Therapeutic Repurposing of Sertraline: Evidence for Its Antifungal Activity from In Vitro, In Vivo, and Clinical Studies
Abstract
1. Introduction
| Focus | Key Findings | Ref. |
|---|---|---|
| Mechanisms of antifungal resistance | Target mutations (ERG11/FKS), efflux (ABC/MFS), biofilm tolerance, Hsp90–calcineurin stress response, genomic plasticity/aneuploidy. | [4] |
| Pharmacodynamics | SSRI; increases synaptic serotonin via reuptake inhibition. | [6] |
| Pharmacokinetics | Metabolized by CYP2B6/CYP2C19/CYP2D6; steady state ~7 days. | [7] |
| Metabolism | N-desmethylsertraline; half-life 24–26 h; once-daily dosing. | [17] |
| Exposure vs. MIC | Conventional doses may not reach antifungal MICs. | [18] |
| Repurposing | Antifungal potential noted; activity vs. Cryptococcus neoformans. | [8] |
2. Materials and Methods
2.1. Study Design and Reporting Framework
2.2. Information Sources and Search Strategy
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Data Extraction
2.6. Outcomes and Operational Definitions
- Synergy (checkerboard): Fractional inhibitory concentration (FIC) index ≤ 0.5; antagonism: FIC ≥ 4.
- Synergy (time–kill): ≥2 log10 CFU/mL reduction in the combination vs. the most active single agent at matched time points.
- Biofilm outcomes: Changes in biomass or viability (e.g., crystal violet/XTT), or inhibition of biofilm formation vs. appropriate controls.
2.7. Risk of Bias and Certainty
2.8. Synthesis Methods
2.9. Ethics
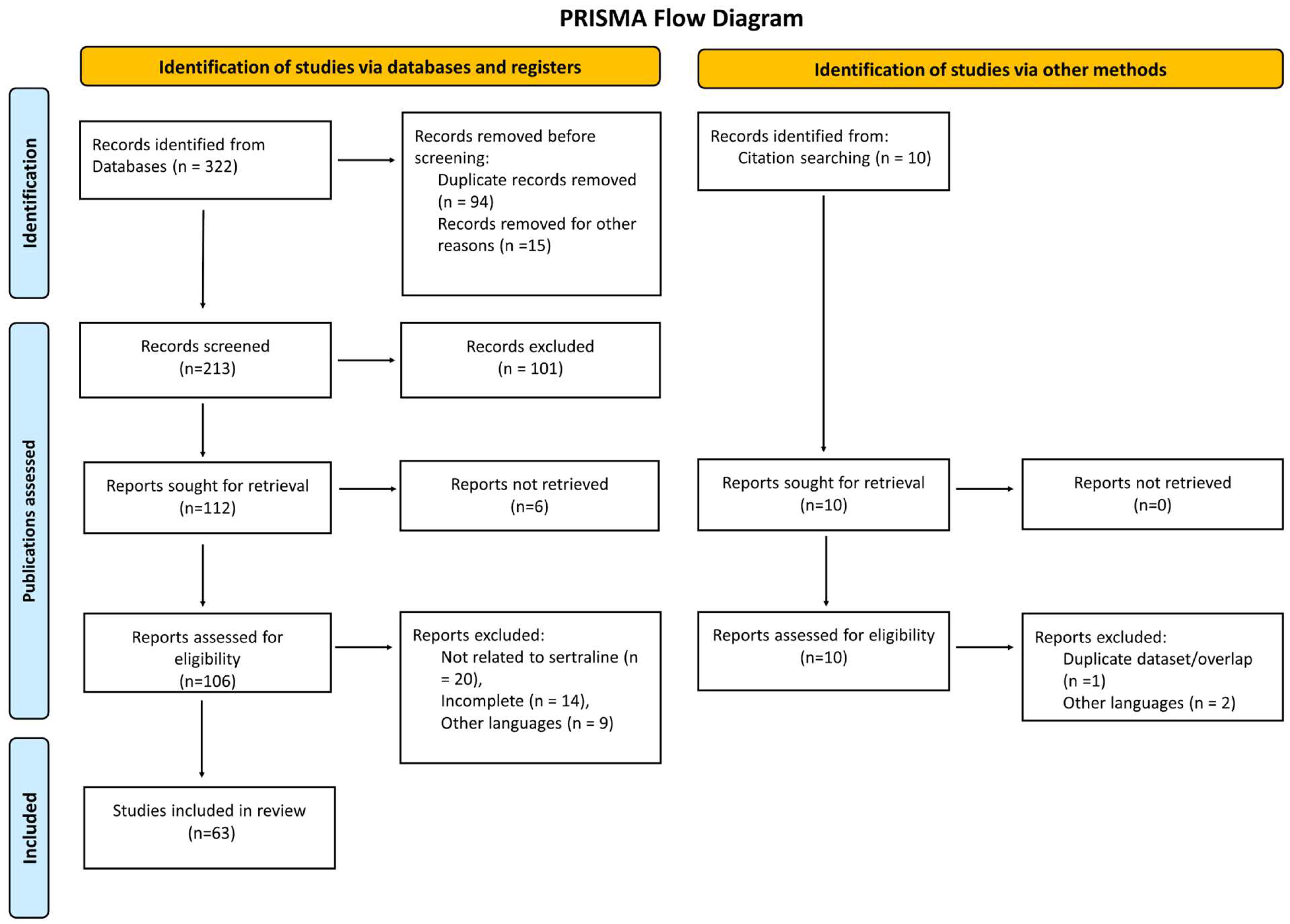
3. Results
| Mechanism of Action | Target Organism(s)/Context | Key Notes | Ref. |
|---|---|---|---|
| β-1,3-glucan synthesis blockade | Candida spp. (cell wall) | Cell wall impairment | [10] |
| Membrane lipid interaction/disruption | Candida spp. | Loss of integrity, lipid bilayer destabilization | [12,14] |
| Oxidative stress induction (↑ ROS) | Candida spp., Cryptococcus neoformans | ROS accumulation | [35] |
| Mitochondrial dysfunction | Candida spp., Cryptococcus spp. | ↓ ATP production, apoptosis induction | [35,36] |
| Efflux pump downregulation (Cdr1p) | C. albicans | ↑ Intracellular azole concentration | [37] |
| Ribosomal inhibition/↓ translation | S. cerevisiae, Cryptococcus spp. | Impaired protein synthesis, ↓ ribosome assembly | [13,38] |
| Calcium homeostasis disruption | Fungal cells | Abnormal Ca2+ influx, signaling alteration | [39,40] |
| Study Type/Setting | Regimen (Alone/Combination) | Main Outcomes/Notes | Ref. |
|---|---|---|---|
| Clinical trial (Tanzania) | AMB 0.7–1 mg/kg × 5 days + SRT 400 mg/day + FLC 1200 mg/day | ↑ Survival; ↑ CSF clearance in cryptococcal meningitis. | [24] |
| Clinical trial | Short-course AMB + SRT 400 mg/day + FLC 1200 mg/day | Improved outcomes in cryptococcal meningitis. | [25] |
| RCT (ASTRO-CM) | Adjunctive sertraline | No survival benefit; dosing/levels issues raised. | [41] |
| Rationale/PK | — | CNS penetration supports adjuvant role where AMB is limited. | [16] |
| Model | Pathogen(s) | Antifungal Partner(s) | Key Findings | Ref. |
|---|---|---|---|---|
| in vitro (biofilm) | Candida spp. | — | ↓ viability > 80%; ALS3-mediated prevention. | [30] |
