Cold–Temperate Betula platyphylla Sukaczev Forest Can Provide More Soil Nutrients to Increase Microbial Alpha Diversity and Microbial Necromass Carbon
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil Sample Collection and Treatment
2.3. Analyses of Soil Physicochemical Properties
2.4. Measurement of Phospholipid Fatty Acids
2.5. Measurement of Microbial Necromass Carbon
2.6. Statistical Analyses
3. Results
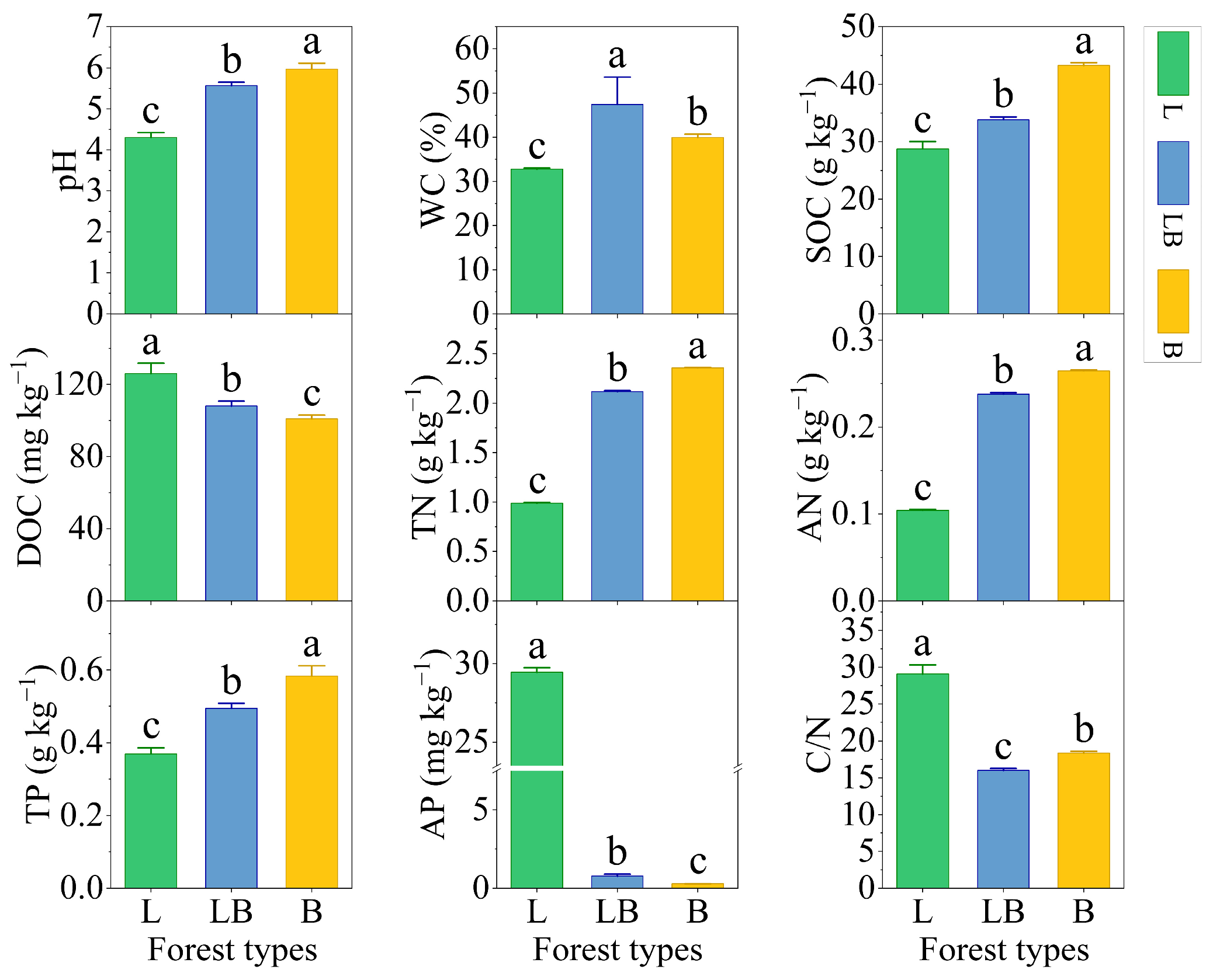
3.1. Soil Physicochemical Properties in Different Forest Types
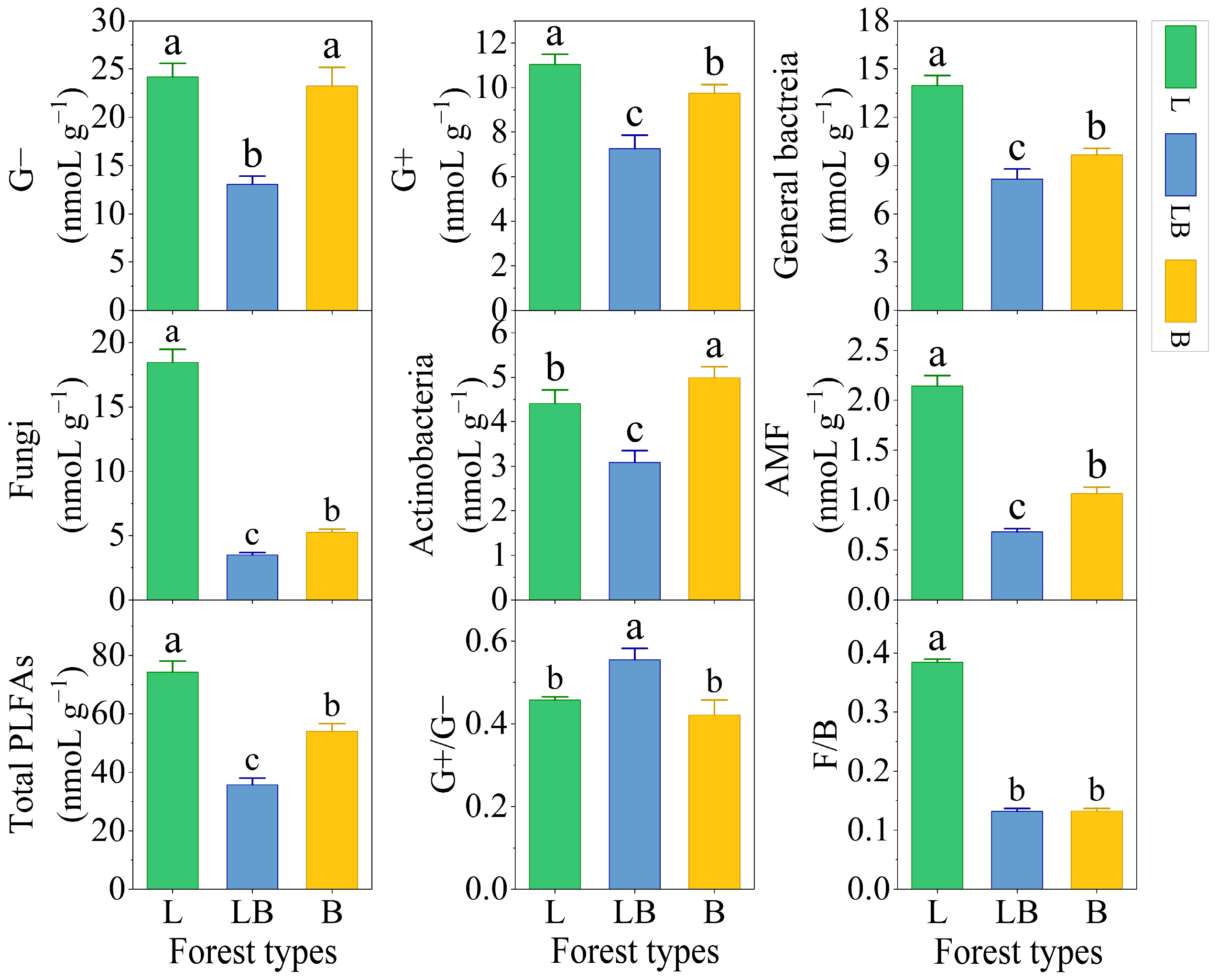
3.2. Soil Microbial Biomass in Different Forest Types
