Abstract
The human gut microbiome represents a complex ecosystem comprising numerous microorganisms critical to basic physiological processes. The gut microbiome’s composition and functionality influence surgical outcomes following orthopedic procedures. The purpose of this study was to evaluate the gut microbiota on critical aspects of orthopedic surgical outcomes. A comprehensive literature search was conducted via PubMed, the Cumulative Index for Nursing and Allied Health Literature (CINAHL), Google Scholar, Cochrane Library, Medline, and Web of Science. A total of 18 research articles of the 599 retrieved results were included in this study. Significant correlations were identified between microbial composition and surgical outcomes, including infection rates, inflammatory responses, and postoperative complications. Bacterial genera like Alistipes and Helicobacter increased postoperative cognitive dysfunction (POCD) risk, while short-chain fatty acid (SCFA)-producing bacteria showed negative correlations with inflammatory markers. Probiotic interventions reduced POCD incidence from 16.4% to 5.1% and modulated inflammatory responses. Additionally, bacterial composition was associated with critical surgical parameters such as bone healing, infection rate, and recovery trajectory. Inflammation, healing processes, and recovery trajectories are influenced by microbial composition in surgical settings. Targeted interventions, such as probiotics, show promise in reducing surgical risks and improving patient recovery.
1. Introduction
The gut microbiota is a complex and dynamic ecosystem consisting of bacteria, viruses, fungi, and protozoa that reside within the gastrointestinal tract [1,2]. It comprises over 1000 species encoding more than three million genes, and its composition varies significantly between individuals [3,4]. Most microorganisms are concentrated in the large intestine, where they perform critical functions in maintaining local intestinal barrier integrity and modulating systemic immune responses [5,6,7]. Disruptions in its diversity and composition have been linked to numerous local and systemic diseases [8].
Gut microbiota evolves in response to host genetic makeup, dietary habits, lifestyle, environment, and medical history [9]. They are involved in various basic physiological aspects, such as the metabolism of nutrition, synthesis of important vitamins, maintenance of the immune system, modulation of inflammation, and protection against pathogenic microorganisms [10,11,12]. Disruptions to this balance, known as dysbiosis, have been linked to inflammatory bowel diseases, metabolic disorders, and impaired immune responses [13]. Microbial dysbiosis may correlate with compromised bone mineral density, increased infection risks in joint replacements, and altered healing mechanisms in fracture repair [14]. Specific bacterial populations have been associated with enhanced or diminished bone regenerative capacities, highlighting potential therapeutic opportunities through microbiome modulation [15].
The intricate relationship between the microbiome, host immunity, and inflammatory regulation emphasizes its critical role in surgical contexts. Surgical interventions disrupt the microbiome’s balance by triggering inflammatory cascades and metabolic changes [16,17,18]. Perioperative factors such as antibiotics and nutritional deprivation can alter the gut microbiome’s diversity, leading to surgical site infections [19,20]. Microbiome alterations significantly impact clinical outcomes in orthopedic surgeries by increasing infection risk and hindering recovery [21]. Joint replacements and fracture repairs are among the most commonly performed procedures worldwide, but infection and delayed healing remain as the leading causes of postoperative complications. The invasive nature of these surgeries and their direct impact on musculoskeletal tissues underscore the urgent need to understand and manage microbiome alterations to optimize patient outcomes [22,23].
Despite the growing interest in the microbiome’s role in inflammatory and surgical contexts, significant gaps in the literature persist. These include the mechanisms behind microbiome-mediated inflammatory responses, long-term impacts of surgical interventions on microbial composition, and variability in microbiome responses across different orthopedic procedures. There is also a need to identify predictive microbiological markers and develop interventional strategies leveraging microbiome insights to improve orthopedic surgical outcomes.
Beyond orthopedics, microbiome testing is of particular interest in specialties such as general surgery, where gut microbial composition has been linked to complications such as anastomotic leaks, malabsorption, ileus, and surgical site infections [24]. Preoperative microbiome profiling offers potential for risk stratification and personalized perioperative management, allowing clinicians to predict and mitigate adverse outcomes more effectively.
Despite its promise, widespread implementation of microbiome diagnostics remains limited by the lack of standardized clinical guidelines and inconsistent insurance coverage. However, recent international efforts to establish regulatory frameworks for microbiome testing may pave the way for broader clinical adoption and reimbursement [25]. Expanding research in this space could shape future surgical guidelines and improve access to microbiome-informed care.
This study summarizes the current text available on the associations between gut microbiota and orthopedic surgical outcomes. It aims to synthesize current evidence on the composition of gut microbiota and its influence on recovery following orthopedic procedures. Furthermore, it will evaluate the relationships between microbial dynamics and key surgical outcomes, identify potential microbiological markers predictive of surgical success, and investigate therapeutic interventions targeting microbiome modulation. This review will provide actionable insights for improving patient outcomes in orthopedic surgery through microbiome-informed strategies.
Research Objectives: The purpose of this study was to comprehensively explore how gut microbiota influences surgical outcomes following orthopedic procedures, and investigate the impact of gut microbiota on postoperative infection rates, bone healing, and recovery.
Research Questions: How do the composition and diversity of gut microbiota influence postoperative outcomes in orthopedic surgery? How do probiotic and prebiotic interventions modulate gut microbiota to influence surgical outcomes in orthopedic surgery?
2. Methodology
The reporting of this study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [26]. For PubMed, search terms included (“gut microbiota” OR “intestinal microbiota” OR “microbiome”) AND (“orthopedic surgery” OR “joint replacement”). Filters for English language peer-reviewed articles were applied. For the Cumulative Index for Nursing and Allied Health Literature (CINAHL), combinations such as “intestinal bacteria AND arthroplasty” were used. Google Scholar searches incorporated broader terms with Boolean operators to include gray literature. Cochrane Library searches focused on randomized controlled trials, using the string “microbiome AND orthopedic outcomes.” Medline and Web of Science searches followed similar patterns, emphasizing human subjects and excluding conference abstracts or non-original research. ChatGPT-4o was used to refine certain ideas within the key takeaways for orthopedic surgeons section to condense and summarize key findings in a practical manner.
2.1. Identification and Selection of Studies
A comprehensive literature search for peer-reviewed original research articles was conducted via PubMed, the CINAHL, Cochrane Library, Medline, and Web of Science.
2.2. Search Strategy
Keywords were identified and used with MeSH terms to formulate search strings. The following keywords were used in different combinations to optimize the search: (“gut microbiota” OR “intestinal microbiota” OR “microbiome” OR “gut flora” OR “intestinal bacteria”) AND (“orthopedic surgery” OR “orthopedic procedures” OR “musculoskeletal surgery” OR “joint replacement” OR “arthroplasty” OR “fracture fixation”).
2.3. Study Selection
A two-reviewer system was used in the screening and article selection for consistency. Discrepancies were resolved through discussion or the intervention of a third reviewer. The retrieved results from various databases were exported to Zotero screening software version 6.0.36. The software automatically detected retracted articles and identified duplicate records, which a reviewer manually merged. Non-duplicate records were then screened using prespecified eligibility criteria.
2.3.1. Eligibility Criteria
This study included research on the role of gut microbiota in orthopedic surgery. Articles fulfilling the modified population intervention comparison primary outcomes study design (PICOS) criteria were selected [25].
The PICOS criteria for eligible studies were defined as follows;
- Population (P): patients undergoing orthopedic surgeries.
- Intervention (I): gut microbiota.
- Comparison (C): placebo groups.
- Primary outcomes (O): pinfection rates, bone healing, microbiota composition changes, incidence of postoperative cognitive dysfunction, and bone mineral density.
- Study Design (S): randomized controlled trials (RCTs), prospective and retrospective cohort studies, case-control studies, observational cohort studies, and any other suitable study designs.
The potential articles were subjected to the following inclusion and exclusion criteria:
2.3.2. Inclusion Criteria
This study included peer-reviewed original research articles published in English. It included studies on human subjects only undergoing orthopedic procedures. In addition, research articles investigating the association between gut microbiota, inflammation markers, bacterial endotoxins, and various clinical outcomes were included.
2.3.3. Exclusion Criteria
Study protocols, reviews, meta-analyses, letters, editorials, conference abstracts, and opinion pieces were excluded.
2.4. Methodological Quality Assessment
The risk of bias visualization tool developed by the Cochrane Collaboration (Rob 2.0) was used to assess the risk of bias in the eligible RCTs. The tool assessed bias arising from the randomization process, deviation from the intended intervention, missing outcome data, measurement of outcome, and selection of reported results [27]. In addition, the eligible cohort studies’ risk of bias was assessed using the Newcastle Ottawa Scale (NOS) evaluating the following domains: the selection of the study groups, the comparability of the groups, and the ascertainment outcome of interest. The National Institutes of Health (NIH) quality assessment tool was used to assess the risk of bias in the eligible case-control studies [28].
2.5. Data Selection and Extraction
Data from the included studies were systematically extracted and tabulated using Microsoft Excel 2024. The following data sets were extracted: authors; study region; sample size; demographics; study design; intervention; primary outcomes: infection rates, bone healing; and secondary outcomes: pain, inflammation, hospital stay, objectives, and findings.