| in vitro (biofilm) | C. auris | — | ~71% biofilm inhibition; morphogenesis/membrane effects. | [31] |
| in vitro | C. auris | FLU/MCF | Inhibition at clinically relevant levels; synergy. | [42] |
| in vivo (murine model) | C. auris | VOR (±) | Reduced fungal burden in vivo. | [43] |
| in vitro | Cryptococcus neoformans | — | High susceptibility to SRT. | [27] |
| Combination (with SRT) | Pathogen(s) | Interaction | Ref. |
|---|---|---|---|
| FLU | C. auris | Synergy | [42] |
| FLU | C. neoformans | Adjunct regimen (no formal synergy metric) | [24] |
| VOR | C. auris | Synergy | [43] |
| MCF | C. auris | Synergy | [42] |
| AMB | C. neoformans | Synergy | [27] |
| VOR | Purpureocillium lilacinum | Antagonism | [32] |
4. Discussion
| Category | Key Note/Recommendation | Ref. |
|---|---|---|
| Safety/tolerability | Generally well tolerated; rare risks: serotonin syndrome, QTc prolongation, hyponatremia. | [54] |
| Safety/interactions | Monitor adverse effects and CYP-mediated interactions in severe infections. | [55] |
| Barrier | Lack of IV formulation limits use in critically ill or rapidly progressive disease. | [58] |
| Future direction | Define concentration–response (PK/PD) for antifungal activity. | [59] |
| Future direction | Biofilm-focused and mixed-community studies. | [63] |
5. Conclusions
6. Future Perspectives (Considering Current Limitations)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeVane, C.L.; Liston, H.L.; Markowitz, J.S. Clinical pharmacokinetics of sertraline. Clin. Pharmacokinet. 2002, 41, 1247–1266. [Google Scholar] [CrossRef] [PubMed]
- McRae, A.L.; Brady, K.T. Review of sertraline and its clinical applications in psychiatric disorders. Expert Opin. Pharmacother. 2001, 2, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Alhadab, A.A.; Brundage, R.C. Population pharmacokinetics of sertraline in healthy subjects: A model-based meta-analysis. AAPS J. 2020, 22, 73. [Google Scholar] [CrossRef]
- Lee, Y.; Robbins, N.; Cowen, L.E. Molecular mechanisms governing antifungal drug resistance. npj Antimicrob. Resist. 2023, 1, 5. [Google Scholar] [CrossRef]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef]
- Cipriani, A.; La Ferla, T.; Furukawa, T.A.; Signoretti, A.; Nakagawa, A.; Churchill, R.; McGuire, H.; Barbui, C. Sertraline versus other antidepressive agents for depression. Cochrane Database Syst. Rev. 2010, 4, CD006117. [Google Scholar] [CrossRef]
- Hiemke, C.; Härtter, S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol. Ther. 2000, 85, 11–28. [Google Scholar] [CrossRef]
- Alhadab, A.A.; Rhein, J.; Tugume, L.; Musubire, A.; Williams, D.A.; Abassi, M.; Nicol, M.R.; Meya, D.B.; Boulware, D.R.; Brundage, R.C.; et al. Pharmacokinetics-pharmacodynamics of sertraline as an antifungal in HIV-infected Ugandans with cryptococcal meningitis. J. Pharmacokinet. Pharmacodyn. 2019, 46, 565–576. [Google Scholar] [CrossRef]
