High-Performance Indigenous Lactiplantibacillus plantarum Strains for Enhanced Malolactic Fermentation and Wine Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Strains and Culture Conditions
2.2. Stress Tolerance Analysis of L. plantarum Strains
- Simulated wine A: 12% (v/v) ethanol, pH 3.60;
- Simulated wine B: 10% (v/v) ethanol, pH 3.30;
- Simulated wine C: 14% (v/v) ethanol, pH 3.80.
2.3. Analysis of L-Malic Acid Content and Viable Bacterial Count
2.4. HPLC Determination Methods for Compounds in Wine
2.5. Analysis of Physicochemical Indices
2.6. Analysis of Anthocyanin Contents and CIELAB Color Parameters
2.7. Analysis of Individual Phenolic Contents in Marselan Wine
2.8. Volatile Compound Analysis
2.9. Statistical Analysis
3. Results and Discussion
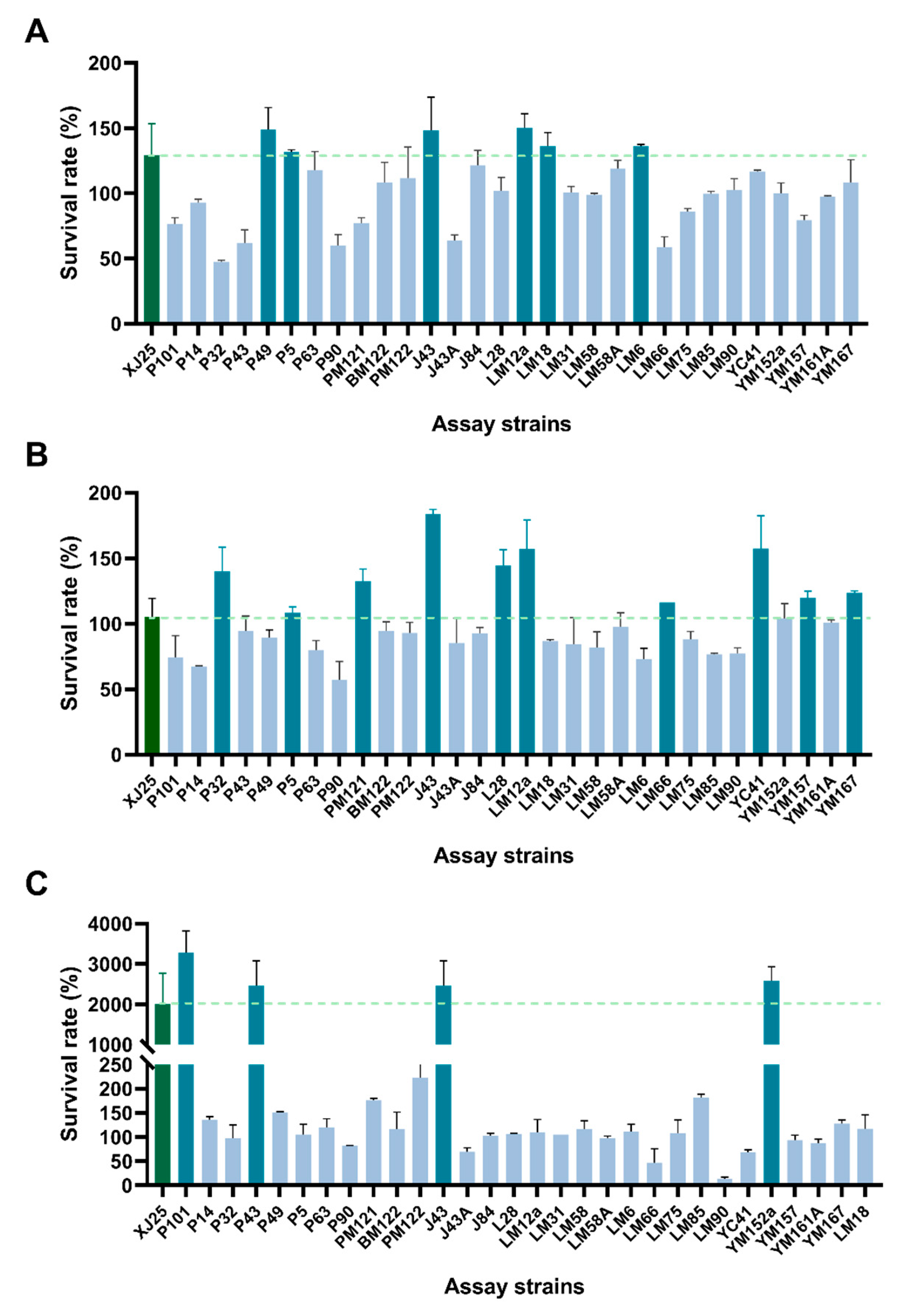
3.1. Combined Stress Tolerance Screening of L. plantarum Strains
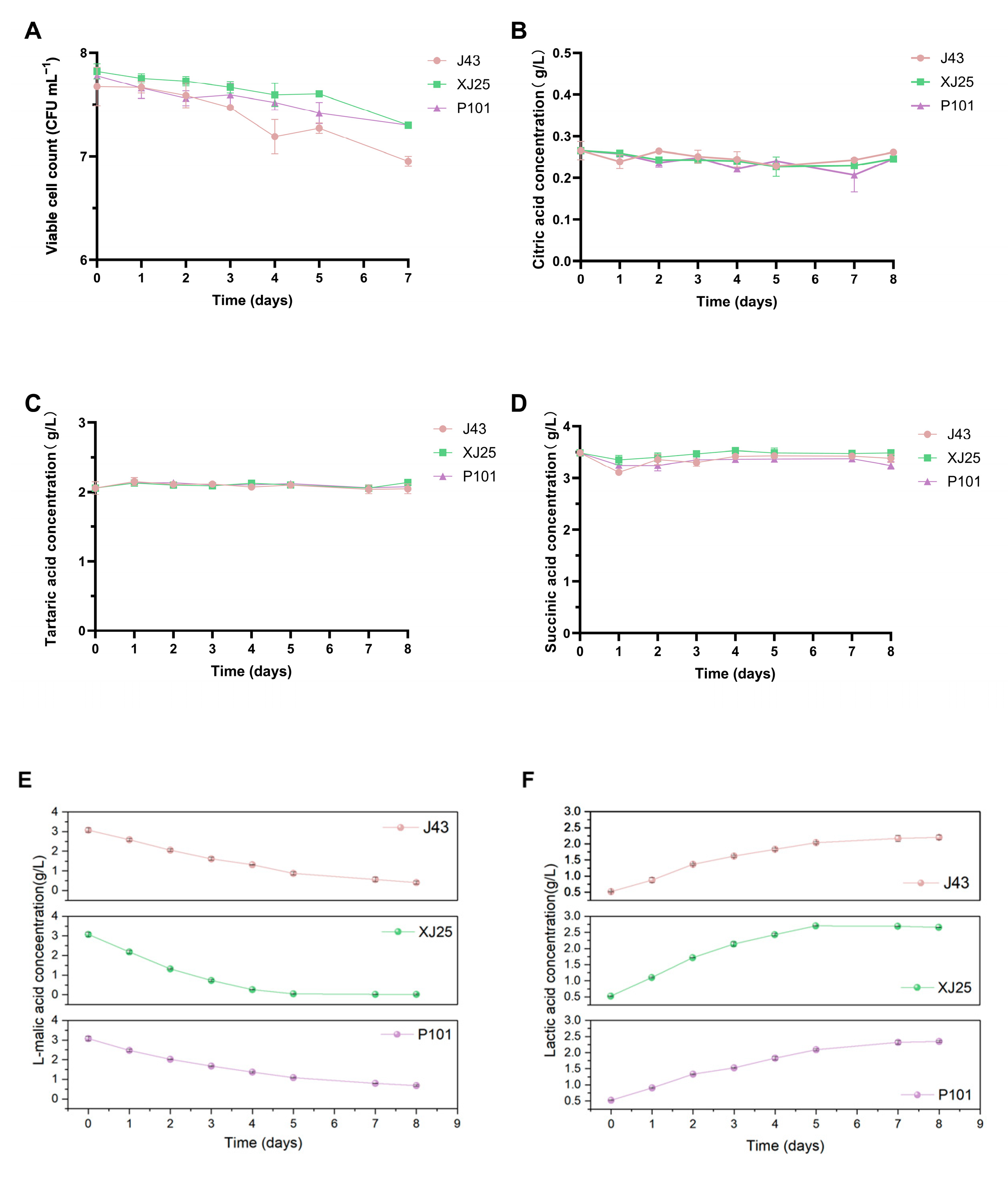
3.2. Viable Cell Counts and L-Malic Acid Consumption During MLF with Different L. plantarum Strains
3.3. Physicochemical Indices of Wines After MLF with Different L. plantarum Strains
3.4. Changes in Viable L. plantarum Counts and Organic Acid Contents in Marselan Wine
3.5. Effects of L. plantarum on Wine Color and Anthocyanin Contents Before and After MLF
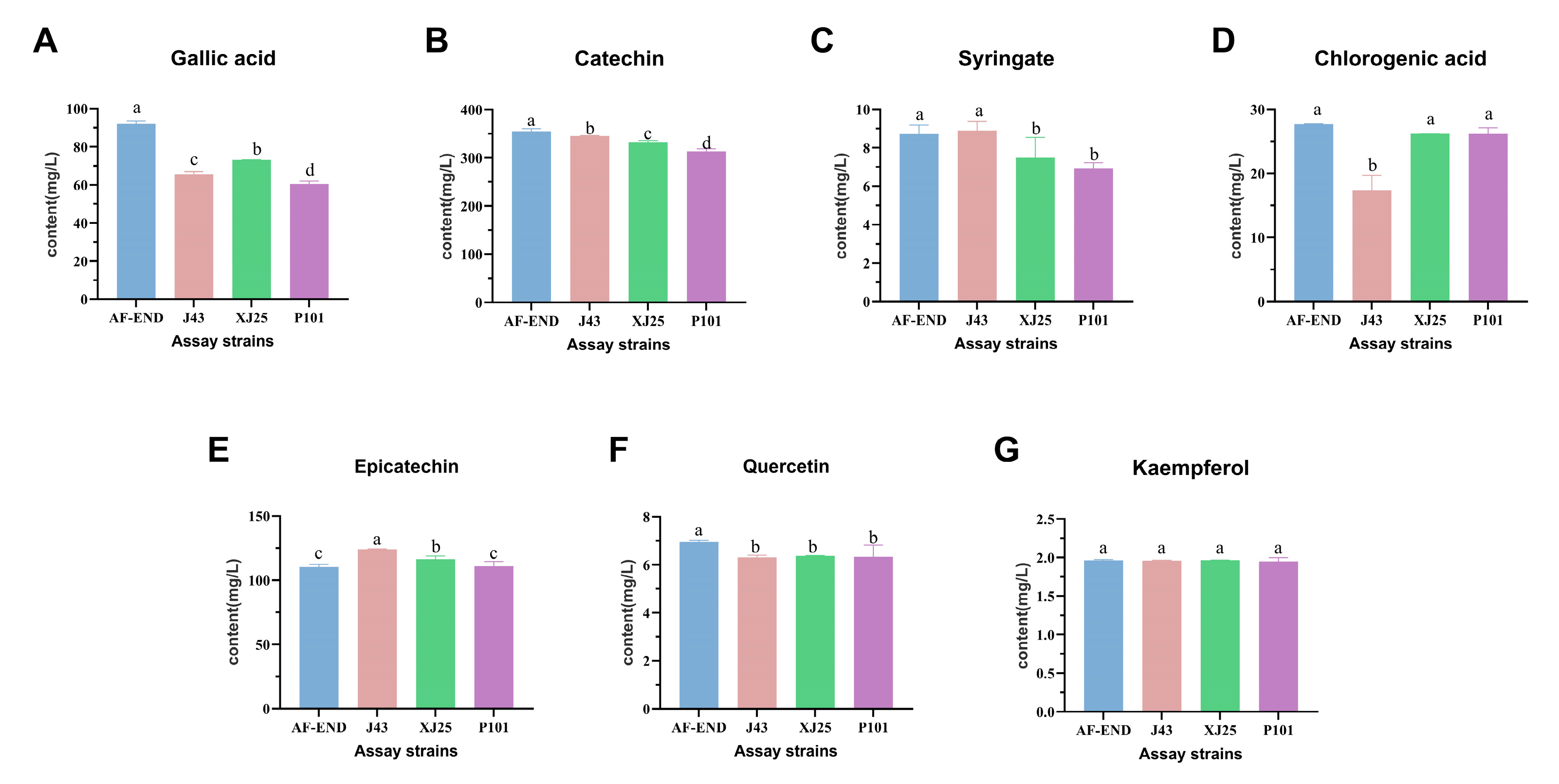
3.6. Individual Phenolic Contents During MLF with Different L. plantarum Strains
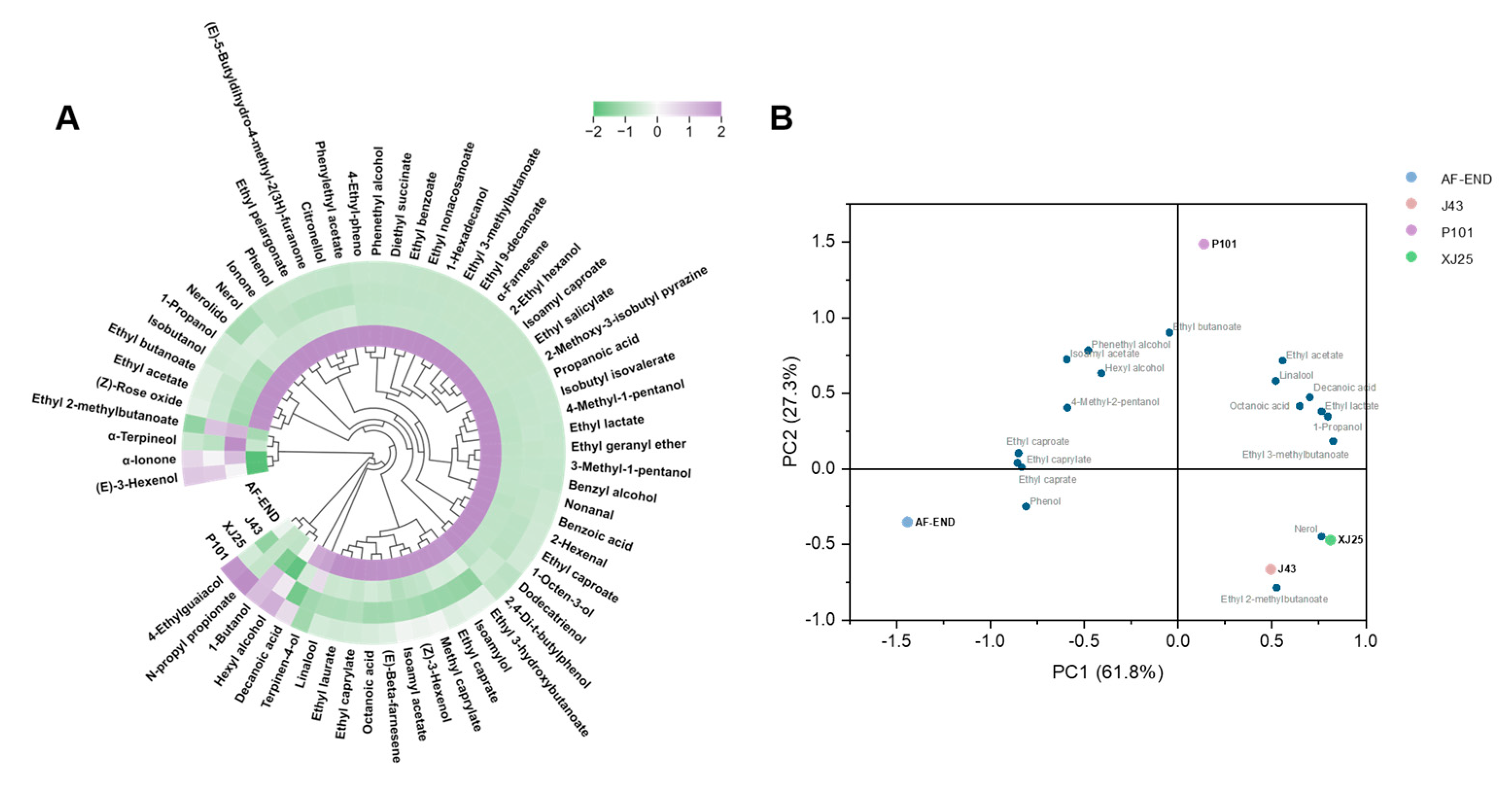
3.7. Volatile Compound and PCA of Marselan Wines Fermented with Different L. plantarum Strains
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daeschel, M.A.; Jung, D.S.; Watson, B.T. Controlling Wine Malolactic Fermentation with Nisin and Nisin-Resistant Strains of Leuconostoc oenos. Appl. Environ. Microbiol. 1991, 57, 601–603. [Google Scholar] [CrossRef]
- Nielsen, J.C.; Richelieu, M. Control of flavor development in wine during and after malolactic fermentation by Oenococcus oeni. Appl. Environ. Microbiol. 1999, 65, 740–745. [Google Scholar] [CrossRef]
- Sun, J.; Ge, Y.; Gu, X.; Li, R.; Ma, W.; Jin, G. Identification and Characterization of Malolactic Bacteria Isolated from the Eastern Foothills of Helan Mountain in China. Foods 2022, 11, 2455. [Google Scholar] [CrossRef]
- Capozzi, V.; Tufariello, M.; De Simone, N.; Fragasso, M.; Grieco, F. Biodiversity of Oenological Lactic Acid Bacteria: Species- and Strain-Dependent Plus/Minus Effects on Wine Quality and Safety. Fermentation 2021, 7, 24. [Google Scholar] [CrossRef]
