Nitric Oxide Contributes to the Pathogenesis of Nocardia farcinica Infection in BALB/c Mice and Alveolar MH-S Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Infection of Mice
2.2. Record of Body Weight Change
2.3. Determination of Bacterial Burden, NO, and Cytokine Concentration in Lung Tissues
2.4. Lung Histopathology
2.5. Cell Culture
2.6. Determination of NO, TNF-α, IFN-γ and Bacterial Replication
2.7. Statistical Analysis
3. Results
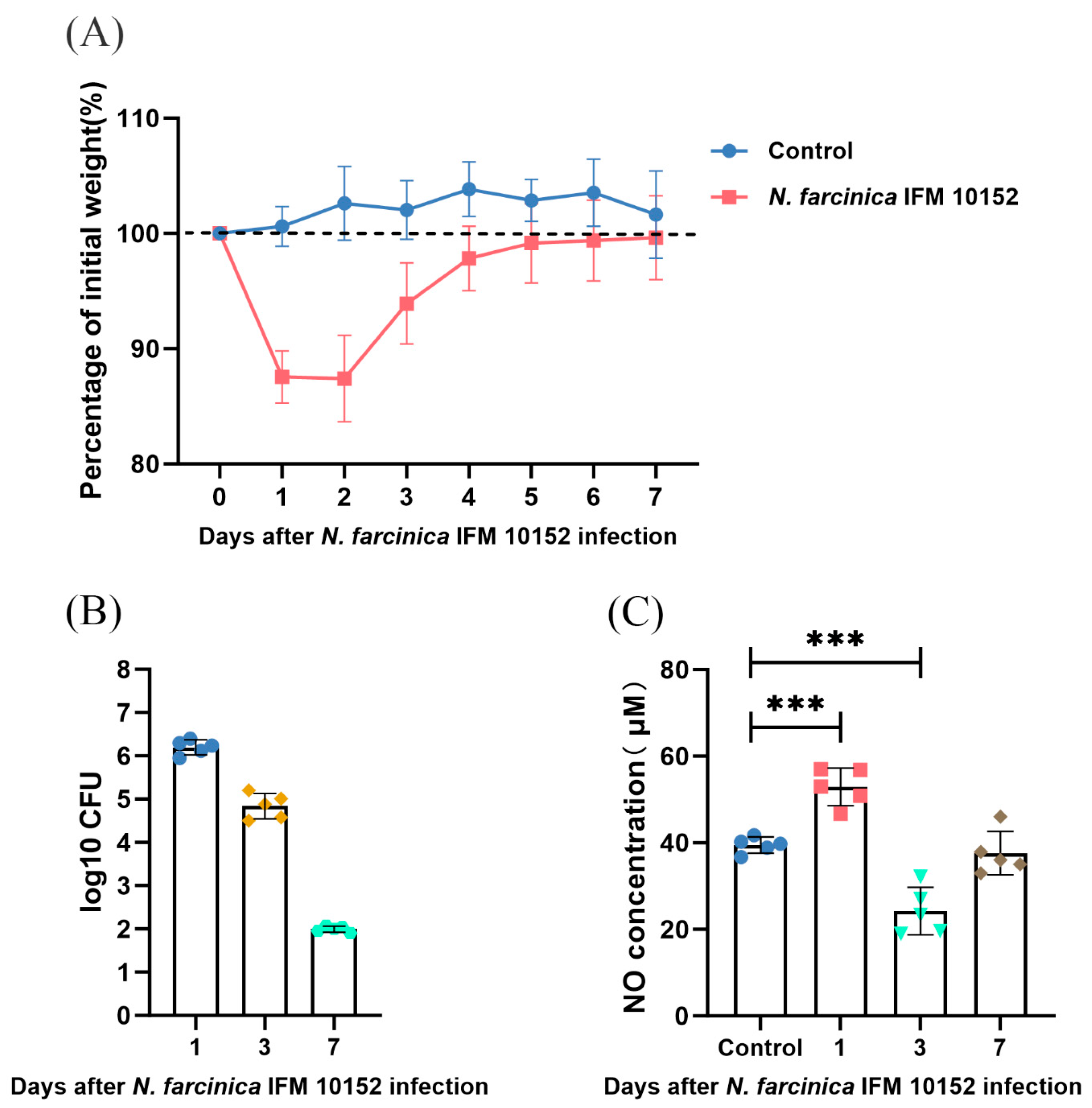
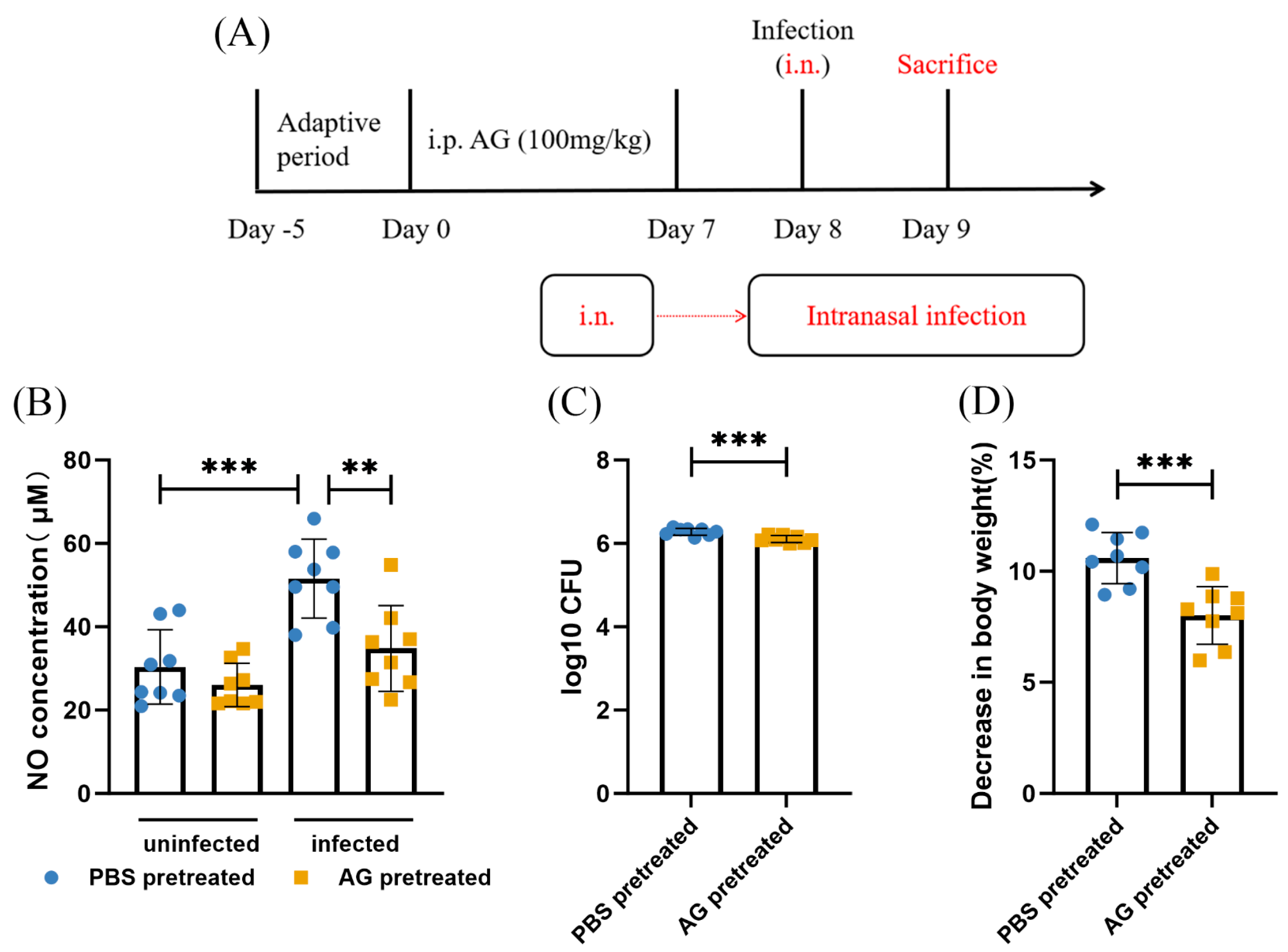
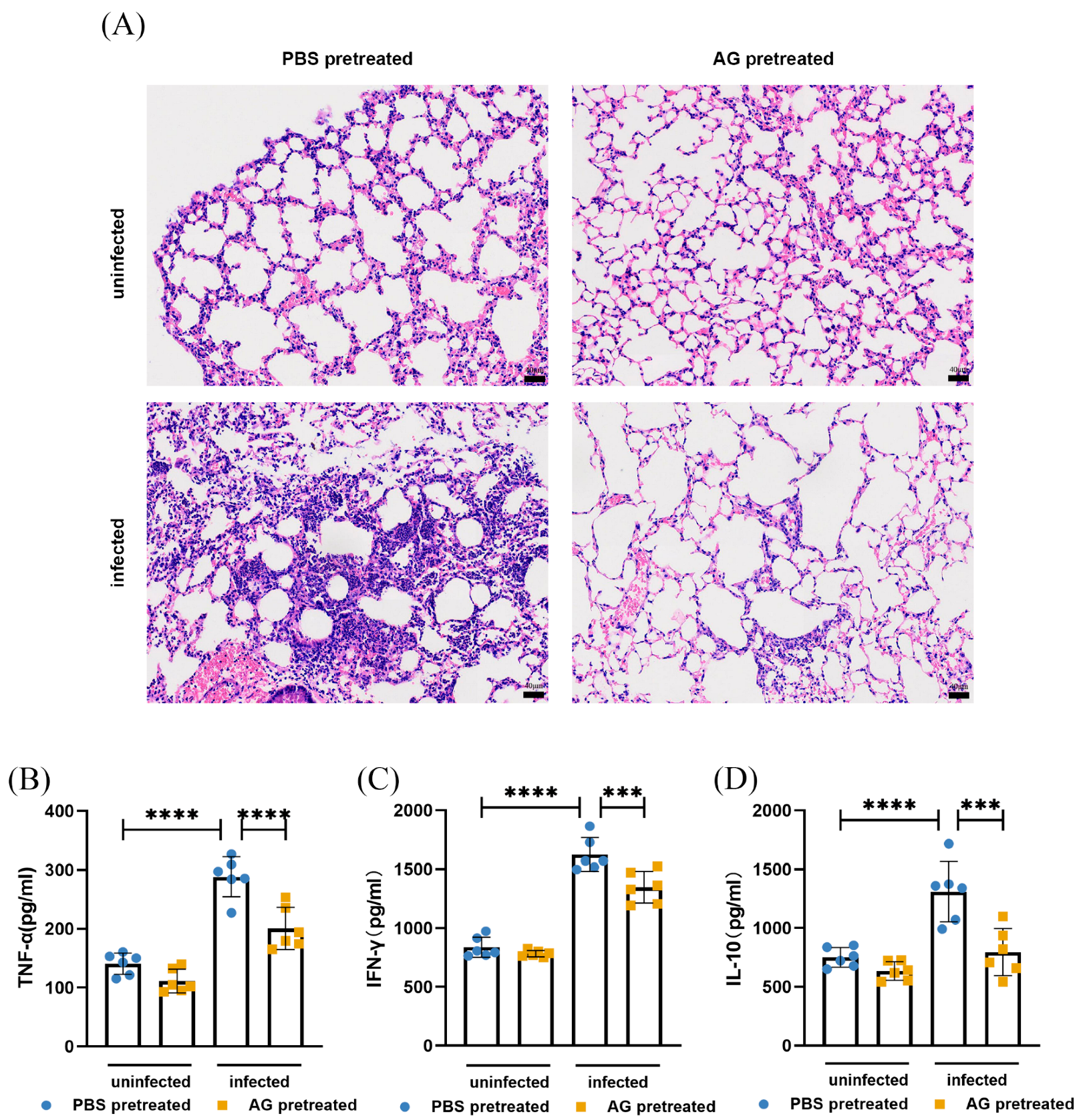
3.1. NO Results in N. farcinica-Induced Acute Pulmonary Disease In Vivo
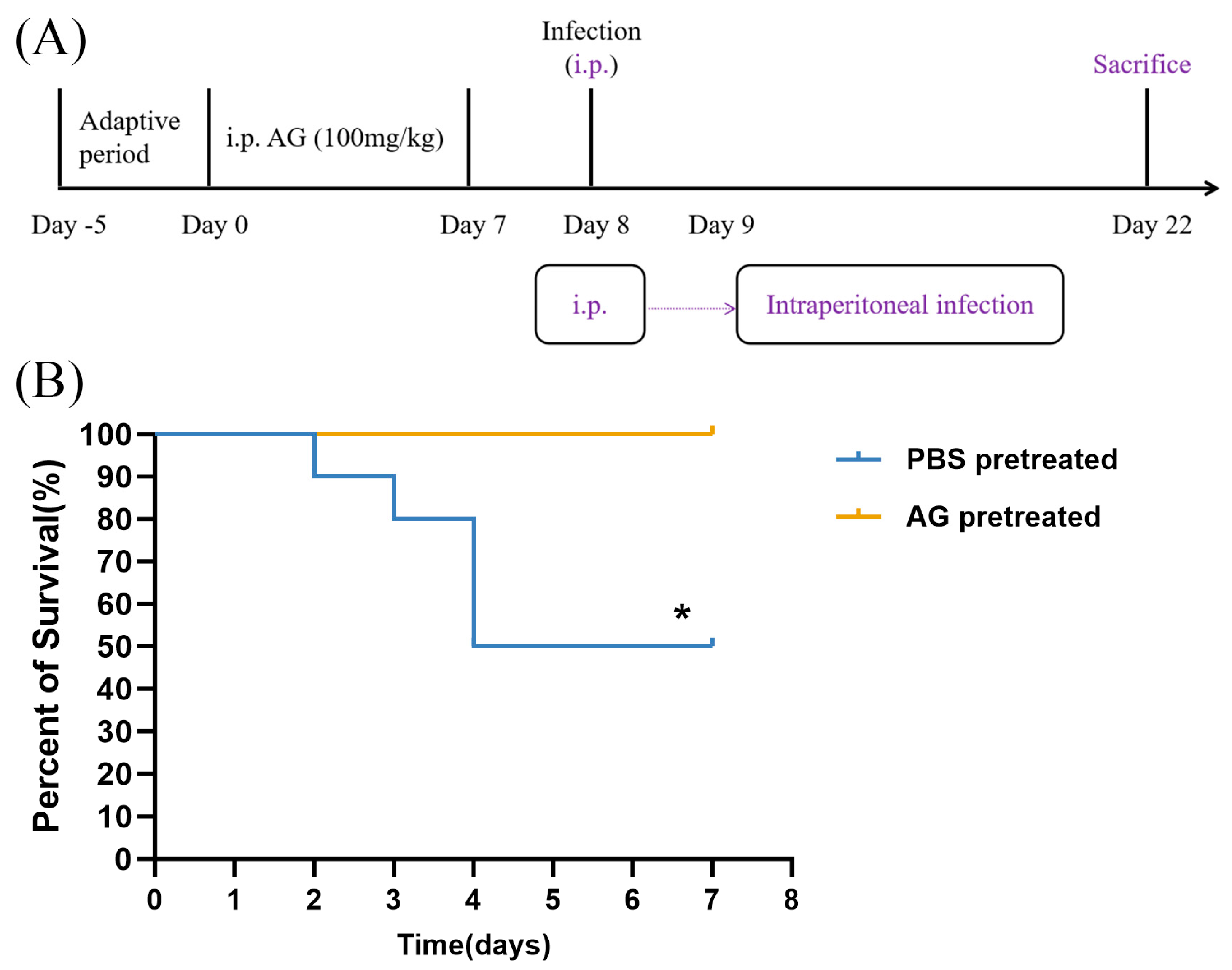
3.2. NO Increases Mortality Rate in N. farcinica-Infected Mice
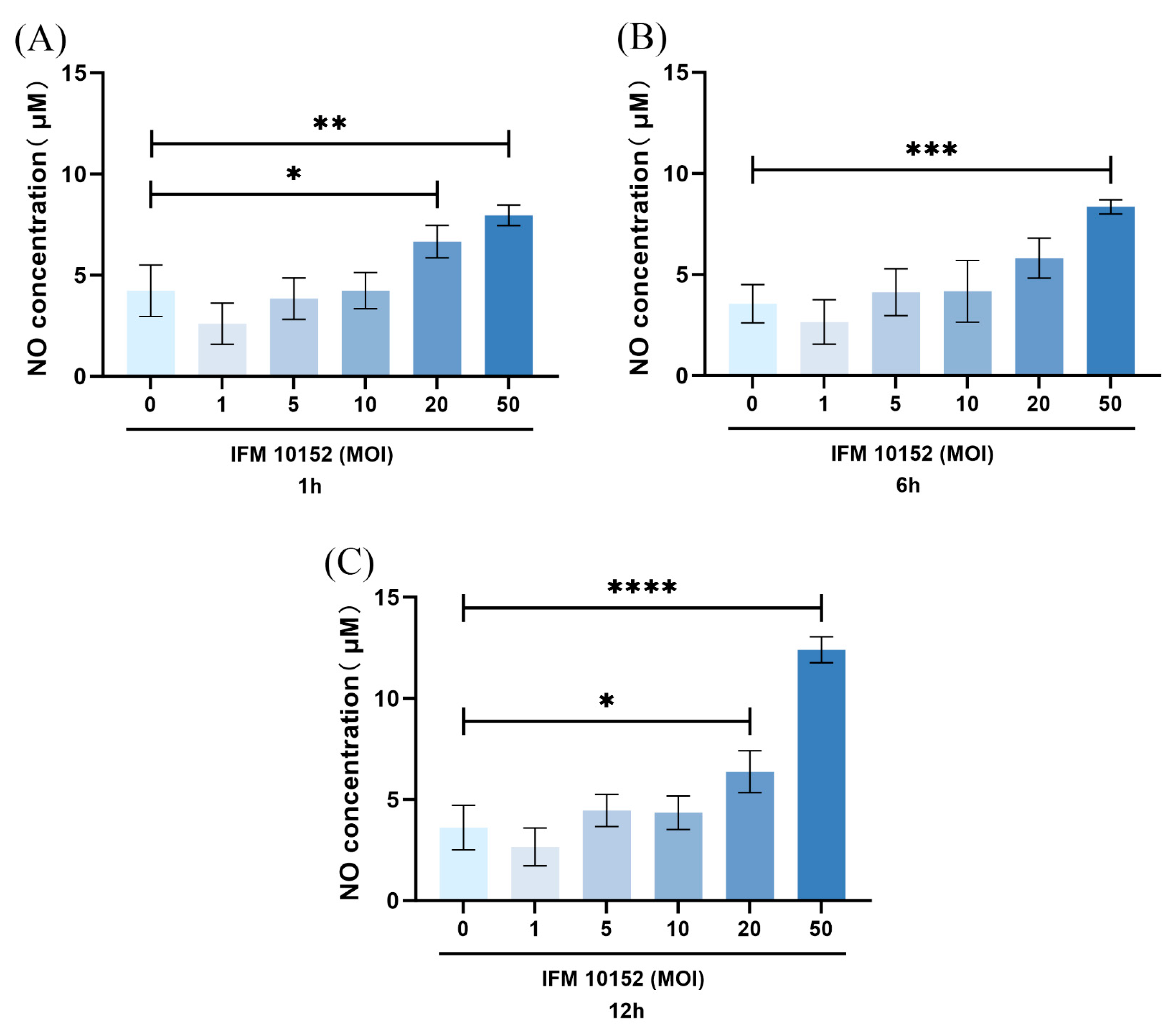
3.3. NO Contributed to N. farcinica-Induced Inflammation and Bacterial Replication In Vitro
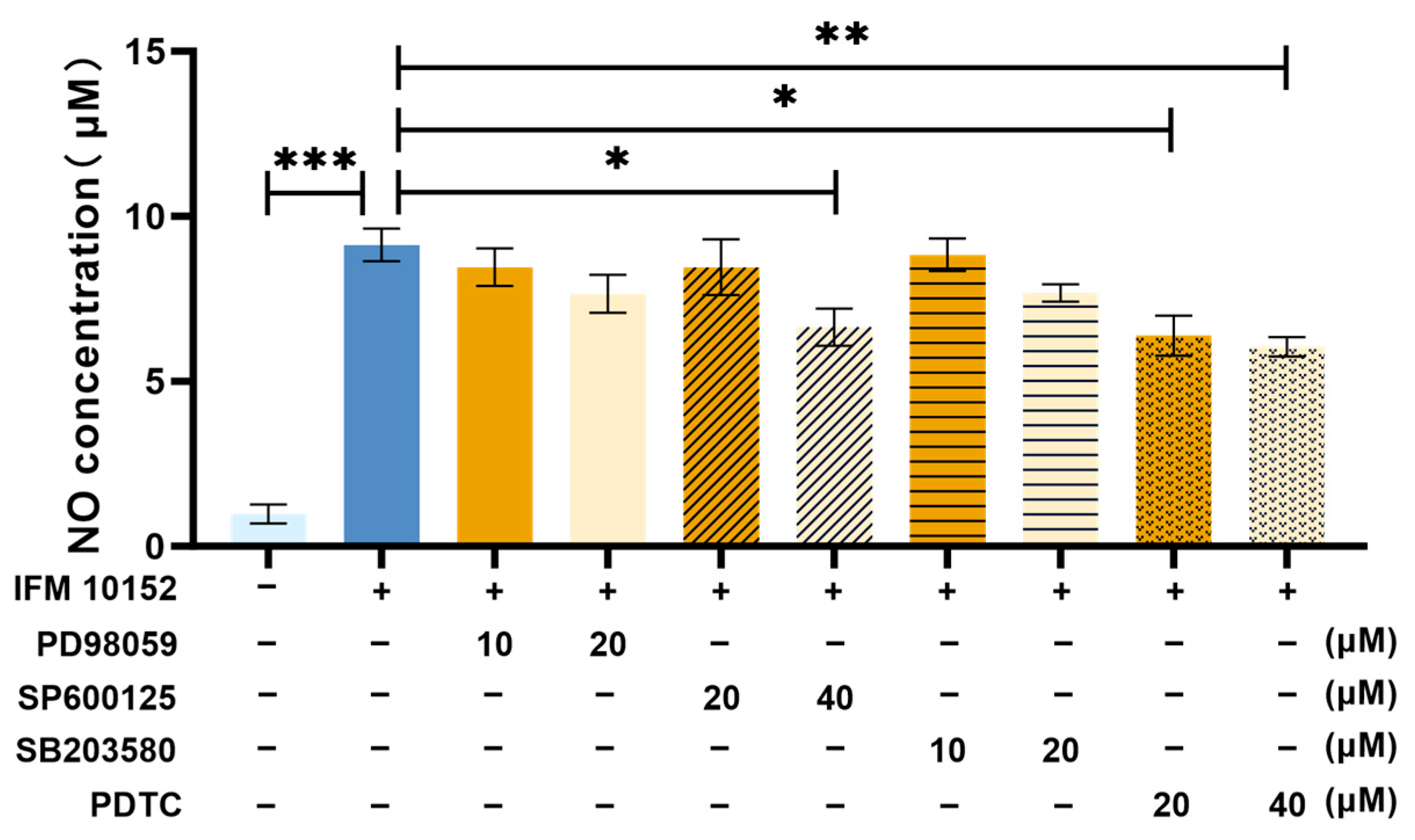
3.4. NO Is Induced by N. farcinica Through MAPK JNK and NF-κB Signaling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Traxler, R.M.; Bell, M.E.; Lasker, B.; Headd, B.; Shieh, W.J.; McQuiston, J.R. Updated Review on Nocardia Species: 2006–2021. Clin. Microbiol. Rev. 2022, 35, e0002721. [Google Scholar] [CrossRef]
