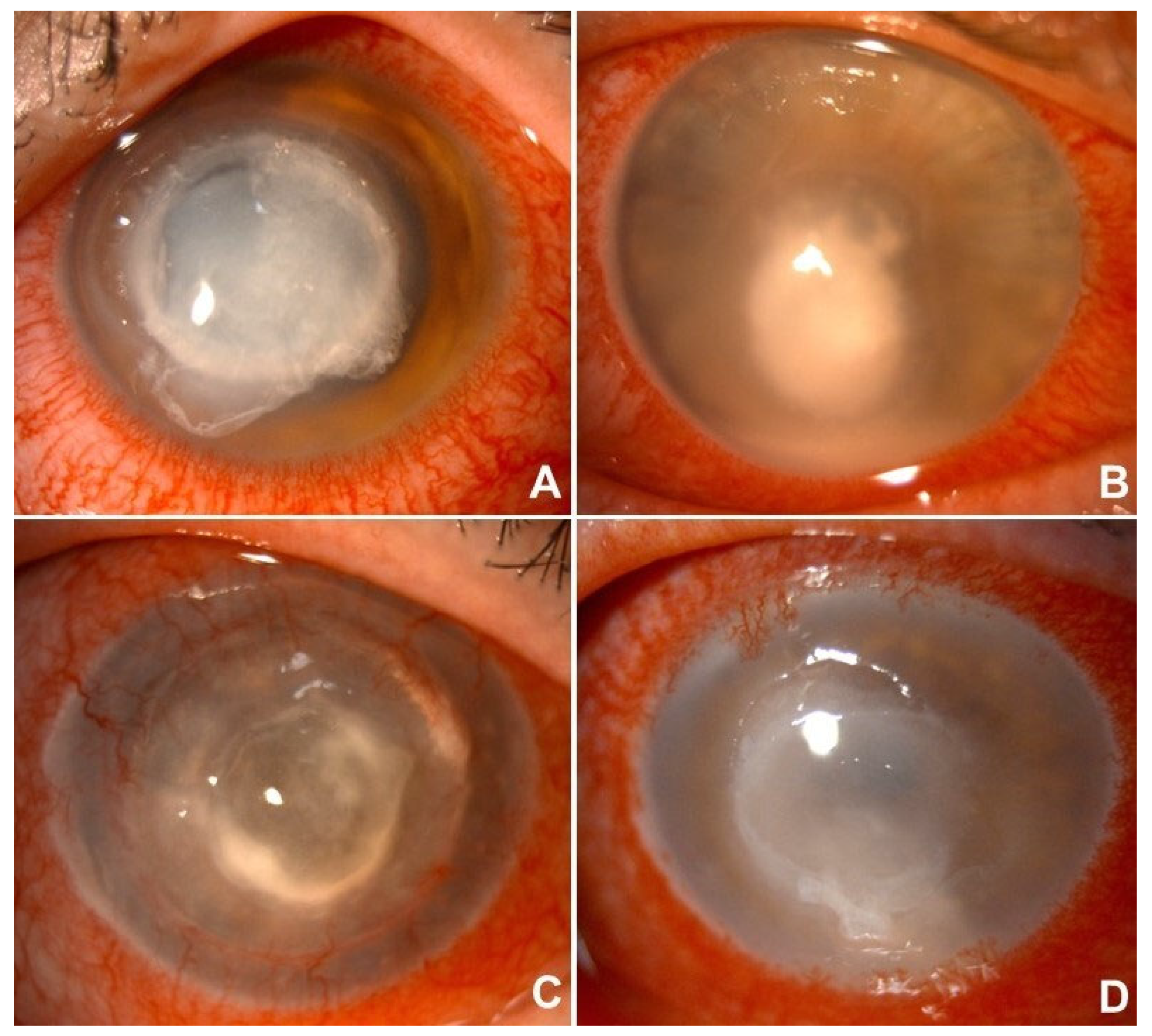
1. Introduction
Microbial keratitis (MK) is an infectious disease of the cornea characterized by ocular pain, conjunctival hyperemia, stromal infiltration, and often corneal ulceration with subsequent vision loss. Globally, MK is the fourth leading cause of blindness, with an estimated 1.5 to 2.0 million new cases reported annually in developing regions [
1,
2,
3]. In contrast, the incidence in developed countries remains considerably lower, ranging from 3.6 to 40.3 cases per 100,000 person-years [
4,
5,
6,
7]. Due to its potential to cause rapid and permanent visual loss, MK is regarded as a major ophthalmic emergency and a significant public health concern. Prompt microbiological diagnosis and early targeted therapy are vital to prevent corneal perforation and severe visual impairment. The range of causative microorganisms—including bacteria, fungi, protozoa, and viruses—varies according to geographic, climatic, socioeconomic, and host-related risk factors [
8,
9,
10].
