrossRef]
- Gong, F.; Yanofsky, C. Instruction of translating ribosome by nascent peptide. Science 2002, 297, 1864–1867. [Google Scholar] [CrossRef] [PubMed]
- Stewart, V.; Yanofsky, C. Evidence for transcription antitermination control of tryptophanase operon expression in Escherichia coli K-12. J. Bacteriol. 1985, 164, 731–740. [Google Scholar] [CrossRef]
- San Martín, A.; Ceballo, S.; Baeza-Lehnert, F.; Lerchundi, R.; Valdebenito, R.; Contreras-Baeza, Y.; Alegría, K.; Barros, L.F. Imaging mitochondrial flux in single cells with a FRET sensor for pyruvate. PLoS ONE 2014, 9, e85780. [Google Scholar] [CrossRef]
- Gong, F.; Yanofsky, C. Reproducing tna operon regulation in vitro in an S-30 system. Tryptophan induction inhibits cleavage of TnaC peptidyl-tRNA. J. Biol. Chem. 2001, 276, 1974–1983. [Google Scholar] [CrossRef]
- van der Stel, A.X.; Gordon, E.R.; Sengupta, A.; Martínez, A.K.; Klepacki, D.; Perry, T.N.; Herrero Del Valle, A.; Vázquez-Laslop, N.; Sachs, M.S.; Cruz-Vera, L.R.; et al. Structural basis for the tryptophan sensitivity of TnaC-mediated ribosome stalling. Nat. Commun. 2021, 12, 5340. [Google Scholar] [CrossRef]
- Breaker, R.R. Prospects for riboswitch discovery and analysis. Mol. Cell 2011, 43, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Findeiß, S.; Etzel, M.; Will, S.; Mörl, M.; Stadler, P.F. Design of Artificial Riboswitches as Biosensors. Sensors 2017, 17, 1990. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, A.A.; Miller, W.G.; Gunsalus, R.P. Escherichia coli tryptophan repressor binds multiple sites within the aroH and trp operators. Genes Dev. 1987, 1, 556–564. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bogosian, G.; Somerville, R.L.; Nishi, K.; Kano, Y.; Imamoto, F. Transcription of the trpR gene of Escherichia coli: An autogeneously regulated system studied by direct measurements of mRNA levels in vivo. Mol. Gen. Genet. 1984, 193, 244–250. [Google Scholar] [CrossRef]
- Jeeves, M.; Evans, P.D.; Parslow, R.A.; Jaseja, M.; Hyde, E.I. Studies of the Escherichia coli Trp repressor binding to its five operators and to variant operator sequences. Eur. J. Biochem. 1999, 265, 919–928. [Google Scholar] [CrossRef]
- Bass, S.; Sugiono, P.; Arvidson, D.N.; Gunsalus, R.P.; Youderian, P. DNA specificity determinants of Escherichia coli tryptophan repressor binding. Genes Dev. 1987, 1, 565–572. [Google Scholar] [CrossRef]
- Gunsalus, R.P.; Yanofsky, C. Nucleotide sequence and expression of Escherichia coli trpR, the structural gene for the trp aporepressor. Proc. Natl. Acad. Sci. USA 1980, 77, 7117–7121. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Arrowsmith, C.H.; Jia, X.; Jardetzky, O. Refined solution structures of the Escherichia coli trp holo- and aporepressor. J. Mol. Biol. 1993, 229, 735–746. [Google Scholar] [CrossRef]
- Herud-Sikimić, O.; Stiel, A.C.; Kolb, M.; Shanmugaratnam, S.; Berendzen, K.W.; Feldhaus, C.; Höcker, B.; Jürgens, G. A biosensor for the direct visualization of auxin. Nature 2021, 592, 768–772. [Google Scholar] [CrossRef]
- Wilkinson, S.P.; Grove, A. HucR, a novel uric acid-responsive member of the MarR family of transcriptional regulators from Deinococcus radiodurans. J. Biol. Chem. 2004, 279, 51442–51450. [Google Scholar] [CrossRef]