- Virdis, C.; Sumby, K.; Bartowsky, E.; Jiranek, V. Lactic Acid Bacteria in Wine: Technological Advances and Evaluation of Their Functional Role. Front. Microbiol. 2020, 11, 612118. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Villena, M.; Bartowsky, E.J.; Capone, D.; Sefton, M.A. Production of indole by wine-associated microorganisms under oenological conditions. Food Microbiol. 2010, 27, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Tofalo, R.; Battistelli, N.; Perpetuini, G.; Valbonetti, L.; Rossetti, A.P.; Perla, C.; Zulli, C.; Arfelli, G. Oenococcus oeni Lifestyle Modulates Wine Volatilome and Malolactic Fermentation Outcome. Front. Microbiol. 2021, 12, 736789. [Google Scholar] [CrossRef]
- E, G.A.; López, I.; Ruiz, J.I.; Sáenz, J.; Fernández, E.; Zarazaga, M.; Dizy, M.; Torres, C.; Ruiz-Larrea, F. High tolerance of wild Lactobacillus plantarum and Oenococcus oeni strains to lyophilisation and stress environmental conditions of acid pH and ethanol. FEMS Microbiol. Lett. 2004, 230, 53–61. [Google Scholar] [CrossRef]
- Chen, Q.; Hao, N.; Zhao, L.; Yang, X.; Yuan, Y.; Zhao, Y.; Wang, F.; Qiu, Z.; He, L.; Shi, K.; et al. Comparative functional analysis of malate metabolism genes in Oenococcus oeni and Lactiplantibacillus plantarum at low pH and their roles in acid stress response. Food Res. Int. 2022, 157, 111235. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhao, H.; Zhang, C.; Yu, J.; Lu, Z. Purification and characterization of plantaricin 163, a novel bacteriocin produced by Lactobacillus plantarum 163 isolated from traditional Chinese fermented vegetables. J. Agric. Food Chem. 2013, 61, 11676–11682. [Google Scholar] [CrossRef]
- Knoll, C.; Divol, B.; du Toit, M. Genetic screening of lactic acid bacteria of oenological origin for bacteriocin-encoding genes. Food Microbiol. 2008, 25, 983–991. [Google Scholar] [CrossRef]
- Matthews, A.; Grimaldi, A.; Walker, M.; Bartowsky, E.; Grbin, P.; Jiranek, V. Lactic acid bacteria as a potential source of enzymes for use in vinification. Appl. Environ. Microbiol. 2004, 70, 5715–5731. [Google Scholar] [CrossRef]
- Mtshali, P.S.; Divol, B.; van Rensburg, P.; du Toit, M. Genetic screening of wine-related enzymes in Lactobacillus species isolated from South African wines. J. Appl. Microbiol. 2010, 108, 1389–1397. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Implications of new research and technologies for malolactic fermentation in wine. Appl. Microbiol. Biotechnol. 2014, 98, 8111–8132. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chen, X.; Cao, Y.; Gao, P.; Xu, T.; Xiong, D.; Zhao, Z. Lactiplantibacillus plantarum exerts strain-specific effects on malolactic fermentation, antioxidant activity, and aroma profile of apple cider. Food Chem. X 2024, 23, 101575. [Google Scholar] [CrossRef] [PubMed]
- Berbegal, C.; Benavent-Gil, Y.; Navascués, E.; Calvo, A.; Albors, C.; Pardo, I.; Ferrer, S. Lowering histamine formation in a red Ribera del Duero wine (Spain) by using an indigenous O. oeni strain as a malolactic starter. Int. J. Food Microbiol. 2017, 244, 11–18. [Google Scholar] [CrossRef]
