Hepatitis E Virus Infection in a Hospital from Southern Romania—New Data About a Threat to Public Health
Abstract
1. Introduction
2. Materials and Methods
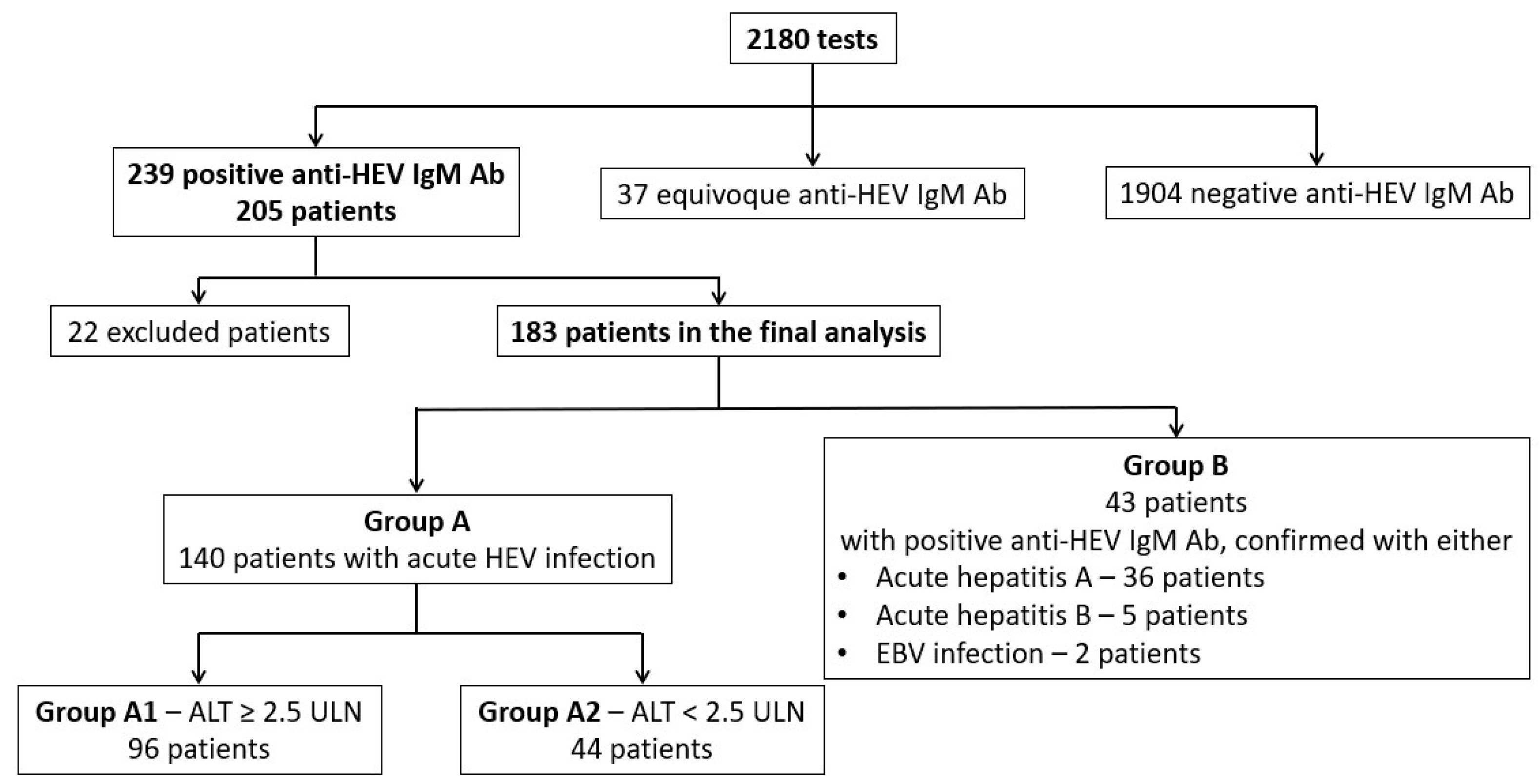
2.1. Characteristics of the Study Groups
- -
- Group A—140 patients with probable acute HEV infection (positive anti-HEV IgM antibodies) and no positive tests for acute infections with any other hepatotropic virus;
- -
- Group B—43 patients with probable acute HEV infection, but also with positive tests for other acute hepatitis viruses: anti-hepatitis A IgM antibodies (36 patients), anti-hepatitis B core IgM antibodies (5 patients), and positive IgM anti-viral capsid antigen of Epstein–Barr virus (EBV) (2 patients). We supposed that anti-HEV IgM antibodies could be false positive for some of these patients.
