Multifaceted Antibiotic Resistance in Diabetic Foot Infections: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
- Studies published between January 2014 and June 2024.
- Studies that provided data on the microbiological profile of diabetic foot infections and reported antibiotic resistance patterns.
- Research conducted on human subjects diagnosed with DFIs, either in hospital or outpatient settings.
- Full-text studies that analyzed bacterial isolates from DFIs, particularly focusing on resistance to antibiotics such as amoxicillin/clavulanate, trimethoprim/sulfamethoxazole, ciprofloxacin, and other commonly used antibiotics.
- Review or meta-analysis papers.
- Case reports, commentaries, and opinion pieces.
- Studies without available full-text or studies not reporting data relevant to antibiotic resistance in DFIs.
- Articles that did not provide conclusive data on DFI-related microbial resistance or clinical outcomes.
2.4. Data Extraction and Analysis
- Study characteristics: author(s), year of publication, country, study design, and sample size.
- Patient characteristics: age, sex, type of diabetes (Type 1 or Type 2), duration of diabetes, and comorbidities such as peripheral neuropathy, peripheral arterial disease, and renal impairment.
- Clinical factors: type of infection (e.g., diabetic foot ulcer, osteomyelitis), severity of the infection (e.g., depth, size of ulcer), and history of previous infections or treatments.
- Microbiological data: prevalence and type of bacterial isolates, including Staphylococcus aureus (MRSA and MSSA), Pseudomonas spp., Proteus spp., Escherichia coli, Enterobacter spp., and others.
- Antibiotic resistance data: rates of resistance to specific antibiotics such as amoxicillin/clavulanate, ciprofloxacin, vancomycin, carbapenems, cephalosporins, and aminoglycosides.
2.5. Quality Assessment and Risk of Bias Analysis
- The clarity of research questions and objectives.
- The appropriateness of study designs and sample sizes.
- The robustness of microbiological testing methods for identifying bacterial isolates and antibiotic resistance.
- The reporting of relevant clinical outcomes, including treatment success and recurrence rates.
- The risk of bias in individual studies was assessed using a modified version of the Cochrane risk of bias tool for non-randomized studies. This included 20 components which were specifically developed for this study under the subheadings: research question, selection criteria, participant characteristics, sample size, outcome, methods, and analysis of which is more relevant for DFIs and antibiotic resistance research conducted as observational prospective studies. The questions were first trialed on the excluded articles to assess the tool validity. Two investigators (P.B. and J.C.) conducted the quality assessment and any discrepancies were resolved by discussion. All 20 questions were equally weighted, and individual scores were calculated based on the proportion of “yes” answers. Studies that scored <50%, 50–75%, and >75% were deemed of high, moderate, and low risk of bias, respectively, based on a collective decision taken by the investigators in line with the ROBINS-E tool guidelines [11,12].
2.6. Statistical Analysis
2.7. Outcomes of Interest
- The impact of comorbidities (e.g., vascular disease, renal impairment) on antibiotic resistance patterns. Correlation analysis was performed using R.
- The relationship between prior antibiotic use, hospitalization, and the development of resistant infections.
- The identification of risk factors for multidrug-resistant organisms (MDROs) in DFIs.
3. Results
3.1. Analysis of Study Populations
3.2. Epidemiological Analysis of Pathogens Related to Comorbidity
3.3. Epidemiological Analysis of Pathogens Related to Antibiotic Resistance
3.4. Correlation Between Types of Comorbidities Found in DFI and Types of Antibiotic Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2016, 49, 106–116. [Google Scholar] [CrossRef]
- Bandyk, D.F. The diabetic foot: Pathophysiology, evaluation, and treatment. Semin. Vasc. Surg. 2018, 31, 43–48. [Google Scholar] [CrossRef]
- Perez-Favila, A.; Martinez-Fierro, M.L.; Rodriguez-Lazalde, J.G.; Cid-Baez, M.A.; Zamudio-Osuna, M.J.; Martinez-Blanco, M.D.R.; Mollinedo-Montaño, F.E.; Rodriguez-Sanchez, I.P.; Castañeda-Miranda, R.; Garza-Veloz, I. Current Therapeutic Strategies in Diabetic Foot Ulcers. Medicina 2019, 55, 714. [Google Scholar] [CrossRef]
- Packer, C.F.; Ali, S.A.; Manna, B. Diabetic Foot Ulcer. [Updated 2023 Jul 17]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Alexiadou, K.; Doupis, J. Management of diabetic foot ulcers. Diabetes Ther. 2012, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.; Armstrong, D.; Krisner, R.; Attinger, C.; Lavery, L.; Lipsky, B.; Mills, J.; Steinberg, J. Diagnosis and management of diabetic foot complications. ADA Clin. Compend. 2018, 2018, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Michael, C.A.; Dominey-Howes, D.; Labbate, M. The antimicrobial resistance crisis: Causes, consequences, and management. Front. Public Health 2014, 2, 145. [Google Scholar] [CrossRef]