- Rodrigues, D.S.; Cabral, V.P.; Barbosa, A.D.; Valente Sá, L.G.; Silva, C.R.; Moreira, L.E.; Neto, J.B.; Silva, J.; Santos, H.S.; Marinho, E.S.; et al. Sertraline has fungicidal activity against Candida spp. and acts by inhibiting membrane and cell wall biosynthesis. Future Microbiol. 2023, 18, 1025–1039. [Google Scholar] [CrossRef]
- Nivoix, Y.; Ledoux, M.P.; Herbrecht, R. Antifungal therapy: New and evolving therapies. Semin. Respir. Crit. Care Med. 2020, 41, 158–174. [Google Scholar] [CrossRef]
- Li, Y.; Couch, L.; Higuchi, M.; Fang, J.L.; Guo, L. Mitochondrial dysfunction induced by sertraline, an antidepressant agent. Toxicol. Sci. 2012, 127, 582–591. [Google Scholar] [CrossRef]
- Barbarossa, A.; Rosato, A.; Carrieri, A.; Fumarola, L.; Tardugno, R.; Corbo, F.; Fracchiolla, G.; Carocci, A. Exploring the antibiofilm effect of sertraline in synergy with Cinnamomum verum essential oil to counteract Candida species. Pharmaceuticals 2024, 17, 1109. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.H.L.; Galvão Rocha, F.M.; Sanches, P.R.; Rossi, A.; Martinez-Rossi, N.M. The antidepressant sertraline modulates gene expression and alternative splicing events in the dermatophyte Trichophyton rubrum: A comprehensive analysis. Genes 2025, 16, 146. [Google Scholar] [CrossRef] [PubMed]
- Then, C.K.; Liu, K.H.; Liao, M.H.; Chung, K.H.; Wang, J.Y.; Shen, S.C. Antidepressants, sertraline and paroxetine, increase calcium influx and induce mitochondrial damage-mediated apoptosis of astrocytes. Oncotarget 2017, 8, 115490–115502. [Google Scholar] [CrossRef] [PubMed]
- Rhein, J.; Huppler Hullsiek, K.; Tugume, L.; Nuwagira, E.; Mpoza, E.; Evans, E.E.; Kiggundu, R.; Pastick, K.A.; Ssebambulidde, K.; Akampurira, A.; et al. Adjunctive sertraline for HIV-associated cryptococcal meningitis: A randomised, placebo-controlled, double-blind phase 3 trial. Lancet Infect. Dis. 2019, 19, 843–851. [Google Scholar] [CrossRef]
- Rhein, J.; Hullsiek, K.H.; Evans, E.E.; Tugume, L.; Nuwagira, E.; Ssebambulidde, K.; Kiggundu, R.; Mpoza, E.; Musubire, A.K.; Bangdiwala, A.S.; et al. Detrimental outcomes of unmasking cryptococcal meningitis with recent ART initiation. Open Forum Infect. Dis. 2018, 5, ofy122. [Google Scholar] [CrossRef]
- Obach, R.S.; Cox, L.M.; Tremaine, L.M. Sertraline is metabolized by multiple cytochrome P450 enzymes, monoamine oxidases, and glucuronyl transferases in humans: An in vitro study. Drug Metab. Dispos. 2005, 33, 262–270. [Google Scholar] [CrossRef]
- Smith, K.D.; Achan, B.; Hullsiek, K.H.; Mcdonald, T.R.; Okagaki, L.H.; Alhadab, A.A.; Akampurira, A.; Rhein, J.R.; Meya, D.B.; Boulware, D.R.; et al. Increased Antifungal Drug Resistance in Clinical Isolates of Cryptococcus neoformans in Uganda. Antimicrob. Agents Chemother. 2015, 59, 7197–7204. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Powderly, W.G. Current approach to the acute management of cryptococcal infections. J. Infect. 2000, 41, 18–22. [Google Scholar] [CrossRef]
- Ahmed, E.I.; Alhuwaydi, A.M.; Taha, A.E.; Abouelkheir, M. Anti-candidal activity of reboxetine and sertraline antidepressants: Effects on pre-formed biofilms. Antibiotics 2023, 12, 881. [Google Scholar] [CrossRef]
