Abstract
This study examined the effects of alfalfa silage versus alfalfa hay in a total mixed ration (TMR) on milk yield, rumen fermentation, and nutrient digestibility in dairy cows. Hydrolyzed tannins (HT) were supplemented individually to assess changes. Thirty-two multiparous Holstein cows (DIM: 94 ± 8 d; milk yield: 41 ± 2 kg) were assigned to four treatments in a 2 × 2 factorial design: basal diet (alfalfa hay, H, or alfalfa silage, S) and additive (control, C, or 100 g/d HT, T). Production performance, rumen fermentation, nutrient digestibility, and blood metabolites were evaluated. Compared with group H, group S had a 0.16% higher milk protein percentage and significantly higher fat-corrected milk yield, milk fat percentage, fat-to-protein ratio, total solids, and milk urea nitrogen. After feeding, the ST group had increased ruminal pH. HT supplementation significantly decreased ruminal NH3-N levels (p < 0.05) and increased microbial crude protein (MCP) content (p < 0.05). Group H showed no significant changes, and the effects of HT were less evident in hay-fed cows than in silage-fed cows. In summary, alfalfa silage feeding increased ruminal microbial populations, while HT supplementation mitigated the post-feeding decline in ruminal pH. Considering the relatively small sample size (n = 32), the results should be viewed as indicative rather than conclusive, and future studies with larger cohorts will be valuable to confirm and extend these findings.
1. Introduction
Alfalfa is widely used as animal forage due to its high content of protein, vitamins, minerals, and other nutrients [1,2,3]. Known for its drought and cold resistance, alfalfa has a broad growing range and high yield [4]. In livestock production, it is often fed to high-producing ruminants (such as dairy cows) in the form of hay or silage. Alfalfa in hay form provides animals with greater satiety, and its high fiber content promotes intestinal motility; however, the drying process increases the risk of protein loss [5,6]. When ensiled at 30–40% dry matter, alfalfa undergoes anaerobic fermentation, which better preserves its nutritional components and generally provides higher energy and digestible protein compared to hay [7]. Incorporating alfalfa into total mixed rations (TMR) can enhance dry matter intake (DMI), as its palatability and digestibility help improve milk yield, as well as protein and fat content [8]. Therefore, it is important to investigate the effects of different forms of alfalfa on digestion and utilization in high-producing dairy cows.
Processing into hay or silage partially modifies alfalfa protein structure, improving palatability and energy use, but digestive degradation remains limiting [9]. Compared with hay, silage typically shows higher crude protein (CP), neutral detergent fiber (NDF), and acid detergent fiber (ADF), with nearly twice the CP degraded into amino acids, peptides, and NH3-N. This raises rumen degradable protein and water-soluble nitrogen, further enhancing digestibility [10]. Rumen microorganisms—bacteria, protozoa, and fungi—are central to energy and protein metabolism [11]. They degrade cellulose and hemicellulose to produce volatile fatty acids (VFAs), mainly acetate and propionate, which supply 70–80% of the cow’s energy. Roughage diets promote cellulolytic microbes, increasing acetate production, a key precursor for milk fat [12]. Propionate, with 20% higher energy efficiency, supports gluconeogenesis and lactose synthesis. In protein metabolism, microbes degrade dietary protein to produce microbial crude protein (MCP), which supplies 60–65% of digestible protein, and assimilate non-protein nitrogen into microbial protein. MCP provides a balanced amino acid profile, with lysine and methionine at ~6.8% and 2.5%, respectively, serving as the main absorbable amino acid source in the small intestine [13]. However, in the rumen, particularly the posterior rumen, a portion of plant-based protein is broken down by microbes, releasing ammonia. Some of this ammonia is subsequently converted into urea and excreted in the urine, leading to environmental pollution. The development of additives capable of leveraging these dynamic microbial functions and interaction mechanisms is crucial for effectively reducing pollutant emissions and enhancing animal production efficiency.
In recent years, a wide range of plant extracts rich in secondary metabolites—including terpenoids, neem extracts, saponins, and flavonoids—have been evaluated for their potential to reduce protein waste [14,15]. Among these, tannins, a class of natural polyphenolic compounds found in shrubs, legumes, cereals, and other plants, have attracted significant attention in protein utilization research. Based on structural differences, tannins are primarily categorized into hydrolyzable tannins (HT) and condensed tannins (CT) [16]. HTs, abundant in gallnuts and chestnut wood, hydrolyze to gallic acid and glucose and generally show stronger biological activity [17]. CTs occur as polymers in legumes [18]. Tannins form stable complexes with proteins, reducing ruminal degradation and traditionally considered anti-nutritional [19,20]. However, they exhibit dual benefits in dairy cattle: ① Protein protection: stable tannin–protein complexes at ruminal pH reduce microbial degradation, allowing more protein to reach the small intestine and improving nitrogen metabolism [21]; ② Microbial modulation: HTs inhibit methanogens while stimulating fibrolytic bacteria, enhancing fermentation and feed digestibility [22]. Appropriate HT supplementation has shown benefits, including reduced methane emissions without impairing protein synthesis. For example, adding 0.72% DM black wattle tannin extract reduced methane by 17% [23,24]. Jones and Mangan (1977) demonstrated that tannins bind with proteins to form complexes that remain stable at ruminal pH but can dissociate under the more acidic pH of the abomasum or the alkaline pH of the duodenum [25]. By reducing protein degradation in the rumen and increasing protein flow to the duodenum, tannins thereby decrease nitrogen loss in urine [26]. Supplementing beef cattle diets with 0.25–1.50% chestnut tannins increased average daily gain by 0.297 kg [18,27].