- Anderson, D.J.; Jenkins, T.C.; Evans, S.R.; Harris, A.D.; Weinstein, R.A.; Tamma, P.D.; Han, J.H.; Banerjee, R.; Patel, R.; Zaoutis, T.; et al. Stewardship and Infection Control Committeea of the Antibacterial Resistance Leadership Group (ARLG) The Role of Stewardship in Addressing Antibacterial Resistance: Stewardship and Infection Control Committee of the Antibacterial Resistance Leadership Group. Clin. Infect. Dis. 2017, 64 (Suppl. S1), S36–S40. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.; May, K.; Hale, T.; Allard, B.; Rowlings, N.; Freeman, A.; Harrison, J.; McCann, J.; Wraight, P. Wound chronicity, inpatient care, and chronic kidney disease predispose to MRSA infection in diabetic foot ulcers. Diabetes Care 2009, 32, 1907–1909. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Wang, Y.; You, J.; Zhu, W.; Liu, C.; Luan, Y.; Li, L.; Li, H. Community-associated methicillin-resistant Staphylococcus aureus infection of diabetic foot ulcers in an eastern diabetic foot center in a tertiary hospital in China: A retrospective study. BMC Infect Dis. 2023, 23, 652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Higgins, J.P.; Morgan, R.L.; Rooney, A.A.; Taylor, K.W.; Thayer, K.A.; Silva, R.A.; Lemeris, C.; Akl, E.A.; Bateson, T.F.; Berkman, N.D.; et al. A tool to assess risk of bias in non-randomized follow-up studies of exposure effects (ROBINS-E). Environ. Int. 2024, 186, 108602. [Google Scholar] [CrossRef] [PubMed]
- Caruso, P.; Maiorino, M.I.; Macera, M.; Signoriello, G.; Castellano, L.; Scappaticcio, L.; Longo, M.; Gicchino, M.; Campitiello, F.; Bellastella, G.; et al. Antibiotic resistance in diabetic foot infection: How it changed with COVID-19 pandemic in a tertiary care center. Diabetes Res. Clin. Pract. 2021, 175, 108797. [Google Scholar] [CrossRef]
- Khan, M.S.; Azam, M.; Khan, M.N.; Syed, F.; Ali, S.H.B.; Malik, T.A.; Alnasser, S.M.A.; Ahmad, A.; Karimulla, S.; Qamar, R. Identification of contributing factors, microorganisms and antimicrobial resistance involved in the complication of diabetic foot ulcer treatment. Microb. Pathog. 2023, 184, 106363. [Google Scholar] [CrossRef]
- Sekhar, S.; Unnikrishnan, M.K.; Rodrigues, G.S.; Vyas, N.; Mukhopadhyay, C. Antimicrobial susceptibility pattern of aerobes in diabetic foot ulcers in a South-Indian tertiary care hospital. Foot 2018, 37, 95–100. [Google Scholar] [CrossRef]
- Saseedharan, S.; Sahu, M.; Chaddha, R.; Pathrose, E.; Bal, A.; Bhalekar, P.; Sekar, P.; Krishnan, P. Epidemiology of diabetic foot infections in a reference tertiary hospital in India. Braz. J. Microbiol. 2018, 49, 401–406. [Google Scholar] [CrossRef]
- Le, T.T.A.; Tran, V.A.; Phan, M.H.; Tran, M.C.; Ngo, H.T. Treatment Outcomes, Antibiotic Selection, and Related Factors in the Management of Diabetic Foot Infections in Vietnam. J. Infect. Public Health 2023, 16, 705–712. [Google Scholar] [CrossRef]
- Najari, H.R.; Karimian, T.; Parsa, H.; QasemiBarqi, R.; Allami, A. Bacteriology of moderate-to-severe diabetic foot infections in two tertiary hospitals of Iran. Foot 2019, 40, 54–58. [Google Scholar] [CrossRef]
- Saltoglu, N.; Surme, S.; Ezirmik, E.; Kadanali, A.; Kurt, A.F.; Ozdemir, M.S.; Ak, O.; Altay, F.A.; Acar, A.; Cakar, Z.S.; et al. The Effects of Antimicrobial Resistance and the Compatibility of Initial Antibiotic Treatment on Clinical Outcomes in Patients With Diabetic Foot Infection. Int. J. Low. Extrem. Wounds 2023, 22, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Jouhar, L.; Jaafar, R.F.; Nasreddine, R.; Itani, O.; Haddad, F.; Rizk, N.; Hoballah, J.J. Microbiological profile and antimicrobial resistance among diabetic foot infections in Lebanon. Int. Wound J. 2020, 17, 1764–1773. [Google Scholar] [CrossRef]
- Hatipoglu, M.; Mutluoglu, M.; Turhan, V.; Uzun, G.; Lipsky, B.A.; Sevim, E.; Demiraslan, H.; Eryilmaz, E.; Ozuguz, C.; Memis, A.; et al. Causative pathogens and antibiotic resistance in diabetic foot infections: A prospective multi-center study. J. Diabetes Its Complicat. 2016, 30, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qi, X.; Yuan, G.; Ju, S.; Yu, Z.; Deng, W.; Liu, Y.; Li, Y.; Bu, X.; Ding, M.; et al. Microbiological profile and clinical characteristics of diabetic foot infection in northern China: A retrospective multicentre survey in the Beijing area. J. Med. Microbiol. 2018, 67, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Pontes, D.G.; Silva, I.T.D.C.E.; Fernandes, J.J.; Monteiro, A.D.F.G.; Gomes, P.H.D.S.; Ferreira, M.G.M.; DE Lima, F.G.; Correia, J.D.O.; DOS Santos, N.J.N.; Cavalcante, L.P. Microbiologic characteristics and antibiotic resistance rates of diabetic foot infections. Rev. Col. Bras. Cir. 2020, 47, e20202471. [Google Scholar] [CrossRef] [PubMed]
