Crithidia fasciculata Shows Non-Pathogenic Behavior in Leishmania Co-Infection Related to Temperature Stress, In Vitro and In Vivo Infections, and Amphotericin B Susceptibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasite Cultivation
2.2. Growth Curves
2.3. Morphological Analysis
2.4. Mitochondrial Membrane Potential (ΔΨm) Assay
2.5. Murine Peritoneal Macrophages In Vitro Infection
2.6. Amphotericin B Sensitivity Assays
2.7. In Vivo Experimental Infection Models
2.8. RT-qPCR Quantification
2.9. Sandflies Experimental Infection
2.10. Statistical Analysis
3. Results
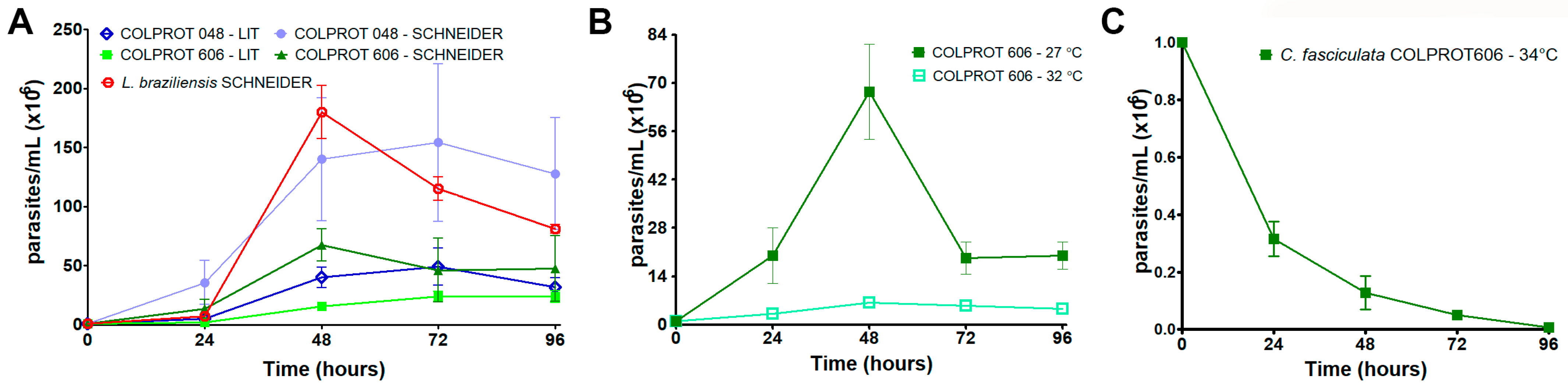
3.1. Growth Kinetics of C. fasciculata COLPROT60
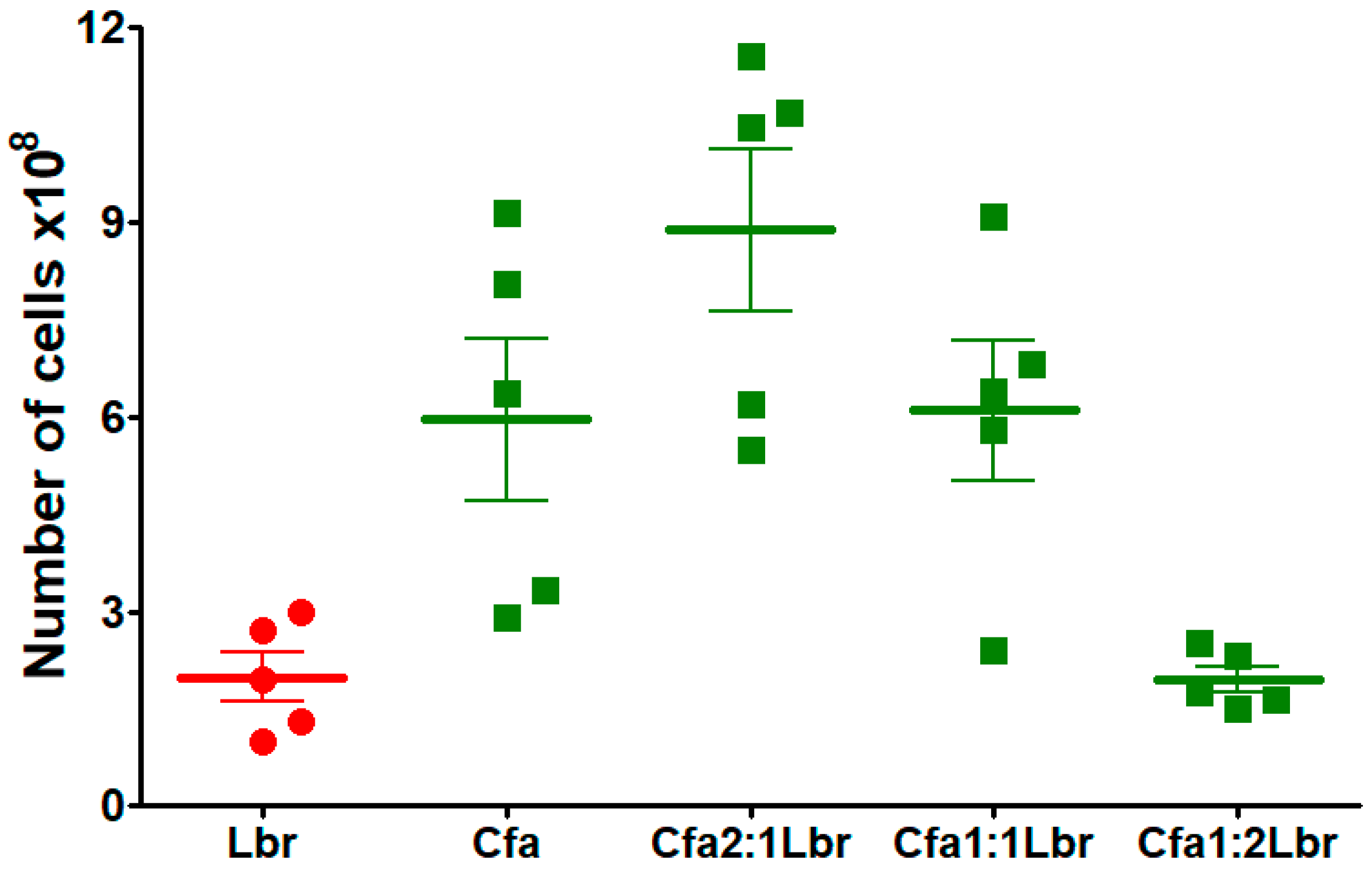
3.2. Cell Growth Evaluation in Leishmania-Crithidia Co-Cultures
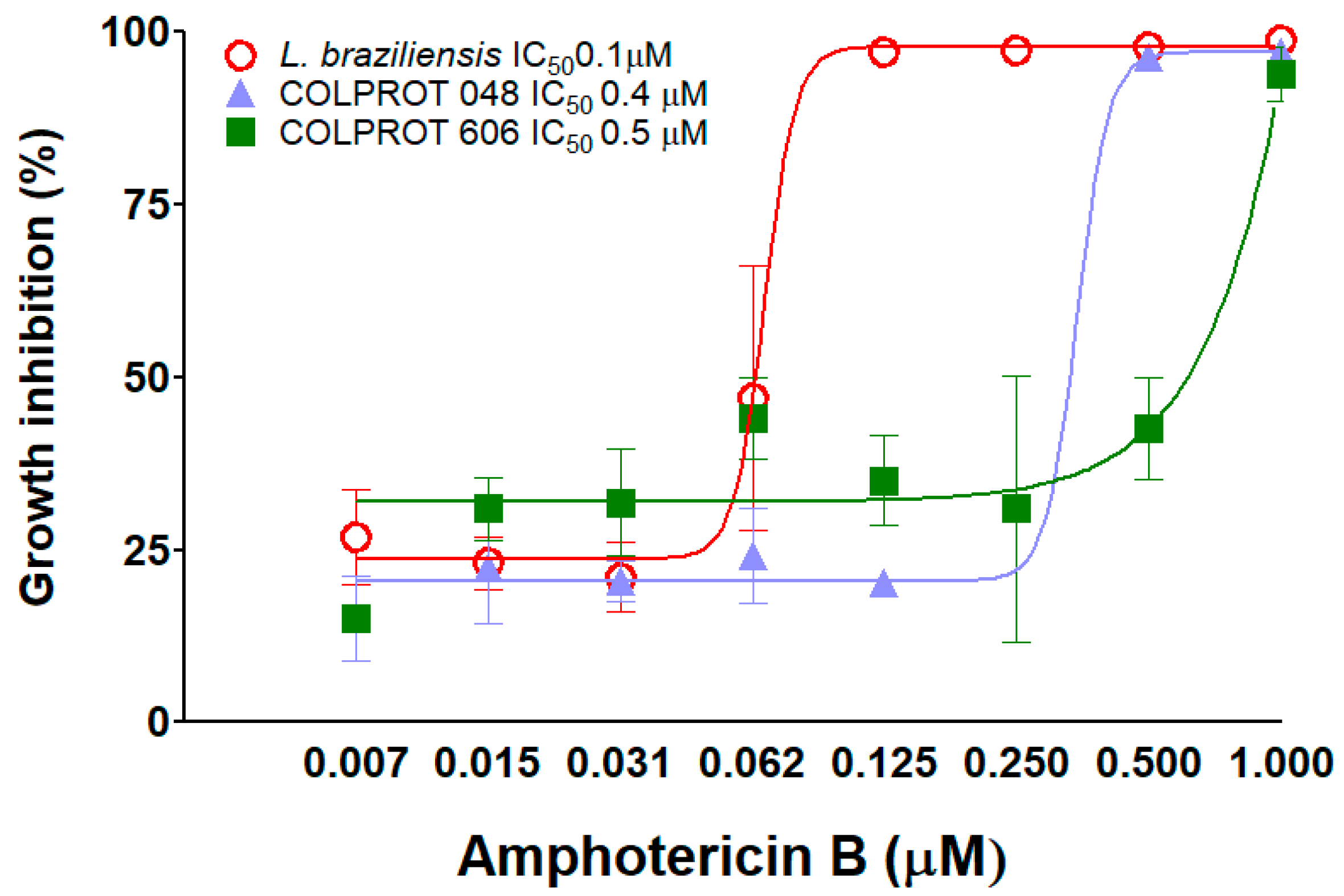
3.3. Susceptibility to Amphotericin B
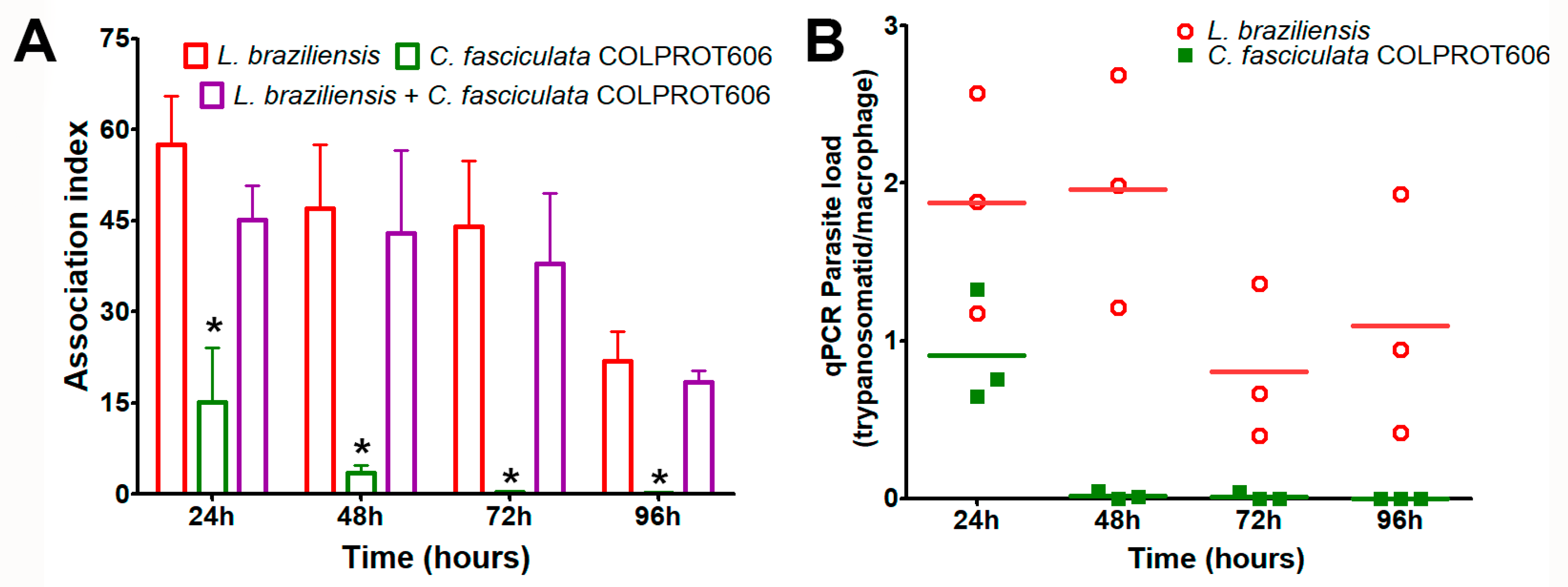
3.4. In Vitro Coinfection of C. fasciculata and L. braziliensis
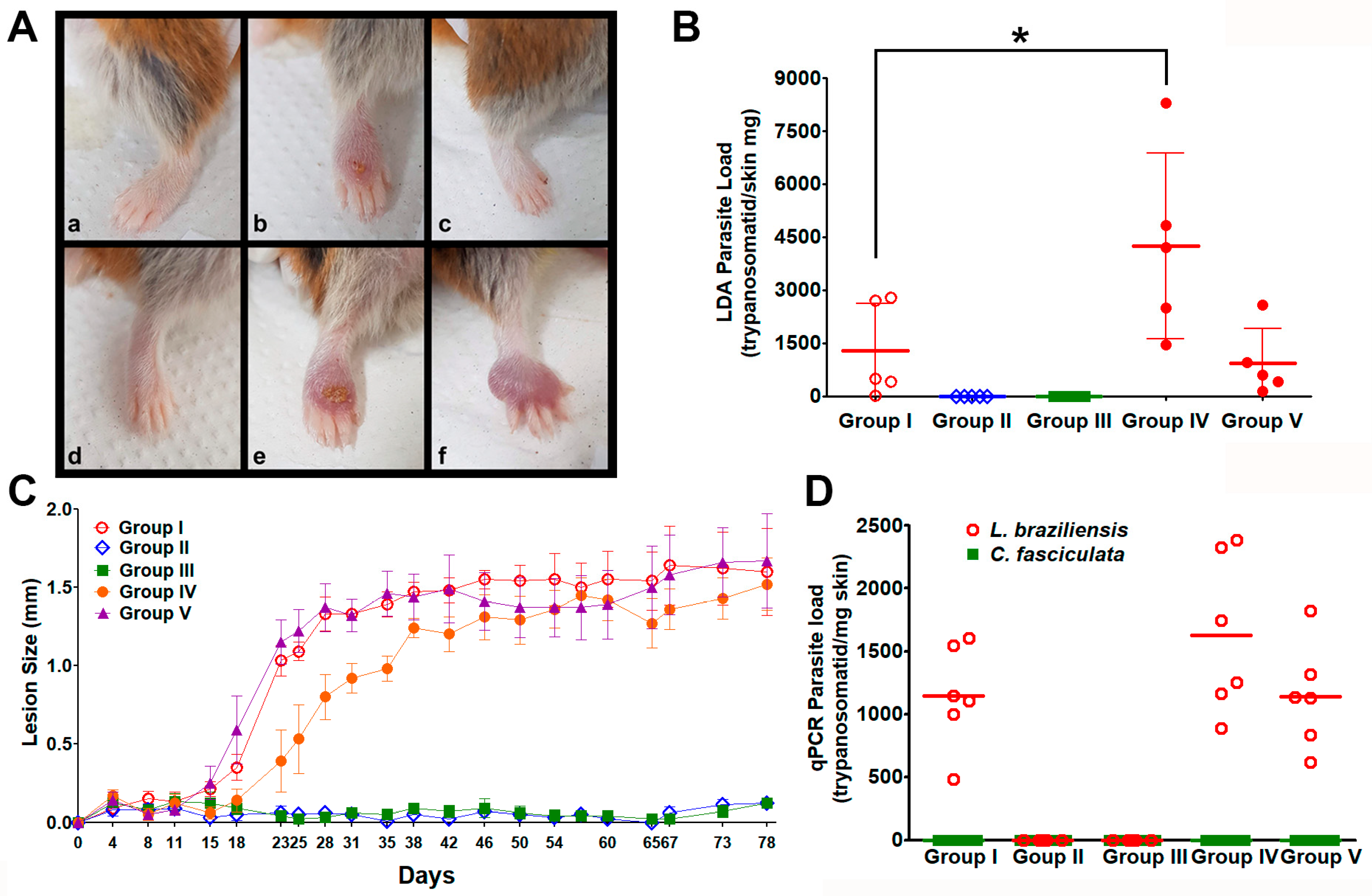
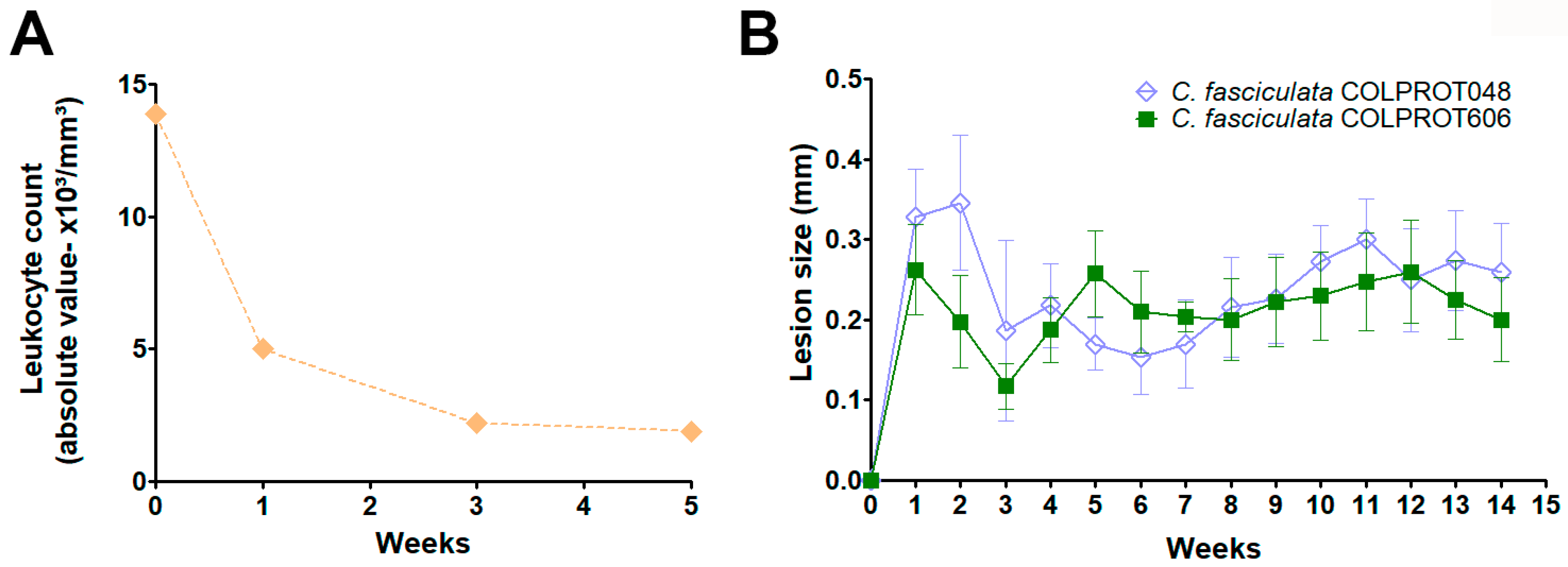
3.5. In Vivo Experimental Infections
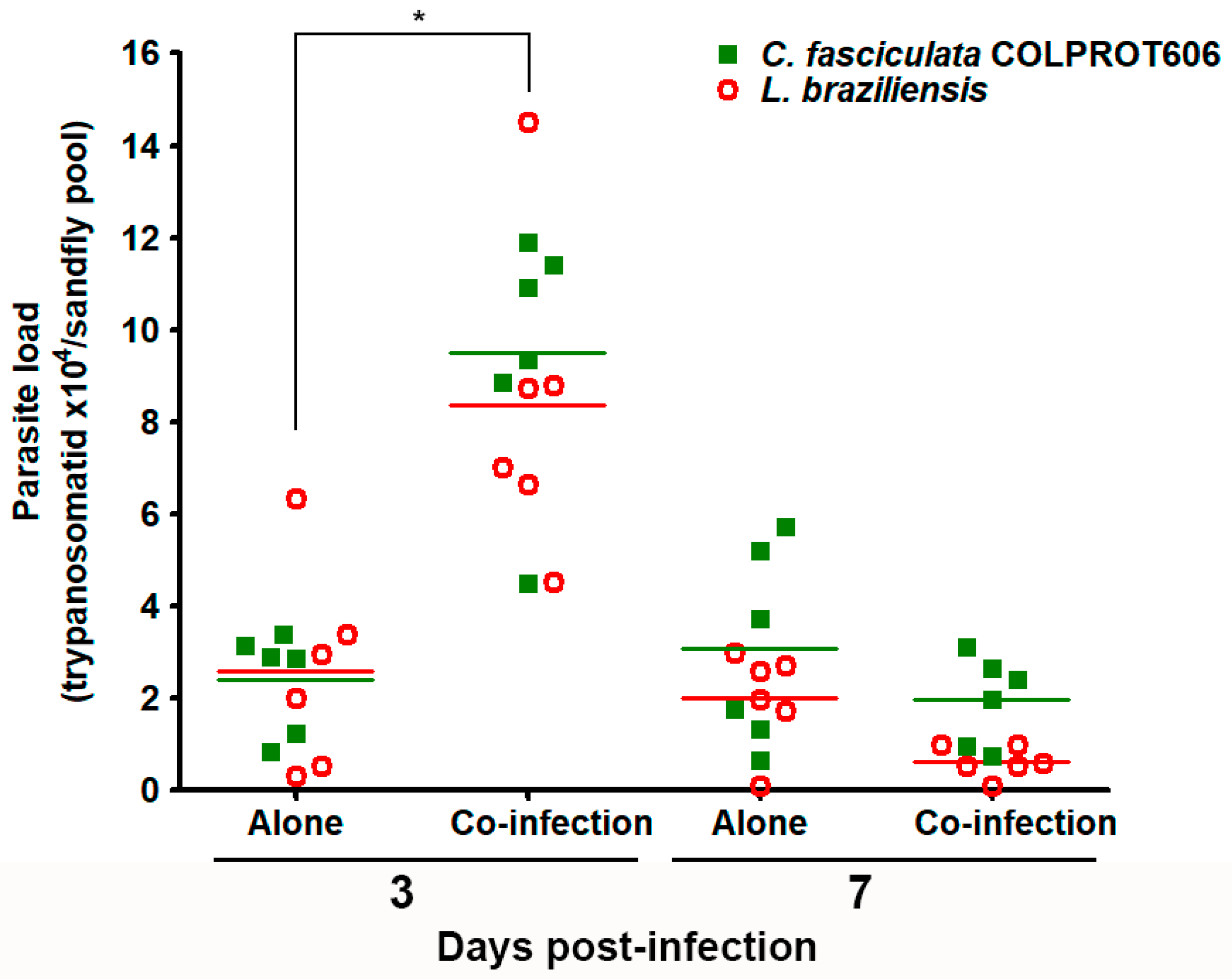
3.6. Co-Infection of Sandflies with L. braziliensis and C. fasciculata COLPROT606
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamida, A.; Ilyas, D.; Zahra, M.; Shehnaz, G. Leishmaniasis: A Neglected Tropical Disease. Glob. Immunol. Infect. Dis. Rev. 2019, 4, 17–23. [Google Scholar] [CrossRef]
- Karmakar, S.; Volpedo, G.; Zhang, W.-W.; Lypaczewski, P.; Ismail, N.; Oliveira, F.; Oristian, J.; Meneses, C.; Gannavaram, S.; Kamhawi, S.; et al. Centrin-deficient Leishmania mexicana confers protection against Old World visceral leishmaniasis. NPJ Vaccines 2022, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Maslov, D.A.; Votýpka, J.; Yurchenko, V.; Lukeš, J. Diversity and phylogeny of insect trypanosomatids: All that is hidden shall be revealed. Trends Parasitol. 2013, 29, 43–52. [Google Scholar] [CrossRef]