This study compared the effects of alfalfa hay versus alfalfa silage on rumen fermentation, production performance, nutrient digestibility, and blood metabolic profiles in dairy cattle. In parallel, it investigated the regulatory mechanisms of HT supplementation on the rumen environment, aiming to assess their potential as an alternative to antibiotics in feed additives. The results are expected to provide a scientific basis for enhancing feed utilization and improving the economic viability of ruminant production.
2. Materials and Methods
2.1. Experimental Animals and Management
The experimental pasture is located at Shanxi Wangxiangyuan Animal Husbandry Co., Ltd., situated west of Gao Village (Beige Town, Taiyuan, China) in December 2022. The animal studies were approved by the Animal Welfare and Ethics Committee of Shanxi Agricultural University. The studies were conducted in accordance with the local legislation and institutional requirements (Approval code. SXAU-EAW-2022C. RD.010025174. Approval Date: 25 November 2022). The alfalfa used in this trial was cultivated in the Yanmen region, Xinzhou, China. The HT additive was purchased from Jiurui Biotechnology Co., Ltd., (Zhangjiajie, China). Each cow received 100 g/day of HT additive with uniform distribution into the TMR.
Thirty-two multiparous Holstein dairy cows (parity 2; DIM: 94 ± 8 d, milk yield: 41 ± 2 kg/d, BW 670 ± 25 kg) were selected for the experiment. They were randomly assigned to four treatment groups in a 2 × 2 factorial design, with eight cows per group (n = 8 per treatment): Alfalfa Hay Control (HC), Alfalfa Hay with 100 g/d Hydrolyzed Tannin (HT), Alfalfa Silage Control (SC), and Alfalfa Silage with 100 g/d Hydrolyzed Tannin (ST). All cows were fed twice daily in stalls and received the additive in two equal doses. The study consisted of a 10 d preliminary period followed by a 30 d main trial. During the preliminary period, feed intake, rumination, and fecal characteristics were monitored. Subsequently, changes in body weight, nutrient digestibility, milk yield, milk quality, rumen fermentation, microbial community structure, enzyme activity, and blood biochemical parameters were measured.
2.2. Experimental Diet
The dry matter of alfalfa silage was approximately 40%, while the dry matter of alfalfa hay was about 90%. The diet was formulated based on the NRC (2021) [28] guidelines, using 2.25 kg of alfalfa silage to replace 1 kg of alfalfa hay, as shown in Table 1.

Table 1.
The composition and nutritional ingredients of the diet.
2.3. Sample Collection and Analytical Methods
This study collected and analyzed multiple indicators in dairy cows to evaluate feed utilization, ruminal fermentation, production performance, and blood metabolic profiles.
2.3.1. Production Performances
Body weight was measured twice: once at the end of the pre-test period and once at the end of the main test period. The body ruler estimation method was used, involving a tape measure to determine chest circumference and body slope length. The body weight was calculated using the formula:
body weight = chest circumference2 × body slope length × 90.
2.3.2. Feed and Fecal Samples Collection and Analysis
On the last day of the pre-feeding period and on days 10, 20, and 30 of the formal trial, 500 g each of feed and ort samples were collected, dried at 55 °C for 48 h until a constant weight was reached, ground, sieved (1 mm aperture), and stored in sealed containers for chemical composition analysis. The following components of the ground feed samples were then analyzed: dry matter (DM), crude protein (CP, Kjeldahl method) [29], crude fiber (CF) [29], neutral detergent fiber (NDF), acid detergent fiber (ADF), crude ash (Van Soest method) [30], calcium, and phosphorus [31].
Fecal samples were collected via rectal sampling on the morning of day 27, at noon on day 28, and in the afternoon of day 29. For every 100 g of fresh feces per cow, 25 mL of a 10% tartaric acid solution was added, and the samples were then frozen at −20 °C for TMR digestibility analysis. Before analysis, manure samples were dried at 55 °C for 72 h until a constant weight was achieved, then ground and passed through a 1 mm sieve. The chemical composition of the fecal samples was then analyzed following the same procedures as for the feed samples.
2.3.3. Rumen Content Sampling and Parameter Analysis
Rumen contents: On the morning of day 30, approximately 100 mL of rumen fluid was collected before feeding and 3 h post-feeding using an oral-nasal probe equipped with a metal filter and a manual pump (Chengdu Huazhi Kaiwu Technology Co., Ltd., Chengdu, China). Immediately after collection, the pH of each sample was measured using a pH meter (LE438, Mettler-Toledo Instrument Co., Ltd., Shanghai, China). The samples were subsequently stored at −20 °C for further analysis of volatile fatty acids (VFAs), ammonia N (NH3-N), microbial crude protein (MCP), enzyme activity and microbial diversity. The concentration of NH3-N in the rumen fluid was determined by the phenol-sodium hypochlorite colorimetric method [32]. The VFA content was measured using gas chromatograph (GC122, Shanghai Precision Scientific Instrument Co., Ltd., Shanghai, China) [33]. Enzymatic activity (Carboxymethyl-cellulase, Pectinase, Cellobiase, Xylanase) was then measured via spectrophotometric colorimetry (UV3000, Shanghai Meipuda Instrument Co., Ltd., Shanghai, China) [34]. Protease activity in rumen fluid was determined by the Folin-phenol method. The MCP content in rumen fluid was measured using the Coomassie brilliant blue colorimetric method [35]. DNA was extracted from rumen microorganisms using the CTAB method. Subsequently, primers synthesized by Beijing Genomics Institute Co., Ltd., (Beijing, China) were used according to the instructions provided with the TaKaRa kit (Beijing Baorui Biological Technology Co., Ltd., Beijing, China). Real-time PCR quantification was carried out using an ABI StepOnePlus instrument (Applied Biosystems, Waltham, MA, USA), achieving amplification efficiencies between 90% and 110% and a standard curve with an R2 ≥ 0.999 [36].