- Kow, R.Y.; Low, C.L.; Ruben, J.K.; Azri, W.M.Z.Z.; Khan, E.S.K.M.J. Microbiology of diabetic foot infections in three district hospital in Malaysia and comparison with South East Asian Countries. Med. J. Malays. 2019, 74, 394–399. [Google Scholar]
- Ji, X.; Jin, P.; Chu, Y.; Feng, S.; Wang, P. Clinical Characteristics and Risk Factors of Diabetic Foot Ulcer With Multidrug-Resistant Organism Infection. Int. J. Low. Extrem. Wounds 2014, 13, 64–71. [Google Scholar] [CrossRef]
- Wu, M.; Guo, F.; He, X.; Zheng, D.; Ye, W.; Li, S.; Lin, Z.; Wang, F. Analysis of Distribution and Drug Susceptibility Test Results of Pathogenic Bacteria in Diabetic Foot Ulcers. Diabetes Ther. 2024, 15, 1627–1637. [Google Scholar] [CrossRef]
- Atlaw, A.; Kebede, H.B.; Abdela, A.A.; Woldeamanuel, Y. Bacterial isolates from diabetic foot ulcers and their antimicrobial resistance profile from selected hospitals in Addis Ababa, Ethiopia. Front. Endocrinol. 2022, 13, 987487. [Google Scholar] [CrossRef] [PubMed]
- Moya-Salazar, J.; Chamana, J.M.; Porras-Rivera, D.; Goicochea-Palomino, E.A.; Salazar, C.R.; Contreras-Pulache, H. Increase in Antibiotic Resistance in Diabetic Foot Infections Among Peruvian Patients: A Single-Center Cross-Sectional Study. Front. Endocrinol. 2023, 14, 1267699. [Google Scholar] [CrossRef]
- Sannathimmappa, M.B.; Nambiar, V.; Aravindakshan, R.; Al Khabori, M.S.J.; Al-Flaiti, A.H.S.; Al-Azri, K.N.M.; Al-Reesi, A.K.S.; Al Kiyumi, A.R.M. Diabetic foot infections: Profile and antibiotic susceptibility patterns of bacterial isolates in a tertiary care hospital of Oman. J. Educ. Health Promot. 2021, 10, 254. [Google Scholar] [CrossRef]
- Ismail, A.A.; Meheissen, M.A.; Elaaty, T.A.A.; Abd-Allatif, N.E.; Kassab, H.S. Microbial profile, antimicrobial resistance, and molecular characterization of diabetic foot infections in a university hospital. Germs 2021, 11, 39–51. [Google Scholar] [CrossRef]
- Bouharkat, B.; Tir Touil, A.; Mullié, C.; Chelli, N.; Meddah, B. Bacterial ecology and antibiotic resistance mechanisms of isolated resistant strains from diabetic foot infections in the north west of Algeria. J. Diabetes Metab. Disord. 2020, 19, 1261–1271. [Google Scholar] [CrossRef]
- Demetriou, M.; Papanas, N.; Panagopoulos, P.; Panopoulou, M.; Maltezos, E. Antibiotic Resistance in Diabetic Foot Soft Tissue Infections: A Series From Greece. Int. J. Low Extrem. Wounds 2017, 16, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Bao, Y.; Ni, L.; Liu, D.; Niu, S.; Lin, H.; Li, H.; Duan, C.; Yan, L.; Huang, S.; et al. Bacterial Profile and Antibiotic Resistance in Patients with Diabetic Foot Ulcer in Guangzhou, Southern China: Focus on the Differences among Different Wagner’s Grades, IDSA/IWGDF Grades, and Ulcer Types. Int. J. Endocrinol. 2017, 2017, 8694903. [Google Scholar] [CrossRef]
- E Costa, T.P.; Duarte, B.; João, A.L.; Coelho, M.; Formiga, A.; Pinto, M.; Neves, J. Multidrug-resistant bacteria in diabetic foot infections: Experience from a portuguese tertiary centre. Int. Wound J. 2020, 17, 1835–1839. [Google Scholar] [CrossRef] [PubMed]
- Boschetti, G.; Sgarabotto, D.; Meloni, M.; Bruseghin, M.; Whisstock, C.; Marin, M.; Ninkovic, S.; Pinfi, M.; Brocco, E. Antimicrobial resistance patterns in diabetic foot infections, an epidemiological study in northeastern Italy. Antibiotics 2021, 10, 1241. [Google Scholar] [CrossRef]
- Goh, T.C.; Bajuri, M.Y.; CNadarajah, S.; Abdul Rashid, A.H.; Baharuddin, S.; Zamri, K.S. Clinical and bacteriological profile of diabetic foot infections in a tertiary care. J. Foot Ankle Res. 2020, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Cezimbra, P.M.; da Costa Borges, J.; Celeste, S.R.C.; de Freitas Orsolin, E.; Mendes, R.R.; Mendes, G.O.; Ferreira, R.L.; Carreiro, S.C.; da Silva Pranchevicius, M.C. Aerobic bacterial profile and antibiotic resistance in patients with diabetic foot infections. Rev. Soc. Bras. Med. Trop. 2015, 48, 546–554. [Google Scholar]
- Turzańska, K.; Adesanya, O.; Rajagopal, A.; Pryce, M.T.; Fitzgerald Hughes, D. Improving the Management and Treatment of Diabetic Foot Infection: Challenges and Research Opportunities. Int. J. Mol. Sci. 2023, 24, 3913. [Google Scholar] [CrossRef]
- Álvaro-Afonso, F.J.; García-Álvarez, Y.; Tardáguila-García, A.; García-Madrid, M.; López-Moral, M.; Lázaro-Martínez, J.L. Bacterial diversity and antibiotic resistance in patients with diabetic foot osteomyelitis. Antibiotics 2023, 12, 212. [Google Scholar] [CrossRef]
- Ray, H.; Weis, C.; Nwaeze, C.; Zhou, V.; Basu, P.; Mitra, A. Development and Control of Biofilms in Diabetic Foot Infections: A Narrative Review. Acta Microbiol. Hell. 2025, 70, 9. [Google Scholar] [CrossRef]
- Dugbartey, G.; Alornyo, K. Association between Diabetic Kidney Disease and Diabetic Foot Ulceration. In Diabetic Foot-Recent Advances; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Eastman, D.M.; Dreyer, M.A. Neuropathic Ulcer. [Updated 2022 Nov 30]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559214/ (accessed on 5 July 2025).