- Toledo, P.D. Comportamiento In Vitro de um Kinetoplastido Trypanosomatidae Aislado de uma Lesion Cutanea. Licenciate Thesis, Universidad Nacional Siglo XX, Llallagua, Bolivia, 2007. [Google Scholar]
- Maruyama, S.R.; de Santana, A.K.; Takamiya, N.T.; Takahashi, T.Y.; Rogerio, L.A.; Oliveira, C.A.; Milanezi, C.M.; Trombela, V.A.; Cruz, A.K.; Jesus, A.R.; et al. Non-Leishmania parasite in fatal visceral leishmaniasis-like disease, Brazil. Emerg. Infect. Dis. 2019, 25, 2088–2092. [Google Scholar] [CrossRef]
- Ghobakhloo, N.; Motazedian, M.H.; Naderi, S.; Ebrahimi, S. Isolation of Crithidia spp. from lesions of immunocompetent patients with suspected cutaneous leishmaniasis in Iran. Trop. Med. Int. Health 2019, 24, 116–126. [Google Scholar] [CrossRef]
- Boucinha, C.; Andrade-Neto, V.V.; Ennes-Vidal, V.; Branquinha, M.H.; Santos, A.L.S.; Torres-Santos, E.C. A stroll through the history of monoxenous trypanosomatids infection in vertebrate hosts. Front. Cell. Infect. Microbiol. 2022, 12, 804707. [Google Scholar] [CrossRef] [PubMed]
- Fakhar, M.; Derakhshani-Nia, M.; Gohardehi, S.; Karamian, M.; Hezarjaribi, H.Z.; Mohebali, M.; Akhoundi, B.; Sharbatkhori, M. Domestic dogs carriers of Leishmania infantum, Leishmania tropica, and Crithidia fasciculata as potential reservoirs for human visceral leishmaniasis in northeastern Iran. Vet. Med. Sci. 2022, 8, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, E.S.; Santos, G.Q.; da Silva, M.V.; Barros, J.H.S.; Bernardo, A.R.; Diniz, R.L.; Rubim, N.M.; Roque, A.L.R.; Jansen, A.M.; Silva, E.D.; et al. Chagas immunochromatographic rapid test in the serological diagnosis of Trypanosoma cruzi infection in wild and domestic canids. Front. Cell. Infect. Microbiol. 2022, 12, 835383. [Google Scholar] [CrossRef]