- McNeil, M.M.; Brown, J.M. The medically important aerobic actinomycetes: Epidemiology and microbiology. Clin. Microbiol. Rev. 1994, 7, 357–417. [Google Scholar] [CrossRef]
- Shen, J.; Du, B.; Liu, Z.; Song, Z.; Yuan, M.; Qiu, X.; Li, Z. Multicenter systematic review of clinical characteristics, diagnostic optimization, and personalized treatment for brain Nocardia infections. Microb. Pathog. 2025, 198, 107147. [Google Scholar] [CrossRef]
- Ji, X.; Han, L.; Zhang, W.; Sun, L.; Xu, S.; Qiu, X.; Fan, S.; Li, Z. Molecular, cellular and neurological consequences of infection by the neglected human pathogen Nocardia. BMC Biol. 2022, 20, 251. [Google Scholar] [CrossRef]
- Steinbrink, J.; Leavens, J.; Kauffman, C.A.; Miceli, M.H. Manifestations and outcomes of nocardia infections: Comparison of immunocompromised and nonimmunocompromised adult patients. Medicine 2018, 97, e12436. [Google Scholar]
- Coussement, J.; Lebeaux, D.; van Delden, C.; Guillot, H.; Freund, R.; Marbus, S.; Melica, G.; Van Wijngaerden, E.; Douvry, B.; Van Laecke, S.; et al. Nocardia Infection in Solid Organ Transplant Recipients: A Multicenter European Case-control Study. Clin. Infect. Dis. 2016, 63, 338–345. [Google Scholar] [CrossRef]