- Zubair, M. Prevalence and interrelationships of foot ulcer, risk-factors and antibiotic resistance in foot ulcers in diabetic populations: A systematic review and meta-analysis. World J. Diabetes 2020, 11, 78–89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, X.; Song, J.; Zhang, L.; Li, X. Analysis of risk factors for multidrug-resistant organisms in diabetic foot infection. BMC Endocr. Disord. 2022, 22, 46. [Google Scholar] [CrossRef] [PubMed]
- Matta-Gutiérrez, G.; García-Morales, E.; García-Álvarez, Y.; Álvaro-Afonso, F.J.; Molines-Barroso, R.J.; Lázaro-Martínez, J.L. The influence of multidrug-resistant bacteria on clinical outcomes of diabetic foot ulcers: A systematic review. J. Clin. Med. 2021, 10, 1948. [Google Scholar] [CrossRef] [PubMed]
- Justin, J.S. The Impact of Smoking and Quitting Smoking on Patients with Diabetes. Diabetes Spectr. 2005, 18, 202–208. [Google Scholar] [CrossRef]
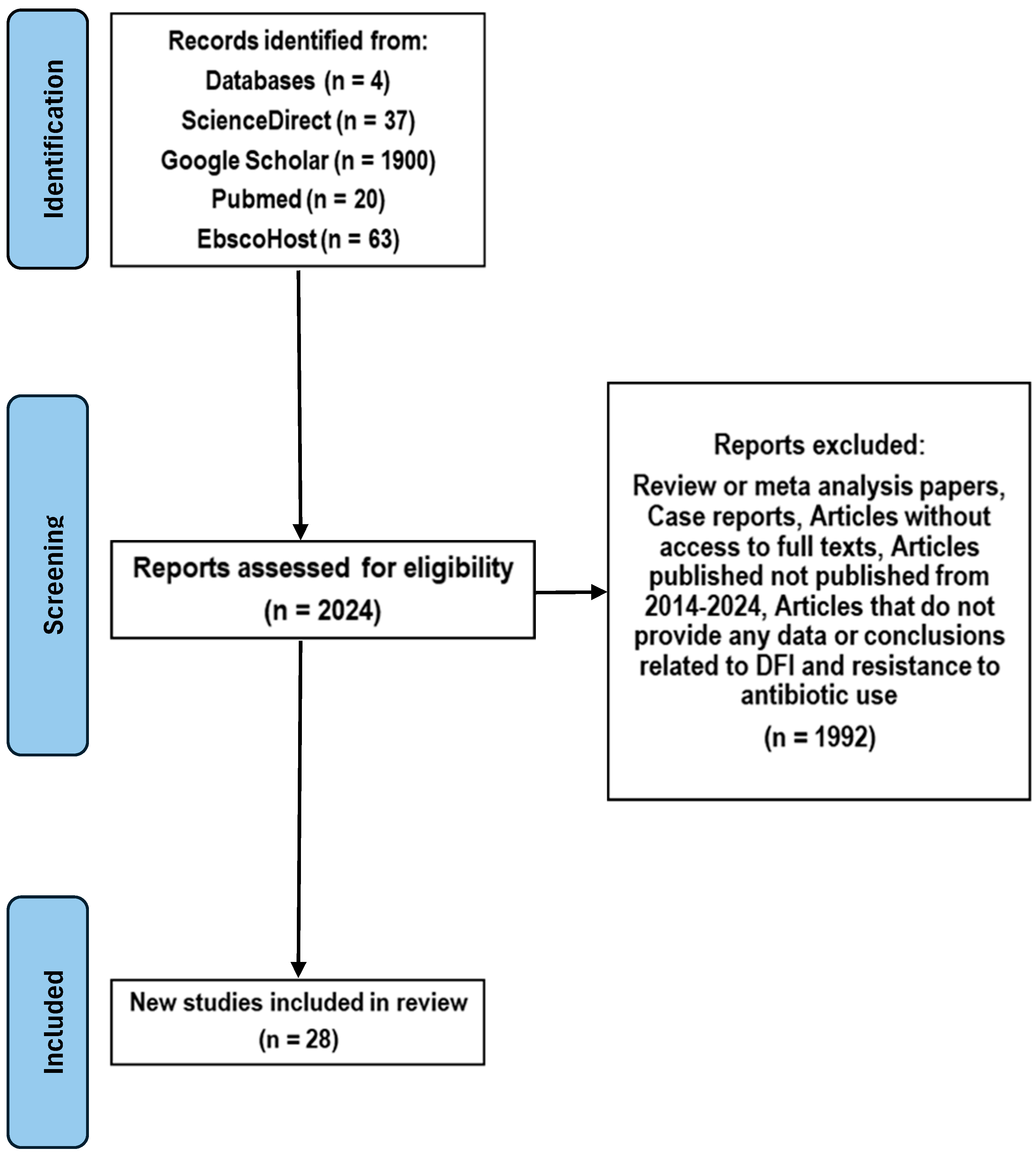
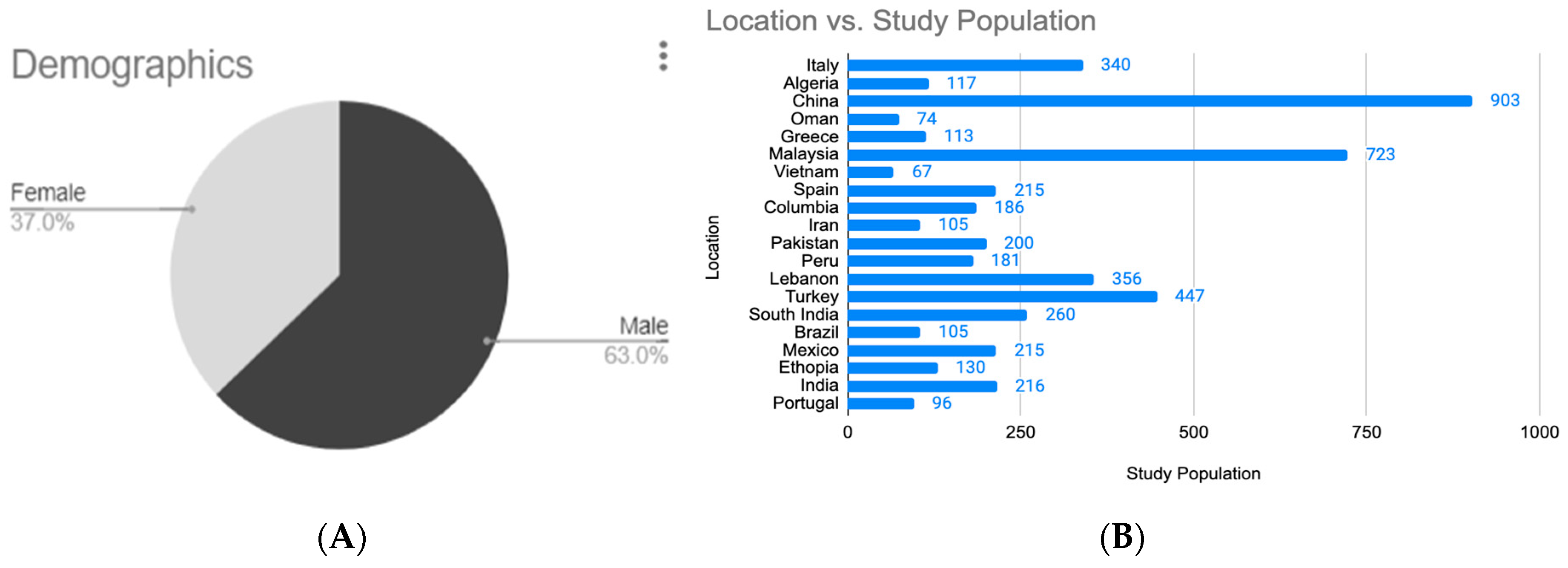
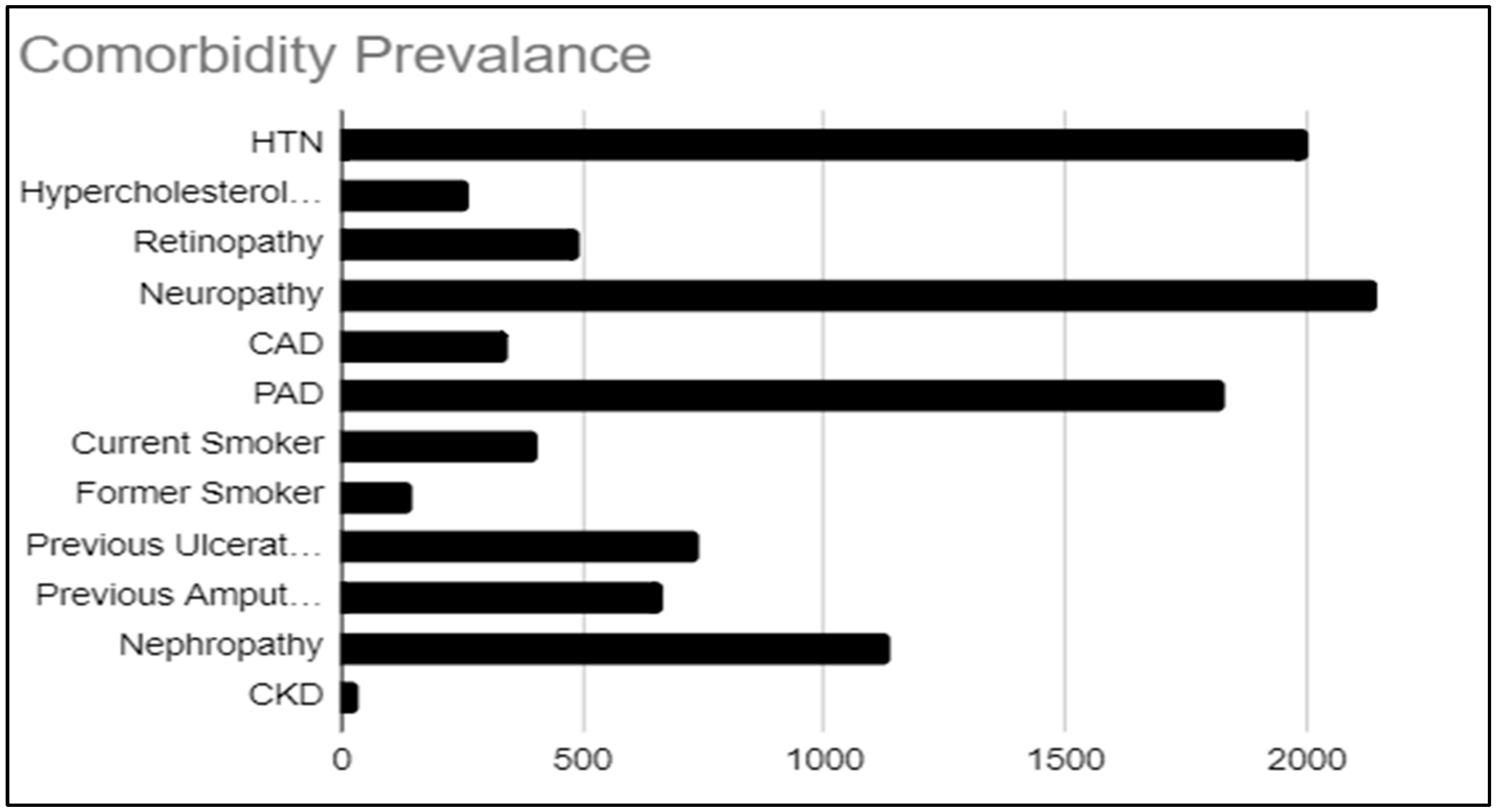
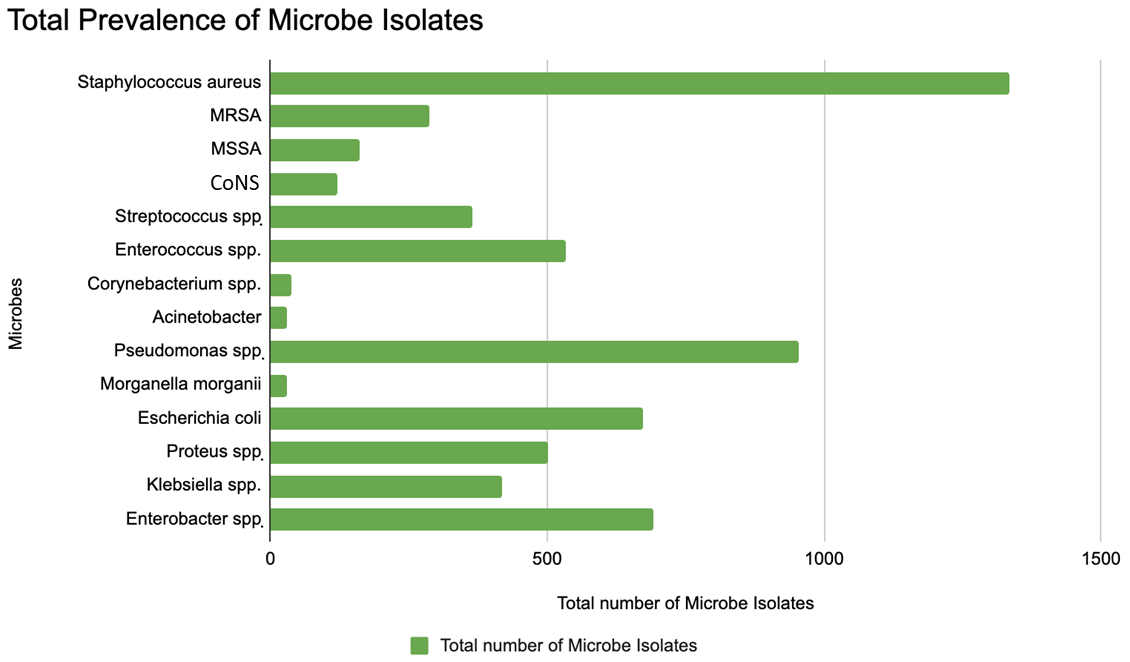
| Search Source | Yield | Articles Included | References |
|---|---|---|---|
| ScienceDirect | 37 | 7 | [1,2,3,4,5,6,7] |
| Pubmed | 20 | 9 | [8,13,14,15,16,17,18,19,20,21] |
| EbscoHost | 63 | 7 | [22,23,24,25,26,27,28] |
| Google Scholar | 1900 | 5 | [29,30,31,32,33] |
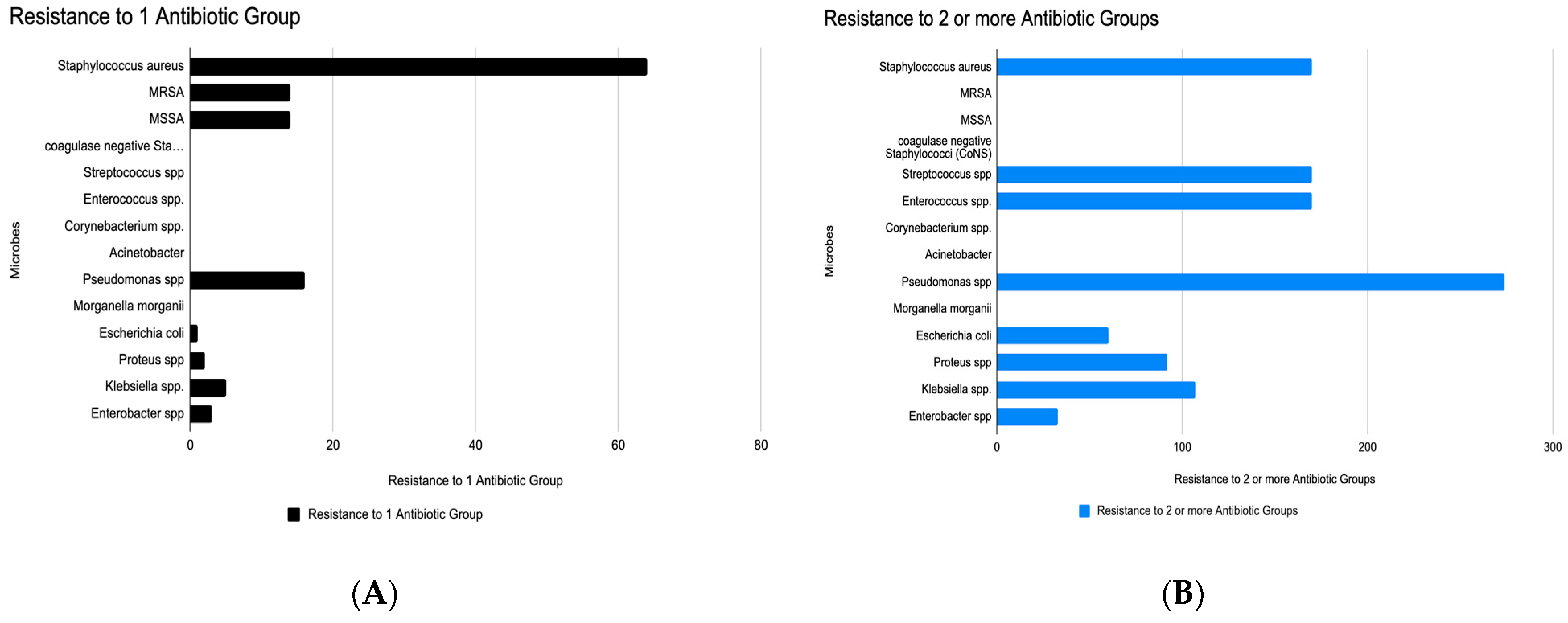