- Rogerio, L.A.; Takahashi, T.Y.; Cardoso, L.; Takamiya, N.T.; de Melo, E.V.; de Jesus, A.R.; de Oliveira, F.A.; Forrester, S.; Jeffares, D.C.; da Silva, J.S.; et al. Co-infection of Leishmania infantum and a Crithidia-related species in a case of refractory relapsed visceral leishmaniasis with non-ulcerated cutaneous manifestation in Brazil. Int. J. Infect. Dis. 2023, 133, 85–88. [Google Scholar] [CrossRef]
- Takamiya, N.T.; Rogerio, L.A.; Torres, C.; Leonel, J.A.F.; Vioti, G.; Oliveira, T.M.F.d.S.; Valeriano, K.C.; Porcino, G.N.; Santos, I.K.F.d.M.; Costa, C.H.N.; et al. Parasite detection in visceral leishmaniasis samples by dye-based qPCR using new gene targets of Leishmania infantum and Crithidia. Trop. Med. Infect. Dis. 2023, 8, 405. [Google Scholar] [CrossRef]
- Fadhil, S.A.; Ali, M.J. Molecular identification of Crithidia sp. from naturally infected dogs. Iraqi J. Vet. Sci. 2024, 38, 831–837. [Google Scholar] [CrossRef]
- Khademi, A.; Mohammadi, Z.; Tohidi, F. Investigating Crithidia spp. in ulcer smear of patients suspected of leishmaniasis in Aq-Qala, Golestan province, Northern Iran, 2019–2020. Jorjani Biomed. J. 2024, 12, 15–17. [Google Scholar]
- Boucinha, C.; Caetano, A.R.; Santos, H.L.; Helaers, R.; Vikkula, M.; Branquinha, M.H.; dos Santos, A.L.S.; Grellier, P.; Morelli, K.A. Analysing ambiguities in trypanosomatids taxonomy by barcoding. Mem. Inst. Oswaldo Cruz 2020, 115, e200504. [Google Scholar] [CrossRef]
- Kraeva, N.; Butenko, A.; Hlaváčová, J.; Kostygov, A.; Myškova, J.; Grybchuk, D.; Leštinová, T.; Votýpka, J.; Volf, P.; Opperdoes, F.; et al. Leptomonas seymouri: Adaptations to the dixenous life cycle analyzed by genome sequencing, transcriptome profiling and co-infection with Leishmania donovani. PLoS Pathog. 2015, 11, e1005127. [Google Scholar] [CrossRef] [PubMed]