- -
- Group A1 (significant cytolysis) with ALT higher than 2.5-fold ULN (96 patients);
- -
- Group A2 (without significant cytolysis) with ALT lower than 2.5-fold ULN (44 patients).
2.2. Serological and Molecular Analysis
2.3. Statistical Analysis
3. Results
3.1. Epidemiological Characteristics of the Study Population
3.2. Clinical Characteristics
3.3. Laboratory Characteristics
3.3.1. Changes in Alanine Transaminase (ALT) Levels
3.3.2. Other Biochemistry Laboratory Markers
3.3.3. Serological Features
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| CO | Cut-off |
| EBV | Epstein–Barr virus |
| ECDC | European Centre for Disease Prevention and Control |
| GGT | Gamma-glutamyl transferase |
| HAV | Hepatitis A virus |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| HDV | Hepatitis D virus |
| HEV | Hepatitis E virus |
| HIV | Human immunodeficiency virus |
| INR | International normalised ratio |
| OD | Optical density |
| PI | Prothrombin index |
| PLT | Platelet count |
| RNA | Ribonucleic acid |
| RT-PCR | Reverse transcription polymerase chain reaction |
| ULN | Upper limit of normal |
| WHO | World Health Organization |
References
- National Centre for Communicable Diseases Surveillance and Control of Romania. Annual Report (2022). Available online: https://insp.gov.ro/download/analiza-bolilor-transmisibile-aflate-in-supraveghere-raport-pentru-anul-2022/ (accessed on 15 July 2024).
- National Centre for Communicable Diseases Surveillance and Control of Romania. Methodologies. Available online: https://insp.gov.ro/centrul-national-de-supraveghere-si-control-al-bolilor-transmisibile-cnscbt/metodologii/ (accessed on 15 July 2024).
- International Committee on Taxonomy of Viruses: ICTV. Current ICTV Taxonomy Release. Available online: https://ictv.global/taxonomy (accessed on 27 September 2025).
- Porea, D.; Anita, A.; Vata, A.; Teodor, D.; Crivei, L.; Raileanu, C.; Gotu, V.; Ratoi, I.; Cozma, A.; Anita, D.; et al. Common European origin of hepatitis E virus in human population from Eastern Romania. Front. Public Health 2020, 8, 578163, GBIF Occurrence Download. Available online: https://doi.org/10.3389/fpubh.2020.578163 (accessed on 15 July 2024).
- Mihai, I.F.; Manciuc, C.; Hunea, I.M.; Lacatusu, G.A.; Leonte, G.E.; Luca, S.; Harja-Alexa, I.A.; Vata, A.; Luca, M.C. Enterically transmitted hepatitis in the third millennium in northeastern Romania. Exp. Ther. Med. 2021, 21, 274, GBIF Occurrence Download. Available online: https://doi.org/10.3892/etm.2021.9705 (accessed on 15 July 2024).
- Istrate, A.; Rădulescu, A.L. A comparison of hepatitis E and A in a teaching hospital in Northwestern Romania. Acute hepatitis E—A mild disease? Med. Pharm. Rep. 2020, 93, 30–38, GBIF Occurrence Download. Available online: https://doi.org/10.15386/mpr-1487 (accessed on 15 July 2024).