- Wilkinson, S.P.; Grove, A. Negative cooperativity of uric acid binding to the transcriptional regulator HucR from Deinococcus radiodurans. J. Mol. Biol. 2005, 350, 617–630. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Tran, N.P.; Cavin, J.F. Genetic and biochemical analysis of PadR-padC promoter interactions during the phenolic acid stress response in Bacillus subtilis 168. J. Bacteriol. 2011, 193, 4180–4191. [Google Scholar] [CrossRef]
- Tran, N.P.; Gury, J.; Dartois, V.; Nguyen, T.K.; Seraut, H.; Barthelmebs, L.; Gervais, P.; Cavin, J.F. Phenolic acid-mediated regulation of the padC gene, encoding the phenolic acid decarboxylase of Bacillus subtilis. J. Bacteriol. 2008, 190, 3213–3224. [Google Scholar] [CrossRef] [PubMed]
- Siedler, S.; Khatri, N.K.; Zsohár, A.; Kjærbølling, I.; Vogt, M.; Hammar, P.; Nielsen, C.F.; Marienhagen, J.; Sommer, M.O.A.; Joensson, H.N. Development of a Bacterial Biosensor for Rapid Screening of Yeast p-Coumaric Acid Production. ACS Synth. Biol. 2017, 6, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.H.; Nishida, R. A review on natural phenylbutanoid attractants: Occurrence, distribution, and role in nature, especially in relation to Dacini fruit fly behavior and pollination. J. Chem. Ecol. 2024, 50, 926–946. [Google Scholar] [CrossRef]
- Koopman, F.; Beekwilder, J.; Crimi, B.; van Houwelingen, A.; Hall, R.D.; Bosch, D.; van Maris, A.J.; Pronk, J.T.; Daran, J.M. De novo production of the flavonoid naringenin in engineered Saccharomyces cerevisiae. Microb. Cell Factories 2012, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Chen, R.; Shaw, W.M.; Hapeta, P.; Jiang, W.; Bell, D.J.; Ellis, T.; Ledesma-Amaro, R. Modular Metabolic Engineering and Synthetic Coculture Strategies for the Production of Aromatic Compounds in Yeast. ACS Synth. Biol. 2023, 12, 1739–1749. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Q.; Yuan, M.; Yang, H.; Deng, Y. Static and Dynamic Regulation of Precursor Supply Pathways to Enhance Raspberry Ketone Synthesis from Glucose in Escherichia coli. J. Agric. Food Chem. 2024, 72, 23411–23421. [Google Scholar] [CrossRef]
- Zhou, P.; Fang, X.; Xu, N.; Yao, Z.; Xie, W.; Ye, L. Development of a Highly Efficient Copper-Inducible GAL Regulation System (CuIGR) in Saccharomyces cerevisiae. ACS Synth. Biol. 2021, 10, 3435–3444. [Google Scholar] [CrossRef]
- Knogge, W.; Schmelzer, E.; Weissenböck, G. The role of chalcone synthase in the regulation of flavonoid biosynthesis in developing oat primary leaves. Arch. Biochem. Biophys. 1986, 250, 364–372. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, P.; Chen, J.; Du, G.; Li, H.; Zhou, J. Characterization of mutants of a tyrosine ammonia-lyase from Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2016, 100, 10443–10452. [Google Scholar] [CrossRef]
- Liu, D.; Sica, M.S.; Mao, J.; Chao, L.F.; Siewers, V. A p-Coumaroyl-CoA Biosensor for Dynamic Regulation of Naringenin Biosynthesis in Saccharomyces cerevisiae. ACS Synth. Biol. 2022, 11, 3228–3238. [Google Scholar] [CrossRef]
- Terán, W.; Felipe, A.; Segura, A.; Rojas, A.; Ramos, J.L.; Gallegos, M.T. Antibiotic-dependent induction of Pseudomonas putida DOT-T1E TtgABC efflux pump is mediated by the drug binding repressor TtgR. Antimicrob. Agents Chemother. 2003, 47, 3067–3072. [Google Scholar] [CrossRef]