Bacterial keratitis (BK) accounts for about 90% of all MK cases [
11]. Major risk factors include contact lens use, ocular trauma, topical corticosteroid use, and a history of previous ocular surgeries [
12]. Fungal keratitis (FK) has a worldwide distribution, with causative agents heavily influenced by geographic location. In tropical and subtropical regions, filamentous fungi are most common, while yeast species are more prevalent in temperate areas.
Acanthamoeba keratitis (AK) is a rare but increasingly recognized cause of MK, caused by free-living amoebae found widely in natural environments [
13]. Over the past decade, AK cases have risen, mainly linked to contact lens wear, which accounts for about 90% of reported cases [
14]. Lastly, viral keratitis (VK) is among the most common types of infectious keratitis [
15,
16]. Of the various viruses causing keratitis, the alpha-herpesvirus herpes simplex virus (HSV) is the most common. Other common viral agents include the beta-herpesvirus cytomegalovirus (CMV), the alpha-herpesvirus varicella-zoster virus (VZV), and the gamma-herpesvirus Epstein–Barr virus (EBV) [
17,
18,
19].
The main aim of our study was to describe the experience of our third-level center in diagnosing and treating infectious keratitis. Particular emphasis was placed on the use of microbiological analysis to identify the microorganism responsible for the infection. Secondary objectives included identifying potential risk factors associated with a higher likelihood of obtaining a positive microbiological result, and comparing the two groups of patients—those with positive and negative microbiological findings—in terms of treatment strategies and clinical outcomes. This analysis may provide insights to help prevent, enable early intervention, and improve the management of infectious keratitis in specialized care settings.
2. Materials and Methods
This study involved 220 patients who presented to the Cornea and Ocular Surface Service at our tertiary referral ophthalmology center over a three-year period (November 2021–January 2025). Patients who had not concluded their therapeutic course yet or were still under clinical surveillance by the end of January 2025 were excluded from the cohort. The study adhered to the principles outlined in the Declaration of Helsinki. Ethical approval was granted by the Institutional Review Board (Registration No. 901/2022/Oss/AOUBo, dated 15 December 2022) of IRCCS AOU Policlinico Sant’Orsola, and informed consent was obtained from all participants prior to enrolment. Clinical data were collected and systematically categorized according to five key stages of the diagnostic-therapeutic process: anamnesis, clinical presentation, microbiological analysis, treatment, and outcomes.
Microbiological samples were collected using various methods, including corneal scraping, conjunctival and corneal swabbing, as well as the collection of contact lenses and their storage media. The Microbiology Laboratory carried out cultures on different media types, such as standard agar, blood agar, Sabouraud agar, and enriched broth media. In some cases, and in every suspect of amoebic keratitis, non-nutrient agar (NNA) overlaid with Escherichia coli and polymerase chain reaction (PCR) assays were also utilized to enhance pathogen detection.
Statistical Analysis
Statistical analyses were performed to investigate the relationship between microbiological findings, treatment strategies, and visual outcomes. All variables were processed based on their measurement scale and clinical significance. BCVA (best corrected visual acuity) improvement was determined as the absolute difference between final and baseline visual acuity, expressed in decimal units.