- World Health Organization. Hepatitis E. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e (accessed on 15 July 2024).
- Adlhoch, C.; Avellon, A.; Baylis, S.A.; Ciccaglione, A.R.; Couturier, E.; de Sousa, R.; Epštein, J.; Ethelberg, S.; Faber, M.; Fehér, Á.; et al. Hepatitis E virus: Assessment of the epidemiological situation in humans in Europe, 2014/15. J. Clin. Virol. 2016, 82, 9–16, GBIF Occurrence Download. Available online: https://doi.org/10.1016/j.jcv.2016.06.010 (accessed on 15 July 2024).
- Izopet, J.; Tremeaux, P.; Marion, O.; Migueres, M.; Capelli, N.; Chapuy-Regaud, S.; Mansuy, J.M.; Abravanel, F.; Kamar, N.; Lhomme, S. Hepatitis E virus infections in Europe. J. Clin. Virol. 2019, 120, 20–26, GBIF Occurrence Download. Available online: https://doi.org/10.1016/j.jcv.2019.09.004 (accessed on 15 July 2024).
- European Centre for Disease Prevention and Control. Options for National Testing and Surveillance for Hepatitis E virus in the EU/EEA—Operational Guidance; ECDC: Stockholm, Sweden, 2019; GBIF Occurrence Download; Available online: https://doi.org/10.2900/417723 (accessed on 15 July 2024).
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernandez Escamez, P.S.; Herman, L.; Koutsoumanis, K.; Lindqvist, R.; Nørrung, B.; et al. Public health risks associated with hepatitis E virus (HEV) as a food-borne pathogen. EFSA J. 2017, 15, 4886–4889, GBIF Occurrence Download. Available online: https://doi.org/10.2903/j.efsa.2017.4886 (accessed on 15 July 2024).
- Domanović, D.; Tedder, R.; Blümel, J.; Zaaijer, H.; Gallian, P.; Niederhauser, C.; Sauleda Oliveras, S.; O’Riordan, J.; Boland, F.; Harritshøj, L.; et al. Hepatitis E and blood donation safety in selected European countries: A shift to screening? Euro Surveill. 2017, 22, 20, GBIF Occurrence Download. Available online: https://doi.org/10.2807/1560-7917.ES.2017.22.16.30514 (accessed on 15 July 2024).
- Murrison, L.B.; Sherman, K.E. The enigma of hepatitis E virus. Gastroenterol. Hepatol. 2017, 13, 484–491. [Google Scholar]
- Savuţa, G.; Aniţă, A.; Aniţă, D.; Ludu, L.; Duca, E.; Pavio, N. Seroepidemiological researches regarding swine human hepatits E in Romania. Lucr. St Med Vet USAMVB Timis 2008, XLI, 309–313. Available online: https://www.researchgate.net/publication/285313425_Seroepidemiological_researches_regarding_swine_and_human_hepatitis_E_in_Romania (accessed on 15 July 2024).
- Anita, A.; Anita, D.; Ludu, L.; Savuta, G. Seroepidemiological investigation human swine hepatitis Botosani County. Bull. UASVM Vet. Med. 2010, 67, 19–22. [Google Scholar]
- Voiculescu, M.; Iliescu, L.; Ionescu, C.; Micu, L.; Ismail, G.; Zilisteanu, D.; Radasan, A.; Micu, G.; Pertache, I. A cross-sectional epidemiological study of HBV, HCV, HDV and HEV prevalence in the SubCarpathian and South-Eastern regions of Romania. J. Gastrointestin Liver Dis. 2010, 19, 43–48, GBIF Occurrence Download. Available online: https://doi.org/10.1007/s11749-009-0177-3 (accessed on 15 July 2024).
- Aniţă, A.; Gorgan, L.; Aniţă, D.; Oşlobanu, L.; Pavio, N.; Savuţa, G. Evidence of hepatitis E infection in swine and humans in the East Region of Romania. Int. J. Infect. Dis. 2014, 29, 232–237, GBIF Occurrence Download. Available online: https://doi.org/10.1016/j.ijid.2014.10.018 (accessed on 15 July 2024).