- Breuer, M.R.; Dasgupta, A.; Vasselli, J.G.; Lin, X.; Shaw, B.D.; Sachs, M.S. The antidepressant sertraline induces the formation of supersized lipid droplets in the human pathogen Cryptococcus neoformans. J. Fungi 2022, 8, 642. [Google Scholar] [CrossRef] [PubMed]
- Wilton, L.; Kollarova, M.; Heeley, E.; Shakir, S. Relative risk of vaginal candidiasis after use of antibiotics compared with antidepressants in women: Postmarketing surveillance data in England. Drug Saf. 2003, 26, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Katende, A.; Mbwanji, G.; Faini, D.; Nyuri, A.; Kalinjuma, A.V.; Mnzava, D.; Hullsiek, K.H.; Rhein, J.; Weisser, M.; Meya, D.B.; et al. Short-course amphotericin B in addition to sertraline and fluconazole for treatment of HIV-associated cryptococcal meningitis in rural Tanzania. Mycoses 2019, 62, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Molloy, S.F.; Kanyama, C.; Heyderman, R.S.; Loyse, A.; Kouanfack, C.; Chanda, D.; Mfinanga, S.; Temfack, E.; Lakhi, S.; Lesikari, S.; et al. Antifungal combinations for treatment of cryptococcal meningitis in Africa. N. Engl. J. Med. 2018, 378, 1004–1017. [Google Scholar] [CrossRef]
- Trpković, A.; Pekmezović, M.; Barać, A.; Crnčević Radović, L.; Arsić Arsenijević, V. In vitro antifungal activities of amphotericin B, 5-fluorocytosine, fluconazole and itraconazole against Cryptococcus neoformans isolated from cerebrospinal fluid and blood from patients in Serbia. J. Mycol. Med. 2012, 22, 243–248. [Google Scholar] [CrossRef]
- Treviño-Rangel, R.d.J.; Villanueva-Lozano, H.; Hernández-Rodríguez, P.; Martínez-Reséndez, M.F.; García-Juárez, J.; Rodríguez-Rocha, H.; González, G.M. Activity of sertraline against Cryptococcus neoformans: In vitro and in vivo assays. Med. Mycol. 2016, 54, 280–286. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Martinez-de-Oliveira, J.; Donders, G.G.G.; Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A. Anti-Candida activity of antidepressants sertraline and fluoxetine: Effect upon pre-formed biofilms. Med. Microbiol. Immunol. 2018, 207, 195–200. [Google Scholar] [CrossRef]
- Hussen, I.; Aliyo, A.; Abbai, M.K.; Dedecha, W. Vaginal candidiasis prevalence, associated factors, and antifungal susceptibility patterns among pregnant women attending antenatal care at Bule Hora University Teaching Hospital, Southern Ethiopia. BMC Pregnancy Childbirth 2024, 24, 619. [Google Scholar] [CrossRef]
- Rodrigues, D.S.; Cabral, V.P.F.; Barbosa, A.D.; Sá, L.G.D.A.V.; Moreira, L.E.A.; de Andrade Neto, J.B.; da Silva, C.R.; de Moraes, M.O.; Silva, J.; Marinho, E.S.; et al. Sertraline has in vitro activity against both mature and forming biofilms of different Candida species. J. Med. Microbiol. 2023, 72, 001664. [Google Scholar] [CrossRef]
- Donlin, M.J.; Meyers, M.J. Repurposing and optimization of drugs for discovery of novel antifungals. Drug Discov. Today 2022, 27, 2008–2014. [Google Scholar] [CrossRef]
- Villanueva-Lozano, H.; González, G.M.; Espinosa-Mora, J.E.; Bodden-Mendoza, B.A.; Andrade, A.; Martínez-Reséndez, M.F.; Treviño-Rangel, R.J. Evaluation of the in vitro interaction of sertraline with voriconazole and amphotericin B against non-Aspergillus filamentous fungi from clinical isolates in northeastern Mexico. J. Infect. Chemother. 2019, 25, 757–764. [Google Scholar]
