Soybean-Bupleurum Rotation System Can Optimize Rhizosphere Soil Microbial Community via Impacting Soil Properties and Enzyme Activities During Bupleurum Seedling Stage
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Soil Samples Collection and Analysis
2.4. High-Throughput Sequencing and Sequence Analysis
2.5. Statistical Analysis
3. Results
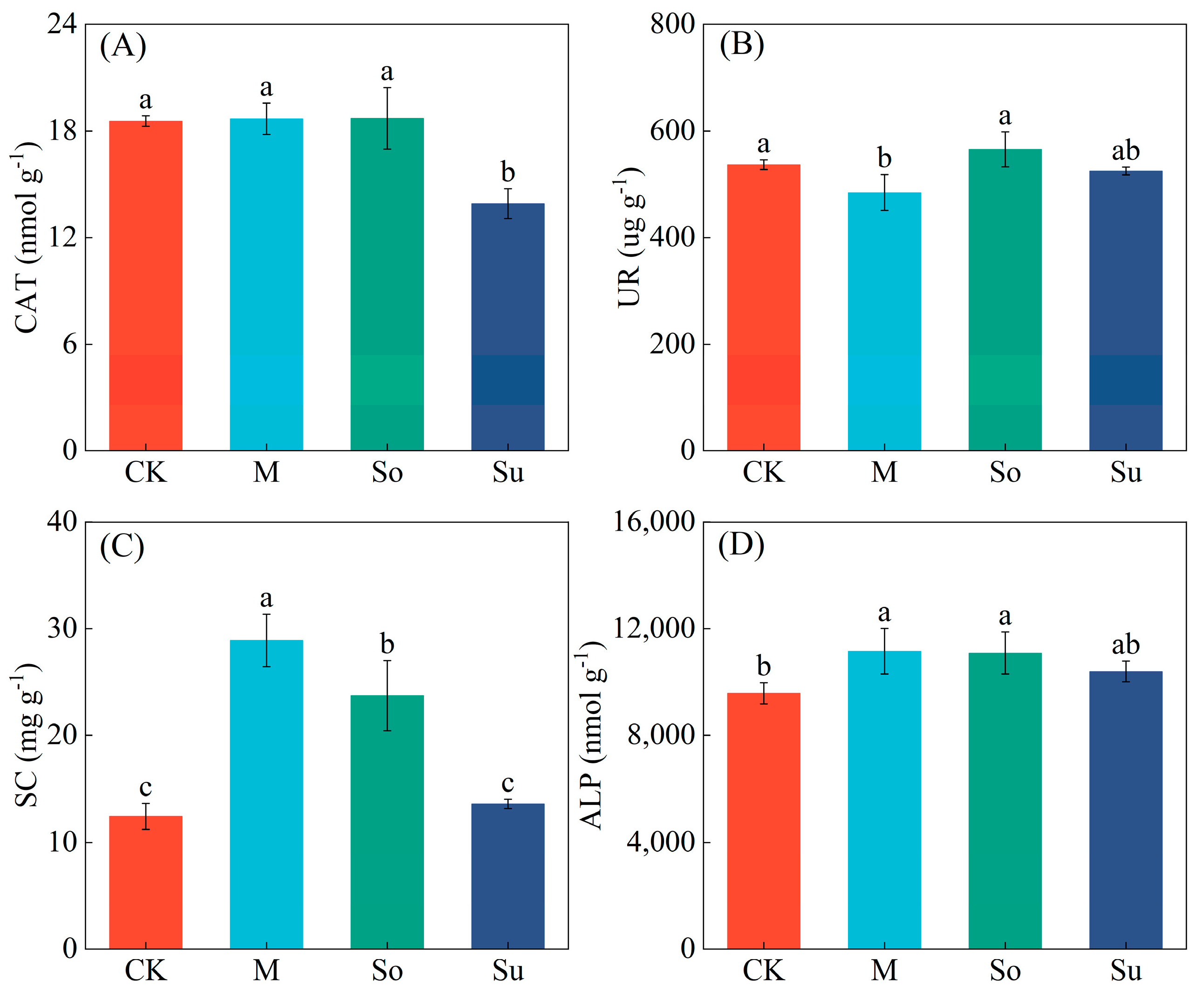
3.1. Soil Properties and Enzyme Activities
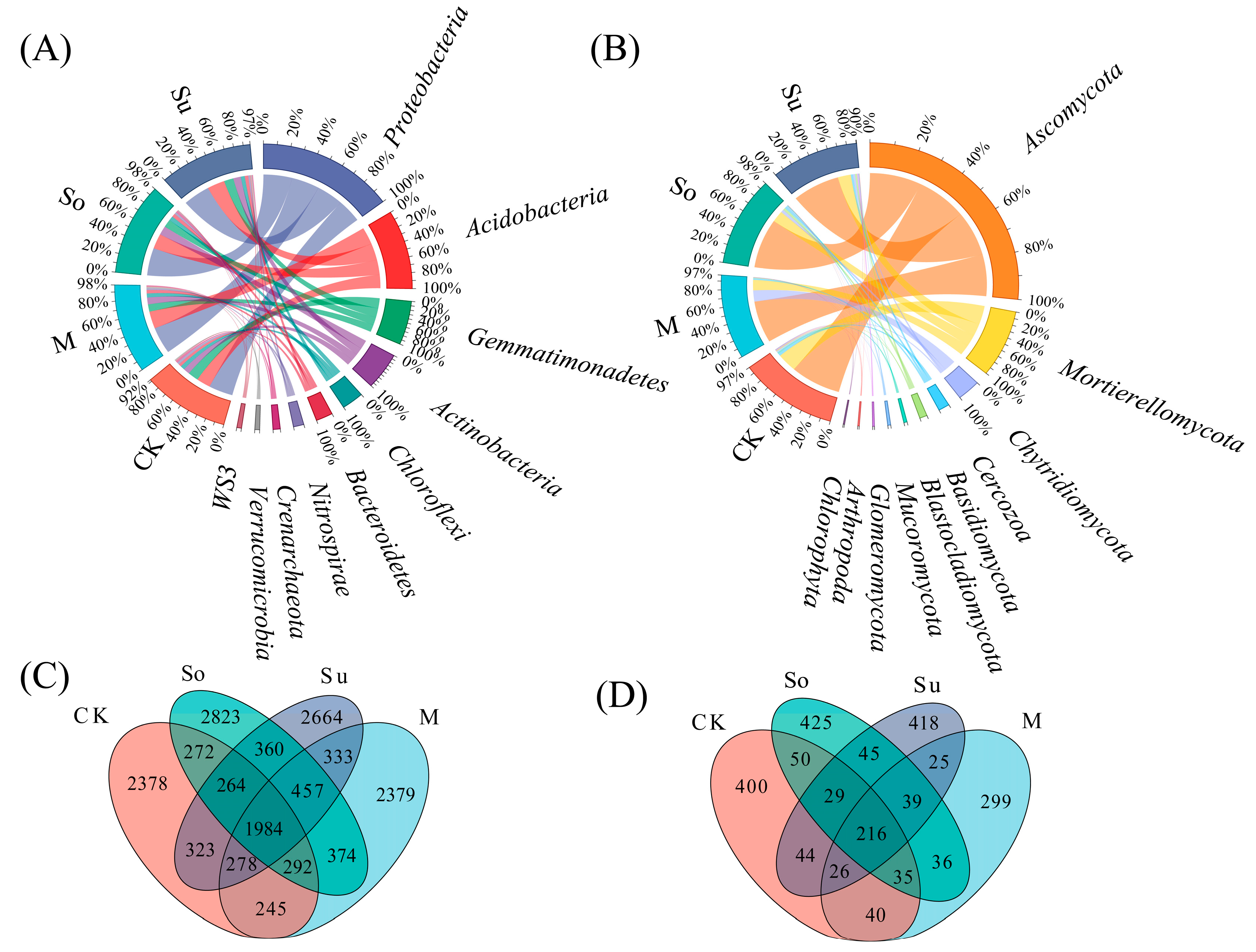
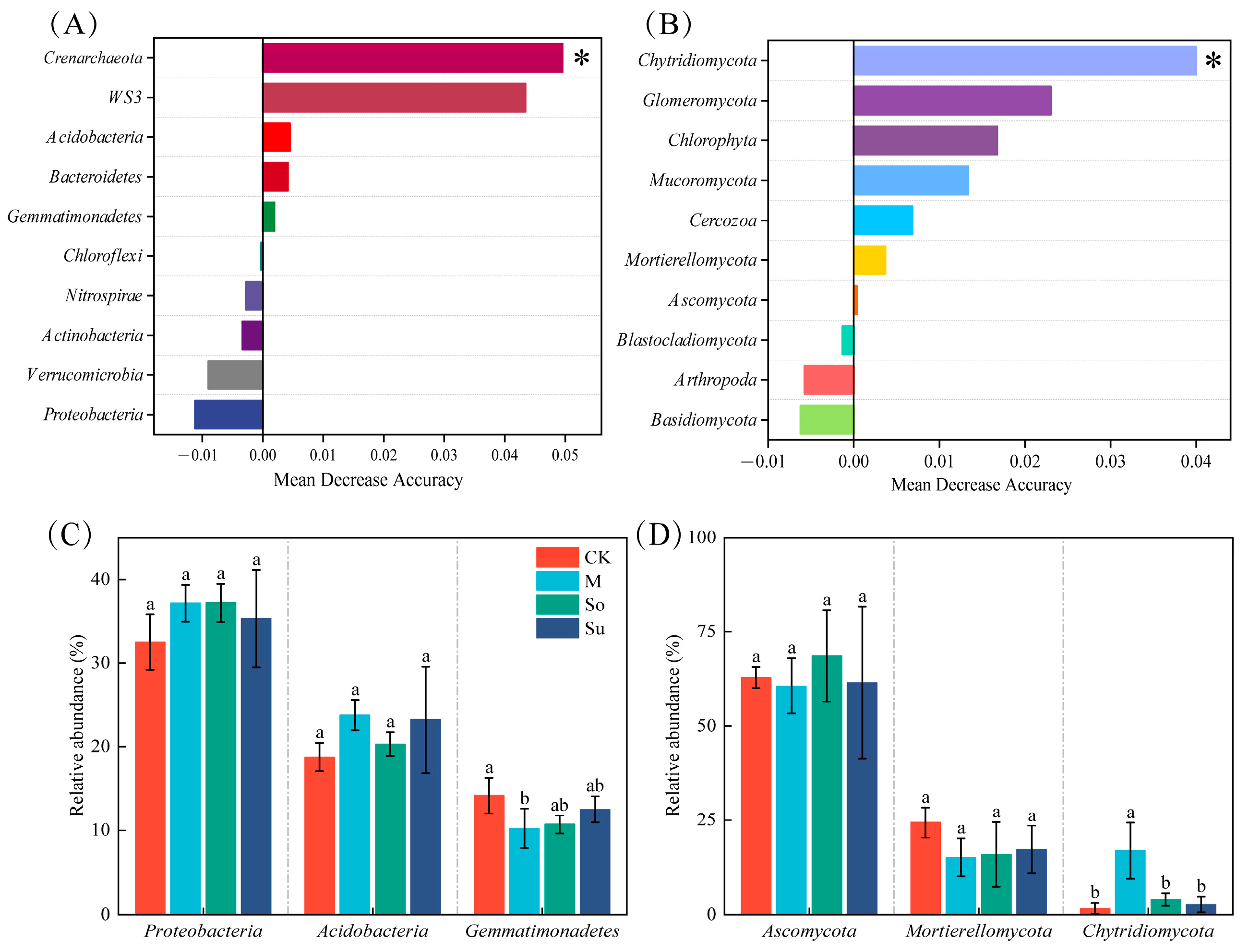
3.2. Soil Microbial Community
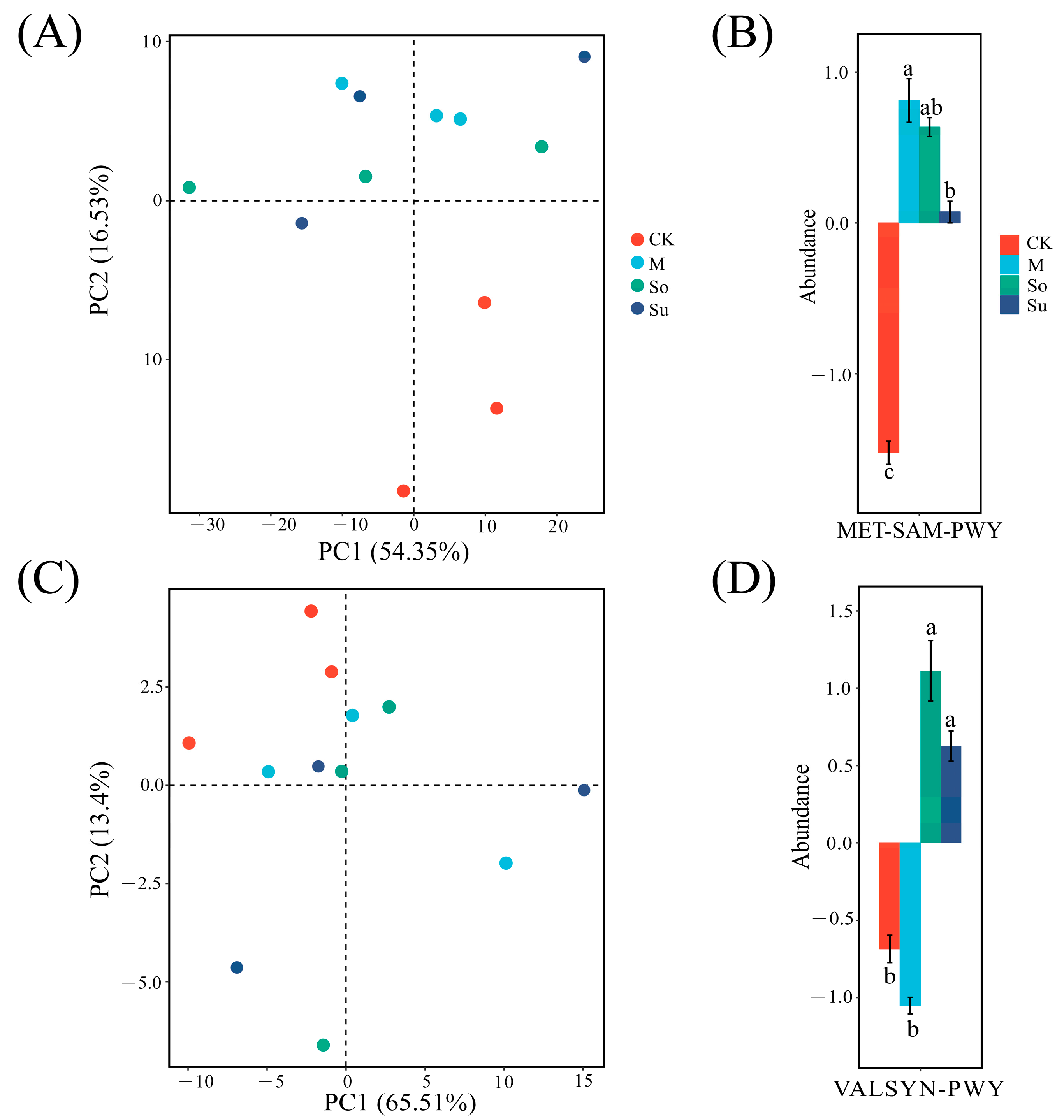
3.3. Soil Microbial Alpha and Beta Diversity
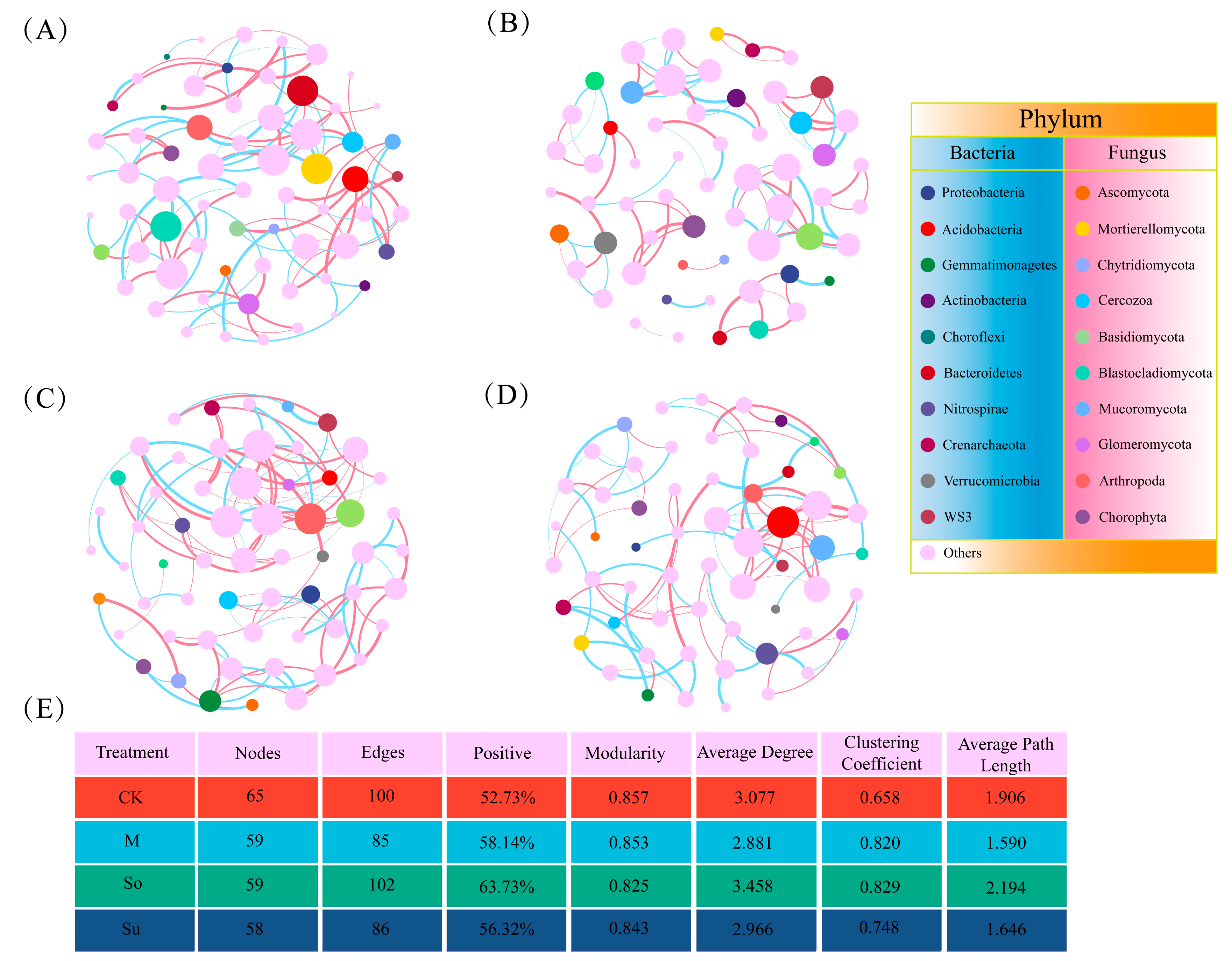
3.4. Co-Occurrences Network Among Microbial Communities
3.5. Metabolic Pathways in Microbial Function Prediction
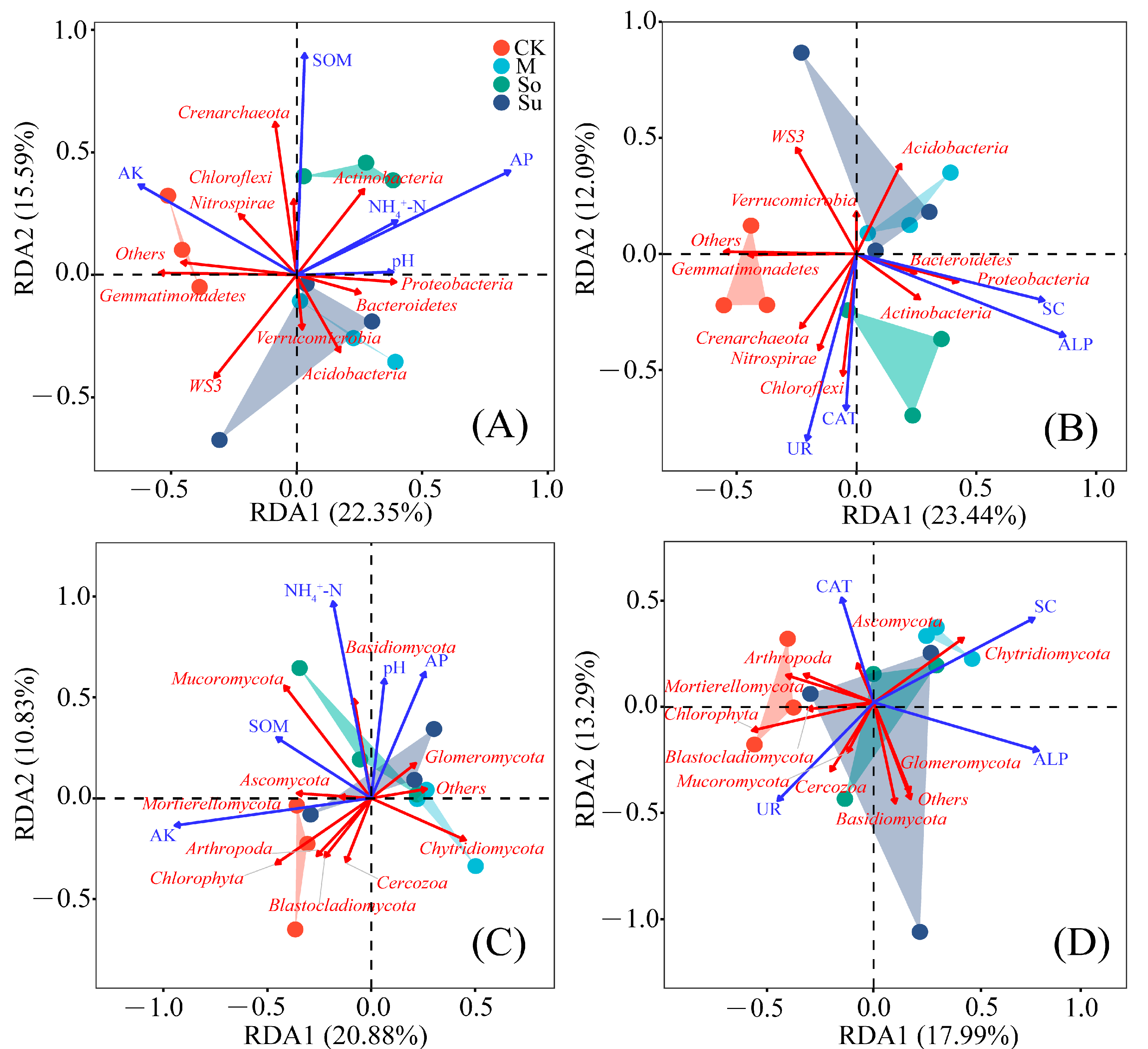
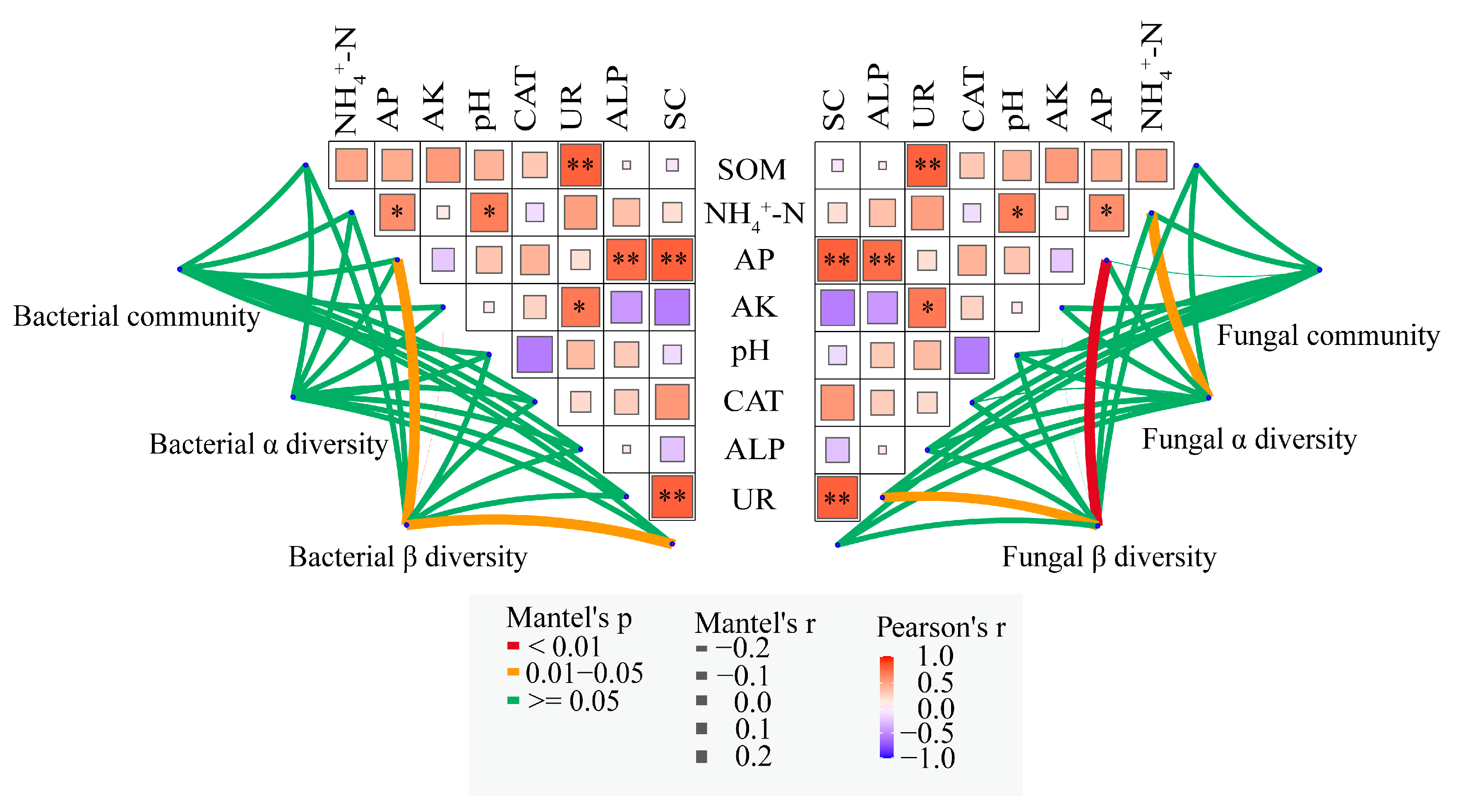
3.6. Correlation Analysis of Soil Properties, Enzyme Activities, and Soil Microbial Characteristics
4. Discussion
4.1. Effects of Different Crop Rotation Patterns on Soil Properties and Enzyme Activities in the Rhizosphere of Bupleurum Seedlings
4.2. Effects of Different Crop Rotation Patterns on the Composition and Diversity of Soil Microbial Communities in the Rhizosphere of Bupleurum Seedlings
4.3. Effects of Different Crop Rotation Patterns on Networks’ Symbiotic Relationships and Functional Prediction of Soil Microbial Community in the Rhizosphere of Bupleurum Seedlings
4.4. Correlation Analysis Between Soil Microorganisms, Soil Properties, and Enzyme Activities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, F.; Dong, X.; Yin, X.; Wang, W.; You, L.; Ni, J. Radix Bupleuri: A review of traditional uses, botany, phytochemistry, pharmacology, and toxicology. Biomed. Res. Int. 2017, 1, 7597596. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, M.; Lam, P.-Y.; Dini-Andreote, F.; Dai, L.; Wei, Z. Multifaceted roles of flavonoids mediating plant-microbe interactions. Microbiome 2022, 10, 233. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Qu, Z.; Wang, H.; Cheng, Y.; Dong, S.; Zhao, H. Comparative proteomic analysis reveals novel insights into the continuous cropping induced response in Scrophularia ningpoensis. J. Sci. Food Agric. 2023, 103, 1832–1845. [Google Scholar] [CrossRef]
- Yu, T.; Hou, X.; Fang, X.; Razavi, B.; Zang, H.; Zeng, Z.; Yang, Y. Short-term continuous monocropping reduces peanut yield mainly via altering soil enzyme activity and fungal community. Environ. Res. 2024, 245, 117977. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Z.; Niu, J.; Dang, K.; Zhang, S.; Wang, S.; Wang, Z. Changes in physicochemical properties, enzymatic activities, and the microbial community of soil significantly influence the continuous cropping of Panax quinquefolius L. (American ginseng). Plant Soil 2021, 463, 427–446. [Google Scholar] [CrossRef]
