The Association Between Maternal Diet and the Human Milk Microbiome: A Review of Evidence and Methodological Challenges
Abstract
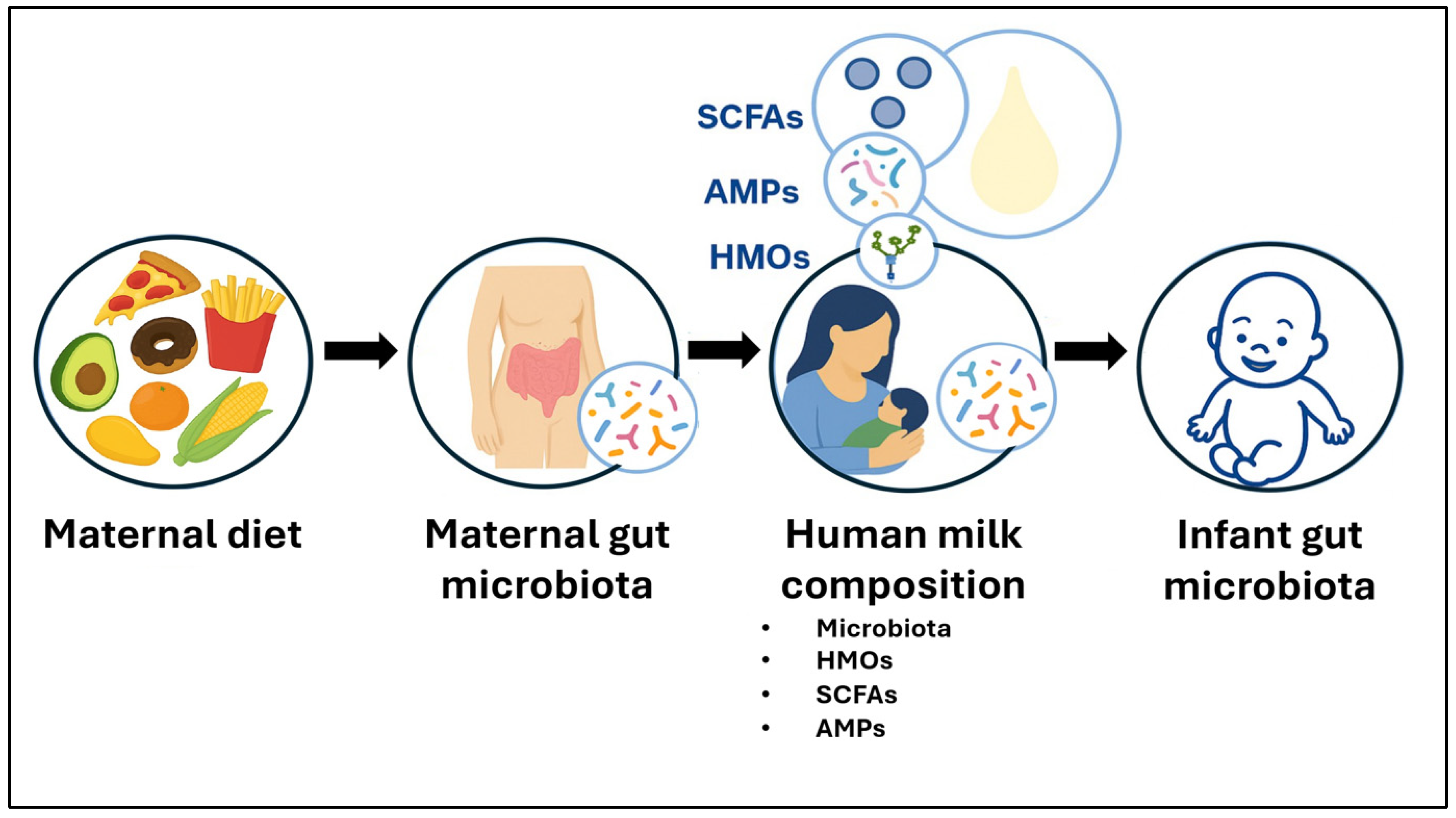
1. Introduction
2. Method
3. The Human Milk Microbiota Composition and Its Determinants
4. Origin of Human Milk Bacteria
5. Studies Investigating the Association Between Maternal Diet and the Human Milk Microbiota
6. Limitations of Current Studies
6.1. Small Sample Sizes and Underpowered Study Designs
6.2. Inconsistent Milk Collection Protocols
6.3. Limitations in Dietary Assessment Methods
6.4. Limitations in Human Milk Microbiota Analysis
6.5. Variability in HM Lactation Stage and Lack of Standardisation
6.6. Inadequate Control for Confounding Variables
7. Biological Mechanisms and Clinical Implications
8. Future Directions
9. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bokulich, N.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.; Wu, F.; Perez-Perez, G.; Chen, Y. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343ra82. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.T.; Li, F.; Lee-Sarwar, K.A.; Tun, H.M.; Brown, B.P.; Pannaraj, P.S.; Bender, J.M.; Azad, M.B.; Thompson, A.L.; Weiss, S.T. Meta-analysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nat. Commun. 2018, 9, 4169. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, H.M.; Rutten, N.B.; Boekhorst, J.; Saulnier, D.M.; Kortman, G.A.; Contractor, N.; Kullen, M.; Floris, E.; Harmsen, H.J.; Vlieger, A.M. Intestinal colonisation patterns in breastfed and formula-fed infants during the first 12 weeks of life reveal sequential microbiota signatures. Sci. Rep. 2017, 7, 8327. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.L.; Monteagudo-Mera, A.; Cadenas, M.B.; Lampl, M.L.; Azcarate-Peril, M.A. Milk-and solid-feeding practices and daycare attendance are associated with differences in bacterial diversity, predominant communities, and metabolic and immune function of the infant gut microbiome. Front. Cell. Infect. Microbiol. 2015, 5, 3. [Google Scholar] [CrossRef]
- Stewart, C.; Ajami, N.; O’Brien, J.; Hutchinson, D.; Smith, D.; Wong, M.; Ross, M.; Lloyd, R.; Doddapaneni, H.; Metcalf, G. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018, 562, 583. [Google Scholar] [CrossRef]
- Ma, J.; Li, Z.; Zhang, W.; Zhang, C.; Zhang, Y.; Mei, H.; Zhuo, N.; Wang, H.; Wang, L.; Wu, D. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: A study of 91 term infants. Sci. Rep. 2020, 10, 15792. [Google Scholar] [CrossRef]
- Olin, A.; Henckel, E.; Chen, Y.; Lakshmikanth, T.; Pou, C.; Mikes, J.; Gustafsson, A.; Bernhardsson, A.K.; Zhang, C.; Bohlin, K. Stereotypic immune system development in newborn children. Cell 2018, 174, 1277–1292.e14. [Google Scholar] [CrossRef]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef]
- Arrieta, M.-C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef]
- Arrieta, M.-C.; Arévalo, A.; Stiemsma, L.; Dimitriu, P.; Chico, M.E.; Loor, S.; Vaca, M.; Boutin, R.C.; Morien, E.; Jin, M. Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J. Allergy Clin. Immunol. 2018, 142, 424–434.e10. [Google Scholar] [CrossRef]
- Walker, W.A. The importance of appropriate initial bacterial colonization of the intestine in newborn, child, and adult health. Pediatr. Res. 2017, 82, 387–395. [Google Scholar] [CrossRef]
- Reddel, S.; Del Chierico, F.; Quagliariello, A.; Giancristoforo, S.; Vernocchi, P.; Russo, A.; Fiocchi, A.; Rossi, P.; Putignani, L.; El Hachem, M. Gut microbiota profile in children affected by atopic dermatitis and evaluation of intestinal persistence of a probiotic mixture. Sci. Rep. 2019, 9, 4996. [Google Scholar] [CrossRef] [PubMed]
- Boutin, R.C.; Sbihi, H.; McLaughlin, R.J.; Hahn, A.S.; Konwar, K.M.; Loo, R.S.; Dai, D.; Petersen, C.; Brinkman, F.S.; Winsor, G.L. Composition and associations of the infant gut fungal microbiota with environmental factors and childhood allergic outcomes. mBio 2021, 12, e03396-20. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Paparo, L.; Nocerino, R.; Della Gatta, G.; Carucci, L.; Russo, R.; Pasolli, E.; Ercolini, D.; Berni Canani, R. Specific gut microbiome signatures and the associated pro-inflamatory functions are linked to pediatric allergy and acquisition of immune tolerance. Nat. Commun. 2021, 12, 5958. [Google Scholar] [CrossRef] [PubMed]
- Scheepers, L.; Penders, J.; Mbakwa, C.; Thijs, C.; Mommers, M.; Arts, I. The intestinal microbiota composition and weight development in children: The KOALA Birth Cohort Study. Int. J. Obes. 2015, 39, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Group, T.S. The Environmental Determinants of Diabetes in the Young (TEDDY) study: Study design. Pediatr. Diabetes 2007, 8, 286–298. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Siljander, H.; Vatanen, T.; Hyötyläinen, T.; Hämäläinen, A.-M.; Peet, A.; Tillmann, V.; Pöhö, P.; Mattila, I. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe 2015, 17, 260–273. [Google Scholar] [CrossRef]