- Brizuela, N.S.; Bravo-Ferrada, B.M.; Curilén, Y.; Delfederico, L.; Caballero, A.; Semorile, L.; Pozo-Bayón, M.; Tymczyszyn, E.E. Advantages of Using Blend Cultures of Native L. plantarum and O. oeni Strains to Induce Malolactic Fermentation of Patagonian Malbec Wine. Front. Microbiol. 2018, 9, 2109. [Google Scholar] [CrossRef] [PubMed]
- Franquès, J.; Araque, I.; El Khoury, M.; Lucas, P.M.; Reguant, C.; Bordons, A. Selection and characterization of autochthonous strains of Oenococcus oeni for vinification in Priorat (Catalonia, Spain). OENO One 2018, 52, 45–56. [Google Scholar] [CrossRef]
- Garofalo, C.; El Khoury, M.; Lucas, P.; Bely, M.; Russo, P.; Spano, G.; Capozzi, V. Autochthonous starter cultures and indigenous grape variety for regional wine production. J. Appl. Microbiol. 2015, 118, 1395–1408. [Google Scholar] [CrossRef]
- Romero, J.; Ilabaca, C.; Ruiz, M.; Jara, C. Oenococcus oeni in Chilean Red Wines: Technological and Genomic Characterization. Front. Microbiol. 2018, 9, 90. [Google Scholar] [CrossRef]
- Ruiz, P.; Izquierdo, P.M.; Seseña, S.; Palop, M.L. Selection of autochthonous Oenococcus oeni strains according to their oenological properties and vinification results. Int. J. Food Microbiol. 2010, 137, 230–235. [Google Scholar] [CrossRef]
- Battistelli, N.; Perpetuini, G.; Perla, C.; Arfelli, G.; Zulli, C.; Rossetti, A.P.; Tofalo, R. Characterization of natural Oenococcus oeni strains for Montepulciano d’Abruzzo organic wine production. Eur. Food Res. Technol. 2020, 246, 1031–1039. [Google Scholar] [CrossRef]
- Liu, X.; Fu, J.; Ma, W.; Jin, G. Screening and evaluation of high stress tolerance, high esterase activity and safety of Oenococcus oeni strains adapt to challenging conditions in Northwest China wine. LWT—Food Sci. Technol. 2024, 213, 116975. [Google Scholar] [CrossRef]
- Xia, N.; Cai, H.; Kou, J.; Xie, Y.; Yao, X.; Li, J.; Zhou, P.; He, F.; Duan, C.; Pan, Q.; et al. Variety-specific flavor characteristics in the Shandong region: Interaction between fermentation and variety. Food Chem. 2025, 478, 143707. [Google Scholar] [CrossRef]
- Wang, C.; Chen, X.; Ren, Y.; Xuan, X.; Pervaiz, T.; Shangguan, L.; Fang, J. Geographical location influence ‘Cabernet Franc’ fruit quality in Shandong province. Sci. Rep. 2024, 14, 2382. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Yuan, Y.; Li, Y.; Wu, S.; Shi, K.; Liu, S. Optimization of Electrotransformation Parameters and Engineered Promoters for Lactobacillus plantarum from Wine. ACS Synth. Biol. 2021, 10, 1728–1738. [Google Scholar] [CrossRef]
- Torriani, S.; Felis, G.E.; Dellaglio, F. Differentiation of Lactobacillus plantarum, L. pentosus, and L. paraplantarum by recA gene sequence analysis and multiplex PCR assay with recA gene-derived primers. Appl. Environ. Microbiol. 2001, 67, 3450–3454. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Orte, P.; Cacho, J.F.; Ferreira, V. Relationship between varietal amino acid profile of grapes and wine aromatic composition. Experiments with model solutions and chemometric study. J. Agric. Food Chem. 2002, 50, 2891–2899. [Google Scholar] [CrossRef] [PubMed]