Welch’s
t-test was used to compare the mean BCVA improvement between patients with positive and negative microbiological results, as it is resilient to violations of the equal-variance assumption [
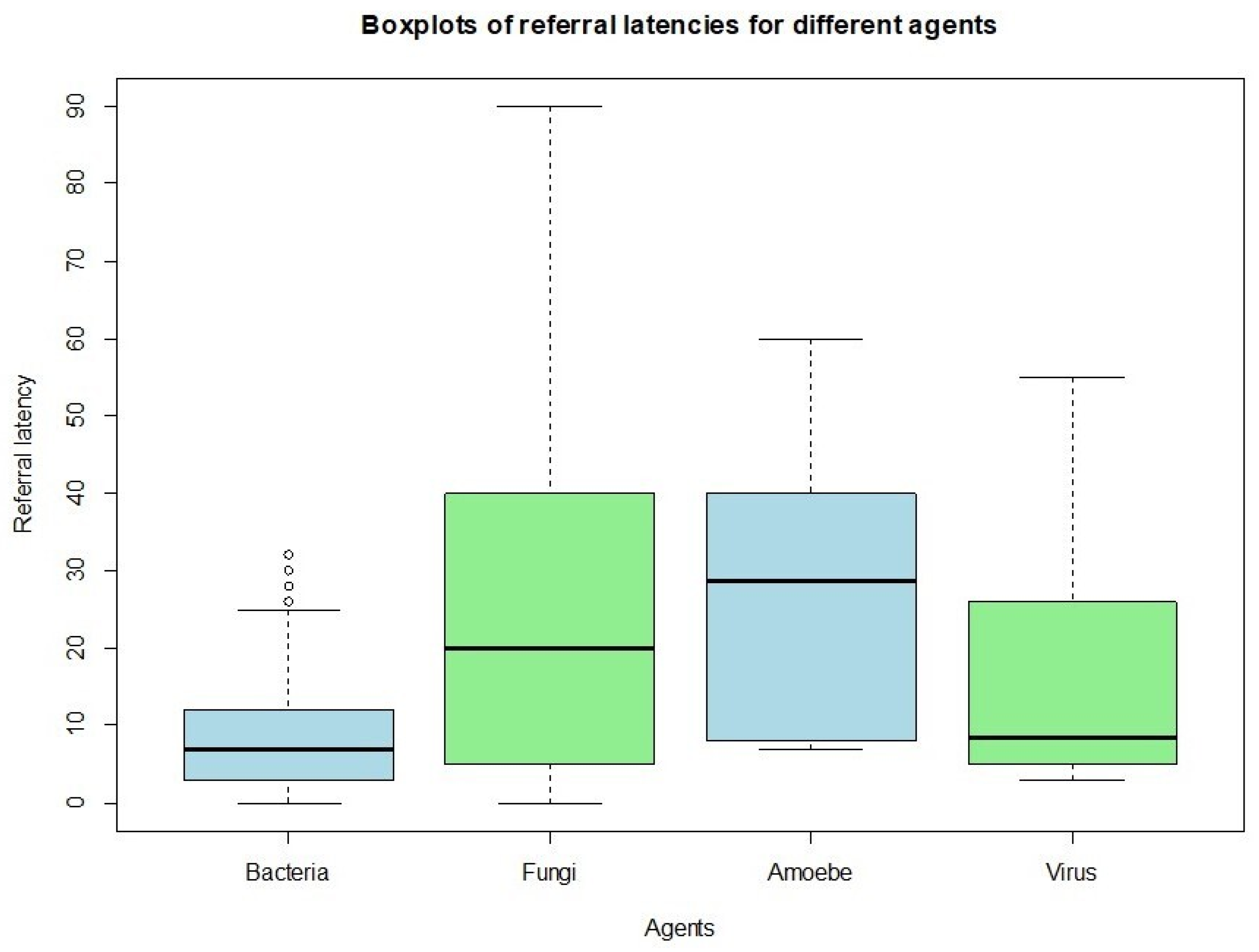
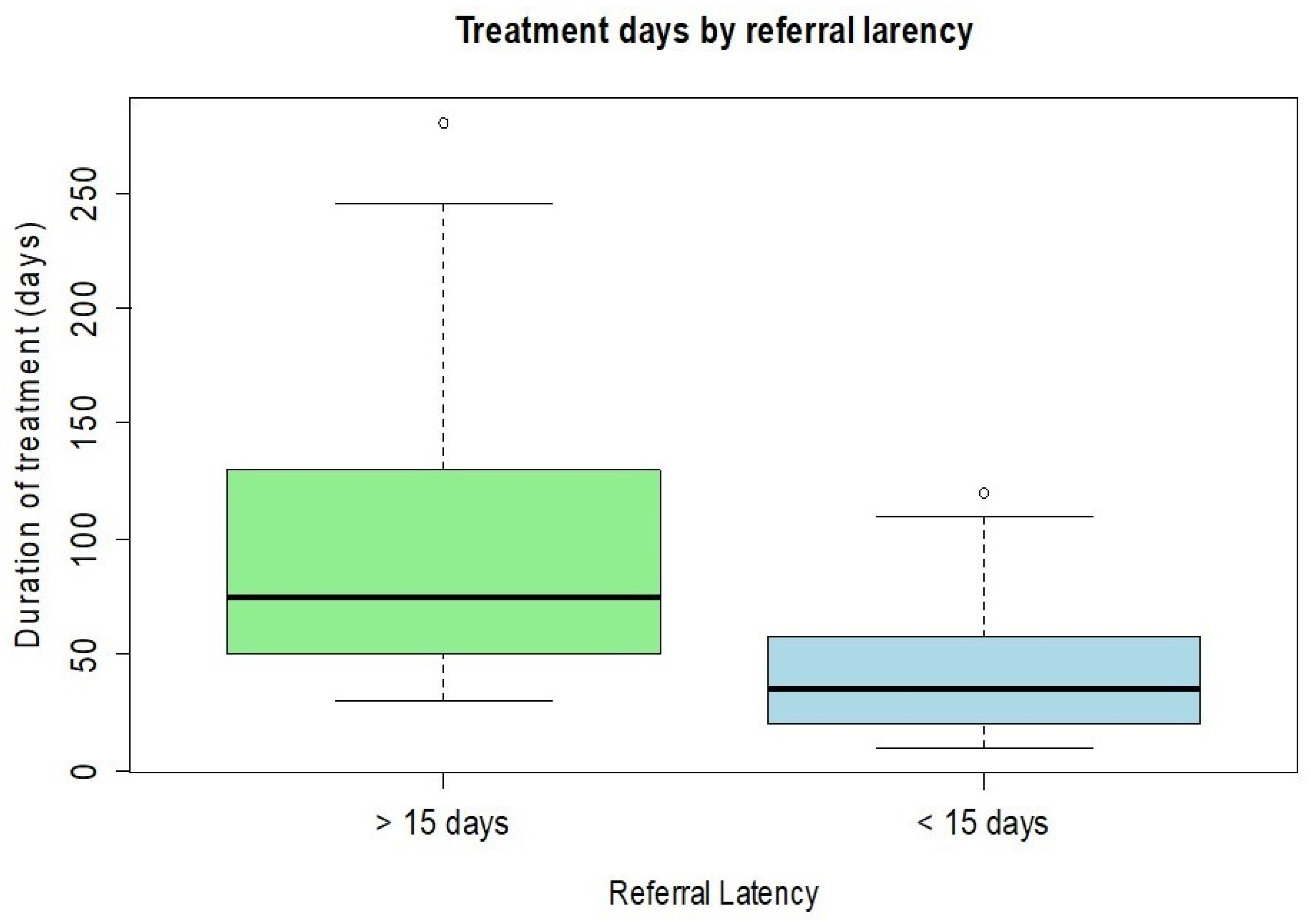
20]. Distributions of continuous outcomes across categorical groups were further explored using boxplots, which provided insights into patterns of BCVA and referral latency (defined as the number of days between symptom onset and presentation at the specialized center) by microbiological status, as well as differences in visual outcomes based on referral timing. The linear relationship between continuous variables—specifically, referral latency and BCVA improvement—was assessed using Pearson’s correlation coefficient. Associations between categorical variables were examined using Fisher’s exact test. These included the relationship between microbiological findings and the performance of rescue surgery, as well as the association between referral latency and both visual improvement and surgical intervention, all treated as dichotomous.
Logistic regression models [
21] were fitted to evaluate two distinct outcomes: first, among patients who underwent microbiological testing, to identify risk factors linked to a positive culture result; and second, whether referral latency was associated with the likelihood of undergoing rescue surgery. Variable selection was performed using a backwards elimination strategy based on the Least Absolute Shrinkage and Selection Operator (LASSO), retaining only predictors with a significance level below 5% [
22,
23].
Furthermore, patients’ medical histories were examined to identify potential risk factors linked to a positive microbiological outcome. The variables reviewed included a history of ocular trauma, contact with a vegetative foreign body, previous ocular surgery, recurrent herpetic keratitis, and contact lens use. A logistic regression model was utilized to assess the association between these risk factors and the likelihood of culture positivity.
All statistical analyses were performed using R, version 4.4.3 [
24].