- Parigi, S.; Eldh, M.; Larssen, P.; Gabrielsson, S.; Villablanca, E. Breast milk and solid food shaping intestinal immunity. Front. Immunol. 2015, 6, 415. [Google Scholar] [CrossRef]
- Asnicar, F.; Manara, S.; Zolfo, M.; Truong, D.T.; Scholz, M.; Armanini, F.; Ferretti, P.; Gorfer, V.; Pedrotti, A.; Tett, A. Studying vertical microbiome transmission from mothers to infants by strain-level metagenomic profiling. mSystems 2017, 2, e00164-16. [Google Scholar] [CrossRef]
- Ward, T.; Hosid, S.; Ioshikhes, I.; Altosaar, I. Human milk metagenome: A functional capacity analysis. BMC Microbiol. 2013, 13, 116. [Google Scholar] [CrossRef]
- Milani, C.; Mancabelli, L.; Lugli, G.; Duranti, S.; Turroni, F.; Ferrario, C.; Mangifesta, M.; Viappiani, A.; Ferretti, P.; Gorfer, V. Exploring vertical transmission of bifidobacteria from mother to child. Appl. Environ. Microbiol. 2015, 81, 7078–7087. [Google Scholar] [CrossRef]
- Duranti, S.; Lugli, G.; Mancabelli, L.; Armanini, F.; Turroni, F.; James, K.; Ferretti, P.; Gorfer, V.; Ferrario, C.; Milani, C.; et al. Maternal inheritance of bifidobacterial communities and bifidophages in infants through vertical transmission. Microbiome 2017, 5, 66. [Google Scholar] [CrossRef]
- Kordy, K.; Gaufin, T.; Mwangi, M.; Li, F.; Cerini, C.; Lee, D.; Adisetiyo, H.; Woodward, C.; Pannaraj, P.; Tobin, N. Contributions to human breast milk microbiome and enteromammary transfer of Bifidobacterium breve. PLoS ONE 2020, 15, e0219633. [Google Scholar] [CrossRef]
- Jost, T.; Lacroix, C.; Braegger, C.; Rochat, F.; Chassard, C. Vertical mother–neonate transfer of maternal gut bacteria via breastfeeding. Environ. Microbiol. 2014, 16, 2891–2904. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, E.; de Andrés, J.; Manrique, M.; Pareja-Tobes, P.; Tobes, R.; Martínez-Blanch, J.F.; Codoñer, F.M.; Ramón, D.; Fernández, L.; Rodríguez, J.M. Metagenomic analysis of milk of healthy and mastitis-suffering women. J. Hum. Lact. 2015, 31, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, M.; Wu, S.; Lebrilla, C.; Chapkin, R.; Ivanov, I.; Donovan, S. Fecal microbiota composition of breast-fed infants is correlated with human milk oligosaccharides consumed. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 825–833. [Google Scholar] [CrossRef]
- Lewis, Z.; Totten, S.; Smilowitz, J.; Popovic, M.; Parker, E.; Lemay, D.; Van Tassell, M.; Miller, M.; Jin, Y.-S.; German, J. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome 2015, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.; Jena, R.; Choudhury, P.; Pattnaik, R.; Mohapatra, S.; Saini, M. Milk derived antimicrobial bioactive peptides: A review. Int. J. Food Prop. 2016, 19, 837–846. [Google Scholar] [CrossRef]
- Hassiotou, F.; Hepworth, A.; Metzger, P.; Tat Lai, C.; Trengove, N.; Hartmann, P.; Filgueira, L. Maternal and infant infections stimulate a rapid leukocyte response in breastmilk. Clin. Transl. Immunol. 2013, 2, e3. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Y.; Zhu, Y.; Yang, J.; Sun, L.; Chai, X.; Wang, Y. Characteristic chromatographic fingerprint study of short-chain fatty acids in human milk, infant formula, pure milk and fermented milk by gas chromatography–mass spectrometry. Int. J. Food Sci. Nutr. 2016, 67, 632–640. [Google Scholar] [CrossRef]
- Odiase, E.; Frank, D.N.; Young, B.E.; Robertson, C.E.; Kofonow, J.M.; Davis, K.N.; Berman, L.M.; Krebs, N.F.; Tang, M. The Gut Microbiota Differ in Exclusively Breastfed and Formula-Fed United States Infants and are Associated with Growth Status. J. Nutr. 2023, 153, 2612–2621. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Rubio, R.; Collado, C.; Laitinen, K.; Salminen, S.; Isolauri, E.; Mira, A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am. J. Clin. Nutr. 2012, 96, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Khodayar-Pardo, P.; Mira-Pascual, L.; Collado, M.; Martinez-Costa, C. Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. J. Perinatol. 2014, 34, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Rubio, R.; Mira-Pascual, L.; Mira, A.; Collado, M. Impact of mode of delivery on the milk microbiota composition of healthy women. J. Dev. Orig. Health Dis. 2016, 7, 54–60. [Google Scholar] [CrossRef]
- Kumar, H.; du Toit, E.; Kulkarni, A.; Aakko, J.; Linderborg, K.M.; Zhang, Y.; Nicol, M.P.; Isolauri, E.; Yang, B.; Collado, M.C. Distinct patterns in human milk microbiota and fatty acid profiles across specific geographic locations. Front. Microbiol. 2016, 7, 1619. [Google Scholar] [CrossRef]
- Toscano, M.; De Grandi, R.; Peroni, D.G.; Grossi, E.; Facchin, V.; Comberiati, P.; Drago, L. Impact of delivery mode on the colostrum microbiota composition. BMC Microbiol. 2017, 17, 205. [Google Scholar] [CrossRef]
- Li, S.-W.; Watanabe, K.; Hsu, C.-C.; Chao, S.-H.; Yang, Z.-H.; Lin, Y.-J.; Chen, C.-C.; Cao, Y.-M.; Huang, H.-C.; Chang, C.-H. Bacterial composition and diversity in breast milk samples from mothers living in Taiwan and mainland China. Front. Microbiol. 2017, 8, 965. [Google Scholar] [CrossRef]