- Espinosa-Urgel, M.; Serrano, L.; Ramos, J.L.; Fernández-Escamilla, A.M. Engineering Biological Approaches for Detection of Toxic Compounds: A New Microbial Biosensor Based on the Pseudomonas putida TtgR Repressor. Mol. Biotechnol. 2015, 57, 558–564. [Google Scholar] [CrossRef]
- Xiong, D.; Lu, S.; Wu, J.; Liang, C.; Wang, W.; Wang, W.; Jin, J.M.; Tang, S.Y. Improving key enzyme activity in phenylpropanoid pathway with a designed biosensor. Metab. Eng. 2017, 40, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, X.; Wu, J.; Li, X.; Li, W.; Sun, X.; Wang, J.; Yan, Y.; Shen, X.; Yuan, Q. Targeting cofactors regeneration in methylation and hydroxylation for high level production of Ferulic acid. Metab. Eng. 2022, 73, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, I.; Lee, J.; Lee, K.L.; Min, B.; Park, C. Transcriptional activation of the aldehyde reductase YqhD by YqhC and its implication in glyoxal metabolism of Escherichia coli K-12. J. Bacteriol. 2010, 192, 4205–4214. [Google Scholar] [CrossRef] [PubMed]
- Merchel Piovesan Pereira, B.; Adil Salim, M.; Rai, N.; Tagkopoulos, I. Tolerance to Glutaraldehyde in Escherichia coli Mediated by Overexpression of the Aldehyde Reductase YqhD by YqhC. Front. Microbiol. 2021, 12, 680553. [Google Scholar] [CrossRef]
- Turner, P.C.; Miller, E.N.; Jarboe, L.R.; Baggett, C.L.; Shanmugam, K.T.; Ingram, L.O. YqhC regulates transcription of the adjacent Escherichia coli genes yqhD and dkgA that are involved in furfural tolerance. J. Ind. Microbiol. Biotechnol. 2011, 38, 431–439. [Google Scholar] [CrossRef]
- Verma, R.; Ellis, J.M.; Mitchell-Koch, K.R. Dynamic Preference for NADP/H Cofactor Binding/Release in E. coli YqhD Oxidoreductase. Molecules 2021, 26, 270. [Google Scholar] [CrossRef]
- Kern, D.; Lapointe, J. The catalytic mechanism of glutamyl-tRNA synthetase of Escherichia coli. A steady-state kinetic investigation. Eur. J. Biochem. 1981, 115, 29–38. [Google Scholar] [CrossRef]
- Moe, J.G.; Piszkiewicz, D. Isoleucyl transfer ribonucleic acid synthetase. Steady-state kinetic analysis. Biochemistry 1979, 18, 2804–2810. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Kim, W.J.; Yoo, S.M.; Choi, J.H.; Ha, S.H.; Lee, M.H.; Lee, S.Y. Repurposing type III polyketide synthase as a malonyl-CoA biosensor for metabolic engineering in bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, 9835–9844. [Google Scholar] [CrossRef] [PubMed]
- Balibar, C.J.; Walsh, C.T. In vitro biosynthesis of violacein from L-tryptophan by the enzymes VioA-E from Chromobacterium violaceum. Biochemistry 2006, 45, 15444–15457. [Google Scholar] [CrossRef]
- Yang, D.; Park, S.Y.; Lee, S.Y. Production of Rainbow Colorants by Metabolically Engineered Escherichia coli. Adv. Sci. 2021, 8, e2100743. [Google Scholar] [CrossRef]
- Lim, H.G.; Jang, S.; Jang, S.; Seo, S.W.; Jung, G.Y. Design and optimization of genetically encoded biosensors for high-throughput screening of chemicals. Curr. Opin. Biotechnol. 2018, 54, 18–25. [Google Scholar] [CrossRef]