2.3.4. Milk Production and DHI Monitor
Milk yield was recorded and milk samples were collected at the end of the pre-test period and on days 9, 19, and 29 of the main test period. A 50 mL composite sample was prepared by mixing milk from the morning, noon, and evening in a 4:3:3 ratio. These samples were then transported at low temperature to the Shanxi DHI Testing Center, where milk components were analyzed using the Fossomatic 5000 series instrument developed by FUCHS in Hellerup, Denmark.
2.3.5. Collection of Blood and Analysis of Serum Indexes
Collection of Blood: On the 30th day of the trial, 30 mL of blood was collected from the tail vein before morning feeding. After allowing the samples to stand at room temperature for 1 h, they were centrifuged at 3000× g for 15 min. The serum was then collected, cryopreserved, and analyzed for routine blood indices using a kit from the Nanjing Jiancheng Institute of Bioengineering (Nanjing, China). Glucose (GLU), total protein (TP), albumin (ALB), globulin (GLO), milk urea nitrogen (MUN), calcium (CA), and phosphorus (P) were determined using a UV3000 spectrophotometer (Shanghai Meipuda Instrument Co., Ltd., Shanghai, China). Additionally, total cholesterol (TC), triglycerides (TG), nonesterified fatty acids (NEFA), β-hydroxybutyrate (β-HB), acetoacetate (ACAC), acetyl-CoA (A-CoA), fatty acid synthase (FAS), adipose triglyceride lipase (ATGL), and other indicators were measured using a microplate reader (Bethon Synergy H1) according to the instructions provided by the Shanghai Duma kit (Shanghai, China).
2.4. Data Processing and Statistical Analysis
Data were analyzed using two-way analysis of variance (ANOVA) with SAS 9.2, where factor one was the form of alfalfa supply and factor two was the application of additives. When the interaction effect of the two factors was significant (p < 0.05), Tukey’s multiple comparison test was used to compare the means of the groups, with significance set at p < 0.05.
3. Results
3.1. Effect of Forage Form and Hydrolysable Tannins on Apparent Nutrient Digestibility
As can be seen from Table 2, when comparing alfalfa silage with alfalfa hay, apparent digestibility was significantly improved. Apparent DM digestibility was higher in the silage group (85.1% for SC vs. 79.4% for HC; p < 0.01). Apparent digestibility of CP, NDF, ADF, and Ca was also greater in the silage group than in the hay group (p < 0.05). Supplementation with 1.5% hydrolysable tannins did not significantly affect apparent digestibility of DM, OM, CP, CF, NDF, ADF, Calcium, or Phosphorus (p > 0.05). Differences in apparent digestibility were driven primarily by forage form, with no significant interaction between forage form and tannin supplementation (p > 0.05).

Table 2.
Effect of alfalfa product form and hydrolysed tannins on the apparent digestibility of nutrients in dairy cows.
3.2. Rumen Microbiota and Fermentation Responses to Forage Form and Tannins
Forage form substantially remodeled the rumen ecosystem. Figure 1 shows that silage-fed cows showed 28% higher fungal counts (p < 0.01) and 17% higher Ruminococcus flavefaciens abundance (p < 0.05); protozoal counts were unaffected by treatments. Tannin-treated groups (both H and S) had significantly lower Prevotella ruminicola populations versus controls (p < 0.01). It can be observed from Table 3 that together with 5–8% higher carboxymethyl-cellulase and xylanase activities (p < 0.01), pectinase activity was lower in S than H (p < 0.05). Hydrolysable tannins did not alter carboxymethyl-cellulase, pectinase, cellobiase, or xylanase activities (p > 0.05).
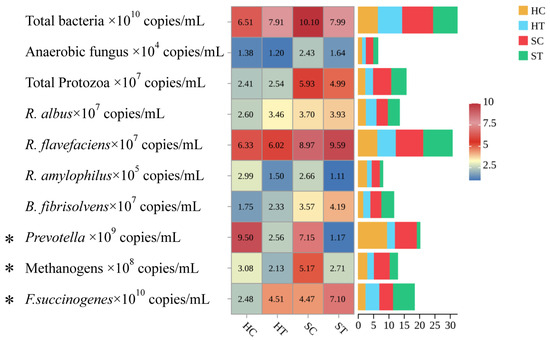
Figure 1.
Effect of alfalfa product form and hydrolysed tannins on rumen microbiota structure. * p < 0.05. The 2.5–10 indicator diagram indicates the left side of the diagram, with darker colors from bottom to top indicating higher values.

Table 3.
Effect of alfalfa product form and hydrolysed tannins on Enzyme Activities in dairy cows.
