Detection of Tick-Borne Microorganisms, Anaplasmataceae and Piroplasmida, in Sorex spp. in Hokkaido, Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Wild Shrew
2.2. Collection of Ticks
2.3. DNA Extraction from Ticks and Spleen of Shrews
2.4. Microorganism Detection in Ticks and Spleens
2.5. Phylogenetic Analysis
2.6. Statistical Test
3. Results
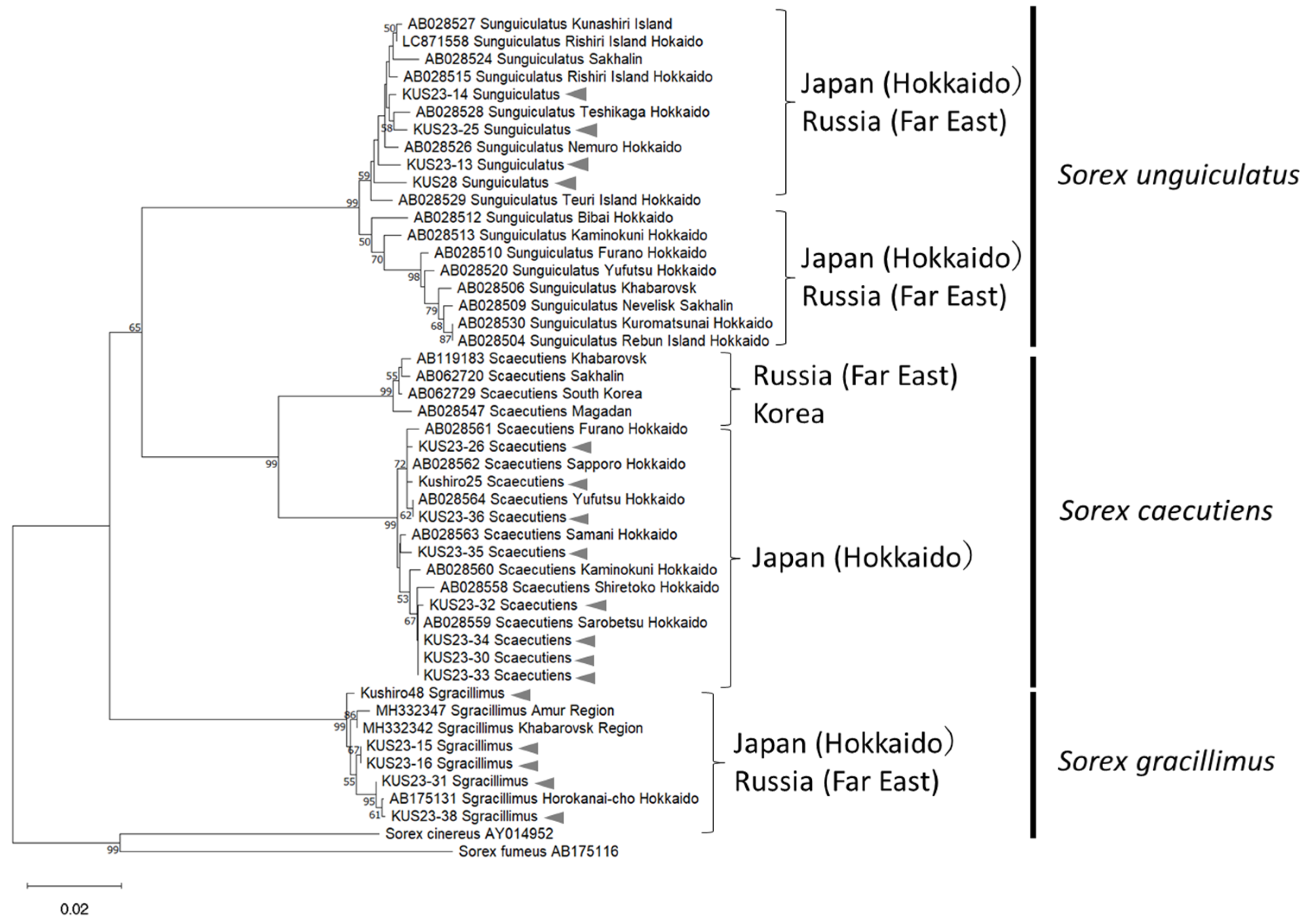
3.1. Collection and Phylogeny of Sorex spp.
3.2. Immature Ixodes ovatus and I. persulcatus Feed on Sorex spp.
3.3. Molecular Detection of Piroplasmida and Anaplasmataceae in Ticks
3.4. Detection of Babesia microti, Neoehrlichia mikurensis, Ehrlichia japonica, and E. muris in Sorex spp. Spleen
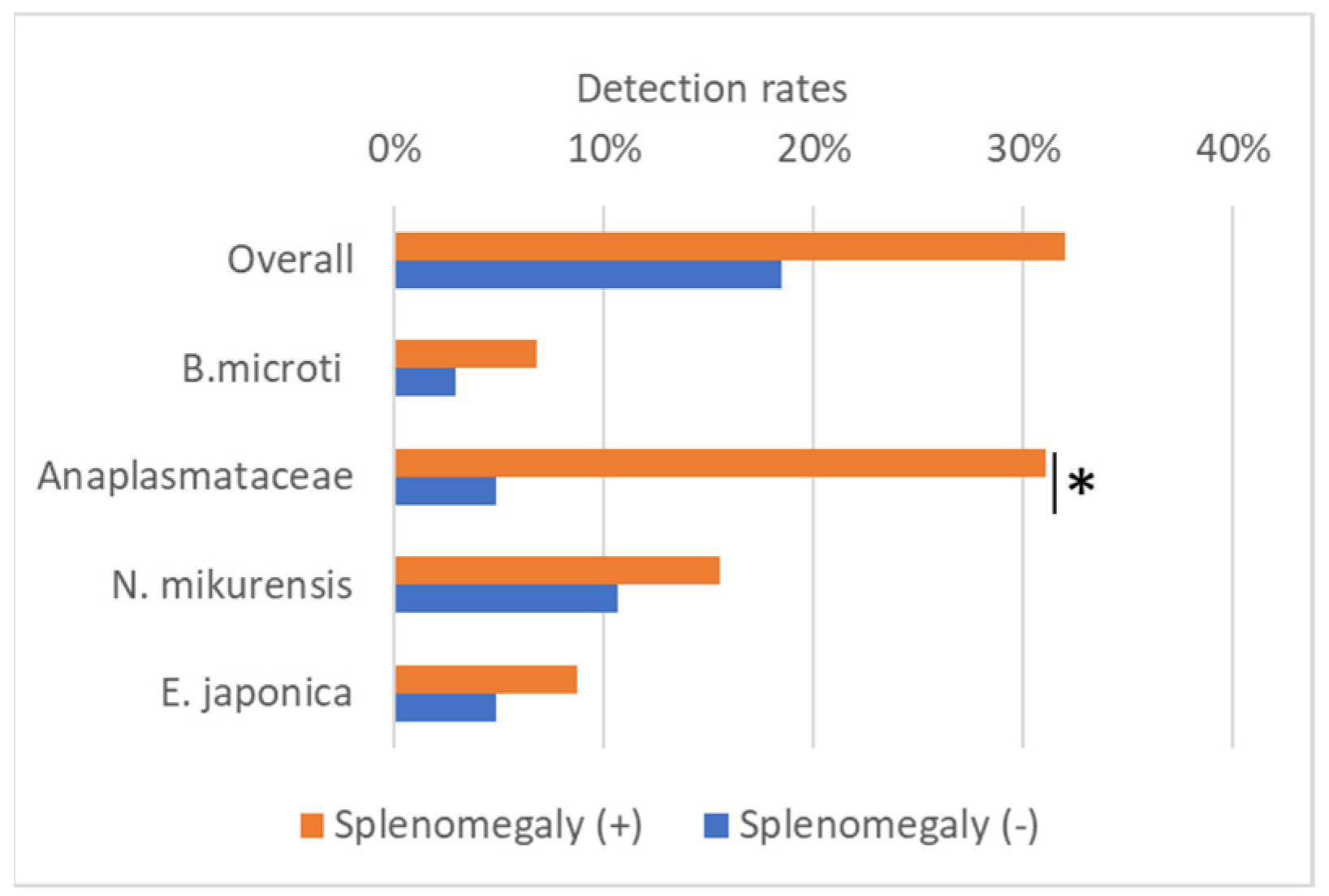
3.5. Detection of Splenomegaly in Shrews
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| PCR | Polymerase chain reaction |
References
- Moraga-Fernández, A.; Muñoz-Hernández, C.; Sánchez-Sánchez, M.; Fernández de Mera, I.G.; de la Fuente, J. Exploring the diversity of tick-borne pathogens: The case of bacteria (Anaplasma, Rickettsia, Coxiella and Borrelia) protozoa (Babesia and Theileria) and viruses (Orthonairovirus, tick-borne encephalitis virus and louping ill virus) in the European continent. Vet. Microbiol. 2023, 286, 109892. [Google Scholar] [CrossRef]
- Xu, A.L.; Xue, H.; Li, Y.; Wang, X.; Zheng, J.X.; Shi, F.Y.; Cui, Q.X.; Lu, Y.; Cun, D.J.; Li, L.H. Comprehensive meta-analysis of severe fever with thrombocytopenia syndrome virus infections in humans, vertebrate hosts and questing ticks. Parasites Vectors 2024, 17, 265. [Google Scholar] [CrossRef]