- GB/T 15038-2006; Analytical Methods of Wine and Fruit Wine. China Standard Publishing House: Beijing, China, 2006.
- Zhang, B.; Liu, D.; Liu, H.; Shen, J.; Zhang, J.; He, L.; Li, J.; Zhou, P.; Guan, X.; Liu, S.; et al. Impact of indigenous Oenococcus oeni and Lactiplantibacillus plantarum species co-culture on Cabernet Sauvignon wine malolactic fermentation: Kinetic parameters, color and aroma. Food Chem. X 2024, 22, 101369. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, S.; He, L.; Tian, Y. Draft Genome Sequence of Lactobacillus plantarum XJ25 Isolated from Chinese Red Wine. Genome Announc. 2016, 4, e01216-16. [Google Scholar] [CrossRef]
- Betteridge, A.; Grbin, P.; Jiranek, V. Improving Oenococcus oeni to overcome challenges of wine malolactic fermentation. Trends Biotechnol. 2015, 33, 547–553. [Google Scholar] [CrossRef]
- Brizuela, N.; Tymczyszyn, E.E.; Semorile, L.C.; Valdes La Hens, D.; Delfederico, L.; Hollmann, A.; Bravo-Ferrada, B. Lactobacillus plantarum as a malolactic starter culture in winemaking: A new (old) player? Electron. J. Biotechnol. 2019, 38, 10–18. [Google Scholar] [CrossRef]
- du Toit, M.; Engelbrecht, L.; Lerm, E.; Krieger-Weber, S. Lactobacillus: The Next Generation of Malolactic Fermentation Starter Cultures—An Overview. Food Bioprocess. Technol. 2011, 4, 876–906. [Google Scholar] [CrossRef]
- Davis, C.R.; Wibowo, D.; Fleet, G.H.; Lee, T.H. Properties of Wine Lactic Acid Bacteria: Their Potential Enological Significance. Am. J. Enol. Vitic. 1988, 39, 137–142. [Google Scholar] [CrossRef]
- Sauer, M.; Russmayer, H.; Grabherr, R.; Peterbauer, C.K.; Marx, H. The Efficient Clade: Lactic Acid Bacteria for Industrial Chemical Production. Trends Biotechnol. 2017, 35, 756–769. [Google Scholar] [CrossRef]
- Pereira, R.; Mohamed, E.T.; Radi, M.S.; Herrgård, M.J.; Feist, A.M.; Nielsen, J.; Chen, Y. Elucidating aromatic acid tolerance at low pH in Saccharomyces cerevisiae using adaptive laboratory evolution. Proc. Natl. Acad. Sci. USA 2020, 117, 27954–27961. [Google Scholar] [CrossRef]
- Lv, H.; Pian, R.; Xing, Y.; Zhou, W.; Yang, F.; Chen, X.; Zhang, Q. Effects of citric acid on fermentation characteristics and bacterial diversity of Amomum villosum silage. Bioresour. Technol. 2020, 307, 123290. [Google Scholar] [CrossRef]
- Wang, S.; Li, S.; Zhao, H.; Gu, P.; Chen, Y.; Zhang, B.; Zhu, B. Acetaldehyde released by Lactobacillus plantarum enhances accumulation of pyranoanthocyanins in wine during malolactic fermentation. Food Res. Int. 2018, 108, 254–263. [Google Scholar] [CrossRef]