2.6. Data Analysis
The extracted data from the studies were thematically analyzed according to the outcome measures of interest by critically reading the data and noting down the initial observations while procedurally coding and grouping them into potential themes. The coded themes were then checked for accuracy [29].
3. Results
3.1. Study Selection
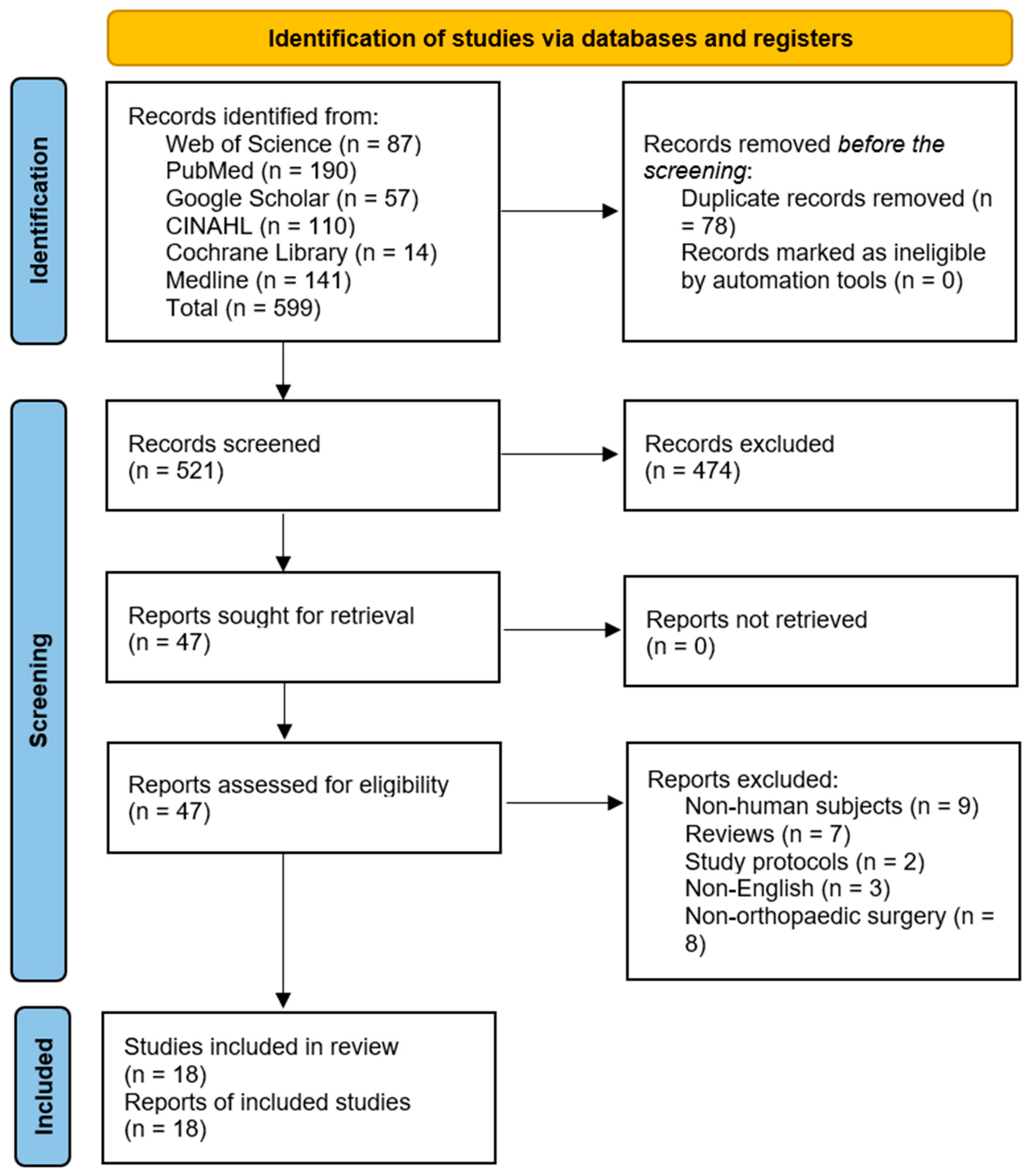
The database search yielded 599 records, of which 78 duplicates were removed. Further, 474 articles were excluded following the title and abstract screening. The remaining 47 articles were sought for retrieval, after which two RCTs, three case-control, and 12 cohort studies that met the eligibility criteria were included. The results are presented in Figure 1.
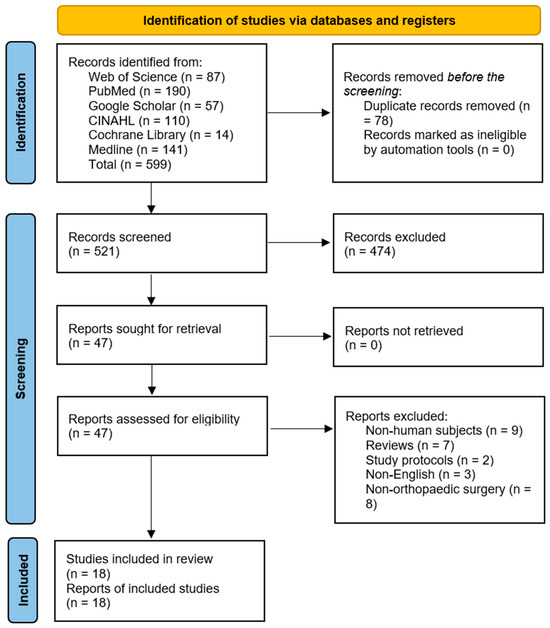
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram [26].
3.2. Methodological Quality Assessment
There was a low overall risk of bias in the included RCTs, as shown in Figure 2 and Figure 3. In addition, the included cohort studies demonstrated good quality, as shown in Table 1. Moreover, the case-control studies included had good overall quality, as shown in Table 2. Table 3 provides a summary of the studies after quality assessment.
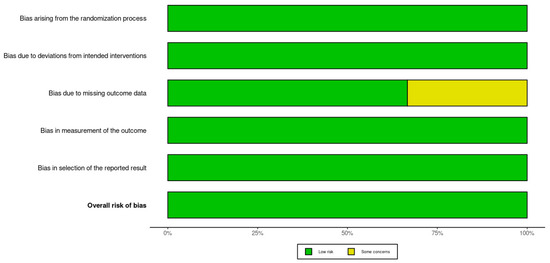
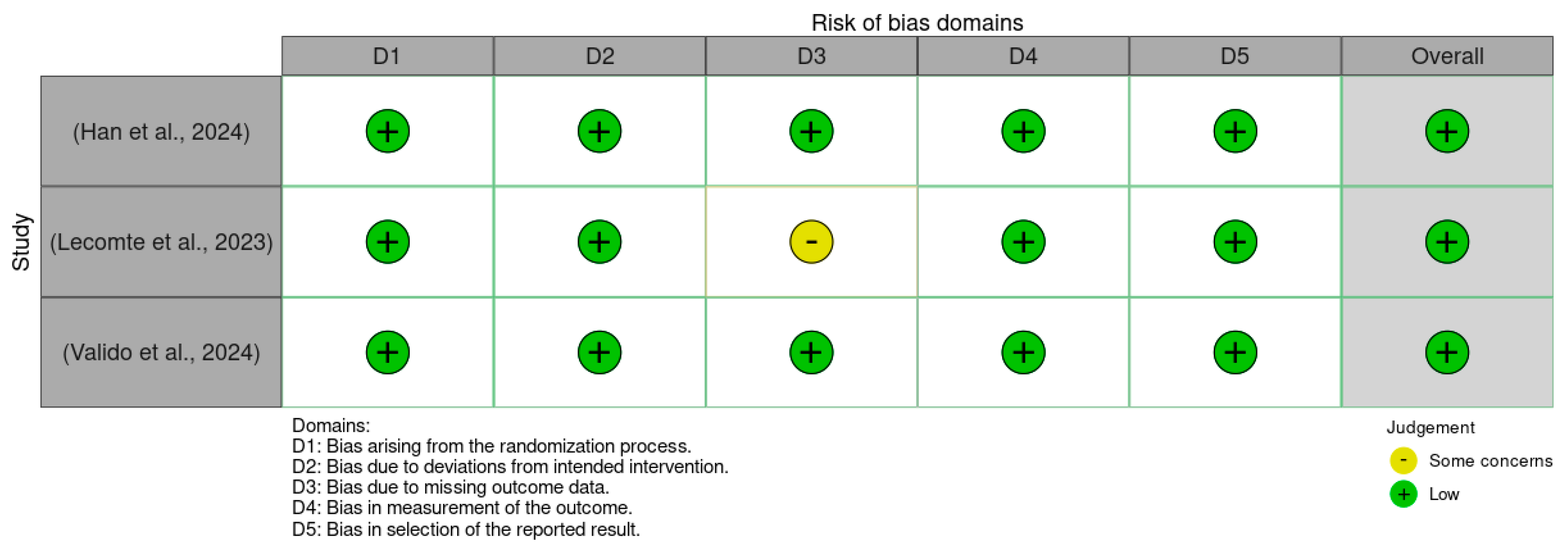
Figure 2.