- He, S.; Lv, M.; Wang, R.; Li, N.; Wang, T.; Shi, W.; Gao, Z.; Li, X. Long-term garlic—maize rotation maintains the stable garlic rhizosphere microecology. Environ. Microbiome 2024, 19, 90. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, R.; Wang, Z.; Feng, Y.; Xue, K.; Liu, K.; Xue, Z.; Cao, W.; Fu, L.; Yin, M. Effects of rotation with a green manure crop on soil quality and microbial nutrient limitation in a tobacco field in Yunnan. Acta Pratacult. Sin. 2024, 33, 147–158. (In Chinese) [Google Scholar]
- Li, B.; Zhang, Q.; Chen, Y.; Su, Y.; Sun, S.; Chen, G. Different crop rotation systems change the rhizosphere bacterial community structure of Astragalus membranaceus (Fisch) Bge. var. mongholicus (Bge.) Hsiao. Appl. Soil Ecol. 2021, 166, 104003. [Google Scholar] [CrossRef]
- Qin, X.; Jiang, J.; Wei, Q.; Zhang, X.; Zhou, C.; Zhao, J.; Huang, L.; Guo, S.; Luo, S.; Ding, J. Effects of rotation and apply bioorganic fertilizer on soil microbial characteristics of pineapple under continuous cropping. Soil Fertil. Sci. China 2024, 12, 50–57. (In Chinese) [Google Scholar]
- Li, X.; Zhang, Y.; Tian, Z.; Wu, G. Difference analysis of soil nutrients, enzymatic activities and microbial community structure between eggplant continuous cropping and rotation. J. Zhejiang Univ. Agric. Life Sci. 2017, 43, 561–569. (in Chinese). [Google Scholar]
- Wang, F.; Zhang, X.; Wei, M.; Wang, Y.; Liang, Z.; Xia, P. Appropriate crop rotation alleviates continuous cropping barriers by changing rhizosphere microorganisms in Panax notoginseng. Rhizosphere 2022, 23, 100568. [Google Scholar] [CrossRef]
- Lu, J.; Liu, Y.; Zou, X.; Zhang, X.; Yu, X.; Wang, Y.; Si, T. Rotational strip peanut/cotton intercropping improves agricultural production through modulating plant growth, root exudates, and soil microbial communities. Agric. Ecosyst. Environ. 2024, 359, 108767. [Google Scholar] [CrossRef]
- Wang, X.; Chi, Y.; Song, S. Important soil microbiota’s effects on plants and soils: A comprehensive 30-year systematic literature review. Front. Microbiol. 2024, 15, 1347745. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, Z.; Liu, J.; Li, X.; Wang, X.; Dai, C.; Zhang, T.; Carrión, V.J.; Wei, Z.; Cao, F. Crop rotation and native microbiome inoculation restore soil capacity to suppress a root disease. Nat. Commun. 2023, 14, 8126. [Google Scholar] [CrossRef]
