Antiviral Activity of Glucosyl Hesperidin Against Feline Calicivirus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Cytotoxicity
2.3. Determination of Viral Infectivity
2.4. Statistical Analyses
3. Results
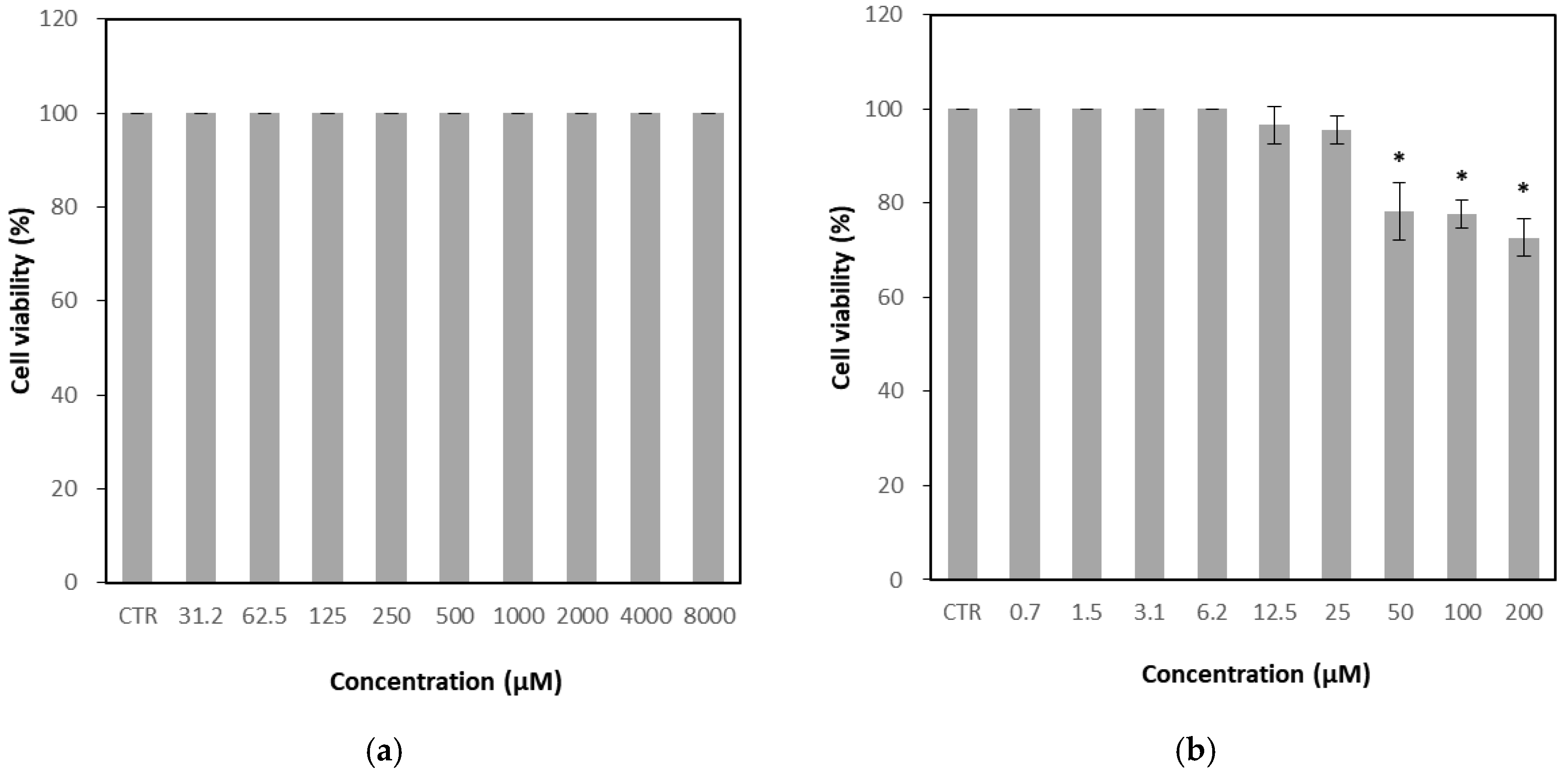
3.1. Determination of Cytotoxicity
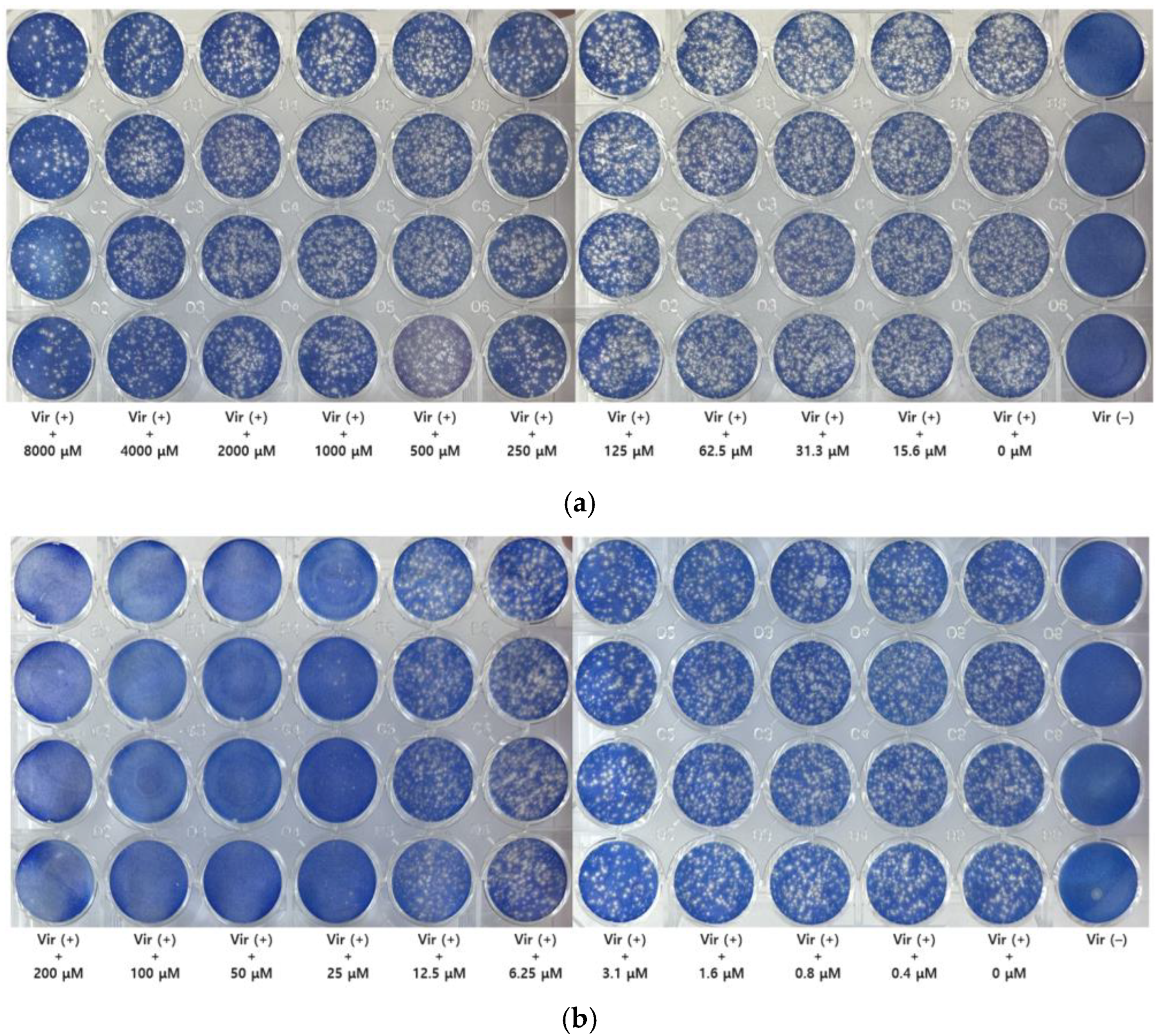
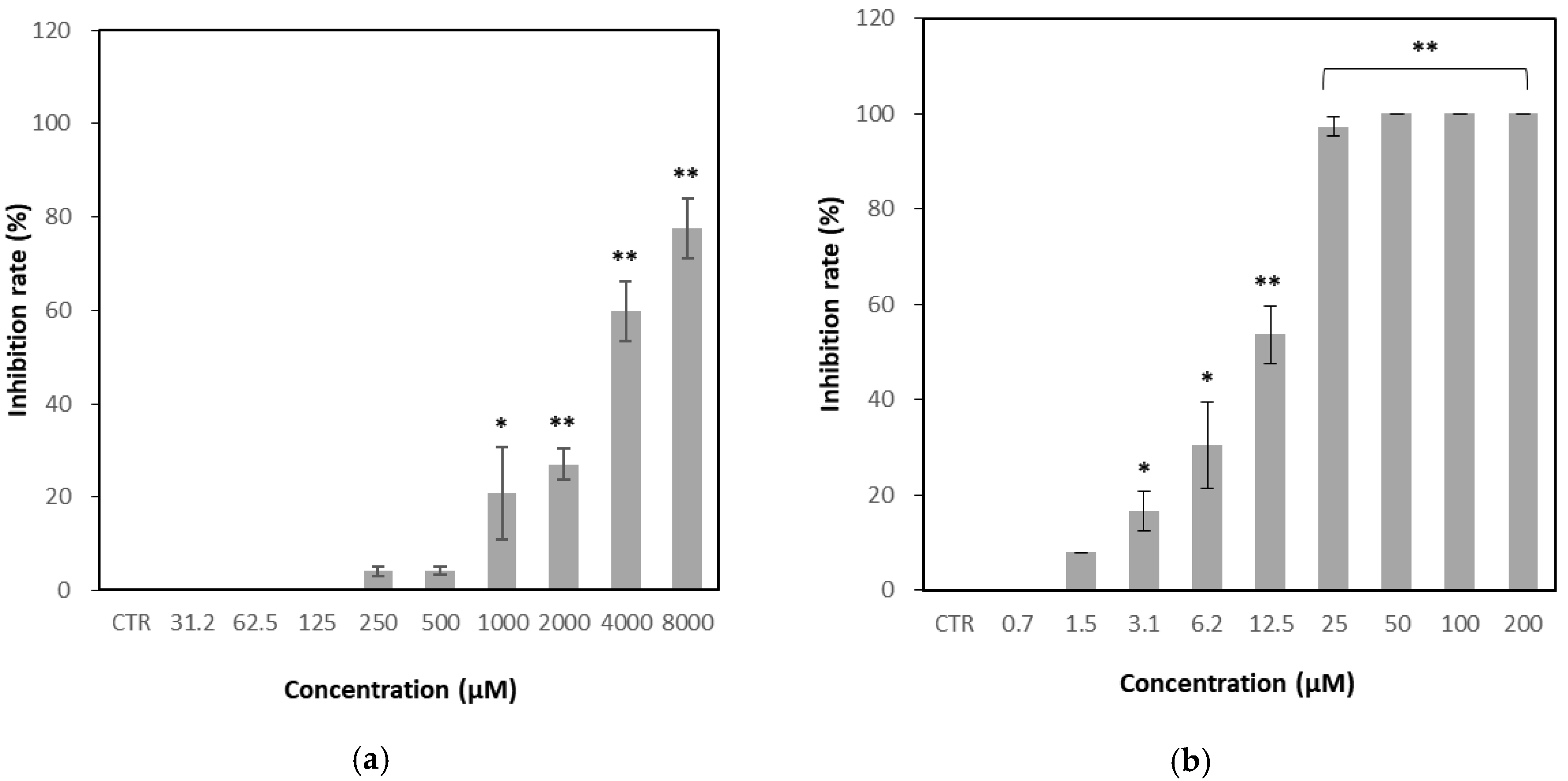
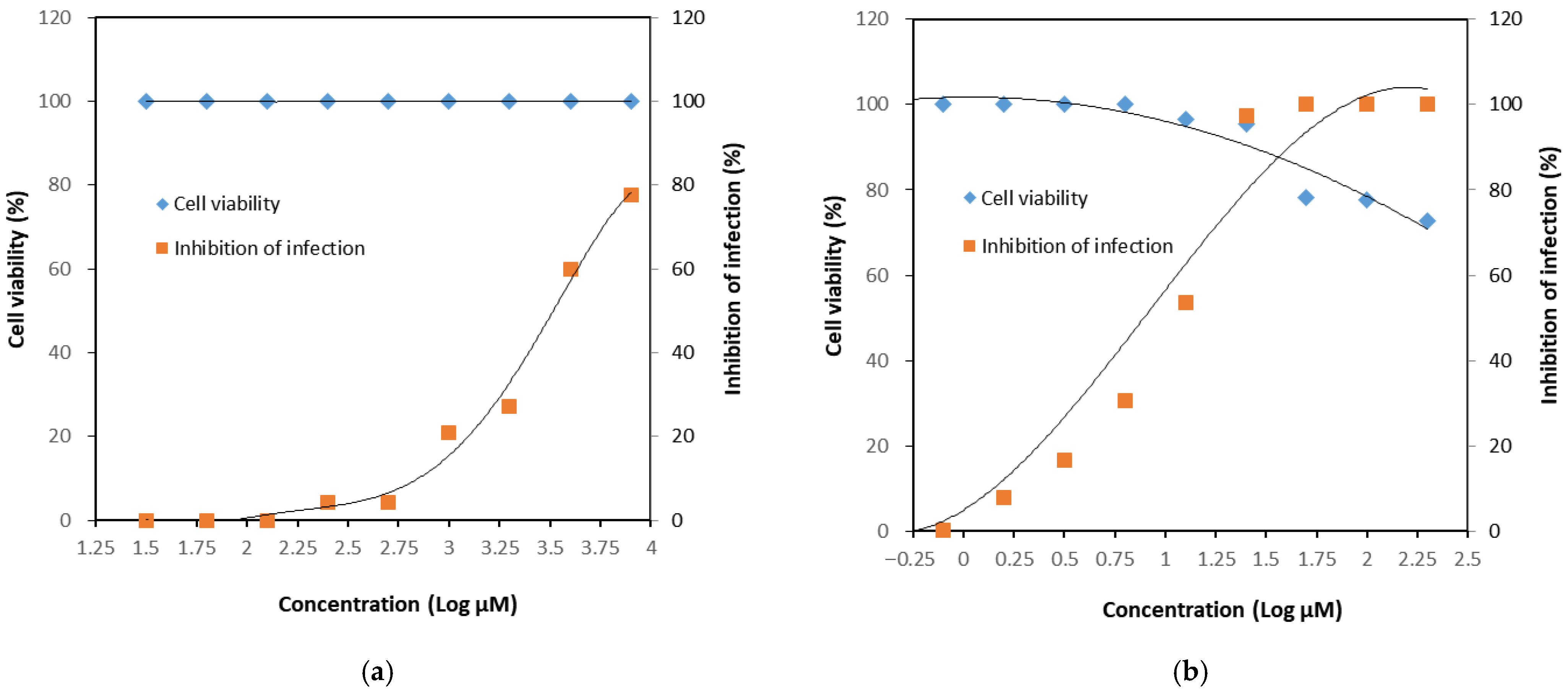
3.2. Determination of Anti-Viral Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GH | Glucosyl hesperidin |
| FCV | Feline calicivirus |
| CRFK | Crandell–Rees feline kidney |
| PFU | Plaque-forming units |
| SI | Selectivity index |
| CC50 | 50% cytotoxic concentration |
| IC50 | 50% inhibitory concentration |
| MOI | Multiplicity of infection |
References
- Widdowson, M.A.; Sulka, A.; Bulens, S.N.; Beard, S.R.; Chaves, S.S. Norovirus and foodborne disease, United States, 1991–2000. Emerg. Infect. Dis. 2005, 11, 95–102. [Google Scholar] [CrossRef]
- Bull, R.A.; Tu, E.T.V.; McIver, C.J.; Rawlinson, W.D.; White, P.A. Emergence of a new norovirus genotype II.4 variant associated with global outbreaks of gastroenteritis. J. Clin. Microbiol. 2006, 44, 327–333. [Google Scholar] [CrossRef]
- Lopman, B.; Vennema, H.; Kohli, E.; Pothier, P.; Sanchez, A.; Negredo, A.; Buesa, J.; Schreier, E.; Reacher, M.; Brown, D.; et al. Increase in viral gastroenteritis outbreaks in Europe and epidemic spread of new norovirus variant. Lancet 2004, 363, 682–688. [Google Scholar] [CrossRef]