Table 4 presents the changes and comparisons of ruminal pH, NH3-N, MCP and VFA among the four groups of dairy cows. Rumen fermentation variables also shifted with forage form. MCP concentration was lower in the S group (35.9 mg/dL) compared with H (p < 0.05). Isovaleric acid (IVA) decreased after replacing hay with silage (p < 0.01), whereas propionic acid (PA), acetate-to-propionate (AA/PA) ratio, and acetate (AA) were higher in S (PA and AA/PA: p < 0.01; AA: p < 0.05). Tannin inclusion produced modest reductions in ruminal NH3-N and methanogen counts (p < 0.05) but did not amplify the microbial or digestive advantages associated with silage; these changes were insufficient to improve milk yield or feed efficiency [37].

Table 4.
Effect of alfalfa product form and hydrolysed tannins on rumen fermentation parameter.
3.3. Effects of Forage Form and Hydrolysable Tannin Supplementation on Intake, Body Weight, and Milk Production
Body weight and production metrics were largely unaffected by forage form or tannin supplementation. As shown in Table 5, the average daily weight gain (DWG) for the HC group was 0.27 kg/d, and for the SC group it was 0.73 kg/d; post-tannin DWG was 0.63 kg/d (HT) and 0.73 kg/d (ST); differences were not significant (p > 0.05), indicating no effect on body weight or lactation length. In Table 6, dry matter intake and milk yield were similar between treatments: HC consumed 22.3 kg DM/d and produced 29.7 kg milk/d, while SC consumed 22.6 kg DM/d and produced 29.8 kg milk/d. Supplementing 1.5% hydrolysable tannins (HT, ST) did not change DMI or daily milk yield.

Table 5.
Lactation period and body weight of dairy cows.

Table 6.
Effects of alfalfa product forms and hydrolyzed tannins on the performance of dairy cows.
Tannins modestly altered milk composition: milk fat increased from 4.12% to 4.28% and total solids from 12.5% to 12.7% (p < 0.05), whereas milk protein remained unchanged. These composition shifts were statistically significant but small and yield-neutral, limiting practical relevance for commercial herds [16].
3.4. Effects of Forage Form and Hydrolysable Tannins on Blood Metabolites
According to the data in Table 7, the serum metabolite profiles remained largely stable across treatment groups. Replacing hay with silage did not induce significant changes in serum glucose (GLU), total protein (TP), albumin (ALB), blood urea nitrogen (BUN), calcium (Ca), or phosphorus (P); similarly, no significant differences were observed in total cholesterol (TC), non-esterified fatty acids (NEFA), β-hydroxybutyrate, or fatty acid synthase (FAS). However, the acetoacetate (ACAC) concentration was significantly higher in the S group compared to the H group (p < 0.05). Hydrolysable tannin supplementation alone had no significant effect on GLU, serum Ca, or P (p > 0.05) but significantly reduced serum TP, globulin (GLO), and BUN (p < 0.05). A significant interaction was observed between alfalfa form and tannin supplementation in terms of BUN (p < 0.05). Tannins did not significantly affect TC, triglycerides (TG), NEFA, β-hydroxybutyrate, acetyl-CoA, FAS, or adipose triglyceride lipase (ATGL) (p > 0.05).

Table 7.
Effect of alfalfa product form and hydrolysed tannins on blood indices in dairy cows.
4. Discussion
The results of the feeding trials show that neither alfalfa silage nor alfalfa hay directly increases total milk production or growth performance, but it does reprogram nutrient partitioning and metabolic fluxes in the rumen. In this study dairy cows fed alfalfa silage often show no significant change in dry matter intake or overall milk output relative to hay-fed cows, yet milk fat and solids content rise markedly [1]. This suggests that ensiling alters the availability and form of nutrients: silage typically has higher soluble fiber and energy, promoting more extensive ruminal fermentation. As a result, silage diets deliver more digestible energy (especially from fiber) and VFAs per unit intake, without increasing gross intake. Mechanistically, improved fiber digestibility with silage “better stimulates rumen fermentation” and produces more acetogenic precursors for milk fat synthesis [38]. Indeed, silage-fed cows tend to have higher ruminal acetate and lower propionate concentrations, reflecting a shift toward acetate-producing fermentation [39]. This acetate-rich milieu directly fuels de novo fatty acid synthesis in the mammary gland, explaining the elevated milk fat observed with silage diets. In short, forage processing (ensiling) reshapes rumen fermentation: it enhances cellulolytic breakdown by microbes and enriches acetate production, thereby enriching milk’s fat fraction without altering total yield [40].
At the microbial level, silage versus hay alters substrate availability and community structure. Ensiling breaks down plant cell walls and frees sugars, favoring growth of genera such as Prevotella that ferment soluble carbohydrates [41]. For example, increased fermentable carbohydrates in silage support Prevotella proliferation, which contributes to both amino acid synthesis and SCFA (acetate/propionate) production. Likewise, fiber-degrading phyla like Fibrobacteres may proliferate under silage feeding, enhancing cellulolysis (as noted by a 166% increase in Fibrobacteres OTUs and a strong correlation between Fibrobacteres abundance and milk yield [42].