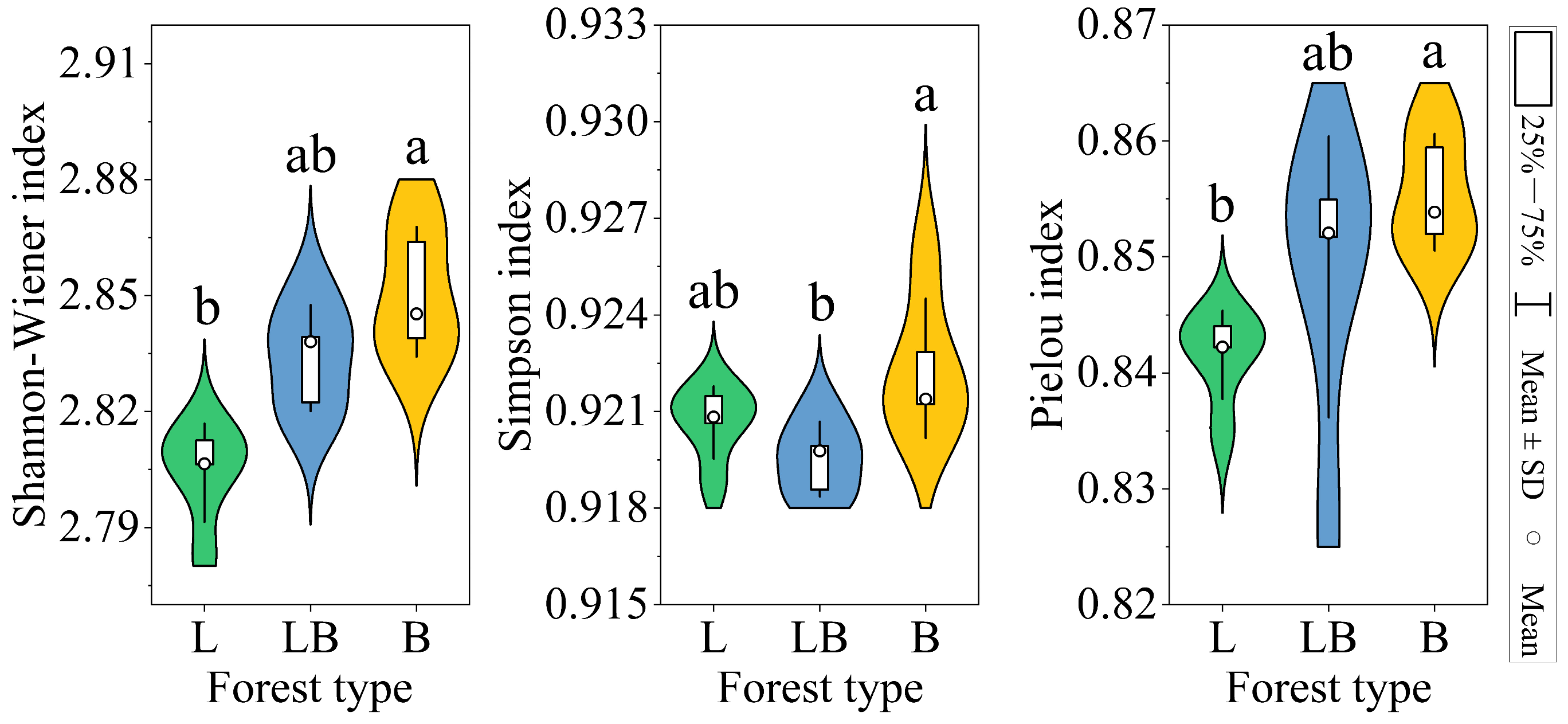
3.3. Soil Microbial Community Diversity and Structure in Different Forest Types
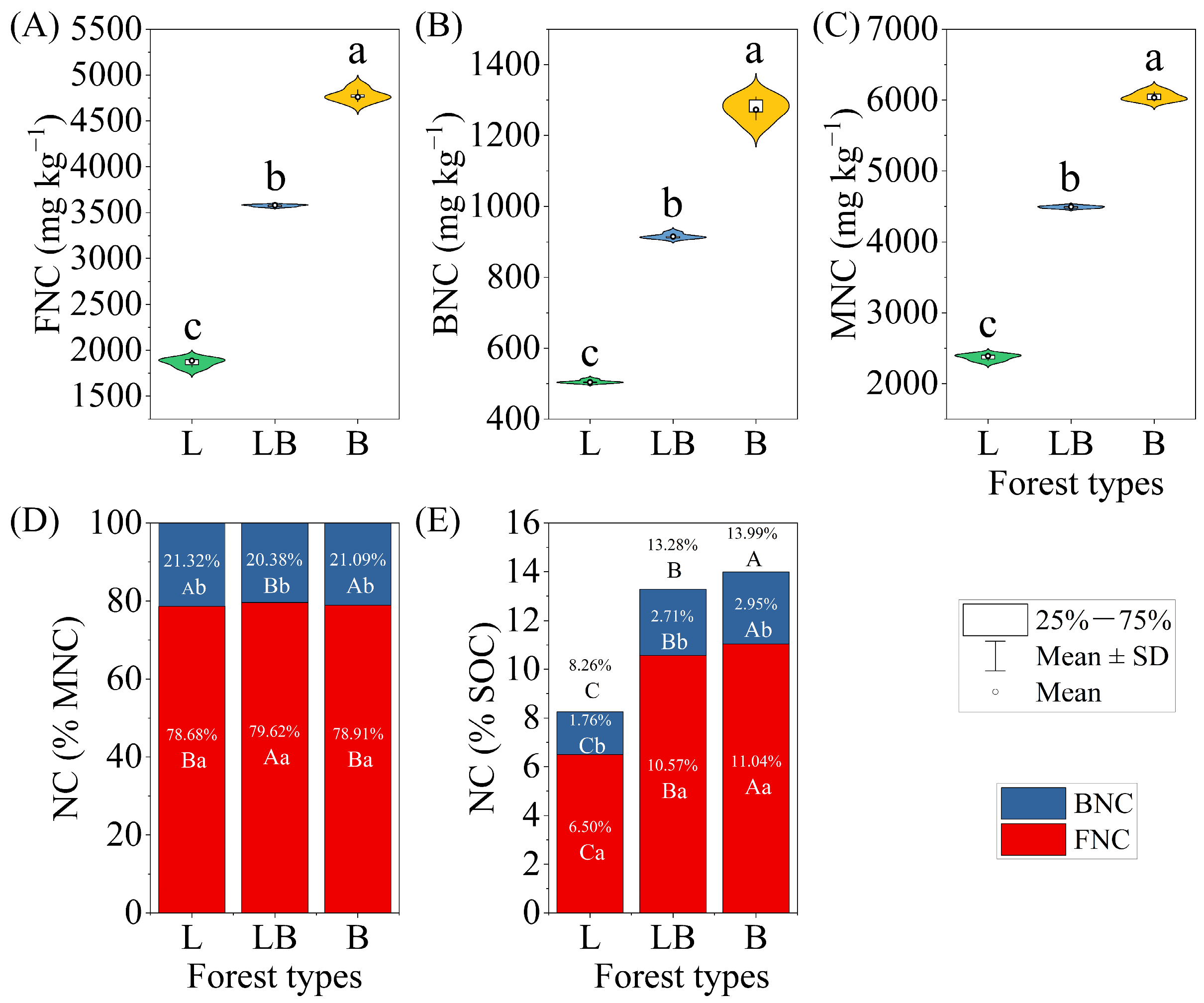
3.4. Soil Microbial Necromass Carbon in Different Forest Types
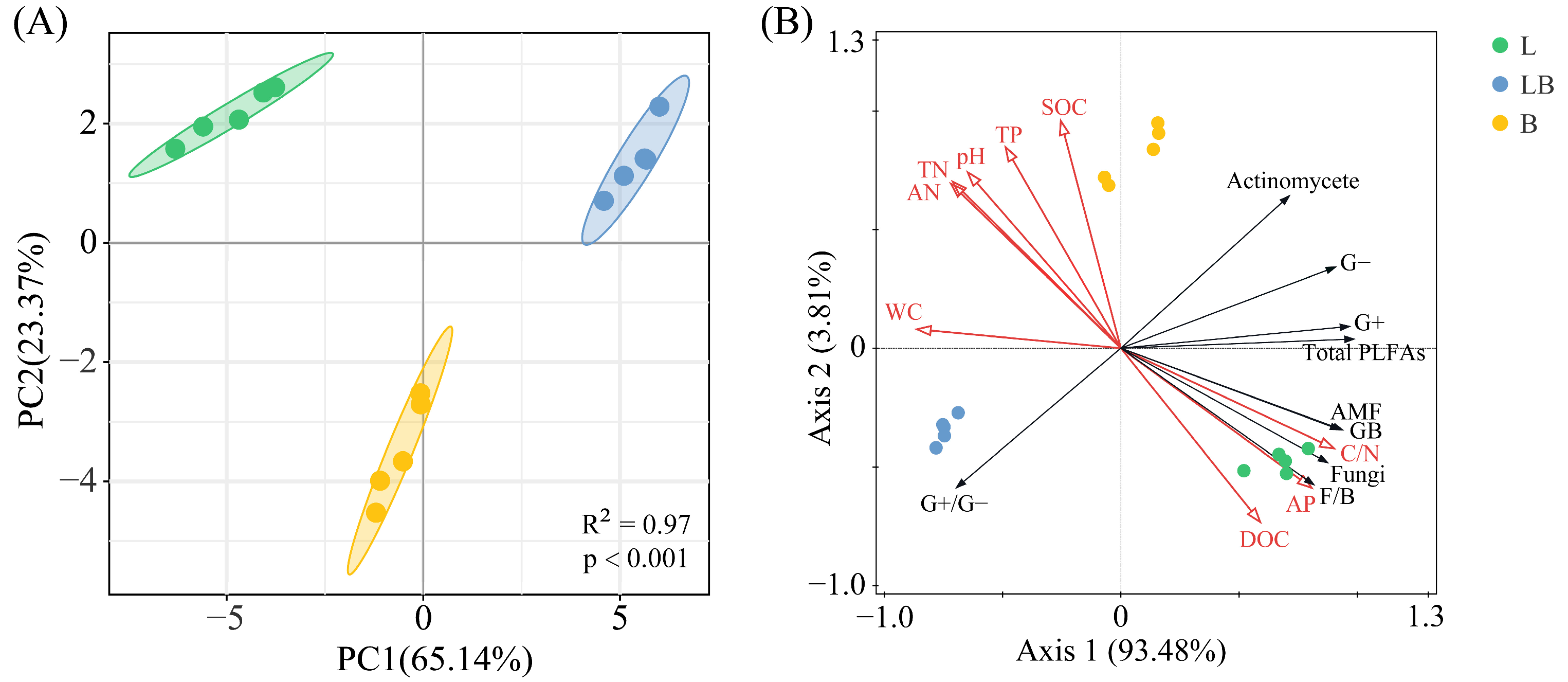
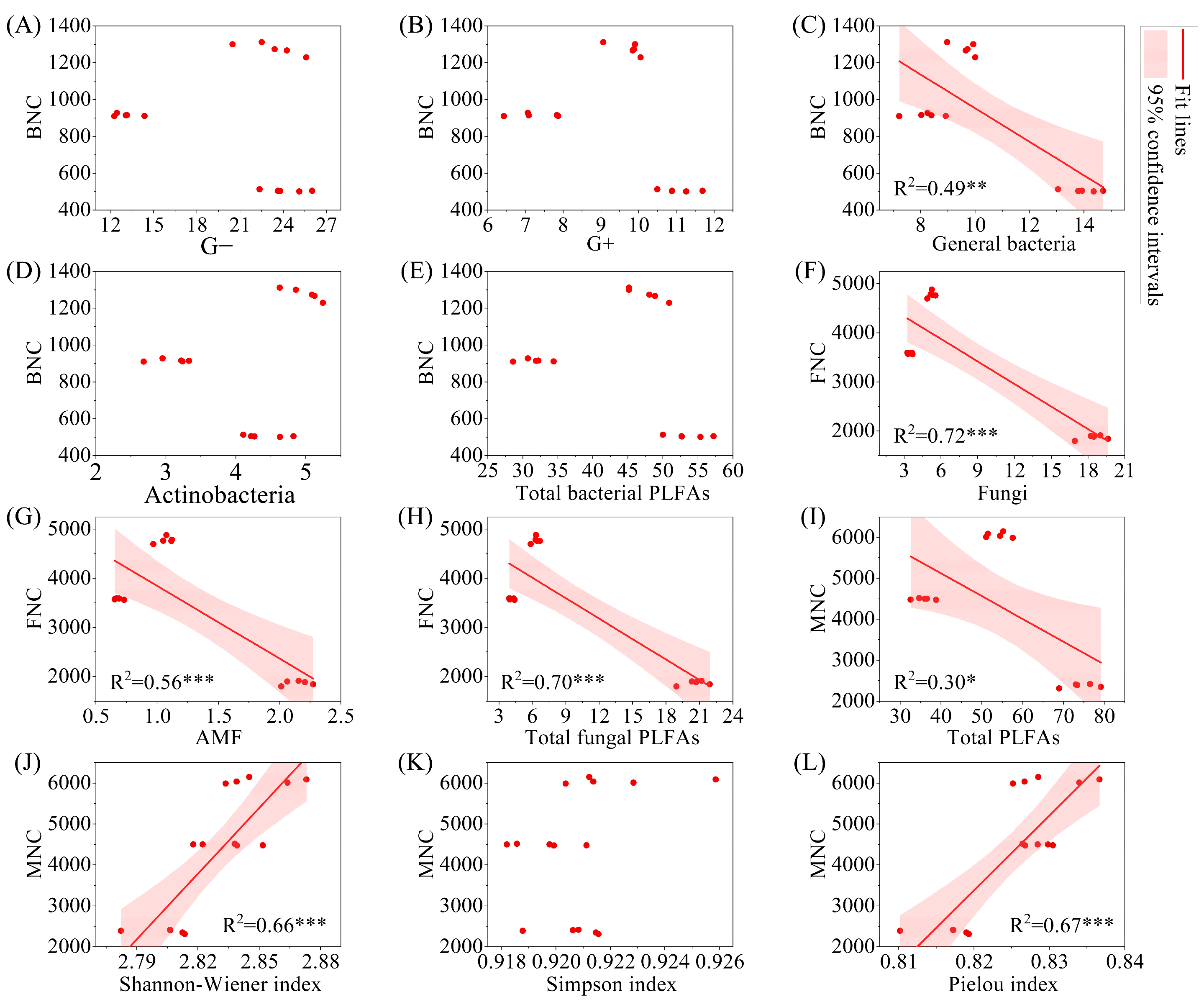
3.5. Correlation and Regression Analysis
4. Discussion
4.1. Changes in Soil Physicochemical Properties in Different Forest Types
4.2. Factors Influencing Changes in the Soil Microbial Community
4.3. Differences in Content of Soil Microbial Necromass Carbon and Influencing Factors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, S.; Song, X.; Zeng, H.; Wang, J. Soil