- Hermansson, H.; Kumar, H.; Collado, M.C.; Salminen, S.; Isolauri, E.; Rautava, S. Breast milk microbiota is shaped by mode of delivery and intrapartum antibiotic exposure. Front. Nutr. 2019, 6, 4. [Google Scholar] [CrossRef]
- Moossavi, S.; Sepehri, S.; Robertson, B.; Bode, L.; Goruk, S.; Field, C.; Lix, L.; de Souza, R.; Becker, A.; Mandhane, P. Composition and variation of the human milk microbiota are influenced by maternal and early-life factors. Cell Host Microbe 2019, 25, 324–335.e4. [Google Scholar] [CrossRef]
- Ding, M.; Qi, C.; Yang, Z.; Jiang, S.; Bi, Y.; Lai, J.; Sun, J. Geographical location specific composition of cultured microbiota and Lactobacillus occurrence in human breast milk in China. Food Funct. 2019, 10, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Jiang, J.; Lu, M.; Tong, W.; Zhou, R.; Li, J.; Yuan, J.; Wang, F.; Li, D. Human milk microbiota development during lactation and its relation to maternal geographic location and gestational hypertensive status. Gut Microbes 2020, 11, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Dinleyici, M.; Pérez-Brocal, V.; Arslanoglu, S.; Aydemir, O.; Sevuk Ozumut, S.; Tekin, N.; Vandenplas, Y.; Moya, A.; Dinleyici, E.C. Human milk virome analysis: Changing pattern regarding mode of delivery, birth weight, and lactational stage. Nutrients 2021, 13, 1779. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.; Brereton, N.J.; Li, C.; Lopez Leyva, L.; Solomons, N.W.; Agellon, L.B.; Scott, M.E.; Koski, K.G. Distinct changes occur in the human breast milk microbiome between early and established lactation in breastfeeding Guatemalan mothers. Front. Microbiol. 2021, 12, 557180. [Google Scholar] [CrossRef]
- Lopez Leyva, L.; Gonzalez, E.; Li, C.; Ajeeb, T.; Solomons, N.W.; Agellon, L.B.; Scott, M.E.; Koski, K.G. Human Milk Microbiota in an Indigenous Population Is Associated with Maternal Factors, Stage of Lactation, and Breastfeeding Practices. Curr. Dev. Nutr. 2021, 5, nzab013. [Google Scholar] [CrossRef]
- Lyons, K.E.; Shea, C.-A.O.; Grimaud, G.; Ryan, C.A.; Dempsey, E.; Kelly, A.L.; Ross, R.P.; Stanton, C. The human milk microbiome aligns with lactation stage and not birth mode. Sci. Rep. 2022, 12, 5598. [Google Scholar] [CrossRef]
- Lackey, K.A.; Williams, J.E.; Meehan, C.L.; Zachek, J.A.; Benda, E.D.; Price, W.J.; Foster, J.A.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W. What’s normal? microbiomes in human milk and infant feces are related to each other but vary geographically: The INSPIRE study. Front. Nutr. 2019, 6, 45. [Google Scholar] [CrossRef]
- Fehr, K.; Moossavi, S.; Sbihi, H.; Boutin, R.C.; Bode, L.; Robertson, B.; Yonemitsu, C.; Field, C.J.; Becker, A.B.; Mandhane, P.J. Breastmilk feeding practices are associated with the co-occurrence of bacteria in mothers’ milk and the infant gut: The CHILD cohort study. Cell Host Microbe 2020, 28, 285–297.e4. [Google Scholar] [CrossRef]
- Cortés-Macías, E.; Selma-Royo, M.; Martínez-Costa, C.; Collado, M.C. Breastfeeding practices influence the breast milk microbiota depending on pre-gestational maternal BMI and weight gain over pregnancy. Nutrients 2021, 13, 1518. [Google Scholar] [CrossRef]
- LeMay-Nedjelski, L.; Asbury, M.; Butcher, J.; Ley, S.; Hanley, A.; Kiss, A.; Unger, S.; Copeland, J.; Wang, P.; Stintzi, A. Maternal Diet and Infant Feeding Practices Are Associated with Variation in the Human Milk Microbiota at 3 Months Postpartum in a Cohort of Women with High Rates of Gestational Glucose Intolerance. J. Nutr. 2021, 151, 320–329. [Google Scholar] [CrossRef]
- Williams, J.; Carrothers, J.; Lackey, K.; Beatty, N.; York, M.; Brooker, S.; Shafii, B.; Price, W.; Settles, M.; McGuire, M. Human milk microbial community structure is relatively stable and related to variations in macronutrient and micronutrient intakes in healthy lactating women. J. Nutr. 2017, 147, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Dzidic, M.; Mira, A.; Artacho, A.; Abrahamsson, T.R.; Jenmalm, M.C.; Collado, M.C. Allergy development is associated with consumption of breastmilk with a reduced microbial richness in the first month of life. Pediatr. Allergy Immunol. 2020, 31, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wei, Y.; Liu, L.; Li, Z. Association between breastmilk microbiota and food allergy in infants. Front. Cell. Infect. Microbiol. 2022, 11, 1396. [Google Scholar] [CrossRef]
- Taylor, R.; Keane, D.; Borrego, P.; Arcaro, K. Effect of maternal diet on maternal Milk and breastfed infant gut microbiomes: A scoping review. Nutrients 2023, 15, 1420. [Google Scholar] [CrossRef] [PubMed]
- Andreas, N.; Kampmann, B.; Le-Doare, K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Aakko, J.; Kumar, H.; Rautava, S.; Wise, A.; Autran, C.; Bode, L.; Isolauri, E.; Salminen, S. Human milk oligosaccharide categories define the microbiota composition in human colostrum. Benef. Microbes 2017, 8, 563–567. [Google Scholar] [CrossRef]
- Ojo-Okunola, A.; Nicol, M.; du Toit, E. Human breast milk bacteriome in health and disease. Nutrients 2018, 10, 1643. [Google Scholar] [CrossRef]