In contrast, the hay diet with more intact fiber may sustain a higher proportion of Firmicutes and Fiber-degraders that operate on slower fermentation. Despite these shifts in specific taxa, the dominant phyla (Bacteroidetes, Firmicutes) remain similar, indicating that overall fiber digestion is maintained across diets [43]. Importantly, beneficial saccharolytic microbes (e.g., Prevotella) increase with silage, while several potentially proteolytic or pathogenic genera (e.g., Erysipelatoclostridium, Pseudoflavonifractor, Candidatus Saccharimonas) decline [44]. Together, these changes suggest that silage feeding enhances the rumen’s fermentative capacity (especially fiber breakdown) and yields more substrates (acetate, glucose) for milk synthesis, even though overall digestion kinetics (apparent digestibility of major nutrients) remain largely unchanged [45].
Supplemental hydrolyzable tannins introduce another layer of ruminal modulation. Tannins are polyphenolic plant compounds that bind dietary proteins and microbes. In the rumen, tannins form indigestible complexes with feed proteins, slowing proteolysis [46]. This action “reduces ruminal protein degradability” [47] and lowers ammonia production, because less dietary protein is deaminated by microbes. The net effect is a shift of more undegraded protein to the intestine (enhancing post-ruminal amino acid supply) and improved nitrogen utilization [48]. At the same time, tannins can suppress certain microbial groups (notably methanogenic archaea and proteolytic bacteria), which helps lower methane output and nitrogen losses. By inhibiting protozoa and archaea, tannins decrease hydrogen availability for methanogenesis and shift VFA proportions toward propionate (a more glucogenic VFA) [49].
These combined effects mean that low-dose tannin supplementation in high-alfalfa diets often does not raise milk yield, but it significantly enhances feed efficiency and sustainability. Remarkably, these ruminal changes do not induce major disruptions in systemic metabolism. Blood metabolite profiles are largely stable across treatments, indicating that the animals’ energy and protein homeostasis remain intact [50]. Studies report no significant differences in serum glucose, total protein or general metabolic enzymes when cows are switched from hay to silage [51]. The one exception is blood urea nitrogen (BUN): tannin-fed cows consistently show reduced BUN concentrations. This reduction is mechanistically consistent with less ammonia absorption from the rumen (and hence less hepatic urea synthesis) when protein degradation is curtailed by tannins [52]. A recent in vivo trial found that chestnut tannin extract (hydrolyzable tannins) fed at 0.18–0.36% of diet significantly lowered BUN, confirming the “suppressive effect of chestnut tannins on rumen protein degradation” [53]. Other blood parameters, glucose, nonesterified fatty acids, β-hydroxybutyrate, and liver enzymes, remain within normal ranges and show no consistent trends under silage or tannin diets. This indicates that the cows accommodate these feeding strategies without overt metabolic stress. (Interestingly, one study noted slightly higher serum triglycerides with silage, perhaps reflecting increased VFA flux toward lipogenesis for milk fat, but overall lipid homeostasis was preserved.) In sum, the primary action of both forage form and tannin additives is confined to the rumen: they fine-tune fermentation and nitrogen cycling, while systemic energy/protein markers are only minimally affected [54]. Nonetheless, it should be acknowledged that the relatively small sample size in each treatment group (n = 8) may limit the statistical power and generalizability of the findings. Future studies involving larger populations and longer feeding periods are warranted to confirm these results and strengthen the evidence base.
5. Conclusions
Collectively, the data demonstrate that first, alfalfa silage can fully replace hay without production loss provided fiber digestibility is enhanced, and second, hydrolysable tannins act as targeted modifiers of ruminal nitrogen metabolism rather than broad enhancers of milk yield in high-alfalfa diets. By integrating protein-binding actions with forage matrix effects, hydrolysable tannins unlock nutritional gains while mitigating nitrogen waste, positioning them as dual-purpose tools for environmentally optimized dairy production.
Author Contributions