Traffic light plot of the Rob 2.0 assessment results [30,31,32].
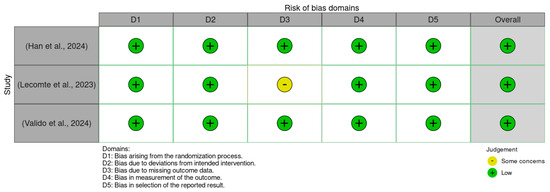
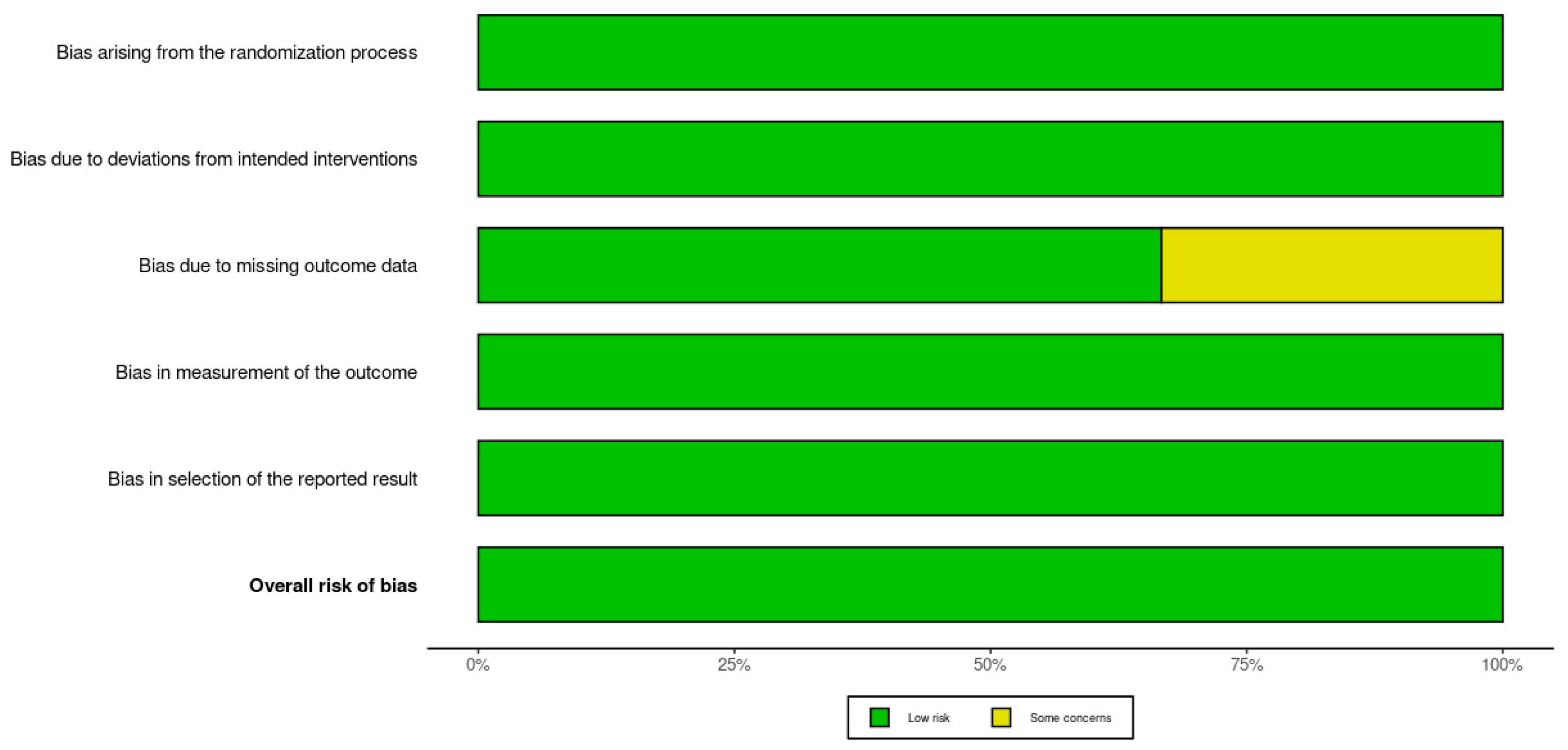
Figure 3.
Summary plot of the Rob 2.0 assessment results for included studies [30,31,32].

Table 1.
Newcastle Ottawa Scale (NOS) assessment results for included studies [33,34,35,36,37,38,39,40,41,42,43,44,45].

Table 2.
National Institutes of Health (NIH) assessment results.

Table 3.
Study characteristics.
Figure 2 presents a traffic light plot summarizing the risk of bias assessments for each included study using the Cochrane Risk of Bias 2.0 (RoB 2.0) tool. Each row corresponds to an individual randomized controlled trial, while the columns represent five specific domains of bias: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, and bias in the selection of the reported result. Color coding is used to visually represent the level of concern within each domain: green indicates a low risk of bias, yellow indicates some concerns, and red indicates a high risk. All three studies were assessed as having an overall low risk of bias [30,31,32].
Figure 3 provides a summary plot that aggregates the domain-specific risk of bias judgments across all included studies. Each bar illustrates the proportion of studies assessed as low risk or having some concerns for each of the five RoB 2.0 domains. The majority of assessments fell into the low-risk category, reflecting overall strong methodological quality across the studies. Notably, the domain concerning missing outcome data was the only category with a study assessed as having some concerns, accounting for approximately one-third of the total sample. This overview reinforces the reliability of the evidence base included in this systematic review while highlighting a specific area that warrants attention in future trials.
3.3. Data Selection and Extraction
The included studies focused on orthopedic and neurological patient populations from the USA, China, Ireland, Switzerland, Korea, and Spain. Most studies utilized advanced microbiological techniques such as 16S rRNA sequencing to analyze gut microbiota composition and its implications for surgical outcomes. The sample sizes ranged from 21 to 551 patients. The studies employed prospective and retrospective cohorts, randomized controlled trials, and case-control research designs.
The studies investigated postoperative cognitive dysfunction, periprosthetic joint infections, bone healing, and neurological outcomes. In addition, they investigated relationships between gut microbiota, inflammation markers, bacterial endotoxins, and clinical outcomes such as infection rates, pain, cognitive function, and bone mineral density. The studies used microbiome analysis, probiotic interventions, metabolite measurements, and biomarker assessments in the analyses.
3.4. Thematic Analysis of Outcomes
3.4.1. Gut Microbiota and Surgical Outcomes
The surgical outcomes for procedures such as spinal fusion and joint replacement are significantly influenced by gut microbiota. This is particularly evident in cases involving surgical site infection (SSI) and wound healing. Dysbiotic microbiota, characterized by an over-abundance of genera such as Alistipes (p = 0.008) and Helicobacter (p = 0.007), was associated with postoperative cognitive dysfunction (POCD). [29] Pathways like glycosaminoglycan biosynthesis were associated with tissue regeneration, suggesting the importance of gut microbiota in recovery post-surgery. Preoperative gut microbiota composition influences infection rates and recovery trajectories following surgery. Patients with pre-existing dysbiosis, such as low Firmicutes–Bacteroidetes ratio, experience delayed recovery and higher infection rates [30].
Gut microbiota composition also significantly influences bone and soft tissue healing. The changes in gut microbiota were found to be significantly associated with the changes in bone mineral density (BMD), hence affecting bone health. In spinal fusion, significant differences in gut microbiota appear between those with low bone mass (T-score ≤ −1.0) and those with normal bone mineral density (p = 0.03) [39]. Das et al. (2019) reported that six genera showed altered abundance in either osteopenia or osteoporosis subjects, five of which remained significant after adjustment for confounders. β-Diversity analysis explained 15–17% of microbiota variance, of which 2% of the variance was contributed by BMD measurements (p ≤ 0.05) [43]. Osteoporosis was enriched by the genera Actinomyces and Lactobacillus, while Veillonella was more abundant in osteopenia.
Despite these significant associations, α-diversity metrics did not differ across groups of BMD [35]. However, elevated levels of gut-derived metabolites, such as trimethylamine N-oxide (TMAO), were linked to adverse outcomes, including delayed wound healing. Trimethylamine N-oxide (TMAO) levels correlate positively with systemic inflammatory markers (e.g., IL-6, p < 0.01) and oxidative stress (ROS, p < 0.001) [31]. Additionally, the Shannon index displayed a lower gut microbiota diversity among the patients in the osteopenia group [33].
3.4.2. Antibiotic Use and Resistance in Orthopedic Surgery
Antibiotic administration is essential in preventing infections during orthopedic surgeries, such as joint arthroplasty and fracture fixation. However, antibiotics can disrupt the delicate balance of the gut microbiota, leading to dysbiosis and systemic complications that affect surgical outcomes. Dysbiosis compromises the gut’s ability to regulate immune responses, increasing susceptibility to opportunistic infections, impaired wound healing, and inflammation. Antibiotic-induced reductions in microbiota diversity diminish the production of short-chain fatty acids (SCFAs). SCFAs are vital for reducing endotoxin translocation, suppressing systemic inflammatory markers like C-reactive protein (CRP), and supporting immune homeostasis [38].