- Ruiz, L.; Bacigalupe, R.; García-Carral, C.; Boix-Amoros, A.; Argüello, H.; Silva, C.B.; de los Angeles Checa, M.; Mira, A.; Rodríguez, J.M. Microbiota of human precolostrum and its potential role as a source of bacteria to the infant mouth. Sci. Rep. 2019, 9, 8435. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef]
- Boix-Amorós, A.; Martinez-Costa, C.; Querol, A.; Collado, M.C.; Mira, A. Multiple Approaches Detect the Presence of Fungi in Human Breastmilk Samples from Healthy Mothers. Sci. Rep. 2017, 7, 13016, Erratum in Sci. Rep. 2018, 8, 16829. https://doi.org/10.1038/s41598-018-35165-1. [Google Scholar] [CrossRef]
- Boix-Amorós, A.; Puente-Sánchez, F.; du Toit, E.; Linderborg, K.M.; Zhang, Y.; Yang, B.; Salminen, S.; Isolauri, E.; Tamames, J.; Mira, A. Mycobiome Profiles in Breast Milk from Healthy Women Depend on Mode of Delivery, Geographic Location, and Interaction with Bacteria. Appl. Environ. Microbiol. 2019, 85, e02994-18. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Ly, M.; Cerini, C.; Saavedra, M.; Aldrovandi, G.M.; Saboory, A.A.; Johnson, K.M.; Pride, D.T. Shared and distinct features of human milk and infant stool viromes. Front. Microbiol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, Y.; Xu, S.; Zhang, R.; Yu, Z.; He, W. Phage diversity in human breast milk: A systematic review. Eur. J. Pediatr. 2025, 184, 334. [Google Scholar] [CrossRef]
- Martín, R.; Heilig, H.G.; Zoetendal, E.G.; Jiménez, E.; Fernández, L.; Smidt, H.; Rodríguez, J.M. Cultivation-independent assessment of the bacterial diversity of breast milk among healthy women. Res. Microbiol. 2007, 158, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.M.; Foster, J.A.; Forney, L.J.; Schütte, U.M.; Beck, D.L.; Abdo, Z.; Fox, L.K.; Williams, J.E.; McGuire, M.K.; McGuire, M.A. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS ONE 2011, 6, e21313. [Google Scholar] [CrossRef]
- Jost, T.; Lacroix, C.; Braegger, C.; Chassard, C. Assessment of bacterial diversity in breast milk using culture-dependent and culture-independent approaches. Br. J. Nutr. 2013, 110, 1253–1262. [Google Scholar] [PubMed]
- Sakwinska, O.; Moine, D.; Delley, M.; Combremont, S.; Rezzonico, E.; Descombes, P.; Vinyes-Pares, G.; Zhang, Y.; Wang, P.; Thakkar, S.K. Microbiota in breast milk of Chinese lactating mothers. PLoS ONE 2016, 11, e0160856. [Google Scholar] [CrossRef]
- Urbaniak, C.; Angelini, M.; Gloor, G.B.; Reid, G. Human milk microbiota profiles in relation to birthing method, gestation and infant gender. Microbiome 2016, 4, 1–9. [Google Scholar] [CrossRef]
- Murphy, K.; Curley, D.; O’Callaghan, T.; O’Shea, C.-A.; Dempsey, E.; O’Toole, P.; Ross, R.; Ryan, C.; Stanton, C. The composition of human milk and infant faecal microbiota over the first three months of life: A pilot study. Sci. Rep. 2017, 7, 40597. [Google Scholar] [CrossRef]
- Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E. Maternal weight and excessive weight gain during pregnancy modify the immunomodulatory potential of breast milk. Pediatr. Res. 2012, 72, 77. [Google Scholar] [CrossRef]
- Lundgren, S.; Madan, J.; Karagas, M.; Morrison, H.; Hoen, A.; Christensen, B. Microbial communities in human milk relate to measures of maternal weight. Front. Microbiol. 2019, 10, 2886. [Google Scholar] [CrossRef] [PubMed]
- Asbury, M.R.; Butcher, J.; Copeland, J.K.; Unger, S.; Bando, N.; Comelli, E.M.; Forte, V.; Kiss, A.; LeMay-Nedjelski, L.; Sherman, P.M. Mothers of preterm infants have individualized breast milk microbiota that changes temporally based on maternal characteristics. Cell Host Microbe 2020, 28, 669–682.e4. [Google Scholar] [CrossRef]
- Cortes-Macías, E.; Selma-Royo, M.; García-Mantrana, I.; Calatayud, M.; González, S.; Martínez-Costa, C.; Collado, M.C. Maternal Diet Shapes the Breast Milk Microbiota Composition and Diversity: Impact of Mode of Delivery and Antibiotic Exposure. J. Nutr. 2021, 151, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Grönlund, M.M.; Gueimonde, M.; Laitinen, K.; Kociubinski, G.; Grönroos, T.; Salminen, S.; Isolauri, E. Maternal breast-milk and intestinal bifidobacteria guide the compositional development of the Bifidobacterium microbiota in infants at risk of allergic disease. Clin. Exp. Allergy 2007, 37, 1764–1772. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.o.; Langa, S.; Reviriego, C.; Jiménez, E.; Marín, M.L.; Olivares, M.; Boza, J.; Jiménez, J.; Fernández, L.; Xaus, J. The commensal microflora of human milk: New perspectives for food bacteriotherapy and probiotics. Trends Food Sci. Technol. 2004, 15, 121–127. [Google Scholar] [CrossRef]
- Fernández, L.; Langa, S.; Martín, V.; Maldonado, A.; Jiménez, E.; Martín, R.; Rodríguez, J.M. The human milk microbiota: Origin and potential roles in health and disease. Pharmacol. Res. 2013, 69, 1–10. [Google Scholar] [CrossRef]
- Stinson, L.; Sindi, A.; Cheema, A.; Lai, C.; Mühlhäusler, B.; Wlodek, M.; Payne, M.; Geddes, D. The human milk microbiome: Who, what, when, where, why, and how? Nutr. Rev. 2020, 8, 51. [Google Scholar] [CrossRef]