Conceptualization, L.C. and G.G.; Methodology, H.D. and C.S.; Software, J.S.; Validation, C.B.; Formal analysis, X.M.; Investigation, C.B. and H.D.; Resources, G.C.; Data curation, J.S.; Writing—original draft, X.M.; Writing—review & editing, C.S. and W.H.; Visualization, C.W. and L.C.; Supervision, Q.L. and C.W.; Project administration, W.H. and Q.L.; Funding acquisition, L.C. and G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the earmarked fund for Modern Agro-industry Technology Research System (2025CYJSTX13-08) and National Natural Science Foundation of China (32001405).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee of Shanxi Agricultural University (protocol code: SXAU-EAW-2022C. RD.010025174; protocol date: 25 November 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Thanks to all participants for their advice and support of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, K.; Chen, M.; Huang, G.; Su, C.; Tang, W.; Li, N.; Yang, J.; Wu, X.; Si, B.; Zhao, S.; et al. Variations in the milk lipidomic profile of lactating dairy cows fed the diets containing alfalfa hay versus alfalfa silage. Anim. Nutr. 2024, 19, 261–271. [Google Scholar] [CrossRef]
- Agarussi, M.C.N.; Pereira, O.G.; da Silva, V.P.; Leandro, E.S.; Ribeiro, K.G.; Santos, S.A. Fermentative profile and lactic acid bacterial dynamics in non-wilted and wilted alfalfa silage in tropical conditions. Mol. Biol. Rep. 2019, 46, 451–460. [Google Scholar] [CrossRef]
- Bao, X.; Feng, H.; Guo, G.; Huo, W.; Li, Q.; Xu, Q.; Liu, Q.; Wang, C.; Chen, L. Effects of laccase and lactic acid bacteria on the fermentation quality, nutrient composition, enzymatic hydrolysis, and bacterial community of alfalfa silage. Front. Microbiol. 2022, 13, 1035942. [Google Scholar] [CrossRef]
- Li, R.; Jiang, D.; Zheng, M.; Tian, P.; Zheng, M.; Xu, C. Microbial community dynamics during alfalfa silage with or without clostridial fermentation. Sci. Rep. 2020, 10, 17782. [Google Scholar] [CrossRef]
- Rufino-Moya, P.J.; Bertolín, J.R.; Blanco, M.; Lobón, S.; Joy, M. Fatty acid profile, secondary compounds and antioxidant activities in the fresh forage, hay and silage of sainfoin (Onobrychis viciifolia) and sulla (Hedysarum coronarium). J. Sci. Food Agric. 2022, 102, 4736–4743. [Google Scholar] [CrossRef]
- Szumacher-Strabel, M.; Stochmal, A.; Cieslak, A.; Kozłowska, M.; Kuznicki, D.; Kowalczyk, M.; Oleszek, W. Structural and quantitative changes of saponins in fresh alfalfa compared to alfalfa silage. J. Sci. Food Agric. 2019, 99, 2243–2250. [Google Scholar] [CrossRef] [PubMed]
- Al-Gaadi, K.A. Impact of raking and baling patterns on alfalfa hay dry matter and quality losses. Saudi J. Biol. Sci. 2018, 25, 1040–1048. [Google Scholar] [CrossRef]
- Nelson, W.F.; Satter, L.D. Impact of alfalfa maturity and preservation method on milk production by cows in early lactation. J. Dairy Sci. 1992, 75, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Yang, G.L.; Jing, Y.Y.; He, Z.N.; Liu, B.; Sun, L.; Zhang, Y.; Gao, F. Alfalfa quality improvement and loss reduction technology advances. Front. Anim. Sci. 2025, 6, 1550492. [Google Scholar] [CrossRef]
- Sikora, M.C.; Hatfield, R.D.; Kalscheur, K.F. Impact of long-term storage on alfalfa leaf and stem silage characteristics. Agronomy 2021, 11, 2505. [Google Scholar] [CrossRef]
- Jia, M.; Zhu, S.; Xue, M.Y.; Chen, H.; Xu, J.; Song, M.; Tang, Y.; Liu, X.; Tao, Y.; Zhang, T.; et al. Single-cell transcriptomics across 2,534 microbial species reveals functional heterogeneity in the rumen microbiome. Nat. Microbiol. 2024, 9, 1884–1898. [Google Scholar] [CrossRef]
- Stergiadis, S.; Cabeza-Luna, I.; Mora-Ortiz, M.; Stewart, R.D.; Dewhurst, R.J.; Humphries, D.J.; Watson, M.; Roehe, R.; Auffret, M.D. Unravelling the role of rumen microbial communities, genes, and activities on milk fatty acid profile using a combination of omics approaches. Front. Microbiol. 2021, 11, 590441. [Google Scholar] [CrossRef]
- Putri, E.M.; World, V. Effects of rumen-degradable-to-undegradable protein ratio in ruminant diet on in vitro digestibility, rumen fermentation, and microbial protein synthesis. Vet. World 2021, 14, 640–648. [Google Scholar] [CrossRef]
- Bagheri, V.M.; Klevenhusen, F.; Zebeli, Q.; Petri, R. Scrophularia striata Extract Supports Rumen Fermentation and Improves Microbial Diversity in vitro Compared to Monensin. Front. Microbiol. 2018, 9, 2164. [Google Scholar] [CrossRef] [PubMed]
- Eom, J.S.; Lee, S.J.; Lee, Y.; Kim, H.S.; Choi, Y.Y.; Kim, H.S.; Kim, D.H.; Lee, S.S. Effects of supplementation levels of Allium fistulosum L. extract on in vitro ruminal fermentation characteristics and methane emission. PeerJ 2020, 8, e9651. [Google Scholar] [CrossRef]
- Jayanegara, A.; Yogianto, Y.; Wina, E.; Sudarman, A.; Kondo, M.; Obitsu, T.; Kreuzer, M. Combination Effects of Plant Extracts Rich in Tannins and Saponins as Feed Additives for Mitigating in Vitro Ruminal Methane and Ammonia Formation. Animals 2020, 10, 1531. [Google Scholar] [CrossRef]
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Ieri, F.; Romani, A. Antimicrobial and antiviral activity of hydrolysable tannins. Mini Rev. Med. Chem. 2008, 8, 1179–1187. [Google Scholar] [CrossRef]