Markers like Zonulin and soluble CD14 (sCD14) provide insight into how gut microbiota disruptions contribute to periprosthetic joint infections (PJIs). Zonulin regulates intestinal tight junctions, but its overexpression compromises gut barrier integrity. Bacterial endotoxins, such as lipopolysaccharides (LPS), enter systemic circulation, and this process triggers systemic inflammation, exacerbates tissue damage, and increases the risk of surgical site infections [34]. Zonulin levels are higher in acute PJIs (10.7 ± 6.2 ng/mL) compared to chronic cases (5.8 ± 4.8 ng/mL, with a p = 0.005) [40]. Similarly, sCD14, a co-receptor for endotoxin recognition, is elevated in acute PJIs (555 ± 216 ng/mL, p = 0.003) [34,48].
Microbial clustering studies identified differences in bacterial profiles between acute and chronic PJIs. Acute cases often involve Staphylococcus-type microbiota (71.4%, p = 0.043), whereas chronic PJIs were linked to Cutibacterium (11.1%, p = 0.036) [35]. Patients with nonunion fractures showed a predominance of gram-positive bacteria (54.3%), but the time to the union did not differ between gram-positive and gram-negative cases (662.3 vs. 446.8 days, p = 0.69). However, three gram-positive cases required amputation or arthroplasty before the union [39].
Antibiotic use also influences diagnostic biomarkers in PJIs. Synovial fluid α-defensin exhibited high diagnostic performance as reflected by an area under the curve (AUC) of 0.93, sensitivity of 94.4%, and specificity of 89.5% at a cut-off of 1580 μg/L (p < 0.001) [34]. Patients with PJIs also had lower operational taxonomic units in microbiota profiles (133 versus 265, p = 0.006), reflecting significant disruptions to microbiota composition [34]. Although biomarkers like α-defensin and leukocyte esterase help identify PJIs, microbiota diversity metrics provide additional insights by highlighting infection-associated microbial changes.
Emerging evidence suggests that supplementing antibiotic regimens with probiotics and prebiotics can restore microbial diversity, support SCFA production, and mitigate inflammatory responses. Strains such as Lactobacillus acidophilus and Bifidobacterium longum have demonstrated efficacy in reducing CRP levels, lowering infection risks, and promoting recovery [40]. This balanced approach highlights the possibility of integrating microbiota-targeted interventions alongside traditional infection prevention strategies to optimize outcomes in orthopedic surgery. Overall, antibiotic use, though detrimental to gut health, is still important to use to prevent infections.
3.4.3. Probiotic and Prebiotic Interventions
Building on the evidence of gut microbiota’s role in surgical outcomes, exploring the benefits of probiotics and prebiotics provide insights into reducing complications. Probiotic use in surgical patients has shown significant effects in reducing infections and improving recovery. Strains like Bifidobacterium longum, Lactobacillus acidophilus, and Streptococcus faecalis effectively alter the gut microbiota composition and reduce postoperative complications, including cognitive dysfunction (p = 0.01). Probiotic administration significantly reduced the incidence of POCD from 16.4% to 5.1% (p = 0.046) [37]. Additionally, elevated plasma LPS and CRP levels in poorer outcome surgery patients highlight their potential to be managed or improved by prebiotic treatment [31].
4. Discussion
This study hypothesized that gut microbiota composition influences orthopedic surgical outcomes, with variations in microbial diversity and specific bacterial populations correlating with inflammation, infection rates, bone healing processes, and postoperative cognitive function.
The findings revealed multifaceted relationships between gut microbiota and orthopedic surgical outcomes. The study demonstrated that microbiota composition plays a critical role in modulating surgical recovery through complex inflammatory and metabolic mechanisms [30,38]. Probiotic and prebiotic interventions have promising potential in reducing postoperative complications, especially POCD [37].
Bacterial genera, such as Alistipes and Helicobacter, were associated with increased risks of POCD, while short-chain fatty acids (SCFAs) producing bacteria had inverse associations with inflammatory markers [30]. The composition of the microbiota was different in patients with divergent surgical outcomes, including divergent bone mineral density, susceptibility to infection, and divergent recovery trajectories [39]. In addition, there were postoperative changes in some plasma markers of inflammation, such as CRP and IL-6 [40]. The most dramatic changes in microbiota occurred in those patients who had cognitive dysfunction, highlighting the complex interaction between gut microbes, neurological function, and surgical stress [37,38,40].
Probiotic strains like Bifidobacterium longum and Lactobacillus acidophilus aid in the postoperative complications and modulation of immune responses [37]. The correlations between gut microbiota and surgical outcomes are complex, multidirectional interactions based on inflammatory pathways, metabolic stress, and immune modulation. Similarly, microbial dysbiosis is an active mediator of the process of surgical recovery apart from a passive marker [38].
The pathways related to glycosaminoglycan biosynthesis point to complex molecular interactions between microbial populations and host tissue recovery [30]. Variations in the composition of microbiota across different patient groups, especially those with cognitive disorders, suggest that surgical stress triggers distinct microbial adaptations [29]. The decline in the Firmicutes–Bacteroidetes ratio in certain patient groups further underscores the dynamic nature of gut microbiome responses to surgical interventions [31].
Malnutrition is a known risk factor associated with orthopedic surgery, which current literature reports as a factor that impedes a patient’s ability to respond to the metabolic demand of surgery. Gut microbiota respond to daily habits such as eating and are involved with nutrition. Disruptions to the microbiota can lead to a delay in immune response [10,11,12,13,49]. Furthermore, nutritional deprivation negatively alters the microbiome, which puts postoperative patients of orthopedic surgery at a higher risk of surgical site infections, renal complications, and mortality [50]. With growing recognition of the role of nutrition in surgical recovery, optimizing key micronutrient levels has become a priority in orthopedic care. Beyond its well-established role in calcium homeostasis and bone mineralization, Vitamin D has been shown to influence gut microbial composition by promoting beneficial bacterial populations and reducing systemic inflammation [51]. The vitamin D receptor, widely expressed in intestinal epithelial cells, is key in maintaining gut barrier integrity and microbial homeostasis [52,53]. Given its relative safety and ease of administration, vitamin D supplementation may be an effective strategy to support both microbiome stability and orthopedic recovery.
Recent literature further supports the critical role of the gut microbiome in skeletal health beyond surgical outcomes. Gut microbes influence bone density, joint integrity, and musculoskeletal resilience through SCFA production, estrogen regulation, and vitamin synthesis [1]. Dysbiosis has been linked to various orthopedic conditions, including osteoarthritis, osteoporosis, intervertebral disc degeneration, neuropathies, and myopathies [47].
In addition to SCFAs and hormonal regulation, gut microbiota contribute to skeletal health through their role in vitamin K metabolism [54]. Certain bacterial strains, including Escherichia coli and Lactobacillus, are major producers of vitamin K2 in the gut [55]. Bacterially synthesized vitamin K2 is absorbed in the ileum and plays a crucial role in activating vitamin K-dependent proteins, such as osteocalcin and matrix Gla protein, which are essential for bone turnover and vascular calcification prevention [56,57]. Dysbiosis may impair this synthesis, contributing to poor bone mineralization and increased fracture risk [58]. Furthermore, microbiome-mediated modulation of osteoblast and osteoclast activity directly affects fracture healing and bone remodeling [31].
Given the observed relationships between gut microbiota composition and surgical outcomes, targeted interventions such as probiotics and prebiotics offer promising strategies to address complications. The significant reduction in POCD from 16.4% to 5.1% with targeted probiotic use represents a clinically meaningful intervention strategy [44,45]. These findings align with existing literature on microbiome-mediated inflammatory responses. The observed correlations between gut microbiota and bone mineral density expand upon limited existing research, providing a more comprehensive understanding of microbial roles in orthopedic healing processes.