- Sukla, S.; Nath, H.; Kamran, M.; Ejazi, S.A.; Ali, N.; Das, P.; Ravichandiran, V.; Roy, S.; Biswas, S. Detection of Leptomonas seymouri in an RNA-like virus in serum samples of visceral leishmaniasis patients and its possible role in disease pathogenesis. Sci. Rep. 2022, 12, 14436. [Google Scholar] [CrossRef]
- Ennes-Vidal, V.; Vitório, B.S.; Menna-Barreto, R.F.S.; Pitaluga, A.N.; Gonçalves-da-Silva, S.A.; Branquinha, M.H.; Santos, A.L.S. Calpains of Leishmania braziliensis: Genome analysis, differential expression, and functional analysis. Mem. Inst. Oswaldo Cruz 2019, 114, e190147. [Google Scholar] [CrossRef] [PubMed]
- Bombaça, A.C.S.; Gandara, A.C.P.; Ennes-Vidal, V.; Bottino-Rojas, V.; Dias, F.A.; Farnesi, L.C.; Sorgine, M.H.; Bahia, A.C.; Bruno, R.V.; Menna-Barreto, R.F.S. Aedes aegypti infection with trypanosomatid Strigomonas culicis alters midgut redox metabolism and reduces mosquito reproductive fitness. Front. Cell. Infect. Microbiol. 2021, 11, 732925. [Google Scholar] [CrossRef] [PubMed]
- Santa-Rita, R.M.; Henriques-Pons, A.; Barbosa, H.S.; De Castro, S.L. Effect of the lysophospholipid analogues edelfosine, ilmofosine and miltefosine against Leishmania amazonensis. J. Antimicrob. Chemother. 2004, 54, 704–710. [Google Scholar] [CrossRef]
- Andrade-Neto, V.V.; Cunha-Júnior, E.F.; Canto-Cavalheiro, M.M.D.; Atella, G.C.; Fernandes, T.d.A.; Costa, P.R.R.; Torres-Santos, E.C. Antileishmanial activity of Ezetimibe: Inhibition of sterol biosynthesis, In Vitro synergy with azoles, and efficacy in experimental cutaneous leishmaniasis. Antimicrob. Agents Chemother. 2016, 60, 6844–6852. [Google Scholar] [CrossRef]