- Tadese, D.A.; Song, C.; Sun, C.; Liu, B.; Liu, B.; Zhou, Q.; Xu, P.; Ge, X.; Liu, M.; Xu, X.; et al. The role of currently used medicinal plants in aquaculture and their action mechanisms: A review. Rev. Aquac. 2022, 14, 816–847. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Beauchemin, K.A. Potential of molecular weight and structure of tannins to reduce methane emissions from ruminants: A review. Animals 2019, 9, 856. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Vaddella, V.; Zhou, D. Effects of chestnut tannins and coconut oil on growth performance, methane emission, ruminal fermentation, and microbial populations in sheep. J. Dairy Sci. 2011, 94, 6069–6077. [Google Scholar] [CrossRef] [PubMed]
- Avila, A.S.; Zambom, M.A.; Faccenda, A.; Werle, C.H.; Almeida, A.R.; Schneider, C.R.; Grunevald, D.G.; Faciola, A.P. Black Wattle (Acacia mearnsii) condensed tannins as feed additives to lactating dairy cows. Animals 2020, 10, 662. [Google Scholar] [CrossRef]
- Costa, M.; Alves, S.P.; Cabo, Â.; Guerreiro, O.; Stilwell, G.; Dentinho, M.T.; Bessa, R.J. Modulation of in vitro rumen biohydrogenation by Cistus ladanifer tannins compared with other tannin sources. J. Sci. Food Agric. 2017, 97, 629–635. [Google Scholar] [CrossRef]
- Perna Junior, F.; Galbiatti Sandoval Nogueira, R.; Ferreira Carvalho, R.; Cuellar Orlandi Cassiano, E.; Mazza Rodrigues, P.H. Use of tannin extract as a strategy to reduce methane in Nellore and Holstein cattle and its effect on intake, digestibility, microbial efficiency and ruminal fermentation. J. Anim. Physiol. Anim. Nutr. 2023, 107, 89–102. [Google Scholar] [CrossRef]
- Jayanegara, A.; Goel, G.; Makkar, H.P.; Becker, K. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Anim. Feed Sci. Technol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- Jones, W.T.; Mangan, J.L. Complexes of the condensed tannins of sainfoin (Onobrychis viciifolia Scop.) with fraction 1 leaf protein and with submaxillary mucoprotein, and their reversal by polyethylene glycol and pH. J. Sci. Food Agric. 1977, 28, 126–136. [Google Scholar] [CrossRef]
- Frutos, P.; Hervas, G.; Giráldez, F.J.; Mantecón, A.R. Tannins and ruminant nutrition. Span. J. Agric. Res. 2004, 2, 191–202. [Google Scholar] [CrossRef]
- Malmuthuge, N.; Griebel, P.J.; Guan, L.L. Taxonomic identification of commensal bacteria associated with the mucosa and digesta throughout the gastrointestinal tracts of preweaned calves. Appl. Environ. Microbiol. 2014, 80, 2021–2028. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Fox, D.G.; Fonseca, M.A.; Cavalcanti, L.F.L. Models of protein and amino acid requirements for cattle. Rev. Bras. Zootec. 2015, 44, 109–132. [Google Scholar] [CrossRef]
- Jung, S.; Rickert, D.A.; Deak, N.A.; Aldin, E.D.; Recknor, J.; Johnson, L.A.; Murphy, P.A. Comparison of Kjeldahl and Dumas methods for determining protein contents of soybean products. J. Am. Oil Chem. Soc. 2003, 80, 1169–1173. [Google Scholar] [CrossRef]
- Paulk, C.B.; Stark, C.R.; Dunmire, K.M. Feed processing technology and quality of feed. In Sustainable Swine Nutrition; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 429–444. [Google Scholar] [CrossRef]
- Venter, K.M.; Angel, R.; Fourie, J.; Plumstead, P.W.; Li, W.; Enting, H.; Dersjant-Li, Y.; Jansen van Rensburg, C. Determination of calcium and phosphorus digestibility of individual feed ingredients as affected by limestone, in the presence and absence of phytase in broilers. Animals 2024, 14, 3603. [Google Scholar] [CrossRef]
- Wang, S.; Ma, T.; Zhao, G.; Zhang, N.; Tu, Y.; Li, F.; Cui, K.; Bi, Y.; Ding, H.; Diao, Q. Effect of age and weaning on growth performance, rumen fermentation, and serum parameters in lambs fed starter with limited ewe–lamb interaction. Animals 2019, 9, 825. [Google Scholar] [CrossRef]
- Li, H.Q.; Liu, Q.; Wang, C.; Guo, G.; Huo, W.J.; Zhang, S.L.; Zhang, Y.L.; Pei, C.X.; Yang, W.Z.; Wang, H. Effects of rumen-protected pantothenate on ruminal fermentation, microbial enzyme activity, cellulolytic bacteria and urinary excretion of purine derivatives in growing beef steers. Livest. Sci. 2017, 202, 159–165. [Google Scholar] [CrossRef]
- Maia, G.G.; Siqueira, L.G.B.; de Paula Vasconcelos, C.O.; Tomich, T.R.; de Almeida Camargo, L.S.; Rodrigues, J.P.P.; de Menezes, R.A.; Gonçalves, L.C.; Teixeira, B.F.; Grando, R.d.O.; et al. Effects of heat stress on rumination activity in Holstein-Gyr dry cows. Livest. Sci. 2020, 239, 104092. [Google Scholar] [CrossRef]
- Önder, A. Anticancer activity of natural coumarins for biological targets. Stud. Nat. Prod. Chem. 2020, 64, 85–109. [Google Scholar] [CrossRef]