- Liu, L.; Ma, L.; Zhu, M.; Liu, B.; Liu, X.; Shi, Y. Rhizosphere microbial community assembly and association networks strongly differ based on vegetation type at a local environment scale. Front. Microbiol. 2023, 14, 1129471. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, Y.; Wang, W.; Wang, Q.; Wang, H.; Chu, F. Effects of Sweet Potato Rotation and Intercropping on the Microbial Community of Rhizosphere Soil. Crops 2021, 37, 153–159. (In Chinese) [Google Scholar]
- Wang, L.; Liu, S.; Tian, G.; Pan, Y.; Wang, H.; Qiu, G.; Li, F.; Pang, Z.; Ding, K.; Zhang, J. Impacts of continuous potato cropping on soil microbial assembly processes and spread of potato common scab. Appl. Soil Ecol. 2025, 206, 105805. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, H.; Wang, M.; Li, H.; Lu, A.; Wang, Y. Effects of rotation and continuous cropping on soil microflora and diversity in tobacco field. Soil Fertil. Sci. China 2018, 6, 84–90. (In Chinese) [Google Scholar]
- Liu, H.; Su, Y.; Fang, L.; Luo, L.; Wang, L.; Zhang, Z.; Zhu, S.; Yang, M. The effect and mechanism of fennel crop rotation on soil bacterial community to alleviate replant failure of Panax notoginseng. Chin. J. Biol. Control 2021, 37, 139–149. (In Chinese) [Google Scholar]
- Wang, Y.; Ji, H.; Wang, R.; Guo, S.; Gao, C. Impact of root diversity upon coupling between soil C and N accumulation and bacterial community dynamics and activity: Result of a 30 year rotation experiment. Geoderma 2017, 292, 87–95. [Google Scholar] [CrossRef]
- Kalembasa, S.J.; Jenkinson, D.S. A comparative study of titrimetric and gravimetric methods for the determination of organic carbon in soil. J. Sci. Food Agric. 1973, 24, 1085–1090. [Google Scholar] [CrossRef]
- Lu, R. Analysis Methods of Soil Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000; Volume 107, pp. 147–150. [Google Scholar]
- Jones, J. Soil Analysis Handbook of Reference Methods; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Guo, C.; Yang, C.; Fu, J.; Song, Y.; Chen, S.; Li, H.; Ma, C. Effects of crop rotation on sugar beet growth through improving soil physicochemical properties and microbiome. Ind. Crops Prod. 2024, 212, 118331. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Dong, Q.; Feng, H.; Siddique, K.H. Plastic mulching significantly improves soil enzyme and microbial activities without mitigating gaseous N emissions in winter wheat-summer maize rotations. Field Crops Res. 2022, 286, 108630. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Zhang, Y.; Fei, J.; Xiangmin, R.; Peng, J.; Luo, G. Crop rotation-driven changes in rhizosphere metabolite profiles regulate soil microbial diversity and functional capacity. Agric. Ecosyst. Environ. 2023, 358, 108716. [Google Scholar] [CrossRef]