- Hamdi, A.M.; Fida, M.; Deml, S.M.; Abu Saleh, O.M.; Wengenack, N.L. Retrospective Analysis of Antimicrobial Susceptibility Profiles of Nocardia Species from a Tertiary Hospital and Reference Laboratory, 2011 to 2017. Antimicrob. Agents Chemother. 2020, 64, e01868-19. [Google Scholar] [CrossRef]
- Conville, P.S.; Brown-Elliott, B.A.; Smith, T.; Zelazny, A.M. The Complexities of Nocardia Taxonomy and Identification. J. Clin. Microbiol. 2018, 56, 10-1128. [Google Scholar] [CrossRef]
- Martínez-Barricarte, R. Isolated Nocardiosis, an Unrecognized Primary Immunodeficiency? Front. Immunol. 2020, 11, 590239. [Google Scholar] [CrossRef]
- Mehta, H.H.; Shamoo, Y. Pathogenic Nocardia: A diverse genus of emerging pathogens or just poorly recognized? PLoS Pathog. 2020, 16, e1008280. [Google Scholar]
- Wang, C.; Sun, Q.; Yan, J.; Liao, X.; Long, S.; Zheng, M.; Zhang, Y.; Yang, X.; Shi, G.; Zhao, Y.; et al. The species distribution and antimicrobial resistance profiles of Nocardia species in China: A systematic review and meta-analysis. PLoS Negl. Trop Dis. 2023, 17, e0011432. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Y.; Cui, Q.; Wu, W.; Li, G.; Chen, D.; Xiang, L.; Qu, J.; Shi, D.; Lu, B. Epidemiology and Antimicrobial Resistance Profiles of the Nocardia Species in China, 2009 to 2021. Microbiol. Spectr. 2022, 10, e0156021. [Google Scholar] [CrossRef]
- Huang, L.; Chen, X.; Xu, H.; Sun, L.; Li, C.; Guo, W.; Xiang, L.; Luo, G.; Cui, Y.; Lu, B. Clinical features, identification, antimicrobial resistance patterns of Nocardia species in China: 2009–2017. Diagn. Microbiol. Infect Dis. 2019, 94, 165–172. [Google Scholar] [CrossRef]
- Ji, X.; Tan, X.; Hou, X.; Si, C.; Xu, S.; Tang, L.; Yuan, X.; Li, Z. Cloning, Expression, Invasion, and Immunological Reactivity of a Mammalian Cell Entry Protein Encoded by the mce1 Operon of Nocardia farcinica. Front. Microbiol. 2017, 8, 281. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, X.; Li, H.; Sun, L.; Hou, X.; Song, H.; Han, L.; Xu, S.; Qiu, X.; Wang, X.; et al. Nfa34810 Facilitates Nocardia farcinica Invasion of Host Cells and Stimulates Tumor Necrosis Factor Alpha Secretion through Activation of the NF-κB and Mitogen-Activated Protein Kinase Pathways via Toll-Like Receptor 4. Infect Immun. 2020, 88, 10-1128. [Google Scholar] [CrossRef]