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and their variation in red wines I. Monomeric anthocyanins and their color expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A Review of the Effect of Winemaking Techniques on Phenolic Extraction in Red Wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar] [CrossRef]
- Ginjom, I.R.; D’Arcy, B.R.; Caffin, N.A.; Gidley, M.J. Phenolic contents and antioxidant activities of major Australian red wines throughout the winemaking process. J. Agric. Food Chem. 2010, 58, 10133–10142. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Míguez, M.J.; Cacho, J.F.; Ferreira, V.; Vicario, I.M.; Heredia, F.J. Volatile components of Zalema white wines. Food Chem. 2007, 100, 1464–1473. [Google Scholar] [CrossRef]
- Aznar, M.; Arroyo, T. Analysis of wine volatile profile by purge-and-trap-gas chromatography-mass spectrometry. Application to the analysis of red and white wines from different Spanish regions. J. Chromatogr. A 2007, 1165, 151–157. [Google Scholar] [CrossRef]
- Rocha, S.l.M.; Rodrigues, F.; Coutinho, P.; Delgadillo, I.; Coimbra, M.A. Volatile composition of Baga red wine: Assessment of the identification of the would-be impact odourants. Anal. Chim. Acta 2004, 513, 257–262. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Liu, X.; Bian, X.; Li, J.; Meng, N.; Liu, M.; Huang, M.; Sun, B.; Li, J. Flavor Interactions in Wine: Current Status and Future Directions From Interdisplinary and Crossmodal Perspectives. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70199. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Yan, X.; Wang, Q.; Zhang, Y.; Tao, Y. Performance of selected P. fermentans and its excellular enzyme in co-inoculation with S. cerevisiae for wine aroma enhancement. LWT—Food Sci. Technol. 2017, 86, 361–370. [Google Scholar] [CrossRef]



| Strain | pH | Total Acid g/L | Glucose g/L | Fructose g/L | Glycerin g/L | Alcohol (%) (v/v) | Total Phenol mg/L |
|---|---|---|---|---|---|---|---|
| AF-END | 3.61 ± 0.00 c | 7.04 ± 0.15 a | 1.39 ± 0.01 c | 7.72 ± 0.10 a | 4.85 ± 0.14 a | 14.26 ± 0.08 a | 3504.96 ± 186.20 a |
| J43 | 3.76 ± 0.00 b | 5.90 ± 0.17 b | 1.37 ± 0.03 c | 6.24 ± 0.17 b | 4.34 ± 0.10 b | 13.68 ± 0.38 b | 3047.19 ± 74.38 b |
| XJ25 | 3.79 ± 0.00 a | 5.30 ± 0.34 c | 1.54 ± 0.02 a | 5.93 ± 0.06 b | 4.42 ± 0.03 b | 14.02 ± 0.06 ab | 3411.33 ± 57.70 a |
| P101 | 3.79 ± 0.00 a | 5.06 ± 0.29 c | 1.46 ± 0.04 b | 6.06 ± 0.30 b | 4.41 ± 0.02 b | 13.99 ± 0.10 ab | 3264.50 ± 168.12 ab |
| Strain | L* | a* | b* | C*ab | h*ab | △E*ab |
|---|---|---|---|---|---|---|
| AF-END | 16.86 ± 0.21 c | 45.25 ± 0.35 a | 20.53 ± 0.26 a | 49.68 ± 0.42 a | 0.43 ± 0.01 a | - |
| J43 | 18.26 ± 0.67 bc | 46.54 ± 1.03 a | 21.58 ± 1.32 a | 51.04 ± 1.25 a | 0.43 ± 0.03 a | 2.17 ± 0.25 b |
| XJ25 | 18.13 ± 0.62 bc | 46.41 ± 0.86 a | 20.85 ± 0.63 a | 50.88 ± 1.04 a | 0.42 ± 0.01 a | 1.75 ± 0.22 c |