- Inacio, J.D.F.; Gervazoni, L.; Canto-Cavalheiro, M.M.; Almeida-Amaral, E.E. The effect of (-)-epigallocatechin 3-O In Vitro and In Vivo in Leishmania braziliensis: Involvement of reactive oxygen species as a mechanism of action. PLoS Negl. Trop. Dis. 2014, 8, e3093. [Google Scholar] [CrossRef]
- Meireles, A.C.A.; Amoretty, P.R.; Souza, N.A.; Kyriacou, C.P.; Peixoto, A.A. Rhythmic expression of the cycle gene in a hematophagous insect vector. BMC Mol. Biol. 2006, 7, 38. [Google Scholar] [CrossRef]
- Gomes-Silva, A.; Valverde, J.G.; Ribeiro-Romão, R.P.; Placido-Pereira, R.M.; Da-Cruz, A.M. Golden hamster (Mesocricetus auratus) as an experimental model for Leishmania (Viannia) braziliensis infection. Parasitology 2013, 140, 771–779. [Google Scholar] [CrossRef]
- Volf, P.; Myskova, J. Sand flies and Leishmania: Specific versus permissive vectors. Trends Parasitol. 2007, 23, 91–92. [Google Scholar] [CrossRef]
- de Melo, L.V.; Vasconcelos dos Santos, T.; Ramos, P.K.; Lima, L.V.; Campos, M.B.; Silveira, F.T. Antigenic reactivity of Leishmania (Viannia) lainsoni axenic amastigote proved to be a suitable alternative for optimizing Montenegro skin test. Parasites Vectors 2024, 17, 402. [Google Scholar] [CrossRef]
- Kaewmee, S.; Mano, C.; Phanitchakun, T.; Ampol, R.; Yasanga, T.; Pattanawong, U.; Junkum, A.; Siriyasatien, P.; Bates, P.A.; Jariyapan, N. Natural infection with Leishmania (Mundinia) martiniquensis supports Culicoides peregrinus (Diptera: Ceratopogonidae) as a potential vector of leishmaniasis and characterization of a Crithidia sp. isolated from the midges. Front. Microbiol. 2023, 14, 1235254. [Google Scholar] [CrossRef]
- Domagalska, M.A.; Dujardin, J. Non-Leishmania parasite in fatal visceral leishmaniasis-like disease, Brazil. Emerg. Infect. Dis. 2020, 26, 388. [Google Scholar] [CrossRef]