- Lee, S.G.; Cho, H.G.; Paik, S.Y. Molecular epidemiology of norovirus in South Korea. BMB Rep. 2015, 48, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Begall, L.F.; Mauroy, A.; Thiry, E. Noroviruses—The State of the Art, Nearly Fifty Years after Their Initial Discovery. Viruses 2021, 13, 1541. [Google Scholar] [CrossRef] [PubMed]
- Robilotti, E.; Deresinski, S.; Pinsky, B.A. Norovirus. Clin. Microbiol. Rev. 2015, 28, 134–164. [Google Scholar] [CrossRef]
- Tian, W.J.; Wang, X.J. Broad-Spectrum Antivirals Derived from Natural Products. Viruses 2023, 15, 1100. [Google Scholar] [CrossRef]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef]
- Duizer, E.; Schwab, K.J.; Neill, F.H.; Atmar, R.L.; Koopman, M.P.G.; Estes, M.K. Laboratory efforts to cultivate noroviruses. J. Gen. Virol. 2004, 85, 79–87. [Google Scholar] [CrossRef]
- Gehrke, C.; Steinmann, J.; Goroncy-Bermes, P. Inactivation of feline calicivirus, a surrogate of norovirus (formerly Norwalk-like viruses), by different types of alcohol in vitro and in vivo. J. Hosp. Infect. 2004, 56, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, J. Surrogate viruses for testing virucidal efficacy of chemical disinfectants. J. Hosp. Infect. 2004, 56, 49–54. [Google Scholar] [CrossRef]
- Beecher, G.R. Overview of dietary flavonoids: Nomenclature, occurrence and intake. J. Nutr. 2003, 133, 3248S–3254S. [Google Scholar] [CrossRef]