| P101 | 19.20 ± 1.22 a | 45.57 ± 3.51 a | 21.50 ± 0.60 a | 49.75 ± 4.12 a | 0.44 ± 0.03 a | 2.55 ± 0.40 a |
| Compounds | Aroma Concentration (μg/L) | Thresholds | OAV | Description | |||
|---|---|---|---|---|---|---|---|
| AF-END | J43 | XJ25 | P101 | ||||
| Esters (20) | |||||||
| Ethyl acetate | 31,039.50 ± 187.59 d | 33,513.88 ± 331.35 c | 37,143.24 ± 107.61 b | 39,475.58 ± 2466.94 a | 7500 | >1 | Banana, Strawberry |
| Ethyl butanoate | 110.39 ± 1.74 b | 101.86 ± 1.36 c | 111.98 ± 2.62 b | 122.11 ± 5.25 a | 400 | >0.1 | Strawberry, Banana, Pineapple |
| Ethyl 2-methylbutanoate | 1.44 ± 0.33 b | 6.71 ± 0.07 a | 6.02 ± 0.91 a | 0.00 ± 0.00 c | >1 | Apple | |
| N-propyl propionate | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 6.69 ± 0.60 a | |||
| Ethyl 3-methylbutanoate | 0.70 ± 0.02 b | 0.94 ± 0.02 a | 0.93 ± 0.03 a | 0.92 ± 0.06 a | 3 | >0.1 | Strawberry, Sweet Fruity |
| Isoamyl acetate | 140.24 ± 4.96 a | 131.82 ± 2.54 a | 130.62 ± 7.22 a | 141.67 ± 0.60 a | 160 | >0.1 | Banana, Fruity |
| Isobutyl isovalerate | 86.09 ± 1.00 a | 85.31 ± 0.04 a | 87.45 ± 4.63 a | 88.51 ± 3.63 a | |||
| Ethyl caproate | 183.10 ± 1.95 a | 126.95 ± 1.86 c | 124.48 ± 1.25 c | 145.04 ± 5.67 b | 14 | >1 | Green apple, Strawberry |
| Ethyl lactate | 5696.61 ± 109.69 c | 8452.46 ± 72.65 b | 10,514.85 ± 890.43 a | 10,125.87 ± 617.30 a | 14,000 | >0.1 | Milk, Butter |
| Methyl caprylate | 41.82 ± 1.17 a | 0.21 ± 0.04 b | 0.01 ± 0.00 b | 0.44 ± 0.06 b | |||
| Ethyl caprylate | 806.04 ± 4.23 a | 483.09 ± 10.60 c | 408.13 ± 17.75 d | 549.00 ± 18.75 b | 5 | >1 | Pineapple, Pear, Floral |
| Ethyl 3-hydroxybutanoate | 259.54 ± 0.03 b | 282.64 ± 3.83 a | 278.41 ± 11.78 ab | 294.31 ± 14.40 a | 200,000 | <0.1 | |
| Ethyl pelargonate | 12.95 ± 0.15 a | 9.89 ± 0.24 b | 7.31 ± 0.62 c | 9.11 ± 0.37 b | 200 | <0.1 | Fruity |
| Ethyl caprate | 551.25 ± 10.49 a | 417.11 ± 4.64 b | 339.74 ± 17.62 c | 426.03 ± 8.34 b | 200 | >1 | Fruity |
| Diethyl succinate | 101.82 ± 1.08 b | 112.89 ± 3.12 a | 105.02 ± 0.91 b | 111.31 ± 0.06 a | 6000 | <0.1 | Fruity, Melon |
| Ethyl benzoate | 3.99 ± 0.00 a | 3.98 ± 0.01 a | 3.95 ± 0.01 b | 3.96 ± 0.00 b | |||
| Ethyl 9-decanoate | 0.00 ± 0.00 c | 1.09 ± 0.12 a | 0.18 ± 0.00 bc | 0.32 ± 0.03 b | |||
| Phenylethyl acetate | 12.41 ± 0.16 a | 12.21 ± 0.39 ab | 10.97 ± 0.34 c | 11.41 ± 0.13 bc | 250 | <0.1 | Rose, Sweet |
| Ethyl laurate | 9.11 ± 0.00 ab | 9.32 ± 0.35 a | 8.43 ± 0.31 b | 9.37 ± 0.27 a | 1500 | <0.1 | Sweet, Beeswax |
| Ethyl nonacosanoate | 5.53 ± 0.02 a | 5.66 ± 0.07 a | 5.64 ± 0.11 a | 5.65 ± 0.06 a | |||
| Alcohols (13) | |||||||
| 1-Propanol | 24,180.88 ± 210.70 ab | 21,199.51 ± 567.61 b | 26,965.99 ± 2592.89 a | 26,482.49 ± 1510.11 a | 306,000 | <0.1 | Mello, Mature fruity, Floral and Green |