- Barral-Netto, M.; Badaró, R.; Barrai, A.; Carvalho, E.M. Imunologia da Leishmaniose Tegumentar. Rev. Soc. Bras. Med. Trop. 1986, 19, 173–191. [Google Scholar] [CrossRef]
- Kaye, P.; Scott, P. Leishmaniasis: Complexity at the host-pathogen interface. Nat. Rev. Microbiol. 2011, 9, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.L.; Freire, M.L.; Troian, I.L.; Morais-Teixeira, E.; Cota, G. Local amphotericin B therapy for cutaneous leishmaniasis: A systematic review. PLoS Negl. Trop. Dis. 2024, 18, e0012127. [Google Scholar] [CrossRef] [PubMed]
- Bacchi, C.J.; Lambros, C.; Goldberg, B.; Hutner, S.H.; De Carvalho, G.D.F. Susceptibility of an insect Leptomonas and Crithidia fasciculata to several established anti-trypanosomatid agents. Antimicrob. Agents Chemother. 1974, 6, 785. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghafli, H.; Barribeau, S.M. Double trouble: Trypanosomatids with two hosts have lower infection prevalence than single host trypanosomatids. Evol. Med. Public Health 2023, 11, 202–218. [Google Scholar] [CrossRef]
- Ferreira, T.d.S.; Minuzzi-Souza, T.T.C.; de Andrade, A.J.; Coelho, T.O.; Rocha, D.d.A.; Obara, M.T.; Hecht, M.; Nitz, N.; Gurgel-Gonçalves, R. Molecular detection of Trypanosoma sp. and Blastocrithidia sp. (Trypanosomatidae) in phlebotomine sand flies (Psychodidae) in the Federal District of Brazil. Rev. Soc. Bras. Med. Trop. 2015, 48, 776–779. [Google Scholar] [CrossRef]
- Kalantari, M.; Motazedian, M.H.; Asgari, Q.; Soltani, Z.; Soltani, A.; Azizi, K. Bionomics of phlebotomine sand flies species (Diptera: Psychodidae) and their natural infection with Leishmania and Crithidia in Fars Province, Southern Iran. J. Parasit. Dis. 2018, 42, 511–518. [Google Scholar] [CrossRef]