- Wennerås, C. Infections with the tick-borne bacterium Candidatus Neoehrlichia mikurensis. Clin. Microbiol. Infect. 2015, 21, 621–630. [Google Scholar] [CrossRef]
- Eisen, L. Rodent-targeted approaches to reduce acarological risk of human exposure to pathogen-infected Ixodes ticks. Ticks Tick Borne Dis. 2023, 14, 102119. [Google Scholar] [CrossRef]
- Krawczyk, A.I.; van Duijvendijk, G.L.A.; Swart, A.; Heylen, D.; Jaarsma, R.I.; Jacobs, F.H.H.; Fonville, M.; Sprong, H.; Takken, W. Effect of rodent density on tick and tick-borne pathogen populations: Consequences for infectious disease risk. Parasites Vectors 2020, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Bown, K.J.; Lambin, X.; Telford, G.; Heyder-Bruckner, D.; Ogden, N.H.; Birtles, R.J. The common shrew (Sorex araneus): A neglected host of tick-borne infections? Vector Borne Zoonotic Dis. 2011, 11, 947–953. [Google Scholar] [CrossRef]
- Goethert, H.K.; Mather, T.N.; Johnson, R.W.; Telford, S.R., 3rd. Incrimination of shrews as a reservoir for Powassan virus. Commun. Biol. 2021, 4, 1319. [Google Scholar] [CrossRef]
- Li, H.; Huang, Z.Y.X.; Lan, J.; Hu, L.; Wei, X.; Wang, Y.; Li, X.; Li, Y.; Becker, D.J.; Wei, F.; et al. Diversity and transmission and zoonotic potential of microbes in true insectivores. Nat. Commun. 2025, 16, 6709. [Google Scholar] [CrossRef]
- Yajima, S.; Nakashima, Y.; Kawahara, A.; Ohdachi, S.D. Utilization of above-ground space in captivity by four sympatric shrew species from Hokkaido, Japan. Mammal Study 2024, 49, 137–143. [Google Scholar] [CrossRef]
- Ohdachi, S. Burrowing habits and earthworm preferences of three species of Sorex in Hokkaido. J. Mamm. Soc. Jpn. 1995, 20, 85–88. [Google Scholar]