| Isobutanol | 20,490.93 ± 572.41 b | 21,429.31 ± 98.03 a | 21,817.10 ± 117.27 a | 21,755.57 ± 438.80 a | 40,000 | >0.1 | Chemical |
| 1-Butanol | 1008.94 ± 5.06 b | 1007.35 ± 0.65 b | 1043.25 ± 15.06 a | 1043.41 ± 3.64 a | 150,000 | <0.1 | Fruity, Green, Malt, Chemical, Alcohol |
| 4-Methyl-2-pentanol | 2066.00 ± 0.00 a | 2066.00 ± 0.00 a | 2066.00 ± 0.00 a | 2066.00 ± 0.00 a | |||
| Isoamylol | 59,476.92 ± 258.64 a | 50,966.47 ± 129.62 c | 26,789.52 ± 816.49 d | 57,480.29 ± 1242.33 b | 30,000 | >1 | Caramel, Lipid |
| 4-Methyl-1-pentanol | 20.08 ± 0.04 ab | 18.84 ± 0.31 c | 19.93 ± 0.16 b | 20.47 ± 0.09 a | 50,000 | <0.1 | |
| 3-Methyl-1-pentanol | 16.08 ± 0.13 a | 13.59 ± 0.24 b | 15.62 ± 0.23 a | 16.43 ± 0.75 a | 500 | <0.1 | |
| Hexyl alcohol | 837.77 ± 1.03 b | 766.70 ± 8.22 c | 817.96 ± 7.20 b | 844.34 ± 14.77 a | 8000 | <0.1 | |
| 1-Heptanol | 28.59 ± 0.08 a | 27.23 ± 0.15 b | 27.49 ± 0.03 b | 27.74 ± 0.22 b | 2500 | <0.1 | |
| Octanol | 5.21 ± 0.20 a | 3.14 ± 0.01 c | 2.84 ± 0.04 c | 3.50 ± 0.21 b | 40 | >0.1 | Floral |
| 1-Decanol | 28.41 ± 2.12 c | 52.91 ± 1.00 a | 46.23 ± 1.94 b | 56.45 ± 2.10 a | 400 | >0.1 | Orange, Fatty |
| Benzyl alcohol | 537.12 ± 2.66 c | 589.82 ± 11.65 b | 630.14 ± 23.13 a | 618.40 ± 20.07 ab | 200,000 | <0.1 | Roast, Fruity |
| Phenethyl alcohol | 12,456.63 ± 35.15 a | 12,143.24 ± 1000.30 a | 11,895.77 ± 395.40 a | 12,682.42 ± 867.55 a | 400 | >1 | Orange, Fatty |
| Terpenes (14) | |||||||
| Limonene | 0.88 ± 0.07 a | 0.30 ± 0.00 b | 0.58 ± 0.04 ab | 0.77 ± 0.16 ab | 10 | >0.1 | Sweet, Citrus, lemon |
| Linalool | 21.66 ± 0.01 c | 21.83 ± 0.03 a | 21.74 ± 0.01 b | 21.87 ± 0.03 a | 25 | >0.1 | Floral, Citrus |
| 1-Octen-3-ol | 15.11 ± 0.10 a | 13.56 ± 0.11 c | 13.30 ± 0.23 c | 14.12 ± 0.34 b | |||
| (E)-3-Hexenol | 38.01 ± 1.45 a | 33.42 ± 0.03 b | 35.02 ± 0.37 b | 35.19 ± 0.65 b | |||
| (Z)-Rose oxide | 3.35 ± 0.00 a | 3.34 ± 0.00 c | 3.35 ± 0.00 d | 3.35 ± 0.00 b | |||
| (Z)-3-Hexenol | 1.64 ± 0.02 d | 20.21 ± 0.10 b | 17.85 ± 0.56 c | 24.47 ± 1.86 a | |||
| α-Terpineol | 12.36 ± 0.12 b | 19.96 ± 6.09 a | 12.13 ± 0.03 b | 12.70 ± 0.11 b | 250 | <0.1 | Lilac |
| Nerol | 127.68 ± 1.17 c | 145.60 ± 10.33 ab | 148.60 ± 2.52 a | 133.38 ± 0.52 b | 400 | >0.1 | Floral, Green |
| Ethyl geranyl ether | 12.12 ± 0.02 a | 12.02 ± 0.03 a | 12.09 ± 0.10 a | 12.16 ± 0.11 a | |||
| Terpinen-4-ol | 9.88 ± 0.02 b | 12.39 ± 1.68 a | 10.38 ± 0.05 ab | 10.38 ± 0.03 ab | |||
| (E)-Beta-farnesene | 3219.34 ± 38.03 a | 2256.72 ± 40.30 c | 1935.72 ± 64.89 d | 2417.84 ± 61.92 b | |||
| Citronellol | 8.40 ± 0.35 a | 6.87 ± 0.69 b | 5.95 ± 0.16 b | 6.50 ± 0.09 b | 100 | <0.1 | Green, Lilac, Rose |
| Nerolido | 9.83 ± 0.02 a | 9.95 ± 0.21 a | 9.97 ± 0.27 a | 9.83 ± 0.02 a | 400 | <0.1 | Green, Floral |
| Dodecatrienol | 81.28 ± 0.00 b | 83.38 ± 1.62 ab | 85.22 ± 0.95 a | 82.61 ± 0.35 b | |||