- Tanure, A.; Rêgo, F.D.; Tonelli, G.B.; Campos, A.M.; Shimabukuro, P.H.F.; Gontijo, C.M.F.; Paz, G.F.; Andrade-Filho, J.D. Diversity of phlebotomine sand flies and molecular detection of trypanosomatids in Brumadinho, Minas Gerais, Brazil. PLoS ONE 2020, 15, e0234445. [Google Scholar] [CrossRef]
- Songumpai, N.; Promrangsee, C.; Noopetch, P.; Siriyasatien, P.; Preativatanyou, K. First evidence of co-circulation of emerging Leishmania martiniquensis, Leishmania orientalis, and Crithidia sp. in Culicoides biting midges (Diptera: Ceratopogonidae), the putative vectors for autochthonous transmission in Southern Thailand. Trop. Med. Infect. Dis. 2022, 7, 379. [Google Scholar] [CrossRef]
- Ennes-Vidal, V.; Pitaluga, A.N.; Britto, C.F.P.C.; Branquinha, M.H.; dos Santos, A.L.S.; Menna-Barreto, R.F.S.; D’aVila-Levy, C.M. Expression and cellular localisation of Trypanosoma cruzi calpains. Mem. Inst. Oswaldo Cruz 2020, 115, e200142. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, S.; Bahrami, F.; Parvizi, P.; Fard-Esfahani, P.; Ajdary, S. An overview of the sand fly salivary proteins in vaccine development against leishmaniases. Iran J. Microbiol. 2022, 14, 792–801. [Google Scholar] [CrossRef]

| Target | Primer/Probe | Sequence (5′-3′) |
|---|---|---|
| C. fasciculata SSU rRNA | Forward | CCGTGCCCTCAAGAACAT |
| Reverse | GGGATGTTCACACCGTACAA | |
| Probe | FAM-TGCACAAGAAGAAGCAGGAGCAGA-3IABkFQ | |
| L. braziliensis SSU rRNA | Forward | TGACGAACCCACACAACAA |
| Reverse | GGTCGCGAATTATCTCCCAATA | |
| Probe | HEX-ACCGAACGAAAGCTGAACCACACT-3IABkFQ | |
| Mouse β-tubulin (Mm.PT.39a.22214835, IDT Inc.) | Forward | GGGTGGAACTGTGTTACGTAG |
| Reverse | TGGTCTTTCTGGTGCTTGTC | |