- Nakao, M.; Miyamoto, K. Long-tailed shrew, Sorex unguiculatus, as a potential reservoir of the spirochetes transmitted by Ixodes ovatus in Hokkaido, Japan. Med. Entomol. Zool. 1993, 44, 237–245. [Google Scholar] [CrossRef]
- Kameyama, Y.; Sato, R.; Asakawa, M.; Ito, T.; Okimoto, K.; Shimoi, G.; Hashizume, R. Parasitic Fauna in Long-Clawed Shrew, Sorex unguiculatus, in Abashiri, Hokkaido. J. Agric. Sci. Tokyo Univ. Agric. 2015, 60, 10–17. [Google Scholar]
- Tsuji, M.; Zamoto, A.; Kawabuchi, T.; Kataoka, T.; Nakajima, R.; Asakawa, M.; Ishihara, C. Babesia microti-like parasites detected in Eurasian red squirrels (Sciurus vulgaris orientis) in Hokkaido, Japan. J. Vet. Med. Sci. 2006, 68, 643–646. [Google Scholar] [CrossRef]
- Zamoto-Niikura, A.; Tsuji, M.; Qiang, W.; Nakao, M.; Hirata, H.; Ishihara, C. Detection of two zoonotic Babesia microti lineages, the Hobetsu and U.S. lineages, in two sympatric tick species, Ixodes ovatus and Ixodes persulcatus, respectively, in Japan. Appl. Environ. Microbiol. 2012, 78, 3424–3430. [Google Scholar] [CrossRef]
- Arai, S.; Tsuji, M.; Kaiho, I.; Murayama, H.; Zamoto, A.; Wei, Q.; Okabe, N.; Kamiyama, T.; Ishihara, C. Retrospective seroepidemiological survey for human babesiosis in an area in Japan where a tick-borne disease is endemic. J. Vet. Med. Sci. 2003, 65, 335–340. [Google Scholar] [CrossRef][Green Version]
- Zamoto, A.; Tsuji, M.; Wei, Q.; Cho, S.H.; Shin, E.H.; Kim, T.S.; Leonova, G.N.; Hagiwara, K.; Asakawa, M.; Kariwa, H.; et al. Epizootiologic survey for Babesia microti among small wild mammals in northeastern Eurasia and a geographic diversity in the beta-tubulin gene sequences. J. Vet. Med. Sci. 2004, 66, 785–792. [Google Scholar] [CrossRef]