microbial necromass carbon in forests: A global synthesis of patterns and controlling factors. Soil Ecol. Lett. 2024, 6, 240237. [Google Scholar] [CrossRef]
- Wang, B.; An, S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial necromass as the source of soil organic carbon in global ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Cabrera-Ariza, A.M.; Rivas, C.A.; Aguilera-Peralta, M.; Navarro-Cerrillo, R.M.; Santelices-Moya, R. Updating the distribution of Nothofagus alessandrii: Impact of deforestation, fragmentation and connectivity. For. Ecosyst. 2025, 12, 100145–100272. [Google Scholar] [CrossRef]
- Amaral, S.; Metzger, J.P.; Rosa, M.; Adorno, B.V.; Gonçalves, G.C.; Pinto, L.F.G. Alarming patterns of mature forest loss in the Brazilian Atlantic Forest. Nat. Sustain. 2025, 8, 256–264. [Google Scholar] [CrossRef]
- Mekonnen, Z.A.; Riley, W.J.; Randerson, J.T.; Grant, R.F.; Rogers, B.M. Expansion of high-latitude deciduous forests driven by interactions between climate warming and fire. Nat. Plants 2019, 5, 952–958. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Hatton, P.; Castanha, C.; Torn, M.S.; Bird, J.A. Litter type control on soil C and N stabilization dynamics in a temperate forest. Glob. Change Biol. 2015, 21, 1358–1367. [Google Scholar] [CrossRef]
- Dai, G.; Zhu, S.; Cai, Y.; Zhu, E.; Jia, Y.; Ji, C.; Tang, Z.; Fang, J.; Feng, X. Plant-derived lipids play a crucial role in forest soil carbon accumulation. Soil Biol. Biochem. 2022, 168, 108645. [Google Scholar] [CrossRef]
- Zhu, Y.; Hui, D.; Wang, Y.P.; Liu, F.; Huang, S.; Li, J.; Zhang, L.; Chen, G.; Chen, J.; Hu, Y.; et al. Linking plant lignin components or microbial necromass to soil organic carbon accumulation across different forest types. Res. Sq. 2022. Available online: https://www.researchsquare.com/article/rs-2353062/v1 (accessed on 18 June 2025).