- Minas, K.; McEwan, N.R.; Newbold, C.J.; Scott, K.P. Optimization of a high-throughput CTAB-based protocol for the extraction of qPCR-grade DNA from rumen fluid, plant and bacterial pure cultures. FEMS Microbiol. Lett. 2011, 325, 162–169. [Google Scholar] [CrossRef]
- Castro-Montoya, J.; Makkar, H.P.S.; Becker, K. Effects of dietary tannins on milk composition: A quantitative synthesis. Anim. Feed Sci. Technol. 2022, 285, 115203. [Google Scholar] [CrossRef]
- Xia, T.; Liu, Z.; Yang, Z.; Jiang, A.; Zhou, C.; Tan, Z. Effects of Partial Replacement of Alfalfa Hay with Alfalfa Silage in Dairy Cows: Impacts on Production Performance and Rumen Microbiota. Animals 2025, 15, 2748. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Meng, Z.; Tan, D.; Datsomor, O.; Zhan, K.; Lin, M.; Zhao, G. Effects of supplementation of sodium acetate on rumen fermentation and microbiota in postpartum dairy cows. Front. Microbiol. 2022, 13, 1053503. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, M.Z.; Ashfaq, M.Y.; Al-Ghouti, M.; Zouari, N. Insights into population adaptation and biodiversity of lactic acid bacteria in challenged date palm leaves silaging, using MALDI–TOF MS. Curr. Res. Microb. Sci. 2024, 6, 100235. [Google Scholar] [CrossRef] [PubMed]
- Caparra, P.; Chies, L.; Scerra, M.; Foti, F.; Bognanno, M.; Cilione, C.; De Caria, P.; Claps, S.; Cifuni, G.F. Effect of dietary ensiled olive cake supplementation on performance and meat quality of Apulo-Calabrese pigs. Animals 2023, 13, 2022. [Google Scholar] [CrossRef]
- Zou, Y.; Zou, X.; Li, X.; Guo, G.; Ji, P.; Wang, Y.; Li, S.; Wang, Y.; Cao, Z. Substituting oat hay or maize silage for portion of alfalfa hay affects growth performance, ruminal fermentation, and nutrient digestibility of weaned calves. Asian-Australas. J. Anim. Sci. 2017, 31, 369. [Google Scholar] [CrossRef]
- Weimer, P.J. Degradation of cellulose and hemicellulose by ruminal microorganisms. Microorganisms 2022, 10, 2345. [Google Scholar] [CrossRef]
- Qu, X.; Raza, S.H.A.; Zhao, Y.; Deng, J.; Ma, J.; Wang, J.; Alkhorayef, N.; Alkhalil, S.S.; Pant, S.D.; Lei, H.; et al. Effect of tea saponins on rumen microbiota and rumen function in Qinchuan beef cattle. Microorganisms 2023, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Jin, M.; Shao, Y.; Yin, J.; Li, H.; Chen, T.; Shi, D.; Zhou, S.; Li, J.; Yang, D. High-sugar, high-fat, and high-protein diets promote antibiotic resistance gene spreading in the mouse intestinal microbiota. Gut Microbes 2022, 14, 2022442. [Google Scholar] [CrossRef]
- Niu, J.; Liu, X.; Xu, J.; Li, F.; Wang, J.; Zhang, X.; Yang, X.; Wang, L.; Ma, S.; Li, D.; et al. Effects of silage diet on meat quality through shaping gut microbiota in finishing pigs. Microbiol. Spectr. 2023, 11, e02416-22. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Muir, J.P.; Naumann, H.D.; Norris, A.B.; Ramírez-Restrepo, C.A.; Mertens-Talcott, S.U. Nutritional aspects of ecologically relevant phytochemicals in ruminant production. Front. Vet. Sci. 2021, 8, 628445. [Google Scholar] [CrossRef] [PubMed]
- Valcl, N.; Lavrenčič, A. Effect of tannins and drying methods on in vitro dry matter and crude protein degradability and digestibility of soybean meal for ruminants. Sci. Rep. 2025, 15, 28612. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Ramírez-Restrepo, C.A.; Muir, J.P. Developing a conceptual model of possible benefits of condensed tannins for ruminant production. Animal 2014, 8, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Salminen, J.P.; Karonen, M.; Sinkkonen, J. Chemical ecology of tannins: Recent developments in tannin chemistry reveal new structures and structure–activity patterns. Chem. Eur. J. 2011, 17, 2806–2816. [Google Scholar] [CrossRef]
- Aguerre, M.J.; Capozzolo, M.C.; Lencioni, P.; Cabral, C.; Wattiaux, M.A. Effect of quebracho-chestnut tannin extracts at 2 dietary crude protein levels on performance, rumen fermentation, and nitrogen partitioning in dairy cows. J. Dairy Sci. 2016, 99, 4476–4486. [Google Scholar] [CrossRef]
- Prodanović, R.; Nedić, S.; Simeunović, P.; Borozan, S.; Nedić, S.; Bojkovski, J.; Kirovski, D.; Vujanac, I. Effects of chestnut tannins supplementation of prepartum moderate yielding dairy cows on metabolic health, antioxidant and colostrum indices. Ann. Anim. Sci. 2021, 21, 609–621. [Google Scholar] [CrossRef]
- Min, B.R.; Barry, T.N.; Attwood, G.T.; McNabb, W.C. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: A review. Anim. Feed Sci. Technol. 2003, 106, 3–19. [Google Scholar] [CrossRef]
- Li, F.; Usman, S.; Huang, W.; Jia, M.; Kharazian, Z.A.; Ran, T.; Li, F.; Ding, Z.; Guo, X. Correction: Effects of inoculating feruloyl esterase-producing Lactiplantibacillus plantarum A1 on ensiling characteristics, in vitro ruminal fermentation and microbiota of alfalfa silage. J. Anim. Sci. Biotechnol. 2023, 14, 53. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).