- Mihai, I.F.; Anita, D.; Dorneanu, O.S.; Luca, C.M.; Manciuc, C.D.; Budacu, C.C.; Roșu, F.M.; Savuta, G.; Anita, A.; Vâţă, A. Seroprevalence of anti-hepatitis E virus antibodies among patients from a tertiary hospital from northeast Romania. Medicina 2022, 58, 1020, GBIF Occurrence Download. Available online: https://doi.org/10.3390/medicina58081020 (accessed on 15 July 2024).
- Komitova, R.; Kevorkyan, A.; Golkocheva-Markova, E.; Atanasova, M.; Rangelova, V.; Raycheva, R.; Ismailova, C.; Stoyanova, A.; Tenev, T. Clinical and virological profile of locally acquired acute hepatitis E in South Bulgaria. J. Infect. Dev. Ctries. 2024, 18, 136–144, GBIF Occurrence Download. Available online: https://doi.org/10.3855/jidc.18341 (accessed on 15 July 2024).
- Wallace, S.J.; Swann, R.; Donnelly, M.; Kemp, L.; Guaci, J.; Murray, A.; Spoor, J.; Lin, N.; Miller, M.; Dalton, H.R.; et al. Mortality and morbidity of locally acquired hepatitis E in the national Scottish cohort: A multicentre retrospective study. Aliment. Pharmacol. Ther. 2020, 51, 974–986, GBIF Occurrence Download. Available online: https://doi.org/10.1111/apt.15704 (accessed on 15 July 2024).
- Schiff, E.R.; Maddrey, W.C.; Reddy, K.R. (Eds.) Schiff’s Diseases of the Liver, 12th ed.; New Delhi Wiley-Blackwell: New Delhi, India, 2017; p. 1232, GBIF Occurrence Download; Available online: https://doi.org/10.1002/9781119251316 (accessed on 15 July 2024).
- HEV IgM. Enzyme Immunoassay (ELISA) for the Determination of IgM Antibodies to Hepatitis E Virus in Human Serum and Plasma. Available online: https://weldonbiotech.com/wp-content/uploads/2018/04/EVM.CE-Insert-Rev.2-0911-Eng.pdf (accessed on 15 July 2024).
- World Health Organization. Publications. Overview. Global Hepatitis Report 2024: Action for Access in low- and Middle-Income Countries. Available online: https://www.who.int/publications/i/item/9789240091672 (accessed on 15 July 2024).
- European Centre for Disease Prevention and Control. Facts about Hepatitis E. Available online: https://www.ecdc.europa.eu/en/hepatitis-e/facts (accessed on 15 July 2024).
- European Centre for Disease Prevention and Control. Hepatitis E virus Infections in the EU/EEA, January 2024. In Weekly Communicable Disease Threats Report, Week 6, 4–10 February 2024; ECDC: Solna, Sweden, 2024; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/communicable-disease-threats-report-week-6-2024.pdf (accessed on 15 July 2024).
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on hepatitis E virus infection. J. Hepatol. 2018, 68, 1256–1271, GBIF Occurrence Download. Available online: https://doi.org/10.1016/j.jhep.2018.03.005 (accessed on 15 July 2024).
- Dalton, H.R.; Seghatchian, J. Hepatitis E virus: Emerging from the shadows in developed countries. Transfus. Apher. Sci. 2016, 55, 271–274, GBIF Occurrence Download. Available online: https://doi.org/10.1016/j.transci.2016.10.016 (accessed on 15 July 2024).
- Ijaz, S.; Said, B.; Boxall, E.; Smit, E.; Morgan, D.; Tedder, R.S. Indigenous hepatitis E in England and Wales from 2003 to 2012: Evidence of an emerging novel phylotype of viruses. J. Infect. Dis. 2014, 209, 1212–1218, GBIF Occurrence Download. Available online: https://doi.org/10.1093/infdis/jit652 (accessed on 15 July 2024).