- Lim, H.G.; Rychel, K.; Sastry, A.V.; Bentley, G.J.; Mueller, J.; Schindel, H.S.; Larsen, P.E.; Laible, P.D.; Guss, A.M.; Niu, W.; et al. Machine-learning from Pseudomonas putida KT2440 transcriptomes reveals its transcriptional regulatory network. Metab. Eng. 2022, 72, 297–310. [Google Scholar] [CrossRef]
- Chubukov, V.; Gerosa, L.; Kochanowski, K.; Sauer, U. Coordination of microbial metabolism. Nat. Rev. Microbiol. 2014, 12, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Camacho, D.M.; Collins, K.M.; Powers, R.K.; Costello, J.C.; Collins, J.J. Next-Generation Machine Learning for Biological Networks. Cell 2018, 173, 1581–1592. [Google Scholar] [CrossRef]
- Hwang, H.G.; Milito, A.; Yang, J.S.; Jang, S.; Jung, G.Y. Riboswitch-guided chalcone synthase engineering and metabolic flux optimization for enhanced production of flavonoids. Metab. Eng. 2023, 75, 143–152. [Google Scholar] [CrossRef]
- Kortmann, M.; Mack, C.; Baumgart, M.; Bott, M. Pyruvate Carboxylase Variants Enabling Improved Lysine Production from Glucose Identified by Biosensor-Based High-Throughput Fluorescence-Activated Cell Sorting Screening. ACS Synth. Biol. 2019, 8, 274–281. [Google Scholar] [CrossRef]
- Yao, J.; He, Y.; Su, N.; Bharath, S.R.; Tao, Y.; Jin, J.M.; Chen, W.; Song, H.; Tang, S.Y. Developing a highly efficient hydroxytyrosol whole-cell catalyst by de-bottlenecking rate-limiting steps. Nat. Commun. 2020, 11, 1515. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.Y.; Noh, M.H.; Moon, J.H.; Milito, A.; Kim, M.; Lee, J.W.; Yang, J.S.; Jung, G.Y. Kinetic compartmentalization by unnatural reaction for itaconate production. Nat. Commun. 2022, 13, 5353. [Google Scholar] [CrossRef]
- Seok, J.Y.; Yang, J.; Choi, S.J.; Lim, H.G.; Choi, U.J.; Kim, K.J.; Park, S.; Yoo, T.H.; Jung, G.Y. Directed evolution of the 3-hydroxypropionic acid production pathway by engineering aldehyde dehydrogenase using a synthetic selection device. Metab. Eng. 2018, 47, 113–120. [Google Scholar] [CrossRef]
- Raghavan, S.S.; Chee, S.; Li, J.; Poschmann, J.; Nagarajan, N.; Jia Wei, S.; Verma, C.S.; Ghadessy, F.J. Development and application of a transcriptional sensor for detection of heterologous acrylic acid production in E. coli. Microb. Cell Factories 2019, 18, 139. [Google Scholar] [CrossRef]
- Xu, X.; Li, X.; Liu, Y.; Zhu, Y.; Li, J.; Du, G.; Chen, J.; Ledesma-Amaro, R.; Liu, L. Pyruvate-responsive genetic circuits for dynamic control of central metabolism. Nat. Chem. Biol. 2020, 16, 1261–1268. [Google Scholar] [CrossRef]
- Chappell, J.; Westbrook, A.; Verosloff, M.; Lucks, J.B. Computational design of small transcription activating RNAs for versatile and dynamic gene regulation. Nat. Commun. 2017, 8, 1051. [Google Scholar] [CrossRef] [PubMed]
- de Jongh, R.P.H.; van Dijk, A.D.J.; Julsing, M.K.; Schaap, P.J.; de Ridder, D. Designing Eukaryotic Gene Expression Regulation Using Machine Learning. Trends Biotechnol. 2020, 38, 191–201. [Google Scholar] [CrossRef]


| Name | Output Signal | Basic Elements | Advantages | References |
|---|---|---|---|---|
| TnaC-based L-tryptophan biosensor | Enhanced Green Fluorescent Protein (eGFP) | pSensor, TrpSEN | High specificity, wide range of applications | [17,18] |