- Treviño-Rangel, R.J.; Villanueva-Lozano, H.; Méndez-Galomo, K.S.; Solís-Villegas, E.M.; Becerril-García, M.A.; Montoya, A.M.; Robledo-Leal, E.R.; González, G.M. In vivo evaluation of the antifungal activity of sertraline against Aspergillus fumigatus. J. Antimicrob. Chemother. 2019, 74, 663–666. [Google Scholar] [CrossRef]
- Farmenks, J.D.; Reed, S.L.; Seidel, D.; Koehler, P.; Cornely, O.A.; Mehta, S.R.; Hoenigl, M. Rare mould infections caused by Mucorales, Lomentospora prolificans and Fusarium, in San Diego, CA: The role of antifungal combination therapy. Int. J. Antimicrob. Agents 2018, 52, 706–712. [Google Scholar] [CrossRef]
- Galvão-Rocha, F.M.; Rocha, C.H.L.; Martins, M.P.; Sanches, P.R.; Bitencourt, T.A.; Sachs, M.S.; Martinez-Rossi, N.M.; Rossi, A. The antidepressant sertraline affects cell signaling and metabolism in Trichophyton rubrum. J. Fungi 2023, 9, 275. [Google Scholar] [CrossRef]
- Krahmer, N.; Guo, Y.; Wilfling, F.; Hilger, M.; Lingrell, S.; Heger, K.; Newman, H.W.; Schmidt-Supprian, M.; Vance, D.E.; Mann, M.; et al. Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP:phosphocholine cytidylyltransferase. Cell Metab. 2011, 14, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.T.; Fink, G.R.; Bartel, D.P. Excised linear introns regulate growth in yeast. Nature 2019, 565, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Rainey, M.M.; Korostyshevsky, D.; Lee, S.; Perlstein, E.O. The antidepressant sertraline targets intracellular vesiculogenic membranes in yeast. Genetics 2010, 185, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Muzafar, S.; Sharma, R.D.; Shah, A.H.; Gaur, N.A.; Dasgupta, U.; Chauhan, N.; Prasad, R. Identification of genomewide alternative splicing events in sequential, isogenic clinical isolates of Candida albicans reveals a novel mechanism of drug resistance and tolerance to cellular stresses. mSphere 2020, 5, e00608–e00620. [Google Scholar] [CrossRef]
- Chinnapaka, S.; Bakthavachalam, V.; Munirathinam, G. Repurposing antidepressant sertraline as a pharmacolog-ical drug to target prostate cancer stem cells: Dual activation of apoptosis and autophagy signaling by deregulating redox balance. Am. J. Cancer Res. 2020, 10, 2043–2065. [Google Scholar]
- Rossato, L.; Loreto, É.S.; Zanette, R.A.; Chassot, F.; Santurio, J.M.; Alves, S.H. In vitro synergistic effects of chlorpromazine and sertraline in combination with amphotericin B against Cryptococcus neoformans var. grubii. Folia Microbiol. 2016, 61, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Costa Silva, R.A.; da Silva, C.R.; de Andrade Neto, J.B.; da Silva, A.R.; Campos, R.S.; Sampaio, L.S.; do Nascimento, F.B.S.A.; da Silva Gaspar, B.; da Cruz Fonseca, S.G.; Josino, M.A.A.; et al. In vitro anti-Candida activity of selective serotonin reuptake inhibitors against fluconazole-resistant strains and their activity against biofilm-forming isolates. Microb. Pathog. 2017, 107, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Alanís-Ríos, S.A.; González, G.M.; Andrade, A.; Becerril-García, M.A.; Bonifaz, A.; Robledo-Leal, E.R.; Montoya, A.M.; Treviño-Rangel, R.J. Evaluation of the synergistic antifungal activity of micafungin and voriconazole plus sertraline against Candida auris. Braz. J. Microbiol. 2022, 53, 2003–2008. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef]