4. Discussion
Microbial keratitis is a significant clinical condition as it can severely impair visual function and, in some cases, cause irreversible vision loss. Microbiological investigations are the gold standard for determining an etiological diagnosis, mainly to guide treatment and improve clinical outcomes. By identifying the causative microorganism and its antimicrobial susceptibility, microbiological testing helps clinicians tailor therapy more accurately, potentially shortening the infection duration, preventing structural damage to the cornea, and increasing chances of visual recovery [
6]. Despite these benefits, microbiological diagnosis is not always routinely performed in all clinical settings, and its true impact on prognosis and the necessity for surgical intervention remains an ongoing area of research.
In our population of 221 eyes, clinical assessment combined with empirical therapy—often supported by microbiological analysis—revealed a predominance of bacterial keratitis (
n = 141, 63.8%), followed by viral keratitis (
n = 44, 19.9%), fungal keratitis (
n = 29, 13,1%), and amoebic keratitis (
n = 7, 3.2%). As a tertiary referral center for corneal pathology, we aimed to analyze the epidemiological characteristics of this patient cohort and to explore how microbiological investigations might influence the disease progression. In our setting, 48.4% of cases (107 out of 221 eyes) involved corneal sample collection to establish a precise etiological diagnosis. The overall culture positivity rate was 75.7% (81/107 patients), aligning with figures reported in other similarly designed studies [
26,
27]. Culture-based methods remain the gold standard for identifying most microbial pathogens, providing reliable sensitivity when corneal specimens are collected and processed correctly [
6]. This highlights the importance of close collaboration between ophthalmologists and microbiology laboratories, as well as the value of a well-structured diagnostic workflow in the initial management of infectious keratitis. Furthermore, PCR-based molecular assays proved to be a helpful adjunct in investigating suspected amoebic and herpetic keratitis, as well as in cases presenting with atypical or overlapping clinical features [
13,
17,
18].
In our study, the most frequently identified bacterial species were Gram-negative organisms, particularly
Pseudomonas aeruginosa (21 cases) and
Klebsiella spp. (5 cases), while Gram-positive bacteria, such as
Staphylococcus aureus and
Staphylococcus epidermidis, were less commonly detected. This distribution aligns with findings from other long-term reviews [
6,
28,
29]. However, other groups investigating bacterial keratitis have reported a predominance of Gram-positive species, including coagulase-negative staphylococci, as described by Lamas-Francis et al. [
30], Kase et al. [
26], and Ting et al. [
11],
Streptococcus pneumoniae as reported by Madduri et al. [
31], and
Staphylococcus epidermidis as noted by Ung et al. [
27]. This discrepancy may partly result from the fact that not all patients in our study underwent microbiological analysis. The decision to perform sampling was left to the clinician’s judgment, based on factors such as clinical severity, presence of risk factors, and response to empirical therapy, similar to the approach used in the study by Russello et al. [
6], conducted in a comparable geographic setting and with a similar design. Consequently, some of our cohort did not contribute to the full etiological spectrum of pathogens.
Among fungal infections, the most frequently isolated organisms were
Candida (4 cases),
Aspergillus (3 cases), and
Fusarium (3 cases).
Acanthamoeba was identified as the causative agent in 7 cases of corneal infection. All viral keratitis cases were attributable to Herpesviridae, specifically
Herpes simplex virus (39 cases) and
Varicella zoster virus (5 cases). Overall, these findings are consistent with existing literature, confirming that fungal and amoebic keratitis represent a minority of infectious keratitis cases in developed countries [
32,
33]. The overall outcome was predictably more favorable for infections of bacterial and viral origin, while fungal and amoebic keratitis more often resulted in low visual acuity and necessitated rescue surgery to manage the infection, in accordance with existing evidence [
13,
17,
18,
27,
34].
Although establishing a direct link between contact lens use or ocular trauma and the increased risk of developing corneal infection was not the primary aim of this study, some notable observations emerged when analyzing the overall data, particularly concerning these two well-known risk factors [
11,
13,
26,
35,
36]. In our cohort, 42.5% of patients (94 out of 221) were contact lens wearers. Importantly, most keratitis cases within this subgroup were caused by
Pseudomonas aeruginosa. Furthermore, all cases of polymicrobial keratitis and every instance of amoebic keratitis occurred in contact lens users, emphasizing their well-documented role as a significant risk factor. Our analysis indicates that contact lens wear was linked to a threefold increase in the likelihood of obtaining a positive microbiological culture, providing a valuable guide for clinicians managing infectious keratitis in patients with such a history [
11,
37]. Seventeen patients reported prior ocular trauma, another established predisposing factor, especially when involving exposure to vegetable or organic material. Consistent with the literature, most keratitis cases in this subgroup were fungal in origin [
11,
26,
27,
38]. In our findings, a history of ocular trauma was associated with a 17-fold increase in the chance of obtaining a positive microbiological result.