- Rodríguez-Rodríguez, J.C.; Fenton, N.J.; Bergeron, Y.; Kembel, S.W. Soil and tree phyllosphere microbial communities differ between coniferous and broadleaf deciduous boreal forests. Plant Soil 2023, 488, 233–253. [Google Scholar] [CrossRef]
- Klotzbücher, T.; Kaiser, K.; Stepper, C.; van Loon, E.; Gerstberger, P.; Kalbitz, K. Long-term litter input manipulation effects on production and properties of dissolved organic matter in the forest floor of a Norway spruce stand. Plant Soil 2012, 355, 407–416. [Google Scholar] [CrossRef]
- Chodak, M.; Beata, K.; Niklińska, M. Composition and activity of soil microbial communities in different types of temperate forests. Biol. Fert. Soils 2016, 52, 1093–1104. [Google Scholar] [CrossRef]
- Labouyrie, M.; Ballabio, C.; Romero, F.; Panagos, P.; Jones, A.; Schmid, M.W.; Mikryukov, V.; Dulya, O.; Tedersoo, L.; Bahram, M.; et al. Patterns in soil microbial diversity across Europe. Nat. Commun. 2023, 14, 3311. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, Y.; Chen, F.; Liu, Y.; Lin, X.; Zong, Y.; Wu, G.; Yu, Z.; Fang, X. Mixing with broad-leaved trees shapes the rhizosphere soil fungal communities of coniferous tree species in subtropical forests. For. Ecol. Manag. 2021, 480, 118664. [Google Scholar] [CrossRef]
- Sawada, K.; Inagaki, Y.; Sugihara, S.; Funakawa, S.; Ritz, K.; Toyota, K. Impacts of conversion from natural forest to cedar plantation on the structure and diversity of root-associated and soil microbial communities. Appl. Soil. Ecol. 2021, 167, 104027. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Song, W.; Slik, J.W.F.; Sukri, R.S.; Jaafar, S.; Dong, K.; Adams, J.M. Distinctive tropical forest variants have unique soil microbial communities, but not always low microbial diversity. Front. Microbiol. 2016, 7, 376. [Google Scholar] [CrossRef]
- Kruse, S.; Wieczorek, M.; Jeltsch, F.; Herzschuh, U. Treeline dynamics in Siberia under changing climates as inferred from an individual-based model for Larix. Ecol. Model. 2016, 338, 101–121. [Google Scholar] [CrossRef]
- Bao, T.; Deng, S.; Yu, K.; Li, W.; Dong, A. Metagenomic insights into seasonal variations in the soil microbial community and function in a Larix gmelinii forest of Mohe, China. J. For. Res. 2021, 32, 371–383. [Google Scholar] [CrossRef]
- Song, D.; Cui, Y.; Ma, D.; Li, X.; Liu, L. Spatial variation of microbial community structure and its driving environmental factors in two forest types in permafrost region of Greater Xing’an Mountains. Sustainability 2022, 14, 9284. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, S.; Yang, L.; Liu, Y.; Gao, M.; Ni, H. Short-term simulated warming changes the beta diversity of bacteria in taiga forests’ permafrost by altering the composition of dominant bacterial phyla. Forest 2024, 15, 693. [Google Scholar] [CrossRef]
- Ade, L.J.; Hu, L.; Zi, H.B.; Wang, C.T.; Lerdau, M.; Dong, S.K. Effect of snowpack on the soil bacteria of alpine meadows in the Qinghai-Tibetan Plateau of China. Catena 2018, 164, 13–22. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yao, S.; Cao, X.; Schmidt-Rohr, K.; Olk, D.C.; Mao, J.; Zhang, B. Structural evidence for soil organic matter turnover following glucose addition and microbial controls over soil carbon change at different horizons of a Mollisol. Soil Biol. Biochem. 2018, 119, 63–73. [Google Scholar] [CrossRef]
- Ma, T.; Zhu, S.; Wang, Z.; Chen, D.; Dai, G.; Feng, B.; Su, X.; Hu, H.; Li, K.; Han, W.; et al. Divergent accumulation of microbial necromass and plant lignin components in grassland soils. Nat. Commun. 2018, 9, 3480. [Google Scholar] [CrossRef]
- Albers, D.; Migge, S.; Schaefer, M.; Scheu, S. Decomposition of beech leaves (Fagus sylvatica) and spruce needles (Picea abies) in pure and mixed stands of beech and spruce. Soil Biol. Biochem. 2004, 36, 155–164. [Google Scholar] [CrossRef]
- Gruba, P.; Mulder, J. Tree species affect cation exchange capacity (CEC) and cation binding properties of organic matter in acid forest soils. Sci. Total Environ. 2015, 511, 655–662. [Google Scholar] [CrossRef]
- Ross, D.S.; Matschonat, G.; Skyllberg, U. Cation exchange in forest soils: The need for a new perspective. Eur. J. Soil. Sci. 2008, 59, 1141–1159. [Google Scholar] [CrossRef]
- Clarholm, M.; Skyllberg, U. Translocation of metals by trees and fungi regulates pH, soil organic matter turnover and nitrogen availability in acidic forest soils. Soil Biol. Biochem. 2013, 63, 142–153. [Google Scholar] [CrossRef]
- Van Nevel, L.; Mertens, J.; De Schrijver, A.; Baeten, L.; De Neve, S.; Tack, F.M.; Meers, E.; Verheyen, K. Forest floor leachate fluxes under six different tree species on a metal contaminated site. Sci. Total Environ. 2013, 447, 99–107. [Google Scholar] [CrossRef]