- Németh, K.; Plumb, G.W.; Berrin, J.-G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell β-glycosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ikoma, Y.; Sugiura, M.; Yano, M.; Hasegawa, Y. Identification and quantification of the conjugated metabolites derived from orally administered hesperidin in rat plasma. J. Agric. Food Chem. 2004, 52, 6653–6659. [Google Scholar] [CrossRef]
- Haidari, F.; Keshavarz, S.A.; Rashidi, M.R.; Shahi, M.M. Orange juice and hesperetin supplementation to hyperuricemic rats alter oxidative stress markers and xanthine oxidoreductase activity. J. Clin. Biochem. Nutr. 2009, 45, 285–291. [Google Scholar] [CrossRef]
- Hirata, A.; Murakami, Y.; Shoji, M.; Kadoma, Y.; Fujisawa, S. Kinetics of radical-scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression. Anticancer Res. 2005, 25, 3367–3374. [Google Scholar]
- Hao, Y.; Wei, Z.; Wang, Z.; Li, G.; Yao, Y.; Dun, B. Biotransformation of flavonoids improves antimicrobial and anti-breast cancer activities in vitro. Foods 2021, 10, 2367. [Google Scholar] [CrossRef]
- Olas, B. A review of in vitro studies of the anti-platelet potential of citrus fruit flavonoids. Food Chem. Toxicol. 2021, 150, 112090. [Google Scholar] [CrossRef]
- Paredes, A.; Aluzuru, M.; Mendez, J.; Rodriguez-Ortega, M. Anti-Sindbis activity of flavanones hesperetin and naringenin. Biol. Pharm. Bull. 2003, 26, 108–109. [Google Scholar] [CrossRef]
- Pyrzynska, K. Hesperidin: A review on extraction methods, stability and biological activities. Nutrients 2022, 14, 2387. [Google Scholar] [CrossRef]