| Aldehydes and Ketones (5) | |||||||
| 2-Hexenal | 401.26 ± 1.77 a | 244.66 ± 11.87 c | 237.31 ± 2.94 c | 291.43 ± 16.09 b | |||
| Nonanal | 0.02 ± 0.01 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.01 ± 0.01 b | 2.5 | <0.1 | Citrus |
| α-Ionone | 10.54 ± 0.25 a | 9.96 ± 0.19 ab | 8.74 ± 0.10 c | 9.40 ± 0.67 bc | |||
| (E)-5-Butyldihydro-4-methyl-2(3H)-furanone | 227.48 ± 0.01 c | 227.84 ± 0.15 a | 227.46 ± 0.12 c | 227.69 ± 0.09 ab | |||
| Ionone | 9.45 ± 0.01 a | 9.47 ± 0.01 a | 9.45 ± 0.01 a | 9.45 ± 0.00 a | |||
| Volatile Phenols (4) | |||||||
| Phenol | 16.16 ± 0.01 a | 15.48 ± 0.73 ab | 15.07 ± 0.10 b | 15.27 ± 0.01 b | 30 | >0.1 | |
| 4-Ethylguaiacol | 125.70 ± 0.03 b | 125.56 ± 0.02 c | 125.62 ± 0.03 c | 125.79 ± 0.04 a | |||
| 4-Ethyl-pheno | 66.10 ± 0.02 a | 66.16 ± 0.09 a | 66.12 ± 0.04 a | 66.21 ± 0.03 a | |||
| 2,4-Di-t-butylphenol | 177.36 ± 0.10 a | 179.34 ± 5.71 a | 185.39 ± 8.94 a | 181.06 ± 2.40 a | |||
| Fatty acids (5) | |||||||
| Propanoic acid | 8232.04 ± 120.08 b | 8853.37 ± 137.58 a | 8869.48 ± 261.78 a | 8880.14 ± 243.06 a | |||
| Isovaleric acid | 80.37 ± 65.62 c | 196.47 ± 16.12 b | 302.09 ± 15.53 a | 202.13 ± 16.33 b | 3000 | <0.1 | Sour, Cheese |
| Octanoic acid | 353.73 ± 7.14 b | 498.00 ± 65.80 a | 435.19 ± 14.94 ab | 501.68 ± 30.48 a | 500 | >1 | Sour, Cheese, Fatty |
| Decanoic acid | 188.28 ± 2.28 c | 245.13 ± 17.88 ab | 232.35 ± 5.00 b | 255.88 ± 5.69 a | 1000 | >0.1 | Sour, Fatty |
| Benzoic acid | 23,418.69 ± 3.04 a | 23,416.63 ± 5.84 a | 23,423.12 ± 9.65 a | 23,433.27 ± 9.87 a | |||
| Other (1) | |||||||
| 2-Methoxy-3-isobutyl pyrazine | 0.94 ± 0.00 a | 0.93 ± 0.00 b | 0.93 ± 0.00 b | 0.93 ± 0.00 b | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Chen, N.; Xu, Z.; Liu, J.; Liu, S.; Shi, K. High-Performance Indigenous Lactiplantibacillus plantarum Strains for Enhanced Malolactic Fermentation and Wine Quality. Microorganisms 2025, 13, 2328. https://doi.org/10.3390/microorganisms13102328
Zhu Y, Chen N, Xu Z, Liu J, Liu S, Shi K. High-Performance Indigenous Lactiplantibacillus plantarum Strains for Enhanced Malolactic Fermentation and Wine Quality. Microorganisms. 2025; 13(10):2328. https://doi.org/10.3390/microorganisms13102328
Chicago/Turabian StyleZhu, Yongzhang, Ni Chen, Zhenghua Xu, Jingyue Liu, Shuwen Liu, and Kan Shi. 2025. "High-Performance Indigenous Lactiplantibacillus plantarum Strains for Enhanced Malolactic Fermentation and Wine Quality" Microorganisms 13, no. 10: 2328. https://doi.org/10.3390/microorganisms13102328
APA StyleZhu, Y., Chen, N., Xu, Z., Liu, J., Liu, S., & Shi, K. (2025). High-Performance Indigenous Lactiplantibacillus plantarum Strains for Enhanced Malolactic Fermentation and Wine Quality. Microorganisms, 13(10), 2328. https://doi.org/10.3390/microorganisms13102328