- Zhou, Q.; Keith, D.M.; Zhou, X.; Cai, M.; Cui, X.; Wei, X.; Luo, Y. Comparing the water-holding characteristics of broadleaved, coniferous, and mixed forest litter layers in a karst region. Mt. Res. Dev. 2018, 38, 220–229. [Google Scholar] [CrossRef]
- Rivero, R.G.; Grunwald, S.; Osborne, T.Z.; Reddy, K.R.; Newman, S. Characterization of the spatial distribution of soil properties in water conservation area 2A, Everglades, Florida. Soil Sci. 2007, 172, 149–166. [Google Scholar] [CrossRef]
- Deng, J.; Zhu, W.; Zhou, Y.; Yin, Y. Soil organic carbon chemical functional groups under different revegetation types are coupled with changes in the microbial community composition and the functional genes. Forests 2019, 10, 240. [Google Scholar] [CrossRef]
- Tian, S.; Man, X. Characteristics of soil microbial biomass carbon and dissolved organic carbon in northern forest region of Daxing’an Montains. Chin. J. Soil Sci. 2016, 47, 838–845. [Google Scholar] [CrossRef]
- Broadbent, A.A.D.; Newbold, L.K.; Pritchard, W.J.; Michas, A.; Goodall, T.; Cordero, I.; Giunta, A.; Snell, H.S.K.; Pepper, V.V.L.H.; Grant, H.K.; et al. Climate change disrupts the seasonal coupling of plant and soil microbial nutrient cycling in an alpine ecosystem. Glob. Change Biol. 2024, 30, e17245. [Google Scholar] [CrossRef] [PubMed]
- Kafle, A.; Cope, K.R.; Raths, R.; Yakha, J.K.; Subramanian, S.; Bücking, H.; Garcia, K. Harnessing soil microbes to improve plant phosphate efficiency in cropping systems. Agronomy 2019, 9, 127. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, P.; Shen, H. Competition intensity affects growing season nutrient dynamics in Korean pine trees and their microhabitat soil in mixed forest. For. Ecol. Manag. 2023, 539, 121018. [Google Scholar] [CrossRef]
- Qi, J.; Liu, Y.; Wang, Z.; Zhao, L.; Zhang, W.; Wang, Y.; Li, X. Variations in microbial functional potential associated with phosphorus and sulfur cycling in biological soil crusts of different ages at the Tengger Desert, China. Appl. Soil Ecol. 2021, 165, 104022. [Google Scholar] [CrossRef]
- Atiwesh, G.; Parrish, C.C.; Banoub, J.; Le, T.T. Lignin degradation by microorganisms: A review. Biotechnol. Prog. 2022, 38, e3226. [Google Scholar] [CrossRef]
- Zhu, W.; Hao, M.; Zhao, W.; Yu, S.; Fan, Z.; Liu, Y.; Dun, X.; Zhang, Z.; Gao, P. Changes of microbial life history strategies to soil nutrient limitations following vegetation restoration and its impact on carbon utilization efficiency. J. Environ. Manag. 2025, 392, 126684. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, X.; Tsolmon, B.; Chen, J.; Wei, W.; Lei, S.; Yang, J.; Bao, Y. The influence of transplanted trees on soil microbial diversity in coal mine subsidence areas in the Loess Plateau of China. Glob. Ecol. Conserv. 2020, 21, e877. [Google Scholar] [CrossRef]
- Rodriguez, D.R.A.; Scheu, S.; Rillig, M.C. Soil microbial responses to multiple global change factors as assessed by metagenomics. Nat. Commun. 2025, 16, 5058. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.W.; Chadwick, D.R.; Bending, G.D.; Collins, C.D.; Whelton, H.L.; Daulton, E.; Covington, J.A.; Bull, I.D.; Jones, D.L. Nutrient (C, N and P) enrichment induces significant changes in the soil metabolite profile and microbial carbon partitioning. Soil Biol. Biochem. 2022, 172, 108779. [Google Scholar] [CrossRef]
- Heděnec, P.; Nilsson, L.O.; Zheng, H.; Gundersen, P.; Schmidt, I.K.; Rousk, J.; Vesterdal, L. Mycorrhizal association of common European tree species shapes biomass and metabolic activity of bacterial and fungal communities in soil. Soil Biol. Biochem. 2020, 149, 107933. [Google Scholar] [CrossRef]
- Camenzind, T.; Hättenschwiler, S.; Treseder, K.K.; Lehmann, A.; Rillig, M.C. Nutrient limitation of soil microbial processes in tropical forests. Ecol. Monogr. 2018, 88, 4–21. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, L.; Qin, S.; Kou, D.; Wang, S.; Zheng, Z.; Penuelas, J.; Yang, Y. Microbial nitrogen and phosphorus co-limitation across permafrost region. Glob. Change Biol. 2023, 29, 3910–3923. [Google Scholar] [CrossRef]
- Liu, G.; Wang, H.; Yan, G.; Wang, M.; Jiang, S.; Wang, X.; Xue, J.; Xu, M.; Xing, Y.; Wang, Q. Soil enzyme activities and microbial nutrient limitation during the secondary succession of boreal forests. Catena 2023, 230, 107268. [Google Scholar] [CrossRef]
- Guo, Y.X.; Yu, G.H.; Hu, S.; Liang, C.; Kappler, A.; Jorgenson, M.T.; Guo, L.; Guggenberger, G. Deciphering the intricate control of minerals on deep soil carbon stability and persistence in Alaskan permafrost. Glob. Change Biol. 2024, 30, e17552. [Google Scholar] [CrossRef]
- Theocharis, A.; Clement, C.; Barka, E.A. Physiological and molecular changes in plants grown at low temperatures. Planta 2012, 235, 1091–1105. [Google Scholar] [CrossRef]