- Cheng, F.J.; Huynh, T.K.; Yang, C.S.; Hu, D.W.; Shen, Y.C.; Tu, C.Y.; Wu, Y.C.; Tang, C.H.; Huang, W.C.; Chen, Y. Hesperidin is a potential inhibitor against SARS-CoV-2 infection. Nutrients 2021, 13, 2800. [Google Scholar] [CrossRef]
- Cao, R.; Zhao, Y.; Zhou, Z.; Zhao, X. Enhancement of the water solubility and antioxidant activity of hesperidin by chitooligosaccharide. J. Sci. Food Agric. 2018, 98, 2422–2427. [Google Scholar] [CrossRef]
- Wdowiak, K.; Rosiak, N.; Tykarska, E.; Zarowski, M.; Płazińska, A.; Płaziński, W.; Cielecka-Piontek, J. Amorphous Inclusion Complexes: Molecular Interactions of Hesperidin and Hesperetin with HP-B-CD and Their Biological Effects. Int. J. Mol. Sci. 2022, 23, 4000. [Google Scholar] [CrossRef]
- Yamamoto, M.; Suzuki, A.; Hase, T. Short-term effects of glucosyl hesperidin and hesperetin on blood pressure and vascular endothelial function in spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 2008, 54, 95–98. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, S.H.; Lee, K.A. A Comparative Study of Hesperetin, Hesperidin and Glucosyl hesperidin: Antioxidant, Anti-Inflammatory, and Antibacterial Activities In Vitro. Antioxidants 2022, 11, 1618. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, S.H.; Hong, S.K.; Gil, B.I.; Lee, K.A. In vitro biological activities of hesperidin-related compounds with different solubility. Antioxidants 2024, 13, 727. [Google Scholar] [CrossRef]