- Salinas-Carmona, M.C.; Longoria-Lozano, O.; Garza-Esquivel, H.R.; López-Ulloa, J.; Reyes-Carrillo, J.; Vázquez-Marmolejo, A.V. Inducible nitric oxide synthase blockade with aminoguanidine, protects mice infected with Nocardia brasiliensis from actinomycetoma development. PLoS Negl. Trop. Dis. 2020, 14, e0008775. [Google Scholar]
- Tsai, H.H.; Lee, W.R.; Wang, P.H.; Cheng, K.T.; Chen, Y.C.; Shen, S.C. Propionibacterium acnes-induced iNOS and COX-2 protein expression via ROS-dependent NF-κB and AP-1 activation in macrophages. J. Dermatol. Sci. 2013, 69, 122–131. [Google Scholar] [CrossRef]
- MacFarlane, A.S.; Schwacha, M.G.; Eisenstein, T.K. In vivo blockage of nitric oxide with aminoguanidine inhibits immunosuppression induced by an attenuated strain of Salmonella typhimurium, potentiates Salmonella infection, and inhibits macrophage and polymorphonuclear leukocyte influx into the spleen. Infect. Immun. 1999, 67, 891–898. [Google Scholar] [CrossRef]
- Shen, J.; Han, L.; Yao, J.; Qiu, X.; Xu, S.; Liu, X.; Li, F.; Li, Z. Infection route influence the consequences of Nocardia farcinica infection in BALB/c mice. BMC Infect. Dis. 2024, 24, 1016. [Google Scholar] [CrossRef]