- Lago-Olveira, S.; Ouhemi, H.; Idrissi, O.; Moreira, M.T.; González-García, S. Promoting more sustainable agriculture in the Moroccan drylands by shifting from conventional wheat monoculture to a rotation with chickpea and lentils. Clean. Environ. Syst. 2024, 12, 100169. [Google Scholar] [CrossRef]
- Dou, Y.; Yu, S.; Liu, S.; Cui, T.; Huang, R.; Wang, Y.; Wang, J.; Tan, K.; Li, X. Crop rotations reduce pathogenic fungi compared to continuous cropping. Rhizosphere 2025, 34, 101074. [Google Scholar] [CrossRef]
- Pituello, C.; Polese, R.; Morari, F.; Berti, A. Outcomes from a long-term study on crop residue effects on plant yield and nitrogen use efficiency in contrasting soils. Eur. J. Agron. 2016, 77, 179–187. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Wang, Y.; Guo, J.; Shao, R.; Wang, S.; Yang, Q. Metagenomic study on microbial function in increasing total nitrogen content in soil under crop rotation systems. Appl. Soil Ecol. 2025, 213, 106331. [Google Scholar] [CrossRef]
- Kalembasa, D.; Szukała, J.; Symanowicz, B.; Kalembasa, S.; Faligowska, A.; Becher, M. Amount of biologically nitrogen fixed by faba bean and its uptake by winter wheat determined by 15N ID method. Arch. Agron. Soil Sci. 2021, 67, 1875–1888. [Google Scholar]
- Li, R.; He, Z.; He, P.; Yang, R.; Ma, D.; Li, Y.; Xiang, Y.; Sun, W.; Zhu, X.; Zhang, Z. Crop diversification improves productivity and soil health by regulating fungal diversity. Soil Tillage Res. 2025, 252, 106628. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, M.; Zhang, R.; Zhang, W.; Liu, Y.; Sun, D.; Wang, X.; Qin, S.; Kang, Y. Legume-potato rotation affects soil physicochemical properties, enzyme activity, and rhizosphere metabolism in continuous potato cropping. Chem. Biol. Technol. Agric. 2023, 10, 132. [Google Scholar] [CrossRef]
- González-Chávez, M.d.C.A.; Aitkenhead-Peterson, J.A.; Gentry, T.J.; Zuberer, D.; Hons, F.; Loeppert, R. Soil microbial community, C, N, and P responses to long-term tillage and crop rotation. Soil Tillage Res. 2010, 106, 285–293. [Google Scholar] [CrossRef]
- Fan, J.; Hao, M. Study on long-term experiment of crop rotation and fertilizationin the Loess PlateauI I. Effect of crop rotation and continuous planting on soil enzyme activities. J. Plant Nutr. Fertil. 2003, 9, 9–13. (In Chinese) [Google Scholar]
- Zhang, H.; Luo, G.; Wang, Y.; Fei, J.; Xiangmin, R.; Peng, J.; Tian, C.; Zhang, Y. Crop rotation-driven change in physicochemical properties regulates microbial diversity, dominant components, and community complexity in paddy soils. Agric. Ecosyst. Environ. 2023, 343, 108278. [Google Scholar] [CrossRef]