- Fares, A. Seasonality of hepatitis: A review update. J. Family Med. Prim. Care 2015, 4, 96–100, GBIF Occurrence Download. Available online: https://doi.org/10.4103/2249-4863.152263 (accessed on 15 July 2024).
- Christou, L.; Kosmidou, M. Hepatitis E virus in the Western world—A pork-related zoonosis. Clin. Microbiol. Infect. 2013, 19, 600–604, GBIF Occurrence Download. Available online: https://doi.org/10.1111/1469-0691.12214 (accessed on 15 July 2024).
- Jang, J.H.; Jung, Y.M.; Kim, J.S.; Lee, S.H.; Kim, J.W.; Hwang, S.G.; Rim, K.S.; Park, S.J.; Park, Y.M.; Kang, S.K.; et al. Coexistence of IgM antihepatitis A virus and IgM antihepatitis E virus in acute viral hepatitis: A prospective, multicentre study in Korea. J. Viral Hepat. 2011, 18, e408-14, GBIF Occurrence Download. Available online: https://doi.org/10.1111/j.1365-2893.2011.01477.x (accessed on 15 July 2024).
- Al-Absi, E.S.; Al-Sadeq, D.W.; Younis, M.H.; Yassine, H.M.; Abdalla, O.M.; Mesleh, A.G.; Hadwan, T.A.; Amimo, J.O.; Thalib, L.; Nasrallah, G.K. Performance evaluation of five commercial assays in assessing seroprevalence of HEV antibodies among blood donors. J. Med. Microbiol. 2018, 67, 1302–1309, GBIF Occurrence Download. Available online: https://doi.org/10.1099/jmm.0.000807 (accessed on 15 July 2024); Erratum in J. Med. Microbiol. 2019, 68, 115. Available online: https://doi.org/10.1099/jmm.0.000880 (accessed on 15 July 2024).
- Pas, S.D.; Streefkerk, R.H.; Pronk, M.; de Man, R.A.; Beersma, M.F.; Osterhaus, A.D.; van der Eijk, A.A. Diagnostic performance of selected commercial HEV IgM and IgG ELISAs for immunocompromised and immunocompetent patients. J. Clin. Virol. 2013, 58, 629–634, GBIF Occurrence Download. Available online: https://doi.org/10.1016/j.jcv.2013.10.010 (accessed on 15 July 2024).
- Lu, Y.H.; Qian, H.Z.; Hu, A.Q.; Ren, H.; Qin, X.; Jiang, Q.W.; Zheng, Y.J. Duration of viraemia in Chinese acute sporadic hepatitis E. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 755–759, GBIF Occurrence Download. Available online: https://doi.org/10.1007/s10096-013-2007-5 (accessed on 15 July 2024).
- Adlhoch, C.; Manďáková, Z.; Ethelberg, S.; Epštein, J.; Rimhanen-Finne, R.; Figoni, J.; Baylis, S.A.; Faber, M.; Mellou, K.; Murphy, N.; et al. Standardising surveillance of hepatitis E virus infection in the EU/EEA: A review of national practices and suggestions for the way forward. J. Clin. Virol. 2019, 120, 63–67, GBIF Occurrence Download. Available online: https://doi.org/10.1016/j.jcv.2019.09.005 (accessed on 15 July 2024).