- Su, X.; Zivanovic, S.; D’Souza, D.H. Effect of chitosan on the infectivity of murine norovirus, feline calicivirus, and bacteriophage MS2. J. Food Prot. 2009, 72, 2623–2628. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Dice, L.; Ailavadi, S.; D’Souza, D.H. Antiviral effects of Quillaja saponaria extracts against human noroviral surrogates. Food Environ. Virol. 2023, 15, 167–175. [Google Scholar] [CrossRef]
- Lanave, G.; Pellegrini, F.; Catella, C.; Mateos, H.; Palazzo, G.; Gentile, A.; Diakoudi, G.; Burgio, M.; Tempesta, M.; Martella, V. Virucidal activity of lemon juice against feline calicivirus, surrogate of norovirus. Antibiotics 2025, 14, 273. [Google Scholar] [CrossRef]
- Triratapiban, C.; Lueangaramkul, V.; Phecharat, N.; Pantanam, A.; Lekcharoensuk, P.; Theerawatanasirikul, S. First study on in vitro antiviral and virucidal effects of flavonoids against feline infectious peritonitis virus at the early stage of infection. Vet. World 2023, 16, 618–630. [Google Scholar] [CrossRef]
- Su, X.; D’Souza, D.H. Naturally occurring flavonoids against human norovirus surrogates. Food Environ. Virol. 2013, 5, 97–102. [Google Scholar] [CrossRef]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising natural compounds against viral infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef]
- Kumara, D.; Harsan, H.S.; Septisetyani, E.P.; Prasetyaningrum, P.W.; Paramitasari, K.A.; Syaifudin, M.; Astirin, O.P.; Ikawati, M.; Meiyanto, E. Inhibitory effects of citrus-derived flavonoids hesperidin and hesperetin on SARS-CoV-2 spike-mediated syncytia formation using in vitro cell model. Adv. Pharm. Bull. 2025, 15, 416–427. [Google Scholar] [CrossRef]