| Microbe | High in Monoresistance | High in MDR | Key Observations & Clinical Implications |
|---|---|---|---|
| Staphylococcus aureus | Very high (~75) | High (~190) | Rapid transition from monoresistance to MDR. Empirical treatment options like β-lactams may fail unless susceptibility is confirmed. |
| MRSA | Moderate (~15) | Low | Shows low MDR count here, possibly underreported or isolated to β-lactam resistance. Still clinically concerning due to treatment limitations. |
| MSSA | Moderate (~15) | Low | Growing evidence that even methicillin-sensitive strains are developing additional resistances. |
| Coagulase-negative Staphylococci | Present | High (~160) | Often dismissed as contaminants, but high MDR burden signals emerging pathogenic roles, especially in device-related infections. |
| Streptococcus spp. | Present | High (~160) | High resistance burdens in both categories signal reduced efficacy of penicillin-class drugs in wound and soft tissue infections. |
| Enterococcus spp. | Low | High (~160) | Not prominent in monoresistance but highly represented in MDR—concerning for vancomycin resistance and nosocomial infections. |
| Corynebacterium spp. | Low | Very high (~280) | A striking MDR surge despite low monoresistance. Often under-recognized, but data suggest it may be a significant reservoir of resistance genes. |
| Acinetobacter spp. | Moderate (~25) | Highest (~280) | Dominates MDR list despite modest monoresistance. Known for rapid resistance development and survival in healthcare settings. |
| Pseudomonas spp. | Moderate (~35) | Moderate (~80) | Common culprit in chronic and biofilm-associated infections. Resistance mechanisms include efflux pumps and porin mutations. |
| Morganella morganii | Low | Moderate (~90) | Rarely spotlighted, but its MDR profile is growing, suggesting need for surveillance in polymicrobial infections. |
| Escherichia coli | Low | Moderate (~110) | Resistance likely due to ESBL or AmpC production. Empirical fluoroquinolone or cephalosporin use may be ineffective. |
| Proteus, Klebsiella, Enterobacter spp. | Low to moderate | Low to moderate | These organisms can serve as reservoirs of plasmid-mediated resistance, with potential to evolve into extended-spectrum or carbapenem-resistant forms. |
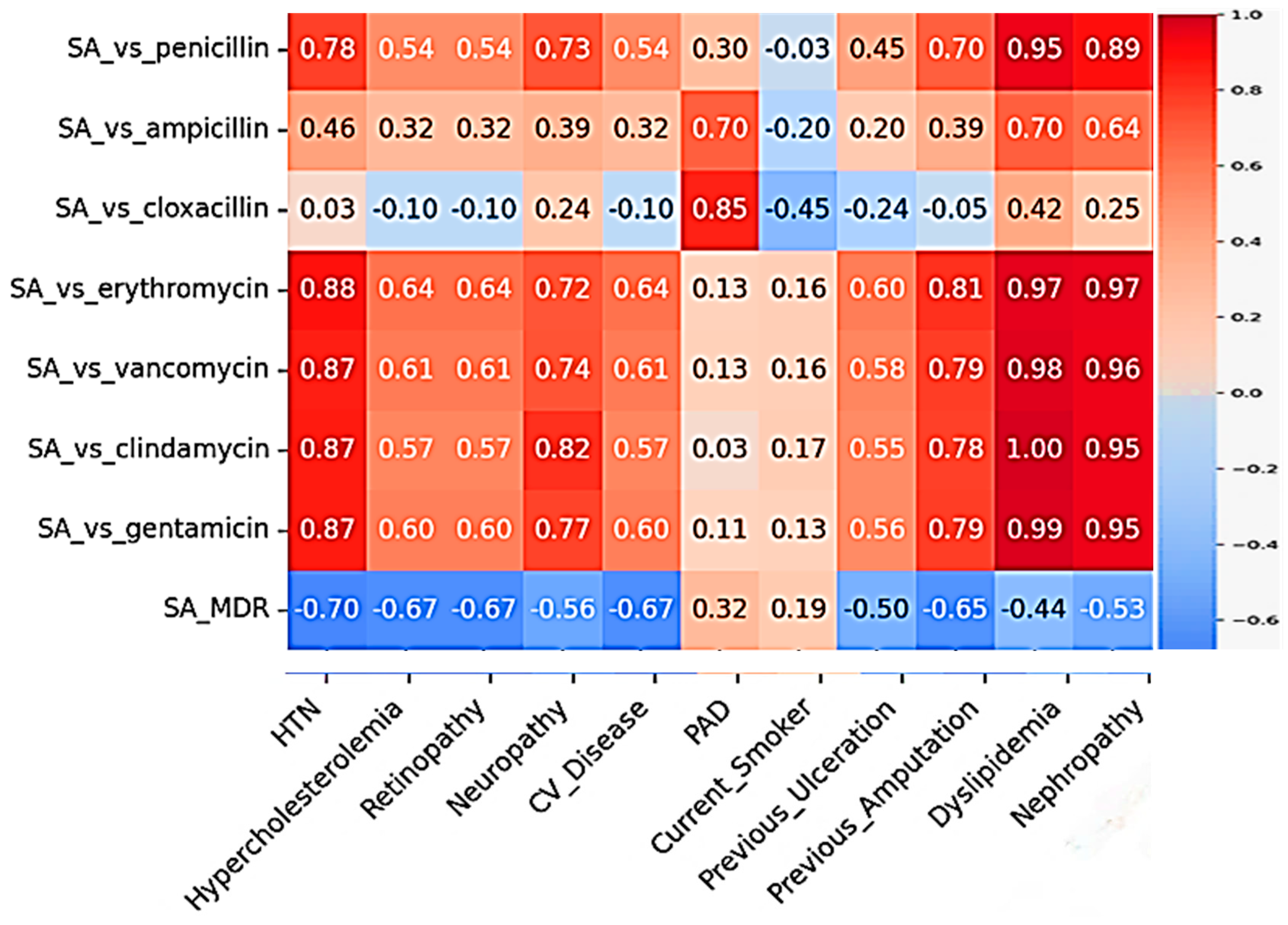
| Comorbidity | Antibiotic | Pearson’s r | p Value | Significance |
|---|---|---|---|---|
| Nephropathy | Penicillin | 0.956 | 0.0051 | Significant correlation |
| Erythromycin | 0.952 | 0.0047 | Significant correlation | |
| Clindamycin | 0.93 | 0.0032 | Significant correlation | |
| Hypertension | Penicillin | 0.90 | 0.0010 | Significant correlation |
| Erythromycin | 0.90 | 0.0037 | Significant correlation | |
| Clindamycin | 0.88 | 0.0045 | Significant correlation | |
| Dyslipidemia | Penicillin | 0.95 | 0.0136 | Significant correlation |