- Yan, H.; Zhu, L.; Wang, Y.; Zhang, S.; Liu, P.; Dong, T.T.; Wu, Q.; Duan, J.-A. Comparative metagenomics analysis of the rhizosphere microbiota influence on Radix Angelica sinensis in different growth soil environments in China. Food Sci. Technol. 2021, 41, 775–784. [Google Scholar] [CrossRef]
- Yuan, Y.; Zuo, J.; Zhang, H.; Zu, M.; Liu, S. The Chinese medicinal plants rhizosphere: Metabolites, microorganisms, and interaction. Rhizosphere 2022, 22, 100540. [Google Scholar] [CrossRef]
- Gong, X.; Feng, Y.; Dang, K.; Jiang, Y.; Qi, H.; Feng, B. Linkages of microbial community structure and root exudates: Evidence from microbial nitrogen limitation in soils of crop families. Sci. Total Environ. 2023, 881, 163536. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Zhang, K.; Jeong, J.; Zeng, Z.; Zang, H. Does crop rotation yield more in China? A meta-analysis. Field Crops Res. 2020, 245, 107659, Erratum in Field Crops Res. 2024, 313, 109404. [Google Scholar] [CrossRef]
- Lyu, H.; Yu, A.; Chai, Q.; Wang, F.; Wang, Y.; Wang, P.; Shang, Y.; Yang, X. Enhancing soil quality and crop yield by increasing dominant bacterial abundance and reducing bacterial diversity under no-tillage with total green manure incorporation. Agric. Ecosyst. Environ. 2025, 378, 109303. [Google Scholar] [CrossRef]
- Oberholster, T.; Vikram, S.; Cowan, D.; Valverde, A. Key microbial taxa in the rhizosphere of sorghum and sunflower grown in crop rotation. Sci. Total Environ. 2018, 624, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Tan, J.; Li, L.; Miao, Y.; Wang, Y. Legumes can increase the yield of subsequent wheat with or without grain harvesting compared to Gramineae crops: A meta-analysis. Eur. J. Agron. 2023, 142, 126643. [Google Scholar] [CrossRef]
- Shu, X.; He, J.; Zhou, Z.; Xia, L.; Hu, Y.; Zhang, Y.; Zhang, Y.; Luo, Y.; Chu, H.; Liu, W. Organic amendments enhance soil microbial diversity, microbial functionality and crop yields: A meta-analysis. Sci. Total Environ. 2022, 829, 154627. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Teng, Y.; Wang, X.; Li, X.; Luo, Y. Microbial diversity drives pyrene dissipation in soil. Sci. Total Environ. 2022, 819, 153082. [Google Scholar] [CrossRef]
- Xie, Z.; Yu, Z.; Li, Y.; Wang, G.; Liu, X.; Tang, C.; Lian, T.; Adams, J.; Liu, J.; Liu, J. Soil microbial metabolism on carbon and nitrogen transformation links the crop-residue contribution to soil organic carbon. Npj Biofilms Microbiomes 2022, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Nannipieri, P.; Hannula, S.E.; Pietramellara, G.; Schloter, M.; Sizmur, T.; Pathan, S.I. Legacy effects of rhizodeposits on soil microbiomes: A perspective. Soil Biol. Biochem. 2023, 184, 109107. [Google Scholar] [CrossRef]