- Kim, J.H.; Cheng, L.W.; Chan, K.L.; Tam, C.C.; Mahoney, N.; Friedman, M.; Shilman, M.M.; Land, K.M. Antifungal drug repurposing. Antibiotics 2020, 9, 812. [Google Scholar] [CrossRef]
- Gowri, M.; Jayashree, B.; Jeyakanthan, J.; Girija, E.K. Sertraline as a promising antifungal agent: Inhibition of growth and biofilm of Candida auris with special focus on the mechanism of action in vitro. J. Appl. Microbiol. 2020, 128, 426–437. [Google Scholar] [CrossRef]
- Lamoth, F.; Juvvadi, P.R.; Steinbach, W.J. Heat shock protein 90 (Hsp90): A novel antifungal target against Aspergillus fumigatus. Crit. Rev. Microbiol. 2016, 42, 310–321. [Google Scholar]
- Lass-Flörl, C.; Dierich, M.P.; Fuchs, D.; Semenitz, E.; Ledochowski, M. Antifungal activity against Candida species of the selective serotonin-reuptake inhibitor, sertraline. Clin. Infect. Dis. 2001, 33, E135–E136. [Google Scholar] [CrossRef]
- Alanís-Ríos, S.A.; González, G.M.; Montoya, A.M.; Villanueva-Lozano, H.; Treviño-Rangel, R.J. Sertraline exhibits in vivo antifungal activity against Candida auris and enhances the effect of voriconazole in combination. Microb. Pathog. 2025, 199, 107212. [Google Scholar] [CrossRef]
- Ngan, N.T.T.; Flower, B.; Day, J.N. Treatment of cryptococcal meningitis: How have we got here and where are we going? Drugs 2022, 82, 1237–1249. [Google Scholar] [CrossRef]
- Tugume, L.; Rhein, J.; Hullsiek, K.H.; Mpoza, E.; Kiggundu, R.; Ssebambulidde, K.; Schutz, C.; Taseera, K.; Williams, D.A.; Abassi, M.; et al. HIV-associated cryptococcal meningitis occurring at relatively higher CD4 counts. J. Infect. Dis. 2019, 219, 877–883. [Google Scholar] [CrossRef]
- Rhein, J.; Morawski, B.M.; Hullsiek, K.H.; Nabeta, H.W.; Kiggundu, R.; Tugume, L.; Musubire, A.; Akampurira, A.; Smith, K.D.; Alhadab, A.; et al. Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: An open-label dose-ranging study. Lancet Infect. Dis. 2016, 16, 809–818. [Google Scholar] [CrossRef]
- Jang, J.H.; Jeong, S.H. Population pharmacokinetic modeling study and discovery of covariates for the antidepressant sertraline, a serotonin selective reuptake inhibitor. Comput. Biol. Med. 2024, 183, 109319. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Han, C.; Bahk, W.M.; Lee, S.J.; Patkar, A.A.; Masand, P.S.; Pae, C.U. Addressing the side effects of contemporary antidepressant drugs: A comprehensive review. Chonnam Med. J. 2018, 54, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Edinoff, A.N.; Akuly, H.A.; Hanna, T.A.; Ochoa, C.O.; Patti, S.J.; Ghaffar, Y.A.; Kaye, A.D.; Viswanath, O.; Urits, I.; Boyer, A.G.; et al. Selective serotonin reuptake inhibitors and adverse effects: A narrative review. Neurol. Int. 2021, 13, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Sauer, W.H.; Berlin, J.A.; Kimmel, S.E. Selective serotonin reuptake inhibitors and myocardial infarction. Circulation 2001, 104, 1894–1898. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifa, B.A.; Bulatova, N.R.; abuRokba, W.; Darwish, R.M. Serotonin reuptake inhibitors effect on fluconazole activity against resistant Candida glabrata strains. J. Glob. Antimicrob. Resist. 2022, 29, 49–54. [Google Scholar] [CrossRef]
- Gintjee, T.J.; Donnelley, M.A.; Thompson, G.R., III. Aspiring antifungals: Review of current antifungal pipeline developments. J. Fungi 2020, 6, 28. [Google Scholar] [CrossRef]
- Cui, X.; Wang, L.; Lü, Y.; Yue, C. Development and research progress of anti-drug resistant fungal drugs. J. Infect. Public Health 2022, 15, 986–1000. [Google Scholar] [CrossRef]
- Eldesouky, H.E.; Li, X.; Abutaleb, N.S.; Mohammad, H.; Seleem, M.N. Synergistic interactions of sulfamethoxazole and azole antifungal drugs against emerging multidrug-resistant Candida auris. Int. J. Antimicrob. Agents 2018, 52, 754–761. [Google Scholar] [CrossRef]
- Stukey, G.J.; Breuer, M.R.; Burchat, N.; Jog, R.; Schultz, K.; Han, G.S.; Sachs, M.S.; Sampath, H.; Marmorstein, R.; Carman, G.M. The antidepressant drug sertraline is a novel inhibitor of yeast Pah1 and human lipin 1 phosphatidic acid phosphatases. J. Lipid Res. 2025, 66, 100711. [Google Scholar] [CrossRef]
- Romanelli, M.M.; da Costa-Silva, T.A.; Cunha-Junior, E.; Ferreira, D.D.; Guerra, J.M.; Galisteo, A.J., Jr.; Pinto, E.G.; Barbosa, L.R.S.; Torres-Santos, E.C.; Tempone, A.G. Sertraline delivered in phosphatidylserine liposomes is effective in an experimental model of visceral leishmaniasis. Front. Cell. Infect. Microbiol. 2019, 9, 353. [Google Scholar] [CrossRef]
- Peyclit, L.; Yousfi, H.; Rolain, J.-M.; Bittar, F. Drug repurposing in medical mycology: Identification of compounds as potential antifungals to overcome the emergence of multidrug-resistant fungi. Pharmaceuticals 2021, 14, 488. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Cerdeira, C.; Eckhardt, W. Therapeutic Repurposing of Sertraline: Evidence for Its Antifungal Activity from In Vitro, In Vivo, and Clinical Studies. Microorganisms 2025, 13, 2334. https://doi.org/10.3390/microorganisms13102334
Rodríguez-Cerdeira C, Eckhardt W. Therapeutic Repurposing of Sertraline: Evidence for Its Antifungal Activity from In Vitro, In Vivo, and Clinical Studies. Microorganisms. 2025; 13(10):2334. https://doi.org/10.3390/microorganisms13102334
Chicago/Turabian StyleRodríguez-Cerdeira, Carmen, and Westley Eckhardt. 2025. "Therapeutic Repurposing of Sertraline: Evidence for Its Antifungal Activity from In Vitro, In Vivo, and Clinical Studies" Microorganisms 13, no. 10: 2334. https://doi.org/10.3390/microorganisms13102334
APA StyleRodríguez-Cerdeira, C., & Eckhardt, W. (2025). Therapeutic Repurposing of Sertraline: Evidence for Its Antifungal Activity from In Vitro, In Vivo, and Clinical Studies. Microorganisms, 13(10), 2334. https://doi.org/10.3390/microorganisms13102334