| Aptamer-based L-tryptophan biosensor | Tetracycline efflux pump encoded gene tetA; GFP; Yellow Fluorescent Protein (YFP); | Tryptophan Riboselector, p15-ribo585, p15-ribo727, pUC19-R151-GFP | Fast response of L-Tryptophan, high specificity and sensitivity | [19,20,21] |
| TF-based tryptophan biosensors | Enhanced Green Fluorescent Protein (eGFP); Yeast-enhanced Green Fluorescent Protein (yeGFP) | TrpR1-PtrpO1, pGAL1-6x-trpO | High dynamic range | [22,23] |
| HucR-V7/PhucR-based vanillin biosensor | Red Fluorescent Protein (RFP) | pPhucR-RFP | Introduce feedback activation and cascading dynamic control strategies | [24] |
| PadR/PpadC-based p-Coumaric acid biosensor | eGFP | pCS-lpp1.0-egfp, pZE-PpadC-egfp | Increased dynamic range and superior sensitivity | [25] |
| TtgR-based (2S)-naringenin biosensor | Monomeric Cherry Red Fluorescent Protein (mCherry) | pPttgR-ttgR, pPttgABC-mCherry | The widest detection range for (2S)-naringenin | [26] |
| MuYqhC-based vanillin biosensor | RFP | pPrrnB-MuYqhC | Screened out MuYqhC, and established a dual-responsive biosensing system | [27] |
| Protein translation elements based amino acids biosensor | Kanamycin resistance gene (KanR) | ptRNATrpCUA | High specificity | [28] |
| Enzyme-coupled tryptophan biosensors | Strain color | VioABCDE enzyme complex | High specificity, easy to be engineered | [29] |
| Biosensors | Sensing Signal | Performance Improvement | Reference |
|---|---|---|---|
| pSensor | L-tryptophan | Increasing the deoxyviolacein titer by 4.4-fold | [17] |
| TrpSEN | L-tryptophan | Increasing the violacein titer by 2.7-fold | [18] |
| p15-ribo727 | L-tryptophan | Obtaining a mutant strain with 155.1% higher L-tryptophan titer | [20] |
| pUC19-R151-GFP | L-tryptophan | The yield of the screening strain was increased by 4.7-fold after modification | [21] |
| pGAL1-6x-trpO | L-tryptophan | Increasing L-tryptophan titer and productivity by up to 74% and 43% respectively | [23] |
| pZE-PpadC-egfp | p-Coumaric acid | The raspberry ketone yield enhanced to 415.56 mg/L, representing a 32.4-fold increase | [25] |
| pPttgABC-mCherry | (2S)-naringenin | Increasing the (2S)-naringenin titer by 65.34% | [26] |
| pPrrnB-MuYqhC | Vanillin | The titer increased by 2.39-fold, vanillin yields ultimately reached 1 mM | [27] |
| ptRNATrpCUA | L-tryptophan | Increasing the L-tryptophan titer by 1.3-fold, achieving the L-tryptophan titer to 20.3 g/L | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Gu, P. Engineering and Application of Biosensors for Aromatic Compounds Production in Escherichia coli. Microorganisms 2025, 13, 2358. https://doi.org/10.3390/microorganisms13102358
Zhao Y, Gu P. Engineering and Application of Biosensors for Aromatic Compounds Production in Escherichia coli. Microorganisms. 2025; 13(10):2358. https://doi.org/10.3390/microorganisms13102358
Chicago/Turabian StyleZhao, Yang, and Pengfei Gu. 2025. "Engineering and Application of Biosensors for Aromatic Compounds Production in Escherichia coli" Microorganisms 13, no. 10: 2358. https://doi.org/10.3390/microorganisms13102358
APA StyleZhao, Y., & Gu, P. (2025). Engineering and Application of Biosensors for Aromatic Compounds Production in Escherichia coli. Microorganisms, 13(10), 2358. https://doi.org/10.3390/microorganisms13102358