| Erythromycin | 0.97 | 0.0051 | Significant correlation | |
| Clindamycin | 0.99 | 0.0002 | Significant correlation |
| Pathogen | Comorbidity | Associated Antibiotic Resistance | Strength of Correlation |
|---|---|---|---|
| S. aureus | Dyslipidemia | Clindamycin, Gentamicin, Vancomycin, Erythromycin, Penicillin | Very Strong |
| Hypertension | Erythromycin | Strong | |
| Pseudomonas spp. | Neuropathy | Cephalosporins, Tetracycline, Gentamicin, Quinolones, Carbapenems | Very Strong |
| Smoking | Erythromycin, Piperacillin-tazobactam, Imipenem | Strong | |
| PAD | Carbapenems, Gentamicin, Piperacillin-tazobactam | Moderate | |
| Nephropathy | Carbapenems, Gentamicin, Piperacillin-tazobactam | Moderate | |
| Hypertension | Carbapenems, Gentamicin, Erythromycin | Moderate | |
| Enterococcus spp. | Smoking | Vancomycin, Tetracycline, Gentamicin | Strong |
| Hypertension | Vancomycin, Tetracycline, Gentamicin | Strong | |
| Nephropathy | Vancomycin, Tetracycline, Gentamicin | Moderate | |
| PAD | Vancomycin, Tetracycline, Gentamicin | Moderate | |
| E. coli | Smoking | Piperacillin-tazobactam, Imipenem, Cephalosporins, Quinolones, Penicillin, Gentamicin | Strong |
| Nephropathy | Aminoglycosides, Carbapenems, Tetracycline, Gentamicin | Moderate | |
| Amputation | Aminoglycosides, Carbapenems, Tetracycline, Gentamicin | Moderate | |
| Hypertension | Penicillin, Tetracycline, Gentamicin | Moderate | |