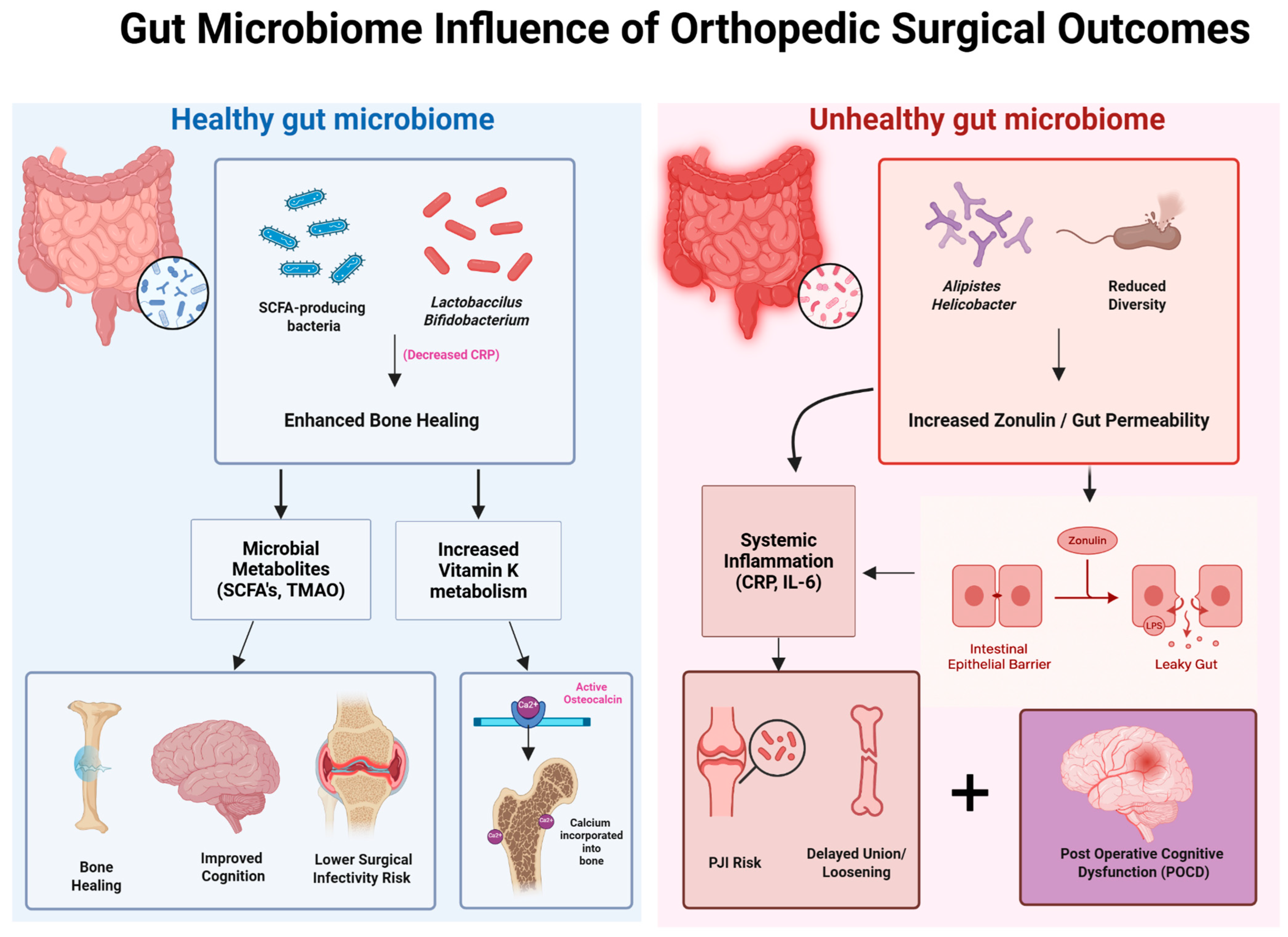
To better visualize the relationship between the gut microbiome and postoperative orthopedic outcomes, Figure 4 presents a side-by-side schematic comparison of healthy versus unhealthy microbial environments and their downstream effects on bone healing, inflammation, and recovery. A balanced gut microbiome—characterized by SCFA-producing species such as Lactobacillus and Bifidobacterium—promotes anti-inflammatory signaling (e.g., reduced CRP), enhanced vitamin K metabolism, and microbial metabolite production, all of which contribute to improved surgical recovery, lower infection risk, and accelerated bone union. In contrast, dysbiosis—marked by reduced microbial diversity and the presence of species such as Alistipes and Helicobacter—leads to increased zonulin-mediated intestinal permeability (“leaky gut”), systemic inflammation (elevated IL-6, CRP), and impaired osseointegration. These disruptions contribute to delayed healing, higher periprosthetic joint infection (PJI) risk, and potential neurocognitive decline following surgery. This visual framework reinforces the critical role of microbial composition in shaping both localized orthopedic outcomes and systemic recovery trajectories.
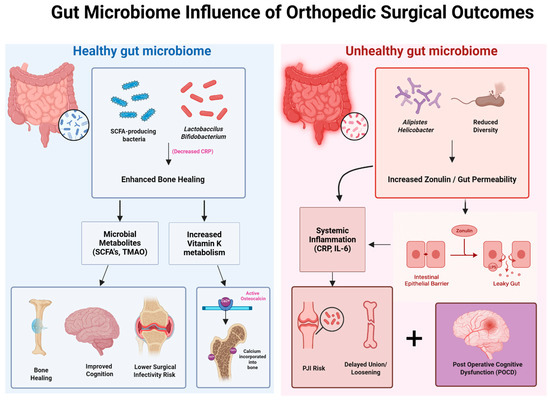
Figure 4.
Impact of gut microbiome composition on orthopedic surgical outcomes. This schematic contrasts the effects of a healthy versus unhealthy gut microbiome on postoperative recovery. A healthy microbiome, rich in SCFA-producing bacteria and Lactobacillus/Bifidobacterium species, promotes enhanced bone healing through microbial metabolites (SCFAs, TMAO) and increased vitamin K metabolism, resulting in improved cognition, reduced infection risk, and better graft incorporation. In contrast, an unhealthy microbiome—marked by reduced diversity and abundance of bacteria such as Alistipes and Helicobacter—leads to increased zonulin expression and gut permeability, driving systemic inflammation (elevated CRP, IL-6), delayed osseous healing, heightened risk of periprosthetic joint infection (PJI), and postoperative cognitive dysfunction (POCD).
4.1. Implications for Orthopedic Surgeons
4.1.1. Preoperative Considerations
Orthopedic surgeons are increasingly recognizing the importance of systemic factors, including gut microbiota, in influencing surgical outcomes. It is worth considering that preoperative assessments should now evaluate gut microbiota status, as dysbiosis—an imbalance in gut bacteria—has been associated with systemic inflammation, impaired wound healing, and elevated surgical complication rates. Emerging tools, such as microbiota profiling and biomarkers like Zonulin and sCD14, can help identify patients at risk for dysbiosis-related complications. Elevated levels of these markers, along with inflammatory cytokines like interleukin-6 (IL-6) and C-reactive protein (CRP), signal gut barrier dysfunction and systemic inflammation, both of which are associated with poorer surgical outcomes [59,60].
Preoperative management of conditions linked to gut dysbiosis, such as diabetes and obesity, can reduce the risk of infections and complications. Studies show that dietary interventions, such as increasing prebiotic and probiotic intake, can improve gut microbiota composition and reduce systemic inflammation. Furthermore, antibiotics, while essential for infection prophylaxis, can disrupt the gut microbiota. Strategies like selective decontamination and the use of microbiome-sparing antibiotics may preserve microbial diversity while preventing surgical site infections. Emerging therapies, such as tailored probiotics, prebiotics, and synbiotics, show promise in enhancing gut health prior to surgery. For example, Lactobacillus rhamnosus and Bifidobacterium longum have demonstrated efficacy in reducing systemic inflammation and promoting mucosal barrier integrity [37].
4.1.2. Postoperative Considerations
Postoperative outcomes in orthopedic surgery are significantly influenced by the interplay between systemic inflammation, immune response, and gut microbiota. Dysbiosis and gut barrier dysfunction are linked to higher rates of SSIs and PJIs. Studies highlight the importance of maintaining gut integrity to reduce bacterial translocation and systemic inflammation. Elevated Trimethylamine N-oxide (TMAO) levels and microbial endotoxins are correlated with delayed wound healing and implant failures [41,59].
Short-chain fatty acids (SCFAs), such as butyrate, produced by gut bacteria, are critical for reducing inflammation and promoting tissue repair. Enhancing SCFA production through dietary or probiotic interventions, post-surgery may accelerate recovery and improve bone healing outcomes. Dysbiosis has been associated with nonunion fractures and lower bone mineral density. Gut microbiota interventions, such as targeting SCFA-producing bacteria and mitigating inflammation, could enhance osteoblast activity and extend prosthetic longevity [39,43].
Fecal microbiota transplantation (FMT) is an emerging, microbiome-restorative therapy with potential relevance to orthopedic surgery. While clinical studies in this population are limited, preclinical evidence suggests that FMT may enhance bone health and reduce systemic inflammation. Ma et al. demonstrated that FMT in aged rats improved gut barrier integrity, restored microbial diversity, and mitigated bone loss [44]. FMT may hold promise for high-risk orthopedic patients with antibiotic-associated dysbiosis or persistent inflammation. Further investigation is warranted to assess its safety, feasibility, and efficacy in surgical settings. Postoperative therapies, such as FMT and tailored probiotics, are gaining traction. These interventions have the potential to mitigate dysbiosis, reduce systemic inflammation, and lower the risk of complications like PJIs and SSIs [44].
As the evidence base continues to grow, several microbiome-targeted strategies can be implemented in recovery, reduce systemic inflammation, and minimize complications. These include probiotics, prebiotics, microbiome testing, and selective antibiotic use. Table 4 summarizes these key interventions, their clinical rationale, recommended applications, and available tools for integration into surgical care.

Table 4.
Evidence-based microbiome-targeted strategies in orthopedic surgery: clinical rationale, recommendations, and tools.