- Nwachukwu, B.C.; Ayangbenro, A.S.; Babalola, O.O. Structural diversity of bacterial communities in two divergent sunflower rhizosphere soils. Ann. Microbiol. 2023, 73, 9. [Google Scholar] [CrossRef]
- Martiny, A.C.; Treseder, K.; Pusch, G. Phylogenetic conservatism of functional traits in microorganisms. ISME J. 2013, 7, 830–838. [Google Scholar] [CrossRef]
- Konopka, A.; Lindemann, S.; Fredrickson, J. Dynamics in microbial communities: Unraveling mechanisms to identify principles. ISME J. 2015, 9, 1488–1495. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Yi, M.; Zhang, L.; Li, Y.; Qian, Y. Structural, metabolic, and functional characteristics of soil microbial communities in response to benzo [a] pyrene stress. J. Hazard. Mater. 2022, 431, 128632. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R.; Joergensen, R.G.; Ludwig, B. Pathways of nitrogen utilization by soil microorganisms–A review. Soil Biol. Biochem. 2010, 42, 2058–2067. [Google Scholar] [CrossRef]
- Zou, X.; Liu, Y.; Huang, M.; Li, F.; Si, T.; Wang, Y.; Yu, X.; Zhang, X.; Wang, H.; Shi, P. Rotational strip intercropping of maize and peanut enhances productivity by improving crop photosynthetic production and optimizing soil nutrients and bacterial communities. Field Crops Res. 2023, 291, 108770. [Google Scholar] [CrossRef]
- Ouyang, Y.; Reeve, J.R.; Norton, J.M. The quality of organic amendments affects soil microbiome and nitrogen-cycling bacteria in an organic farming system. Front. Soil Sci. 2022, 2, 869136. [Google Scholar] [CrossRef]
- Kumar, M.; Ansari, W.A.; Zeyad, M.T.; Singh, A.; Chakdar, H.; Kumar, A.; Farooqi, M.S.; Sharma, A.; Srivastava, S.; Srivastava, A.K. Core microbiota of wheat rhizosphere under Upper Indo-Gangetic plains and their response to soil physicochemical properties. Front. Plant Sci. 2023, 14, 1186162. [Google Scholar] [CrossRef]
- Yuan, L.; Gao, Y.; Mei, Y.; Liu, J.; Kalkhajeh, Y.K.; Hu, H.; Huang, J. Effects of continuous straw returning on bacterial community structure and enzyme activities in rape-rice soil aggregates. Sci. Rep. 2023, 13, 2357. [Google Scholar] [CrossRef]
- Yang, H.; Cheng, L.; Che, L.; Su, Y.; Li, Y. Nutrients addition decreases soil fungal diversity and alters fungal guilds and co-occurrence networks in a semi-arid grassland in northern China. Sci. Total Environ. 2024, 926, 172100. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; Van Der Heijden, M.G. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, J.; He, Y.; Yu, X.; Chen, S.; Penttinen, P.; Liu, S.; Yang, Y.; Zhao, K.; Zou, L. Organic fertilizers shape soil microbial communities and increase soil amino acid metabolites content in a blueberry orchard. Microb. Ecol. 2023, 85, 232–246. [Google Scholar] [CrossRef]