- Rodríguez, J. The origin of human milk bacteria: Is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv. Nutr. 2014, 5, 779–784. [Google Scholar] [CrossRef]
- Gardner, H.; Kent, J.C.; Lai, C.T.; Mitoulas, L.R.; Cregan, M.D.; Hartmann, P.E.; Geddes, D.T. Milk ejection patterns: An intra-individual comparison of breastfeeding and pumping. BMC Pregnancy Childbirth 2015, 15, 156. [Google Scholar] [CrossRef]
- Johns, H.M.; Forster, D.A.; Amir, L.H.; McLachlan, H.L. Prevalence and outcomes of breast milk expressing in women with healthy term infants: A systematic review. BMC Pregnancy Childbirth 2013, 13, 212. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, Y.; Ning, P.; Ma, S.-G.; He, P.-Y.; Wang, Y. Maternal risk factors for lactation mastitis: A meta-analysis. West. J. Nurs. Res. 2021, 43, 698–708. [Google Scholar] [CrossRef]
- Stinson, L.F.; Geddes, D.T. Typical skin and oral bacterial species present in human milk are not the result of contamination during the sampling process. Lett. Appl. Microbiol. 2025, 78, ovaf084. [Google Scholar] [CrossRef]
- Dewanto, N.E.; Firmansyah, A.; Sungkar, A.; Dharmasetiawani, N.; Sastroasmoro, S.; Kresno, S.B.; Suradi, R.; Bardosono, S.; Prasetyo, D. The effect of Bifidobacterium animalis lactis HNO19 supplementation among pregnant and lactating women on interleukin-8 level in breast milk and infant’s gut mucosal integrity. Med. J. Indones. 2017, 26, 204–211. [Google Scholar]
- Abrahamsson, T.R.; Sinkiewicz, G.; Jakobsson, T.; Fredrikson, M.; Björkstén, B. Probiotic lactobacilli in breast milk and infant stool in relation to oral intake during the first year of life. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, S.J.; Boyle, R.J.; Kivivuori, S.; Oppedisano, F.; Smith, K.R.; Robins-Browne, R.; Salminen, S.J.; Tang, M.L. Prenatal probiotic administration can influence Bifidobacterium microbiota development in infants at high risk of allergy. J. Allergy Clin. Immunol. 2009, 123, 499–501.e8. [Google Scholar] [CrossRef]
- Simpson, M.R.; Avershina, E.; Storrø, O.; Johnsen, R.; Rudi, K.; Øien, T. Breastfeeding-associated microbiota in human milk following supplementation with Lactobacillus rhamnosus GG, Lactobacillus acidophilus La-5, and Bifidobacterium animalis ssp. lactis Bb-12. J. Dairy Sci. 2018, 101, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Wickens, K.; Barthow, C.; Mitchell, E.A.; Stanley, T.V.; Purdie, G.; Rowden, J.; Kang, J.; Hood, F.; van den Elsen, L.; Forbes-Blom, E. Maternal supplementation alone with Lactobacillus rhamnosus HN 001 during pregnancy and breastfeeding does not reduce infant eczema. Pediatr. Allergy Immunol. 2018, 29, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Cárdenas, N.; Arroyo, R.; Manzano, S.; Jiménez, E.; Martín, V.; Rodríguez, J.M. Prevention of infectious mastitis by oral administration of Lactobacillus salivarius PS2 during late pregnancy. Clin. Infect. Dis. 2016, 62, 568–573. [Google Scholar] [CrossRef]
- Mastromarino, P.; Capobianco, D.; Miccheli, A.; Praticò, G.; Campagna, G.; Laforgia, N.; Capursi, T.; Baldassarre, M.E. Administration of a multistrain probiotic product (VSL# 3) to women in the perinatal period differentially affects breast milk beneficial microbiota in relation to mode of delivery. Pharmacol. Res. 2015, 95, 63–70. [Google Scholar]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.-Z. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 2018, 174, 1388–1405.e21. [Google Scholar] [CrossRef]
- Perez, P.; Dore, J.; Leclerc, M.; Levenez, F.; Benyacoub, J.; Serrant, P.; Segura-Roggero, I.; Schiffrin, E.; Donnet-Hughes, A. Bacterial imprinting of the neonatal immune system: Lessons from maternal cells? Pediatrics 2007, 119, e724–e732. [Google Scholar] [CrossRef]
- Civardi, E.; Garofoli, F.; Tzialla, C.; Paolillo, P.; Bollani, L.; Stronati, M. Microorganisms in human milk: Lights and shadows. J. Matern. Fetal Neonatal Med. 2013, 26, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Langa, S.; Martin, V.; Jiménez, E.; Martin, R.; Rodriguez, J. The microbiota of human milk in healthy women. Cell. Mol. Biol. 2013, 59, 31–42. [Google Scholar] [PubMed]
- Rescigno, M.; Urbano, M.; Valzasina, B.; Francolini, M.; Rotta, G.; Bonasio, R.; Granucci, F.; Kraehenbuhl, J.-P.; Ricciardi-Castagnoli, P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001, 2, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Neville, M.; Allen, J.; Archer, P.; Casey, C.; Seacat, J.; Keller, R.; Lutes, V.; Rasbach, J.; Neifert, M. Studies in human lactation: Milk volume and nutrient composition during weaning and lactogenesis. Am. J. Clin. Nutr. 1991, 54, 81–92. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Stinson, L.; Gay, M.; Koleva, P.; Eggesbø, M.; Johnson, C.; Wegienka, G.; du Toit, E.; Shimojo, N.; Munblit, D.; Campbell, D.; et al. Human Milk From Atopic Mothers Has Lower Levels of Short Chain Fatty Acids. Front. Immunol. 2020, 11, 1427. [Google Scholar] [CrossRef]