4.1.3. Microbiome Testing Protocols: Current Practices and Future Directions
PJIs and other SSIs are devastating complications in orthopedics as they may lead to prolonged treatments and revision surgeries. One major risk factor includes microbial colonization, but current practices to detect and address this vary. There is therefore a need for standardized microbial screening protocols conducted before orthopedic procedures in order to identify high-risk patients and implement targeted measures. Screening and decolonizing S. aureus carriers with mupirocin and chlorhexidine halved hospital-acquired infections (3.4% vs. 7.7%, RR 0.42). Additionally, routine S. aureus screening and decolonization significantly reduced SSIs and PJIs in joint arthroplasty patients [59]. Despite these advantages, these practices are not universal, so formalizing protocols could ultimately reduce these risk factors. Postoperative surveillance in high-risk patients is also essential. Closer monitoring through wound cultures or inflammatory markers can facilitate prompt detection and treatment of infections.
Orthopedic surgery can utilize microbial risk management protocols already present in other surgical fields. When considering solid organ transplant programs, there is a rigorous infection screening of donors and recipients as part of preoperative workup. This is attributed to checking for pathogens and colonization with multidrug-resistant organisms. Within cardiothoracic surgery, patients are often screened for S. aureus carriage prior to procedures such as the implantation of cardiac devices to reduce the risk of endocarditis and deep sternal wounds [61]. Colorectal surgery protocols also highlight the vital role of the microbiome as colorectal surgeons perform preoperative bowel decontamination to reduce intraoperative contamination. Across transplant, cardiac, and colorectal domains, microbiological screening and measures have been conducted to reduce infection risks, and orthopedic surgery can also benefit from a similar approach [62].
The growing interest in microbial testing in orthopedic surgery reflects a broader movement across medicine to establish formal guidelines for perioperative microbiome management. In recent years, leading professional societies in infectious disease and surgery have issued recommendations addressing the Staphylococcus aureus screening, antibiotic stewardship, and the management of patients colonized with multidrug-resistant organisms. For example, updated guidelines from the American Society of Health-System Pharmacists (ASHP) recommend adding vancomycin prophylaxis for patients known to carry MRSA or those deemed high-risk. Similarly, the International Consensus Group on Orthopedic Infections reached 85% agreement that nasal screening and decolonization should be standard practice to reduce periprosthetic joint infections (PJIs), an approach already adopted in many hospital protocols [63].
Research efforts are also underway to develop tools such as a microbiome risk index for use in preoperative assessments, and pilot programs are evaluating the integration of gut microbiome analysis into enhanced recovery pathways. As these initiatives evolve, more detailed guidelines tailored specifically to orthopedic surgery will likely emerge. A future framework could include mandatory S. aureus screening and decolonization for implant-based procedures, targeted prophylaxis for patients colonized with resistant organisms, and microbiome-based optimization strategies in high-risk cases. Although comprehensive guidelines are still in development, orthopedic surgery is shifting toward a preventive, microbiome-informed model. Implementing standardized microbial screening and surveillance now positions orthopedic teams to improve surgical outcomes and align with evolving clinical best practices [64].
Although formal microbiome protocols in orthopedic surgery are still emerging, the field is steadily moving toward a microbiome-conscious model. Incorporating standardized screening and surveillance practices positions orthopedic teams to improve surgical outcomes. To place this shift in a broader context, Table 5 draws on examples from general surgery, where microbiome-driven strategies have already shown clinical benefit. These cross-disciplinary insights reinforce the relevance of microbiome management beyond orthopedics and offer a practical framework for guiding future protocol development.

Table 5.
Impact of Microbiome Composition on Surgical Outcomes in Orthopedic and General Surgery.
4.2. Study Strengths and Limitations
This review emphasizes the relevance of the gut microbiome in orthopedic surgical outcomes and examines its role in postoperative recovery, infection risk, and inflammatory responses. The synthesis of current literature indicates that shifts in microbial composition may influence surgical success. Tailored probiotic interventions may affect microbiome profiles, and predictive markers could help assess the risk of surgical complications. Preoperative optimization protocols may also be used to reduce the risk of adverse outcomes. Although the field is still maturing, the evidence points toward a future where microbiome-informed strategies may help reduce complications and enhance surgical recovery.
A strength of this review is its interdisciplinary approach by drawing from both surgical literature and microbiome research to identify patterns that may be overlooked when the two are studied in isolation. This study offers a foundation for hypothesis generation and future clinical investigation. It also highlights underexplored areas. such as cognitive dysfunction and the inflammatory cascade, where microbiome-targeted strategies hold promise.
Despite its strengths, this study faces limitations that reflect the nature of systematic reviews. Much of the available literature consists of observational studies, case series, and retrospective cohort analyses, which introduce potential biases and limit the ability to draw firm causal relationships. Many of the studies reviewed did not include large patient populations or randomized controlled trials (RCTs), which are the gold standard for clinical research. In addition, publication bias is also a concern in any systematic review, especially in fields where negative studies may remain unpublished.
The literature also demonstrates considerable methodological heterogeneity. Differences in sequencing platforms, sample processing, taxonomic classification, and reference databases may make direct comparisons between studies difficult. The lack of standardization makes it challenging to determine which findings are clinically meaningful. Furthermore, the association between microbiome composition and surgical outcomes may be confounded by the wide variations in patient populations. Some studies focus on young, relatively healthy individuals undergoing elective procedures, while others include older patients with pre-existing dysbiosis due to chronic illness or diet.
The timing of microbiome assessments also represents a limitation. Most studies assess the gut microbiota at a single time point—often preoperatively or within a short postoperative window. However, the microbiome is highly dynamic and can undergo significant shifts due to perioperative factors such as anesthesia, antibiotic administration, dietary changes, and hospitalization. Longitudinal studies tracking microbiome composition across multiple perioperative time points are necessary to better understand its role in recovery and complications. This is especially important as the literature on microbiome-targeted interventions remains in its early stages. Similarly, very few studies have tested direct interventions, such as the administration of probiotics, in orthopedic populations. Although these interventions are well-explored in fields like gastroenterology, their translation to surgical care remains limited.
4.3. Future Directions
Advancing microbiome research in orthopedic surgery will require a shift from observational associations to well-designed clinical trials. Most existing studies are descriptive or retrospective, and this limits their ability to guide practice. Further work should prioritize randomized controlled trials evaluating direct microbiome targeted interventions, such as preoperative probiotics or dietary modification. These trials should also consider the patient subgroups more likely to benefit, including those with high antibiotic exposure or baseline dysbiosis.
More rigorous and standardized methodologies should also be adopted. Current studies assess microbiome composition at a single perioperative time point. This fails to capture the dynamic changes that occur in response to surgery, anesthesia, antibiotics, and hospitalization. Longitudinal designs that track microbial shifts from baseline through recovery are essential to understanding causality. Methodological inconsistencies, such as variation in sequencing platforms, sample processing, and taxonomic pipelines, continue to limit comparability across studies. Establishing consensus protocols for sample collection, analysis, and interpretation will be critical for ensuring reproducibility and advancing clinical integration.
Research should ultimately account for patient-level variability that influences both microbiome composition and response to intervention. Age, comorbidities, nutrition, and prior antibiotic use all affect microbial resilience. Stratified study designs can help clarify who benefits most and under what conditions. With interdisciplinary collaboration and continued advances in sequencing and computational tools, the microbiome may emerge as a modifiable factor in surgical optimization, helping to bridge basic science and clinical care in meaningful and measurable ways.
5. Conclusions
Gut microbiota has influence in orthopedic surgical outcomes and is worth further exploration for possible interventions to improve care. The gut microbiota is one of the critical dynamic variables in the outcomes of orthopedic surgeries. However, microbial composition influences not only inflammation and healing but also recovery trajectories. Targeted modulation is essential in understanding the potential utility of interventions, especially probiotics, in mitigating surgical risk and improving recovery. With the evolution of orthopedic surgery, microbiological insights into the perioperative care of patients may allow personalized and precision medicine approaches aimed at optimizing patient recovery and minimizing surgical complications.