- Liu, B.; Cheng, X.; He, X.; Bei, Q.; Dai, Y.; Wang, Y.; Zhu, B.; Zhang, K.; Tian, X.; Duan, M. Effects of bio-mulching on wheat soil microbial community and carbon utilization efficiency in southwest China. Catena 2022, 214, 106260. [Google Scholar] [CrossRef]
- Araujo, A.S.F.; de Pereira, A.P.d.A.; Antunes, J.E.L.; Oliveira, L.M.d.S.; de Melo, W.J.; Rocha, S.M.B.; do Amorim, M.R.; Araujo, F.F.; Melo, V.M.M.; Mendes, L.W. Dynamics of bacterial and archaeal communities along the composting of tannery sludge. Environ. Sci. Pollut. Res. 2021, 28, 64295–64306. [Google Scholar] [CrossRef]
- Ji, L.; Nasir, F.; Tian, L.; Chang, J.; Sun, Y.; Zhang, J.; Li, X.; Tian, C. Outbreaks of root rot disease in different aged American ginseng plants are associated with field microbial dynamics. Front. Microbiol. 2021, 12, 676880. [Google Scholar] [CrossRef] [PubMed]
- Tarin, M.W.K.; Fan, L.; Xie, D.; Tayyab, M.; Rong, J.; Chen, L.; Muneer, M.A.; Zheng, Y. Response of soil fungal diversity and community composition to varying levels of bamboo biochar in red soils. Microorganisms 2021, 9, 1385. [Google Scholar] [CrossRef] [PubMed]


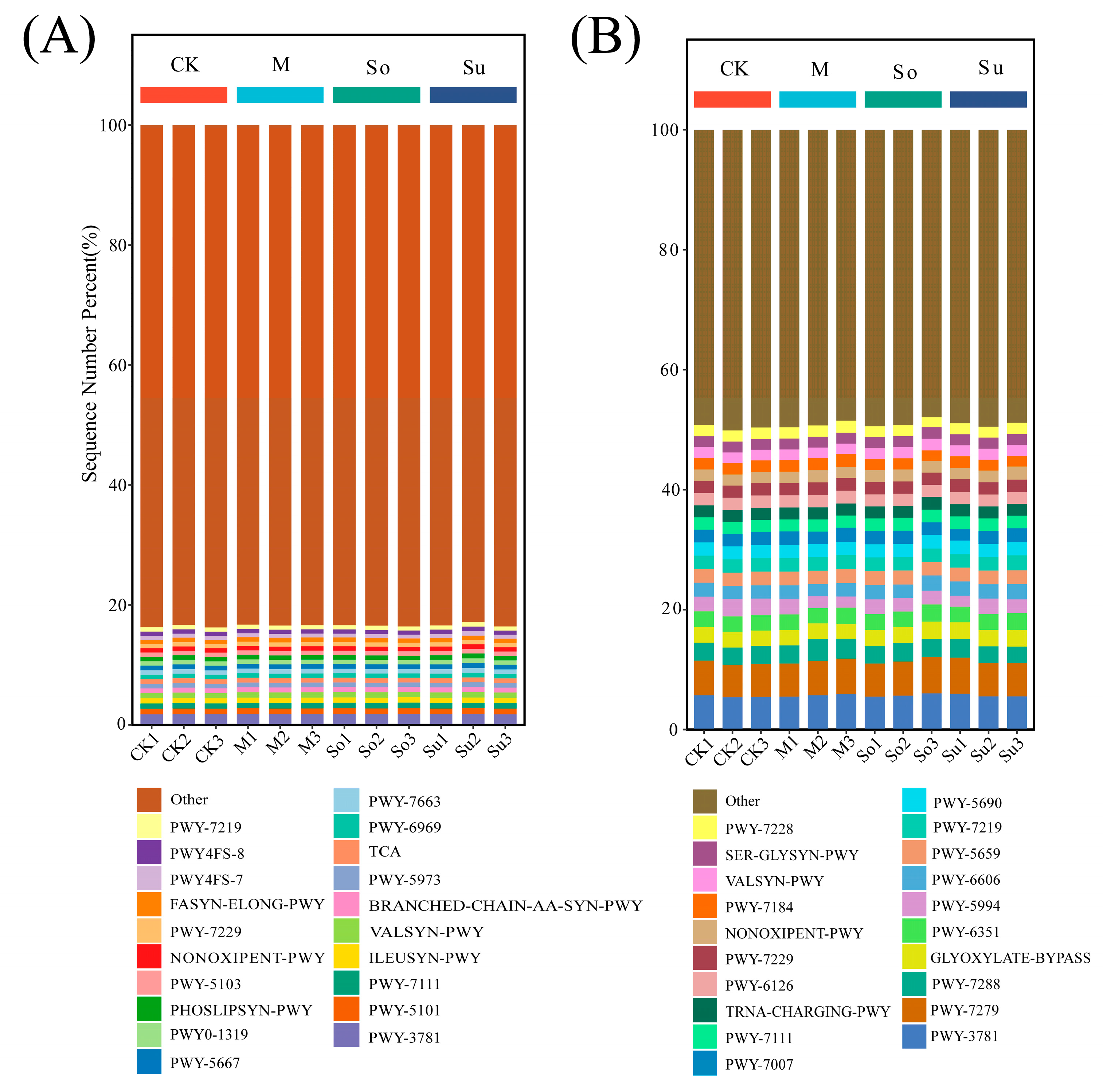
| Pathway | Description |
|---|---|
| BRANCHED-CHAIN-AA-SYN-PWY | superpathway of branched amino acid biosynthesis |
| FASYN-ELONG-PWY | fatty acid elongation saturated |
| ILEUSYN-PWY | L-isoleucine biosynthesis I (from threonine) |
| NONOXIPENT-PWY | pentose phosphate pathway (non-oxidative branch) |
| PHOSLIPSYN-PWY | superpathway of phospholipid biosynthesis I (bacteria) |
| PWY-5101 | L-isoleucine biosynthesis II |
| PWY-5103 | L-isoleucine biosynthesis III |
| PWY-5667 | CDP-diacylglycerol biosynthesis I |
| PWY-5973 | cis-vaccenate biosynthesis |
| PWY-6969 | TCA cycle V (2-oxoglutarate:ferredoxin oxidoreductase) |
| PWY-7111 | pyruvate fermentation to isobutanol (engineered) |
| PWY-7211 | superpathway of pyrimidine deoxyribonucleotides de novo biosynthesis |
| PWY-7219 | adenosine ribonucleotides de novo biosynthesis |
| PWY-7229 | superpathway of adenosine nucleotides de novo biosynthesis I |
| PWY-7663 | gondoate biosynthesis (anaerobic) |
| PWY0-1319 | CDP-diacylglycerol biosynthesis II |
| PWY4FS-7 | phosphatidylglycerol biosynthesis I (plastidic) |
| PWY4FS-8 | phosphatidylglycerol biosynthesis II (non-plastidic) |
| TCA | TCA cycle I (prokaryotic) |
| VALSYN-PWY | L-valine biosynthesis |
| Pathway | Description |