- Tarantino, M.; Dionisi, A.M.; Pistoia, C.; Petrucci, P.; Luzzi, I.; Pasquali, P. Involvement of nitric oxide in the control of a mouse model of Campylobacter jejuni infection. FEMS Immunol. Med. Microbiol. 2009, 56, 98–101. [Google Scholar] [CrossRef]
- de Souza Ferreira, C.; Pennacchi, P.C.; Araújo, T.H.; Taniwaki, N.N.; de Araújo Paula, F.B.; da Silveira Duarte, S.M.; Rodrigues, M.R. Aminoguanidine treatment increased NOX2 response in diabetic rats: Improved phagocytosis and killing of Candida albicans by neutrophils. Eur. J. Pharmacol. 2016, 772, 83–91. [Google Scholar] [CrossRef]
- Ishikawa, J.; Yamashita, A.; Mikami, Y.; Hoshino, Y.; Kurita, H.; Hotta, K.; Shiba, T.; Hattori, M. The complete genomic sequence of Nocardia farcinica IFM 10152. Proc. Natl. Acad. Sci. USA 2004, 101, 14925–14930. [Google Scholar] [CrossRef]
- Jung, J.Y.; Madan-Lala, R.; Georgieva, M.; Rengarajan, J.; Sohaskey, C.D.; Bange, F.C.; Robinson, C.M. The intracellular environment of human macrophages that produce nitric oxide promotes growth of mycobacteria. Infect. Immun. 2013, 81, 3198–3209. [Google Scholar] [CrossRef] [PubMed]