Author Contributions
Conceptualization, A.N.-T., S.K., M.M. and C.F.; methodology, A.N.-T.; formal analysis, A.N.-T.; investigation, A.N.-T., S.K. and C.F.; data curation, A.N.-T.; writing—original draft preparation, A.N.-T.—abstract, introduction, microbiome–bone axis, perioperative considerations, microbial dysbiosis in orthopedic surgery, immune-microbiota interactions, probiotics and prebiotics in surgical recovery, antimicrobial stewardship, limitations, implications for orthopedic surgeons, and future directions; writing—review and editing, S.K.—Vitamin D and the microbiome, contribution to limitations and future directions, refinement of abstract, citation integration, assistance with figure and table layout; M.M.—minor revisions to introduction and probiotics sections, proofreading, reference formatting, and terminology consistency; visualization, A.N.-T. (Figure 1, Figure 2, Figure 3 and Figure 4; Table 1, Table 2, Table 3 and Table 4), M.M. (Table 5); supervision, C.F., S.M. and K.N.; project administration, C.F., S.M. and K.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Anwar, H.; Iftikhar, A.; Muzaffar, H.; Almatroudi, A.; Allemailem, K.S.; Navaid, S.; Saleem, S.; Khurshid, M. Biodiversity of Gut Microbiota: Impact of Various Host and Environmental Factors. BioMed Res. Int. 2021, 2021, 5575245. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, G.L.; Andrews, J.M.; Weyrich, L.S. The Neglected Gut Microbiome: Fungi, Protozoa, and Bacteriophages in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2022, 28, 1112–1122. [Google Scholar] [CrossRef]
- Mani, I.; Singh, V. Multi-omics in Gut Microbiome. In Multi-Omics Analysis of the Human Microbiome; ResearchGate: Berlin, Germany, 2024. [Google Scholar] [CrossRef]
- Pant, A.; Maiti, T.K.; Mahajan, D.; Das, B. Human Gut Microbiota and Drug Metabolism. Microb. Ecol. 2023, 86, 97–111. [Google Scholar] [CrossRef]
- Khan, I.; Bai, Y.; Zha, L.; Ullah, N.; Ullah, H.; Shah, S.R.H.; Sun, H.; Zhang, C. Mechanism of the gut microbiota colonization resistance and enteric pathogen infection. Front. Cell. Infect. Microbiol. 2021, 11, 716299. [Google Scholar] [CrossRef]
- Fakhoury, H.M.A.; Kvietys, P.R.; AlKattan, W.; Anouti, F.A.; Elahi, M.A.; Karras, S.N.; Grant, W.B. Vitamin D and intestinal homeostasis: Barrier, microbiota, and immune modulation. J. Steroid Biochem. Mol. Biol. 2020, 200, 105663. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, C.; Perillo, F.; Strati, F.; Fantini, M.; Caprioli, F.; Facciotti, F. The Role of Gut Microbiota Biomodulators on Mucosal Immunity and Intestinal Inflammation. Cells 2020, 9, 1234. [Google Scholar] [CrossRef] [PubMed]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 356589. [Google Scholar] [CrossRef]
- Parizadeh, M.; Arrieta, M.C. The global human gut microbiome: Genes, lifestyles, and diet. Trends Mol. Med. 2023, 29, 789–801. [Google Scholar] [CrossRef]
- Seely, K.D.; Kotelko, C.A.; Douglas, H.; Bealer, B.; Brooks, A.E. The Human Gut Microbiota: A Key Mediator of Osteoporosis and Osteogenesis. Int. J. Mol. Sci. 2021, 22, 9452. [Google Scholar] [CrossRef]
- Vernocchi, P.; Del Chierico, F.; Putignani, L. Gut Microbiota Metabolism and Interaction with Food Components. Int. J. Mol. Sci. 2020, 21, 3688. [Google Scholar] [CrossRef]
- Wiertsema, S.P.; van Bergenhenegouwen, J.; Garssen, J.; Knippels, L.M.J. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients 2021, 13, 886. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, S.B.; Essa, M.M.; Rathipriya, A.G.; Bishir, M.; Ray, B.; Mahalakshmi, A.M.; Tousif, A.H.; Sakharkar, M.K.; Kashyap, R.S.; Friedland, R.P.; et al. Gut dysbiosis, defective autophagy and altered immune responses in neurodegenerative diseases: Tales of a vicious cycle. Pharmacol. Ther. 2022, 231, 107988. [Google Scholar] [CrossRef]
- Jones, R.M.; Mulle, J.G.; Pacifici, R. Osteomicrobiology: The influence of gut microbiota on bone in health and disease. Bone 2018, 115, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, A.; Saeedi, P.; Gérard, P.; Jalalvandi, E.; Cannella, D.; Bekhit, A.E.D. The role of microbiota in tissue repair and regeneration. J. Tissue Eng. Regen. Med. 2020, 14, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Park, H. Inflammation and Impaired Gut Physiology in Post-operative Ileus: Mechanisms and the Treatment Options. J. Neurogastroenterol. Motil. 2022, 28, 517–530. [Google Scholar] [CrossRef]
- Mustansir Dawoodbhoy, F.; Patel, B.K.; Patel, K.; Bhatia, M.; Lee, C.N.; Moochhala, S.M. Gut Microbiota Dysbiosis as a Target for Improved Post-Surgical Outcomes and Improved Patient Care: A Review of Current Literature. Shock 2021, 55, 441–454. [Google Scholar] [CrossRef]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef]
- Koliarakis, I.; Athanasakis, E.; Sgantzos, M.; Mariolis-Sapsakos, T.; Xynos, E.; Chrysos, E.; Souglakos, J.; Tsiaoussis, J. Intestinal microbiota in colorectal cancer surgery. Cancers 2020, 12, 3011. [Google Scholar] [CrossRef]
- Stavrou, G.; Kotzampassi, K. Gut microbiome, surgical complications and probiotics. Ann. Gastroenterol. 2017, 30, 45–53. [Google Scholar] [CrossRef]
- Long, D.R.; Alverdy, J.C.; Vavilala, M.S. Emerging Paradigms in the Prevention of Surgical Site Infection: The Patient Microbiome and Antimicrobial Resistance. Anesthesiology 2022, 137, 252–262. [Google Scholar] [CrossRef]
- Buis, N.; Esfandiari, H.; Hoch, A.; Fürnstahl, P. Overview of Methods to Quantify Invasiveness of Surgical Approaches in Orthopedic Surgery—A Scoping Review. Front. Surg. 2022, 8, 771275. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Yan, Z.; Ji, S.; Xiao, S.; Gao, J. Metal nanoparticle hybrid hydrogels: The state-of-the-art of combining hard and soft materials to promote wound healing. Theranostics 2024, 14, 1534–1560. [Google Scholar] [CrossRef] [PubMed]
- Lederer, A.K.; Chikhladze, S.; Kohnert, E.; Huber, R.; Müller, A. Current Insights: The Impact of Gut Microbiota on Postoperative Complications in Visceral Surgery—A Narrative Review. Diagnostics 2021, 11, 2099. [Google Scholar] [CrossRef]
- Porcari, S.; Mullish, B.H.; Asnicar, F.; Ng, S.C.; Zhao, L.; Hansen, R.; O’Toole, P.W.; Raes, J.; Hold, G.; Putignani, L.; et al. International consensus statement on microbiome testing in clinical practice. Lancet Gastroenterol. Hepatol. 2025, 10, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Clarke, V.; Braun, V. Thematic analysis. J. Posit. Psychol. 2017, 12, 297–298. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, Y.; Xu, X.; Chen, S.; Zhang, S.; Jiang, N.; Liu, Z.; Zhang, J.; Luo, Z.; Zhang, X.; et al. Improvement of post-surgery constipation in patients with fractures by Lactobacillus rhamnosus JYLR-127: A single-blind randomized controlled trial. Nutrients 2024, 16, 1505. [Google Scholar] [CrossRef]
- Valido, E.; Capossela, S.; Glisic, M.; Hertig-Godeschalk, A.; Bertolo, A.; Stucki, G.; Flueck, J.L.; Stoyanov, J. Gut microbiome and inflammation among athletes in wheelchair in a crossover randomized pilot trial of probiotic and prebiotic interventions. Sci. Rep. 2024, 14, 12838. [Google Scholar] [CrossRef]
- Lecomte, M.; Tomassi, D.; Rizzoli, R.; Tenon, M.; Berton, T.; Harney, S.; Fança-Berthon, P. Effect of a hop extract standardized in 8-prenylnaringenin on bone health and gut microbiome in postmenopausal women with osteopenia: A one-year randomized, double-blind, placebo-controlled trial. Nutrients 2023, 15, 2688. [Google Scholar] [CrossRef]
- Aboushaala, K.; Chee, A.V.; Adnan, D.; Toro, S.J.; Singh, H.; Savoia, A.; Dhillon, E.S.; Yuh, C.; Dourdourekas, J.; Patel, I.K.; et al. Gut microbiome dysbiosis is associated with lumbar degenerative spondylolisthesis in symptomatic patients. JOR Spine 2024, 7, e70005. [Google Scholar] [CrossRef] [PubMed]
- Chisari, E.; Cho, J.; Wouthuyzen-Bakker, M.; Parvizi, J. Gut permeability may be associated with periprosthetic joint infection after total hip and knee arthroplasty. Sci. Rep. 2022, 12, 15094. [Google Scholar] [CrossRef] [PubMed]