| Probe | Cy5-CCGGAGAATGGGAAGCCGAACATAc-3IAbRQSp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.F.B.d.; Martins, C.B.; Andrade-Neto, V.V.; Lemos-Silva, T.; dos Santos, R.F.; Gonçalves da-Silva, S.A.; Traub-Csekö, Y.M.; Menna-Barreto, R.F.S.; Torres-Santos, E.C.; d’Avila, C.M.; et al. Crithidia fasciculata Shows Non-Pathogenic Behavior in Leishmania Co-Infection Related to Temperature Stress, In Vitro and In Vivo Infections, and Amphotericin B Susceptibility. Microorganisms 2025, 13, 2335. https://doi.org/10.3390/microorganisms13102335
Santos JFBd, Martins CB, Andrade-Neto VV, Lemos-Silva T, dos Santos RF, Gonçalves da-Silva SA, Traub-Csekö YM, Menna-Barreto RFS, Torres-Santos EC, d’Avila CM, et al. Crithidia fasciculata Shows Non-Pathogenic Behavior in Leishmania Co-Infection Related to Temperature Stress, In Vitro and In Vivo Infections, and Amphotericin B Susceptibility. Microorganisms. 2025; 13(10):2335. https://doi.org/10.3390/microorganisms13102335
Chicago/Turabian StyleSantos, Julia Fernandes Barbosa dos, Carolina Boucinha Martins, Valter Viana Andrade-Neto, Thais Lemos-Silva, Rosiane Freire dos Santos, Silvia Amaral Gonçalves da-Silva, Yara Maria Traub-Csekö, Rubem Figueiredo Sadok Menna-Barreto, Eduardo Caio Torres-Santos, Claudia Masini d’Avila, and et al. 2025. "Crithidia fasciculata Shows Non-Pathogenic Behavior in Leishmania Co-Infection Related to Temperature Stress, In Vitro and In Vivo Infections, and Amphotericin B Susceptibility" Microorganisms 13, no. 10: 2335. https://doi.org/10.3390/microorganisms13102335
APA StyleSantos, J. F. B. d., Martins, C. B., Andrade-Neto, V. V., Lemos-Silva, T., dos Santos, R. F., Gonçalves da-Silva, S. A., Traub-Csekö, Y. M., Menna-Barreto, R. F. S., Torres-Santos, E. C., d’Avila, C. M., & Ennes-Vidal, V. (2025). Crithidia fasciculata Shows Non-Pathogenic Behavior in Leishmania Co-Infection Related to Temperature Stress, In Vitro and In Vivo Infections, and Amphotericin B Susceptibility. Microorganisms, 13(10), 2335. https://doi.org/10.3390/microorganisms13102335