- Eberle, R.J.; Olivier, D.S.; Pacca, C.C.; Avilla, C.M.S.; Nogueira, M.L.; Amaral, M.S.; Willbold, D.; Arni, R.K.; Coronado, M.A. In vitro study of hesperetin and hesperidin as inhibitors of zika and chikungunya virus proteases. PLoS ONE 2021, 16, e0246319. [Google Scholar] [CrossRef]
- Lee, K.A. Cell recovery, anti-inflammatory, and melanogenesis inhibitory activity of water soluble hesperidin in vitro. J. Korean Appl. Sci. Technol. 2023, 40, 1278–1288. [Google Scholar]
- Man, M.Q.; Yang, B.; Elias, P.M. Benefits of hesperidin for cutaneous functions. Evid. Based Complement. Altern. Med. 2019, 2019, 266307. [Google Scholar] [CrossRef]
- Park, C.; Yoo, J.H.; Park, S.U. Recent insights into the biological functions of hesperidin. EXCLI J. 2025, 24, 8730. [Google Scholar]
- McDonagh, P.; Sheehy, P.A.; Fawcett, A.; Norris, J.M. Antiviral effect of mefloquine on feline calicivirus in vitro. Vet. Microbiol. 2015, 176, 370–377. [Google Scholar] [CrossRef]
- Mellou, F.; Loutrari, H.; Stamatis, H.; Roussos, C.; Kolisis, F.N. Enzymatic esterification of flavonoids with unsaturated fatty acids: Effect of the novel esters on vascular endothelial growth factor release from K562 cells. Process Biochem. 2006, 41, 2029–2034. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Lai, X.; Nong, J.; Zhao, G.; Xiao, X. One-pot biocatalytic synthesis and antioxidant activities of highly lipophilic naringin derivatives by using bi-functional whole-cells. Food Res. Int. 2020, 136, 109291. [Google Scholar] [CrossRef]