- Ernakovich, J.G.; Wallenstein, M.D. Permafrost microbial community traits and functional diversity indicate low activity at in situ thaw temperatures. Soil Biol. Biochem. 2015, 87, 78–89. [Google Scholar] [CrossRef]
- Maillard, F.; Klinghammer, F.; Jassey, V.E.J.; Zhang, B.; Kennedy, P.G.; Lara, E.; Geisen, S.; Tranvik, L.; Hammer, E.; Tunlid, A. Hidden decomposers: Revisiting saprotrophy among soil protists and its potential impact on carbon cycling. Soil Biol. Biochem. 2025, 205, 109786. [Google Scholar] [CrossRef]
- Maillard, F.; Michaud, T.J.; See, C.R.; DeLancey, L.C.; Blazewicz, S.J.; Kimbrel, J.A.; Pett-Ridge, J.; Kennedy, P.G. Melanization slows the rapid movement of fungal necromass carbon and nitrogen into both bacterial and fungal decomposer communities and soils. Msystems 2023, 8, e39023. [Google Scholar] [CrossRef]
- Buckeridge, K.M.; Creamer, C.; Whitaker, J. Deconstructing the microbial necromass continuum to inform soil carbon sequestration. Funct. Ecol. 2022, 36, 1396–1410. [Google Scholar] [CrossRef]
- Angst, G.; Angst, Š.; Frouz, J.; Jabinski, S.; Jílková, V.; Kukla, J.; Li, M.; Meador, T.B.; Angel, R. Stabilized microbial necromass in soil is more strongly coupled with microbial diversity than the bioavailability of plant inputs. Soil Biol. Biochem. 2024, 190, 109323. [Google Scholar] [CrossRef]
- Yang, L.; Lyu, M.; Li, X.; Xiong, X.; Lin, W.; Yang, Y.; Xie, J. Decline in the contribution of microbial residues to soil organic carbon along a subtropical elevation gradient. Sci. Total Environ. 2020, 749, 141583. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Pang, X.; Yin, C. Soil fungal community composition drives forest-specific and seasonal dynamics of microbial carbon use efficiency in subalpine ecosystems. J. Plant Ecol. 2025. accepted. [Google Scholar] [CrossRef]
- Soong, J.L.; Fuchslueger, L.; Maranon-Jimenez, S.; Torn, M.S.; Janssens, I.A.; Penuelas, J.; Richter, A. Microbial carbon limitation: The need for integrating microorganisms into our understanding of ecosystem carbon cycling. Glob. Change Biol. 2020, 26, 1953–1961. [Google Scholar] [CrossRef]
- Hu, J.; Du, M.; Chen, J.; Tie, L.; Zhou, S.; Buckeridge, K.M.; Cornelissen, J.; Huang, C.; Kuzyakov, Y. Microbial necromass under global change and implications for soil organic matter. Glob. Change Biol. 2023, 29, 3503–3515. [Google Scholar] [CrossRef]
- Malik, A.A.; Puissant, J.; Buckeridge, K.M.; Goodall, T.; Jehmlich, N.; Chowdhury, S.; Gweon, H.S.; Peyton, J.M.; Mason, K.E.; van Agtmaal, M.; et al. Land use driven change in soil pH affects microbial carbon cycling processes. Nat. Commun. 2018, 9, 3591. [Google Scholar] [CrossRef]
- Song, J.; Zhang, H.; Gunina, A.; Mganga, K.Z.; Chang, F.; Yu, R.; Zhou, J.; Chen, A.; Li, Y. Subsurface application of organic ameliorant in saline soils increases microbial necromass accumulation in mineral-associated organic matter. Carbon Res. 2025, 4, 39. [Google Scholar] [CrossRef]
- Wang, B.; Dou, Y.; Liang, C.; Liu, C.; Ao, D.; Yao, H.; Yang, E.; An, S.; Wen, Z. Microbial necromass in soil profiles increases less efficiently than root biomass in long-term fenced grassland: Effects of microbial nitrogen limitation and soil depth. Sci. Total Environ. 2024, 956, 177058. [Google Scholar] [CrossRef]


| Factors | Explains (%) | p |
|---|---|---|
| C/N | 76.6 | <0.01 |
| TP | 17.8 | <0.01 |
| pH | 1.0 | >0.05 |
| AP | 0.6 | >0.05 |
| SOC | 0.8 | >0.05 |
| AN | 0.4 | >0.05 |
| DOC | <0.1 | >0.05 |
| TN | <0.1 | >0.05 |
| WC | <0.1 | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Gao, M.; Yang, L.; Cheng, Z.; Liu, S.; Liu, Y. Cold–Temperate Betula platyphylla Sukaczev Forest Can Provide More Soil Nutrients to Increase Microbial Alpha Diversity and Microbial Necromass Carbon. Microorganisms 2025, 13, 2291. https://doi.org/10.3390/microorganisms13102291
Jiang Y, Gao M, Yang L, Cheng Z, Liu S, Liu Y. Cold–Temperate Betula platyphylla Sukaczev Forest Can Provide More Soil Nutrients to Increase Microbial Alpha Diversity and Microbial Necromass Carbon. Microorganisms. 2025; 13(10):2291. https://doi.org/10.3390/microorganisms13102291
Chicago/Turabian StyleJiang, Yunbing, Mingliang Gao, Libin Yang, Zhichao Cheng, Siyuan Liu, and Yongzhi Liu. 2025. "Cold–Temperate Betula platyphylla Sukaczev Forest Can Provide More Soil Nutrients to Increase Microbial Alpha Diversity and Microbial Necromass Carbon" Microorganisms 13, no. 10: 2291. https://doi.org/10.3390/microorganisms13102291
APA StyleJiang, Y., Gao, M., Yang, L., Cheng, Z., Liu, S., & Liu, Y. (2025). Cold–Temperate Betula platyphylla Sukaczev Forest Can Provide More Soil Nutrients to Increase Microbial Alpha Diversity and Microbial Necromass Carbon. Microorganisms, 13(10), 2291. https://doi.org/10.3390/microorganisms13102291