| Proteus spp. | Current Smoking | Erythromycin, Amoxicillin/clavulanate, Piperacillin-tazobactam, Imipenem, Cephalosporins, Quinolones, Penicillin, Gentamicin | Very Strong |
| Previous Amputation | Gentamicin, Penicillin, Erythromycin, multiple others | Very Strong | |
| Nephropathy | Aminoglycosides | Strong | |
| Former Smoker | Tetracycline | Strong |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Sadeh, O.; Chakraborty, J.; Yang, E.; Basu, P.; Kumar, P. Multifaceted Antibiotic Resistance in Diabetic Foot Infections: A Systematic Review. Microorganisms 2025, 13, 2311. https://doi.org/10.3390/microorganisms13102311
Li W, Sadeh O, Chakraborty J, Yang E, Basu P, Kumar P. Multifaceted Antibiotic Resistance in Diabetic Foot Infections: A Systematic Review. Microorganisms. 2025; 13(10):2311. https://doi.org/10.3390/microorganisms13102311
Chicago/Turabian StyleLi, Weiqi, Oren Sadeh, Jina Chakraborty, Emily Yang, Paramita Basu, and Priyank Kumar. 2025. "Multifaceted Antibiotic Resistance in Diabetic Foot Infections: A Systematic Review" Microorganisms 13, no. 10: 2311. https://doi.org/10.3390/microorganisms13102311
APA StyleLi, W., Sadeh, O., Chakraborty, J., Yang, E., Basu, P., & Kumar, P. (2025). Multifaceted Antibiotic Resistance in Diabetic Foot Infections: A Systematic Review. Microorganisms, 13(10), 2311. https://doi.org/10.3390/microorganisms13102311