- Prentice, P.; Schoemaker, M.; Vervoort, J.; Hettinga, K.; Lambers, T.; Van Tol, E.; Acerini, C.; Olga, L.; Petry, C.; Hughes, I. Human Milk Short-Chain Fatty Acid Composition is Associated with Adiposity Outcomes in Infants. J. Nutr. 2019, 149, 716–722. [Google Scholar] [CrossRef]
- Xi, M.; Yan, Y.; Duan, S.; Li, T.; Szeto, I.M.-Y.; Zhao, A. Short-chain fatty acids in breast milk and their relationship with the infant gut microbiota. Front. Microbiol. 2024, 15, 1356462. [Google Scholar] [CrossRef]
- Tarini, J.; Wolever, T. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl. Physiol. Nutr. Metab. 2010, 35, 9–16. [Google Scholar] [CrossRef]
- Schneider, S.; Girard-Pipau, F.; Anty, R.; van der Linde, E.; Philipsen-Geerling, B.; Knol, J.; Filippi, J.; Arab, K.; Hébuterne, X. Effects of total enteral nutrition supplemented with a multi-fibre mix on faecal short-chain fatty acids and microbiota. Clin. Nutr. 2006, 25, 82–90. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- David, L.; Maurice, C.; Carmody, R.; Gootenberg, D.; Button, J.; Wolfe, B.; Ling, A.; Devlin, A.; Varma, Y.; Fischbach, M. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Brinkworth, G.; Noakes, M.; Clifton, P.; Bird, A. Comparative effects of very low-carbohydrate, high-fat and high-carbohydrate, low-fat weight-loss diets on bowel habit and faecal short-chain fatty acids and bacterial populations. Br. J. Nutr. 2009, 101, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Abell, G.; Cooke, C.; Bennett, C.; Conlon, M.; McOrist, A. Phylotypes related to Ruminococcus bromii are abundant in the large bowel of humans and increase in response to a diet high in resistant starch. FEMS Microbiol. Ecol. 2008, 66, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Padilha, M.; Danneskiold-Samsøe, N.; Brejnrod, A.; Hoffmann, C.; Cabral, V.; Iaucci, J.; Sales, C.; Fisberg, R.; Cortez, R.; Brix, S. The human milk microbiota is modulated by maternal diet. Microorganisms 2019, 7, 502. [Google Scholar] [CrossRef] [PubMed]
- Babakobi, M.; Reshef, L.; Gihaz, S.; Belgorodsky, B.; Fishman, A.; Bujanover, Y.; Gophna, U. Effect of Maternal Diet and Milk Lipid Composition on the Infant Gut and Maternal Milk Microbiomes. Nutrients 2020, 12, 2539. [Google Scholar] [CrossRef]
- Marsh, A.J.; Azcarate-Peril, M.A.; Aljumaah, M.R.; Neville, J.; Perrin, M.T.; Dean, L.L.; Wheeler, M.D.; Hines, I.N.; Pawlak, R. Fatty acid profile driven by maternal diet is associated with the composition of human milk microbiota. Front. Microbiomes 2022, 1, 1041752. [Google Scholar] [CrossRef]
- Henning, S.M.; Yang, J.; Lee, R.-P.; Huang, J.; Thames, G.; Korn, M.; Ben-Nissan, D.; Heber, D.; Li, Z. Pomegranate juice alters the microbiota in breast milk and infant stool: A pilot study. Food Funct. 2022, 13, 5680–5689. [Google Scholar] [CrossRef]
- Londoño-Sierra, D.C.; Mesa, V.; Guzmán, N.C.; Bolívar Parra, L.; Montoya-Campuzano, O.I.; Restrepo-Mesa, S.L. Maternal Diet May Modulate Breast Milk Microbiota—A Case Study in a Group of Colombian Women. Microorganisms 2023, 11, 1812. [Google Scholar] [CrossRef]
- Sindi, A.S.; Stinson, L.F.; Gridneva, Z.; Leghi, G.E.; Netting, M.J.; Wlodek, M.E.; Muhlhausler, B.S.; Rea, A.; Trevenen, M.L.; Geddes, D.T.; et al. Maternal dietary intervention during lactation impacts the maternal faecal and human milk microbiota. J. Appl. Microbiol. 2024, 135, lxae024. [Google Scholar] [CrossRef] [PubMed]
- Ajeeb, T.T.; Gonzalez, E.; Solomons, N.W.; Vossenaar, M.; Koski, K.G. Human milk microbiome: Associations with maternal diet and infant growth. Front. Nutr. 2024, 11, 1341777. [Google Scholar] [CrossRef]
- Bzikowska-Jura, A.; Koryszewska-Bagińska, A.; Konieczna, M.; Gawor, J.; Gromadka, R.; Wesołowska, A.; Olędzka, G. The Impact of Dietary Habits and Maternal Body Composition on Human Milk Microbiota—Polish Pilot Study. Molecules 2025, 30, 2723. [Google Scholar] [CrossRef] [PubMed]
- Bisanz, J.E.; Enos, M.K.; PrayGod, G.; Seney, S.; Macklaim, J.M.; Chilton, S.; Willner, D.; Knight, R.; Fusch, C.; Fusch, G.; et al. Microbiota at Multiple Body Sites during Pregnancy in a Rural Tanzanian Population and Effects of Moringa-Supplemented Probiotic Yogurt. Appl. Environ. Microbiol. 2015, 81, 4965–4975. [Google Scholar] [CrossRef] [PubMed]
- Shenker, N.S.; Perdones-Montero, A.; Burke, A.; Stickland, S.; McDonald, J.A.K.; Alexander-Hardiman, K.; Flanagan, J.; Takats, Z.; Cameron, S.J.S. Metabolomic and Metataxonomic Fingerprinting of Human Milk Suggests Compositional Stability over a Natural Term of Breastfeeding to 24 Months. Nutrients 2020, 12, 3450. [Google Scholar] [CrossRef]
- Seferovic, M.D.; Mohammad, M.; Pace, R.M.; Engevik, M.; Versalovic, J.; Bode, L.; Haymond, M.; Aagaard, K.M. Maternal diet alters human milk oligosaccharide composition with implications for the milk metagenome. Sci. Rep. 2020, 10, 22092. [Google Scholar] [CrossRef]
- Boix-Amorós, A.; Collado, M.C.; Mira, A. Relationship between Milk Microbiota, Bacterial Load, Macronutrients, and Human Cells during Lactation. Front. Microbiol. 2016, 7, 492. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Kweon, M.-N. The gut microbiota: A key regulator of metabolic diseases. BMB Rep. 2016, 49, 536. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar]