- Pasten, C.; Lozano, M.; Rocco, J.; Carrión, F.; Alvarado, C.; Liberona, J.; Michea, L.; Irarrázabal, C.E. Aminoguanidine Prevents the Oxidative Stress, Inhibiting Elements of Inflammation, Endothelial Activation, Mesenchymal Markers, and Confers a Renoprotective Effect in Renal Ischemia and Reperfusion Injury. Antioxidants 2021, 10, 1724. [Google Scholar] [CrossRef]
- Li, Q.; Song, W.; Tian, Z.; Wang, P. Aminoguanidine alleviated MMA-induced impairment of cognitive ability in rats by downregulating oxidative stress and inflammatory reaction. Neurotoxicology 2017, 59, 121–130, Erratum in Neurotoxicology 2017, 93, 355–356. [Google Scholar]
- Saadat, S.; Beheshti, F.; Askari, V.R.; Hosseini, M.; Mohamadian Roshan, N.; Boskabady, M.H. Aminoguanidine affects systemic and lung inflammation induced by lipopolysaccharide in rats. Respir. Res. 2019, 20, 96. [Google Scholar] [CrossRef]
- Okamoto, T.; Gohil, K.; Finkelstein, E.I.; Bove, P.; Akaike, T.; van der Vliet, A. Multiple contributing roles for NOS2 in LPS-induced acute airway inflammation in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 286, L198–L209. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Han, L.; Shen, J.; Du, B.; Song, Z.; Yuan, M.; Xu, S.; Qiu, X.; Liu, X.; Li, F.; et al. Nitric Oxide Contributes to the Pathogenesis of Nocardia farcinica Infection in BALB/c Mice and Alveolar MH-S Macrophages. Microorganisms 2025, 13, 2336. https://doi.org/10.3390/microorganisms13102336
Yao J, Han L, Shen J, Du B, Song Z, Yuan M, Xu S, Qiu X, Liu X, Li F, et al. Nitric Oxide Contributes to the Pathogenesis of Nocardia farcinica Infection in BALB/c Mice and Alveolar MH-S Macrophages. Microorganisms. 2025; 13(10):2336. https://doi.org/10.3390/microorganisms13102336
Chicago/Turabian StyleYao, Jiang, Lichao Han, Jirao Shen, Bingqian Du, Ziyu Song, Min Yuan, Shuai Xu, Xiaotong Qiu, Xueping Liu, Fang Li, and et al. 2025. "Nitric Oxide Contributes to the Pathogenesis of Nocardia farcinica Infection in BALB/c Mice and Alveolar MH-S Macrophages" Microorganisms 13, no. 10: 2336. https://doi.org/10.3390/microorganisms13102336
APA StyleYao, J., Han, L., Shen, J., Du, B., Song, Z., Yuan, M., Xu, S., Qiu, X., Liu, X., Li, F., Liang, Y., Guan, W., & Li, Z. (2025). Nitric Oxide Contributes to the Pathogenesis of Nocardia farcinica Infection in BALB/c Mice and Alveolar MH-S Macrophages. Microorganisms, 13(10), 2336. https://doi.org/10.3390/microorganisms13102336