| Aetiology | Year | Total | ||||
|---|---|---|---|---|---|---|
| 2019 | 2020 | 2021 | 2022 | 2023 | ||
| HAV | 75 | 13 | 7 | 142 | 55 | 292 (45.5%) |
| HBV | 39 | 9 | 8 | 8 | 18 | 82 (12.8%) |
| HCV | 14 | 2 | 0 | 4 | 1 | 21 (3.3%) |
| HDV | 35 | 7 | 8 | 10 | 4 | 64 (9.9%) |
| HEV | 82 | 18 | 13 | 49 | 21 | 183 (28.5%) |
| Total | 245 | 49 | 36 | 213 | 99 | 642 (100%) |
| Demographic Feature | Group A | Group B | Group A vs. Group B |
|---|---|---|---|
| Sex (male) (N, %) | 69 (49.3%) | 32 (74.4%) | ꭓ2 = 8.4, p < 0.01 |
| Age (years) (mean, SD) | 47.26 (SD = 15.13) | 35.95 (SD = 14.83) | t(181) = 4.3, p < 0.01 |
| Urban environment (N, %) | 102 (75%) | 29 (67.4%) | ꭓ2 = 0.95, p = 0.33 |
| Residence in the metropolitan area of Bucharest (N, %) | 88 (64.7%) | 25 (58.1%) | ꭓ2 = 0.6, p = 0.47 |
| Clinical Feature | Group A (%) | Group B (%) | Group A vs. Group B |
|---|---|---|---|
| Digestive symptoms | 69.6% | 97.2% | ꭓ2 = 10.59, p < 0.01 |
| Fever | 64.8% | 55.6% | ꭓ2 = 0.77, p = 0.38 |
| Jaundice | 31.8% | 88.4% | ꭓ2 = 41.8, p < 0.01 |
| Hepatomegaly | 64.6% | 88.6% | ꭓ2 = 6.16, p = 0.02 |
| Laboratory Feature | Group A | Group B | Group A vs. Group B |
|---|---|---|---|
| PLT < 105 cells/mm3 (N, %) | 8 (5.8%) | 1 (2.3%) | ꭓ2 = 0.85, p = 0.68 |
| PI < 50% (N, %) | 10 (8.1%) | 4 (9.8%) | ꭓ2 = 0.11, p = 0.75 |
| Total bilirubin (mg/dL) (mean, SD) | 3.08 (SD = 5.2) | 7.82 (SD = 5.25) | t(178) = 5.2, p < 0.01 |
| ALP > ULN (N, %) | 63 (49.2%) | 37 (92.5%) | ꭓ2 = 23.69, p < 0.01 |
| GGT > ULN (N, %) | 106 (78.5%) | 41 (95.3%) | ꭓ2 = 6.42, p = 0.01 |
| Lipase > ULN (N, %) | 15 (18.8%) | 2 (6.9%) | ꭓ2 = 2.27, p = 0.23 |
| Albumin < 3.5 g/dL (N, %) | 8 (10.3%) | 2 (7.7%) | ꭓ2 = 0.14, p = 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, C.; Cireșă, A.; Popescu, G.A.; Vasile, C.C.; Bănică, L.M.; Florea, D. Hepatitis E Virus Infection in a Hospital from Southern Romania—New Data About a Threat to Public Health. Microorganisms 2025, 13, 2290. https://doi.org/10.3390/microorganisms13102290
Popescu C, Cireșă A, Popescu GA, Vasile CC, Bănică LM, Florea D. Hepatitis E Virus Infection in a Hospital from Southern Romania—New Data About a Threat to Public Health. Microorganisms. 2025; 13(10):2290. https://doi.org/10.3390/microorganisms13102290
Chicago/Turabian StylePopescu, Cristina, Alexandra Cireșă, Gabriel Adrian Popescu, Carmen Cristina Vasile, Leontina Mirela Bănică, and Dragoș Florea. 2025. "Hepatitis E Virus Infection in a Hospital from Southern Romania—New Data About a Threat to Public Health" Microorganisms 13, no. 10: 2290. https://doi.org/10.3390/microorganisms13102290
APA StylePopescu, C., Cireșă, A., Popescu, G. A., Vasile, C. C., Bănică, L. M., & Florea, D. (2025). Hepatitis E Virus Infection in a Hospital from Southern Romania—New Data About a Threat to Public Health. Microorganisms, 13(10), 2290. https://doi.org/10.3390/microorganisms13102290