- Lopez Leyva, L.; Gonzalez, E.; Solomons, N.W.; Koski, K.G. Human milk microbiome is shaped by breastfeeding practices. Front. Microbiol. 2022, 13, 885588. [Google Scholar] [CrossRef]
- Stinson, L.F.; Ma, J.; Sindi, A.S.; Geddes, D.T. Methodological approaches for studying the human milk microbiome. Nutr. Rev. 2023, 81, 705–715. [Google Scholar] [CrossRef]
- Ojo-Okunola, A.; Claassen-Weitz, S.; Mwaikono, K.S.; Gardner-Lubbe, S.; Zar, H.J.; Nicol, M.P.; du Toit, E. The influence of DNA extraction and lipid removal on human milk bacterial profiles. Methods Protoc. 2020, 3, 39. [Google Scholar] [CrossRef]
- Moossavi, S.; Fontes, M.E.; Rossi, L.; Fusch, G.; Surette, M.G.; Azad, M.B. Capturing the diversity of the human milk microbiota through culture-enriched molecular profiling: A feasibility study. FEMS Microbiol. Lett. 2021, 368, fnab001. [Google Scholar] [CrossRef]
- Stinson, L.F.; Ma, J.; Rea, A.; Dymock, M.; Geddes, D.T. Centrifugation does not remove bacteria from the fat fraction of human milk. Sci. Rep. 2021, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.A.; Güllert, S.; Neulinger, S.C.; Streit, W.R.; Schmitz, R.A. Evaluation of 16S rRNA gene primer pairs for monitoring microbial community structures showed high reproducibility within and low comparability between datasets generated with multiple archaeal and bacterial primer pairs. Front. Microbiol. 2016, 7, 1297. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Brooker, M.R.; Dowd, S.E.; Camerlengo, T. Target region selection is a critical determinant of community fingerprints generated by 16S pyrosequencing. PLoS ONE 2011, 6, e20956. [Google Scholar] [CrossRef] [PubMed]
- Matchado, M.S.; Rühlemann, M.; Reitmeier, S.; Kacprowski, T.; Frost, F.; Haller, D.; Baumbach, J.; List, M. On the limits of 16S rRNA gene-based metagenome prediction and functional profiling. Microb. Genom. 2024, 10, 001203. [Google Scholar] [CrossRef]
- Eisenhofer, R.; Minich, J.J.; Marotz, C.; Cooper, A.; Knight, R.; Weyrich, L.S. Contamination in Low Microbial Biomass Microbiome Studies: Issues and Recommendations. Trends Microbiol. 2019, 27, 105–117. [Google Scholar] [CrossRef]
- Liu, Y.; Elworth, R.A.L.; Jochum, M.D.; Aagaard, K.M.; Treangen, T.J. De novo identification of microbial contaminants in low microbial biomass microbiomes with Squeegee. Nat. Commun. 2022, 13, 6799. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- VanderWeele, T.J. Principles of confounder selection. Eur. J. Epidemiol. 2019, 34, 211–219. [Google Scholar] [CrossRef]
- Laursen, M.F.; Pekmez, C.T.; Larsson, M.W.; Lind, M.V.; Yonemitsu, C.; Larnkjær, A.; Mølgaard, C.; Bode, L.; Dragsted, L.O.; Michaelsen, K.F.; et al. Maternal milk microbiota and oligosaccharides contribute to the infant gut microbiota assembly. ISME Commun. 2021, 1, 21. [Google Scholar] [CrossRef]
- Sindi, A.S.; Stinson, L.F.; Lean, S.S.; Chooi, Y.-H.; Leghi, G.E.; Netting, M.J.; Wlodek, M.E.; Muhlhausler, B.S.; Geddes, D.T.; Payne, M.S. Effect of a reduced fat and sugar maternal dietary intervention during lactation on the infant gut microbiome. Front. Microbiol. 2022, 13, 900702. [Google Scholar] [CrossRef]
- Barber, C.; Mego, M.; Sabater, C.; Vallejo, F.; Bendezu, R.A.; Masihy, M.; Guarner, F.; Espín, J.C.; Margolles, A.; Azpiroz, F. Differential Effects of Western and Mediterranean-Type Diets on Gut Microbiota: A Metagenomics and Metabolomics Approach. Nutrients 2021, 13, 2638. [Google Scholar] [CrossRef]
- Louis, S.; Tappu, R.-M.; Damms-Machado, A.; Huson, D.H.; Bischoff, S.C. Characterization of the gut microbial community of obese patients following a weight-loss intervention using whole metagenome shotgun sequencing. PLoS ONE 2016, 11, e0149564. [Google Scholar] [CrossRef] [PubMed]
- Jost, T.; Lacroix, C.; Braegger, C.; Chassard, C. Stability of the Maternal Gut Microbiota During Late Pregnancy and Early Lactation. Curr. Microbiol. 2014, 68, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Cheema, A.S.; Trevenen, M.L.; Turlach, B.A.; Furst, A.J.; Roman, A.S.; Bode, L.; Gridneva, Z.; Lai, C.T.; Stinson, L.F.; Payne, M.S. Exclusively breastfed infant microbiota develops over time and is associated with human milk oligosaccharide intakes. Int. J. Mol. Sci. 2022, 23, 2804. [Google Scholar] [CrossRef] [PubMed]
- Carrothers, J.M.; York, M.A.; Brooker, S.L.; Lackey, K.A.; Williams, J.E.; Shafii, B.; Price, W.J.; Settles, M.L.; McGuire, M.A.; McGuire, M.K. Fecal microbial community structure is stable over time and related to variation in macronutrient and micronutrient intakes in lactating women. J. Nutr. 2015, 145, 2379–2388. [Google Scholar] [CrossRef]