Additionally, our research focused on comparing the clinical outcomes of two groups of patients who underwent microbiological or molecular analysis (
n = 107). Patients in whom a pathogen was identified (
n = 81, 75.7%) demonstrated a better final visual acuity compared to those with negative microbiological results (
n = 26, 24.3%). Moreover, the group with positive cultures required rescue surgery less frequently. In contrast, similarly designed studies conducted by Yarimada et al. [
34] and Bhadange et al. [
39] reported no significant difference in final best-corrected visual acuity between the culture-positive and culture-negative groups. Additionally, in those studies, the culture-positive group more often required rescue surgical intervention and/or evisceration. These contrasting findings may be partially explained by differences in the microbiological profiles of the two groups. In our study, culture-positive cases were often associated with more aggressive pathogens, particularly Gram-negative bacteria such as
Pseudomonas aeruginosa, which are known for their rapid progression and tissue-destructive potential. This aligns with the observations reported by Bhadange et al. [
39] and Yarimada et al. [
34], who found that culture-positive infections were more frequently associated with severe complications and a higher need for surgical intervention. Conversely, the culture-negative group likely included infections caused by less aggressive or rapidly resolving pathogens, such as Gram-positive bacteria or viruses, which may respond well to empirical treatment. Furthermore, it is important to consider that culture negativity does not equate to diagnostic failure, as prior antibiotic use, low microbial load, or fastidious organisms may contribute to negative results despite ongoing infection. These factors may help explain why, in our cohort, differences in visual outcomes between the two groups, although statistically significant, were not as dramatic as one might expect.
In addition to microbiological factors, the timing of referral appears to be crucial. In many of our culture-negative cases, patients were referred to our center only after receiving empirical treatment in peripheral settings, often following a delay of more than 15 days. This prior exposure to topical or systemic antimicrobials may have decreased the effectiveness of microbiological investigations, and the absence of a confirmed cause may have contributed to a delay in starting targeted therapy—ultimately leading to worse clinical outcomes. Furthermore, in our cohort, patients with clinical signs suggestive of Gram-positive bacterial infections—typically associated with milder courses—were less often selected for microbiological sampling. As a result, this subgroup is likely underrepresented among culture-negative cases in our analysis, unlike in previous studies where such patients might have been included in the culture-negative group. This difference in selection criteria may also help explain why our culture-negative group showed relatively poorer clinical outcomes compared to those reported by Yarimada et al. [
34] and Bhadange et al. [
39].
Lastly, patients referred to our tertiary care center were stratified according to the interval between the initial diagnosis of keratitis and the baseline examination performed at our facility. Patients who presented within 15 days of their initial diagnosis experienced a shorter overall treatment duration, achieved higher final visual acuity, and demonstrated a lower need for rescue surgical interventions compared to those who were referred after more than 15 days. These findings suggest that early specialist evaluation and timely initiation of appropriate therapy play a critical role in improving outcomes in infectious keratitis. Furthermore, our analysis revealed that cases of bacterial keratitis tended to be referred to our center significantly earlier than infections caused by fungi or Acanthamoeba. This delay in presentation for fungal and amoebic keratitis may be explained by their often more insidious and less distinctive clinical manifestations, which can result in delayed recognition, prolonged empirical treatment in peripheral centers, and a consequent deterioration of the infection before specialist assessment is sought. Overall, these observations emphasize the importance of prompt referral and early microbiological and ophthalmological evaluation, particularly in cases with atypical or slowly progressive presentations, to optimize prognosis and reduce the risk of irreversible visual impairment.
This study presents some limitations that should be considered. Given its retrospective and observational design, the accuracy of certain data points—such as the exact onset of symptoms or prior treatments received before referral—may be subject to variability across clinical records. Additionally, the sample size for less common forms of keratitis, such as fungal and amoebic infections, was relatively small, which may limit the strength of conclusions drawn for these subgroups. Finally, as the research was conducted in a single tertiary care center, the findings may reflect specific referral patterns and clinical practices that are not necessarily generalizable to all ophthalmology settings.