|---|---|
| GLYOXYLATE-BYPASS | glyoxylate cycle |
| NONOXIPENT-PWY | pentose phosphate pathway (non-oxidative branch) |
| PWY-3781 | aerobic respiration I (cytochrome c) |
| PWY-5659 | GDP-mannose biosynthesis |
| PWY-5690 | TCA cycle II (plants and fungi) |
| PWY-5994 | palmitate biosynthesis I (animals and fungi) |
| PWY-6126 | superpathway of adenosine nucleotides de novo biosynthesisII |
| PWY-6351 | D-myo-inositol (1,4,5)-trisphosphate biosynthesis |
| PWY-6606 | guanosine nucleotides degradation II |
| PWY-7007 | methyl ketone biosynthesis |
| PWY-7111 | pyruvate fermentation to isobutanol (engineered) |
| PWY-7184 | pyrimidine deoxyribonucleotides de novo biosynthesis I |
| PWY-7219 | adenosine ribonucleotides de novo biosynthesis |
| PWY-7228 | superpathway of guanosine nucleotides de novo biosynthesis I |
| PWY-7229 | superpathway of adenosine nucleotides de novo biosynthesis I |
| PWY-7279 | aerobic respiration II (cytochrome c) (yeast) |
| PWY-7288 | fatty acid β-oxidation (peroxisome, yeast) |
| SER-GLYSYN-PWY | superpathway of L-serine and glycine biosynthesis I |
| TRNA-CHARGING-PWY | tRNA charging |
| VALSYN-PWY | L-valine biosynthesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Dong, P.; Chen, M.; Wang, H.; Wang, L.; Yuan, J.; Hu, C.; Liu, Z.; Li, Y.; Fan, Q. Soybean-Bupleurum Rotation System Can Optimize Rhizosphere Soil Microbial Community via Impacting Soil Properties and Enzyme Activities During Bupleurum Seedling Stage. Microorganisms 2025, 13, 2346. https://doi.org/10.3390/microorganisms13102346
Yang Q, Dong P, Chen M, Wang H, Wang L, Yuan J, Hu C, Liu Z, Li Y, Fan Q. Soybean-Bupleurum Rotation System Can Optimize Rhizosphere Soil Microbial Community via Impacting Soil Properties and Enzyme Activities During Bupleurum Seedling Stage. Microorganisms. 2025; 13(10):2346. https://doi.org/10.3390/microorganisms13102346
Chicago/Turabian StyleYang, Qingshan, Peng Dong, Mengni Chen, Hui Wang, Lu Wang, Jiawei Yuan, Chengyu Hu, Zhen Liu, Yongshan Li, and Qiaolan Fan. 2025. "Soybean-Bupleurum Rotation System Can Optimize Rhizosphere Soil Microbial Community via Impacting Soil Properties and Enzyme Activities During Bupleurum Seedling Stage" Microorganisms 13, no. 10: 2346. https://doi.org/10.3390/microorganisms13102346
APA StyleYang, Q., Dong, P., Chen, M., Wang, H., Wang, L., Yuan, J., Hu, C., Liu, Z., Li, Y., & Fan, Q. (2025). Soybean-Bupleurum Rotation System Can Optimize Rhizosphere Soil Microbial Community via Impacting Soil Properties and Enzyme Activities During Bupleurum Seedling Stage. Microorganisms, 13(10), 2346. https://doi.org/10.3390/microorganisms13102346