- Williams, J.E.; Carrothers, J.M.; Lackey, K.A.; Beatty, N.F.; Brooker, S.L.; Peterson, H.K.; Steinkamp, K.M.; York, M.A.; Shafii, B.; Price, W.J. Strong multivariate relations exist among milk, oral, and fecal microbiomes in mother-infant dyads during the first six months postpartum. J. Nutr. 2019, 149, 902–914. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; Romo-Vaquero, M.; García-Mantrana, I.; Rodríguez-Varela, A.; Collado, M.C.; Espín, J.C.; Selma, M.V. Urolithin Metabotypes can Anticipate the Different Restoration of the Gut Microbiota and Anthropometric Profiles during the First Year Postpartum. Nutrients 2019, 11, 2079. [Google Scholar] [CrossRef]
- Moya, A.; Ferrer, M. Functional redundancy-induced stability of gut microbiota subjected to disturbance. Trends Microbiol. 2016, 24, 402–413. [Google Scholar] [CrossRef]
- Morgan, X.C.; Segata, N.; Huttenhower, C. Biodiversity and functional genomics in the human microbiome. Trends Genet. 2013, 29, 51–58. [Google Scholar] [CrossRef]
- Sindi, A.S.; Stinson, L.F.; Lai, C.T.; Gridneva, Z.; Leghi, G.E.; Netting, M.J.; Wlodek, M.E.; Muhlhausler, B.S.; Zhou, X.; Payne, M.S. Human milk lactoferrin and lysozyme concentrations vary in response to a dietary intervention. J. Nutr. Biochem. 2025, 135, 109760. [Google Scholar] [CrossRef]
- González-Chávez, S.A.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents 2009, 33, 301.e1–301.e8. [Google Scholar] [CrossRef]
- Legrand, D. Lactoferrin, a key molecule in immune and inflammatory processes. Biochem. Cell Biol. 2012, 90, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.P.; Conneely, O.M. Lactoferrin: Role in iron homeostasis and host defense against microbial infection. Biometals 2004, 17, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Ostan, N.K.; Yu, R.-H.; Ng, D.; Lai, C.C.-L.; Pogoutse, A.K.; Sarpe, V.; Hepburn, M.; Sheff, J.; Raval, S.; Schriemer, D.C. Lactoferrin binding protein B–a bi-functional bacterial receptor protein. PLoS Pathog. 2017, 13, e1006244. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-S.; Ohashi, M.; Tanaka, T.; Kumura, H.; Kim, G.-Y.; Kwon, I.-K.; Goh, J.-S.; Shimazaki, K.-i. Growth-promoting effects of lactoferrin on L. acidophilus and Bifidobacterium spp. Biometals 2004, 17, 279–283. [Google Scholar]
- Rahman, M.M.; Kim, W.-S.; Ito, T.; Kumura, H.; Shimazaki, K.-i. Growth promotion and cell binding ability of bovine lactoferrin to Bifidobacterium longum. Anaerobe 2009, 15, 133–137. [Google Scholar] [CrossRef]
- Chipman, D.M.; Sharon, N. Mechanism of Lysozyme Action: Lysozyme is the first enzyme for which the relation between structure and function has become clear. Science 1969, 165, 454–465. [Google Scholar] [CrossRef]
- Ellison, R., 3rd; Giehl, T.J. Killing of gram-negative bacteria by lactoferrin and lysozyme. J. Clin. Investig. 1991, 88, 1080–1091. [Google Scholar] [CrossRef]
- Biddulph, C.; Holmes, M.; Kuballa, A.; Davies, P.S.; Koorts, P.; Carter, R.J.; Maher, J. Human milk oligosaccharide profiles and associations with maternal nutritional factors: A scoping review. Nutrients 2021, 13, 965. [Google Scholar] [CrossRef]
- Selma-Royo, M.; González, S.; Gueimonde, M.; Chang, M.; Fürst, A.; Martínez-Costa, C.; Bode, L.; Collado, M.C. Maternal diet is associated with human milk oligosaccharide profile. Mol. Nutr. Food Res. 2022, 66, 2200058. [Google Scholar] [CrossRef]
- Mokhtari, P.; Schmidt, K.A.; Zamanian, H.; Babaei, M.; Machle, C.J.; Trifonova, D.; Alderete, T.L.; Holzhausen, E.A.; Ottino-González, J.; Chalifour, B.N. Maternal Diet Associated with Oligosaccharide Abundances in Human Milk from Latina Mothers. Nutrients 2024, 16, 1795. [Google Scholar] [CrossRef]
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B. The human serum metabolome. PLoS ONE 2011, 6, e16957. [Google Scholar] [CrossRef]
- Cummings, J.H.; Pomare, E.; Branch, W.; Naylor, C.; MacFarlane, G. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Liu, S.; Chen, L.; Shen, J.; Niu, Y.; Wang, T.; Zhang, W.; Fu, L. Effect of exercise and butyrate supplementation on microbiota composition and lipid metabolism. J. Endocrinol. 2019, 243, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, C.; Jiang, Q.; Yin, Y. Butyrate in energy metabolism: There is still more to learn. Trends Endocrinol. Metab. 2021, 32, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sindi, A.S. The Association Between Maternal Diet and the Human Milk Microbiome: A Review of Evidence and Methodological Challenges. Microorganisms 2025, 13, 2347. https://doi.org/10.3390/microorganisms13102347
Sindi AS. The Association Between Maternal Diet and the Human Milk Microbiome: A Review of Evidence and Methodological Challenges. Microorganisms. 2025; 13(10):2347. https://doi.org/10.3390/microorganisms13102347
Chicago/Turabian StyleSindi, Azhar S. 2025. "The Association Between Maternal Diet and the Human Milk Microbiome: A Review of Evidence and Methodological Challenges" Microorganisms 13, no. 10: 2347. https://doi.org/10.3390/microorganisms13102347
APA StyleSindi, A. S. (2025). The Association Between Maternal Diet and the Human Milk Microbiome: A Review of Evidence and Methodological Challenges. Microorganisms, 13(10), 2347. https://doi.org/10.3390/microorganisms13102347