- Ganta, A.; Tong, Y.; Boadi, B.I.; Konda, S.R.; Egol, K.A. Microbiome of infected fracture nonunion: Does it affect outcomes? J. Orthop. Sci. 2024, 29, 1270–1273. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Zhang, W.; Zhang, S.; Chen, J.; He, K.; Zhang, C.; Yuan, X.; Xie, B. The gut microbiota and metabolite profiles are altered in patients with spinal cord injury. Mol. Brain 2023, 16, 26. [Google Scholar] [CrossRef]
- Li, H.; Fu, J.; Erlong, N.; Li, R.; Xu, C.; Hao, L.; Chen, J.; Chai, W. Characterization of periprosthetic environment microbiome in patients after total joint arthroplasty and its potential correlation with inflammation. BMC Infect. Dis. 2023, 23, 423. [Google Scholar] [CrossRef]
- Liu, F.; Duan, M.; Fu, H.; Zhao, G.; Han, Y.; Lan, F.; Ahmed, Z.; Cao, G.; Li, Z.; Ma, D.; et al. Orthopedic Surgery Causes Gut Microbiome Dysbiosis and Intestinal Barrier Dysfunction in Prodromal Alzheimer Disease Patients: A Prospective Observational Cohort Study. Ann. Surg. 2022, 276, 270–280. [Google Scholar] [CrossRef]
- Cyphert, E.L.; Clare, S.; Dash, A.; Nixon, J.C.; Raphael, J.; Harrison, J.; Heilbronner, A.; Kim, H.J.; Cunningham, M.; Lebl, D.; et al. A pilot study of the gut microbiota in spine fusion surgery patients. HSS J. 2025, 21, 65–72. [Google Scholar] [CrossRef]
- Lin, H.; Liu, T.; Li, X.; Gao, X.; Wu, T.; Li, P. The role of gut microbiota metabolite trimethylamine N-oxide in functional impairment of bone marrow mesenchymal stem cells in osteoporosis disease. Ann. Transl. Med. 2020, 8, 1009. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.; Xu, Y.; Ling, C.; Tang, Z.; Kiram, A.; Hu, Z.; Zhu, Z.; Qiu, Y.; Liu, Z. Gut microbiota alterations in adolescent idiopathic scoliosis are associated with aberrant bone homeostasis. Orthop. Surg. 2024, 16, 965–975. [Google Scholar] [CrossRef]
- Baek, Y.J.; Lee, Y.J.; Lee, J.A.; Kim, S.Y.; Park, J.H.; Choi, H.J.; Lim, J.Y.; Han, S.H.; Park, Y.S.; Kim, Y.H.; et al. Role of α-defensin and the microbiome in prosthetic joint infection: A prospective cohort study in Korea. J. Clin. Med. 2023, 12, 5964. [Google Scholar] [CrossRef]
- Das, M.; Cronin, O.; Keohane, D.M.; Cormac, E.M.; Nugent, H.; Nugent, M.; Molloy, C.; O’Toole, P.W.; Shanahan, F.; Molloy, M.G.; et al. Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology 2019, 58, 2295–2304. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, N.; Zhang, P.; Wu, W.; Fu, L. Fecal microbiota transplantation mitigates bone loss by improving gut microbiome composition and gut barrier function in aged rats. PeerJ 2021, 9, e12293. [Google Scholar] [CrossRef]
- Duan, M.; Liu, F.; Fu, H.; Lu, S.; Wang, T. Preoperative Microbiomes and Intestinal Barrier Function Can Differentiate Prodromal Alzheimer’s Disease From Normal Neurocognition in Elderly Patients Scheduled to Undergo Orthopedic Surgery. Front. Cell. Infect. Microbiol. 2021, 11, 592842. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Xu, Y.; Li, S.; Zhan, G.; Hua, D.; Tan, J.; Chi, X.; Xiang, H.; Guo, F.; Luo, A. Contribution of preoperative gut microbiota in postoperative neurocognitive dysfunction in elderly patients undergoing orthopedic surgery. Front. Aging Neurosci. 2023, 15, 1108205. [Google Scholar] [CrossRef] [PubMed]
- Roselló-Añón, A.; Chiappe, C.; Valverde-Vázquez, M.R.; Sangüesa-Nebot, M.J.; Gómez-Cabrera, M.C.; Pérez-Martínez, G.; Doménech-Fernández, J. Pilot study to determine the association between gut microbiota and fragility hip fracture. Evista Esp. Cir. Ortop. Traumatol. 2023, 67, 279–289. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Zhang, C.; Liu, Z.; Li, C.; Ren, Z. Gut Microbiota and Bone Diseases: A Growing Partnership. Front. Microbiol. 2022, 13, 877776. [Google Scholar] [CrossRef]
- Morales, F.; Montserrat-de la Paz, S.; Leon, M.J.; Rivero-Pino, F. Effects of Malnutrition on the Immune System and Infection and the Role of Nutritional Strategies Regarding Improvements in Children’s Health Status: A Literature Review. Nutrients 2024, 16, 1. [Google Scholar] [CrossRef]
- L Bishop, E.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. JBMR Plus 2020, 5, e10405. [Google Scholar] [CrossRef]
- Moise, A.; Balescu-Arion, C. Vitamin D and the Immune System. When? Why? How? Cent. Eur. Ann. Clin. Res. 2020, 2, 1. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol. Nutr. Food Res. 2011, 55, 96–108. [Google Scholar] [CrossRef]
- Yan, H.; Chen, Y.; Zhu, H.; Huang, W.H.; Cai, X.H.; Li, D.; Lv, Y.J.; Zhao, -S.; Zhou, H.H.; Luo, F.Y.; et al. The relationship among intestinal bacteria, vitamin K and response of vitamin K antagonist: A review of evidence and potential mechanism. Front. Med. 2022, 9, 829304. [Google Scholar] [CrossRef]
- Conly, J.M.; Stein, K.; Worobetz, L.; Rutledge-Harding, S. The contribution of vitamin K2 (menaquinones) produced by the intestinal microflora to human nutritional requirements for vitamin K. Am. J. Gastroenterol. 1994, 89, 915–923. [Google Scholar]
- Shearer, M.J. Role of vitamin K and Gla proteins in the pathophysiology of osteoporosis and vascular calcification. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 433–438. [Google Scholar] [CrossRef]
- Merra, G.; Dominici, F.; Gualtieri, P.; Capacci, A.; Cenname, G.; Esposito, E.; Dri, M.; Di Renzo, L.; Marchetti, M. Role of vitamin K2 in bone-vascular crosstalk. Int. J. Vitam. Nutr. Res. 2024, 94, 143–152. [Google Scholar] [CrossRef]
- Weber, P. Vitamin K and bone health. Nutrition 2001, 17, 880–887. [Google Scholar] [CrossRef]
- Luna, M.; Guss, J.D.; Vasquez-Bolanos, L.S.; Castaneda, M.; Rojas, M.V.; Strong, J.M.; Alabi, D.A.; Dornevil, S.D.; Nixon, J.C.; Taylor, E.A.; et al. Components of the gut microbiome that influence bone tissue-level strength. J. Bone Miner. Res. 2021, 36, 1823–1834. [Google Scholar] [CrossRef]
- George, S.; Leasure, A.R.; Horstmanshof, D. Effectiveness of Decolonization With Chlorhexidine and Mupirocin in Reducing Surgical Site Infections: A Systematic Review. Dimens. Crit. Care Nurs. 2016, 35, 204–222. [Google Scholar] [CrossRef]
- Zukowska, A.; Zukowski, M. Surgical Site Infection in Cardiac Surgery. J. Clin. Med. 2022, 11, 6991. [Google Scholar] [CrossRef]
- Wei, X.; Xing, F.; Xu, Y.; Zhang, F.; Cheng, D.; Zhou, Y.; Zheng, F.; Zhao, J.; Wang, Y.; Chen, L.; et al. Preoperative gut microbiota of POCD patients induces pre- and postoperative cognitive impairment and systemic inflammation in rats. J. Neuroinflamm. 2024, 21, 221. [Google Scholar] [CrossRef]
- Weaver, L.; Troester, A.; Jahansouz, C. The Impact of Surgical Bowel Preparation on the Microbiome in Colon and Rectal Surgery. Antibiotics 2024, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D.; et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am. J. Health Syst. Pharm. 2013, 70, 195–283. [Google Scholar] [CrossRef] [PubMed]
- Ontario Health (Quality). Pre-surgical Nasal Decolonization of Staphylococcus aureus: A Health Technology Assessment. Ont. Health Technol. Assess. Ser. 2022, 22, 1–165. [Google Scholar]
- Guerrero, M.A.; Anderson, B.; Carr, G.; Snyder, K.L.; Boyle, P.; Ugwu, S.A.; Davis, M.; Bohnenkamp, S.K.; Nfonsam, V.; Riall, T.S. Adherence to a standardized infection reduction bundle decreases surgical site infections after colon surgery: A retrospective cohort study on 526 patients. Patient Saf. Surg. 2021, 15, 15. [Google Scholar] [CrossRef]
- Song, D.W.; Park, B.K.; Suh, S.W.; Lee, S.E.; Kim, J.W.; Park, J.M.; Kim, H.R.; Lee, M.K.; Choi, Y.S.; Kim, B.G.; et al. Bacterial culture and antibiotic susceptibility in patients with acute appendicitis. Int. J. Colorectal Dis. 2018, 33, 441–447. [Google Scholar] [CrossRef]
- Effenberger, M.; Al-Zoairy, R.; Gstir, R.; Graziadei, I.; Schwaighofer, H.; Tilg, H.; Zoller, H. Transmission of oral microbiota to the biliary tract during endoscopic retrograde cholangiography. BMC Gastroenterol. 2023, 23, 103. [Google Scholar] [CrossRef]
- Khoury, F.; Pezzone, M.; Aijazi, M.; Fons, I.; Araujo, D.; Kondaveeti, B.; Ahuja, A.; Yassin, M. Gastrointestinal endoscopy 30-day-associated bacteremia: Nonoutbreak 5-year review in an inner-city, tertiary-care hospital. Am. J. Infect. Control. 2024, 52, 1166–1169. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).