- Schnitzler, P.; Schuhmacher, A.; Astani, A.; Reichling, J. Melissa officinalis oil affects infectivity of enveloped herpesviruses. Phytomedicine 2008, 15, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, D.; Sarkar, M.C.; Chatterjee, T.; Sharma Dey, R.; Bag, P.; Chakraborti, S.; Khan, M.T. Recent advancements for the evaluation of anti-viral activities of natural products. New Biotechnol. 2009, 25, 347–368. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Meng, S.; Li, Z.R.; Peng, Z.G.; Han, Y.X.; Guo, S.S.; Cui, X.L.; Li, Y.H.; Jiang, J.D. The antiviral effect of 7-hydroxyisoflavone against Enterovirus 71 in vitro. J. Asian Nat. Prod. Res. 2013, 15, 382–389. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-S.; Lee, S.-H.; Lee, K.-A. Antiviral Activity of Glucosyl Hesperidin Against Feline Calicivirus. Microorganisms 2025, 13, 2332. https://doi.org/10.3390/microorganisms13102332
Choi S-S, Lee S-H, Lee K-A. Antiviral Activity of Glucosyl Hesperidin Against Feline Calicivirus. Microorganisms. 2025; 13(10):2332. https://doi.org/10.3390/microorganisms13102332
Chicago/Turabian StyleChoi, Sung-Sook, Sun-Hyung Lee, and Kyung-Ae Lee. 2025. "Antiviral Activity of Glucosyl Hesperidin Against Feline Calicivirus" Microorganisms 13, no. 10: 2332. https://doi.org/10.3390/microorganisms13102332
APA StyleChoi, S.-S., Lee, S.-H., & Lee, K.-A. (2025). Antiviral Activity of Glucosyl Hesperidin Against Feline Calicivirus. Microorganisms, 13(10), 2332. https://doi.org/10.3390/microorganisms13102332