- Inayoshi, M.; Naitou, H.; Kawamori, F.; Masuzawa, T.; Ohashi, N. Characterization of Ehrlichia species from Ixodes ovatus ticks at the foot of Mt. Fuji, Japan. Microbiol. Immunol. 2004, 48, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, J.F.; Liu, W.; Zheng, Y.C.; Huo, Q.B.; Tang, K.; Zuo, S.Y.; Liu, K.; Jiang, B.G.; Yang, H.; et al. Human infection with Candidatus Neoehrlichia mikurensis, China. Emerg. Infect. Dis. 2012, 18, 1636–1639. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Kawahara, M.; Rikihisa, Y.; Fujita, H.; Watanabe, Y.; Suto, C.; Ito, T. New Ehrlichia species closely related to Ehrlichia chaffeensis isolated from Ixodes ovatus ticks in Japan. J. Clin. Microbiol. 2000, 38, 1331–1338. [Google Scholar] [CrossRef]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Rocha, S.C.; Moustafa, M.A.M.; Velásquez, C.V.; Azuama, O.C.; Zafar, K.; Meyer, C.; Araujo, M.; Taylor, K.; Parveen, N. Long-term survival of Babesia microti and Borrelia burgdorferi in C3H/HeJ mice and their effect on Lyme arthritis and babesiosis manifestations. Microbiol. Spectr. 2025, 13, e0025225. [Google Scholar] [CrossRef] [PubMed]
- McGill, J.L.; Wang, Y.; Ganta, C.K.; Boorgula, G.D.Y.; Ganta, R.R. Antigen-specific CD4+CD8+ double-positive T cells are increased in the blood and spleen during Ehrlichia chaffeensis infection in the canine host. Front. Immunol. 2018, 9, 1585. [Google Scholar] [CrossRef] [PubMed]
- Gynthersen, R.M.M.; Stensvold, C.R.; Nielsen, S.L.; Møller, H.J.; Nielsen, H.V.; Lebech, A.M.; Christensen, J.R.; Mens, H.; El Fassi, D. Neoehrlichia mikurensis-An emerging opportunistic tick-borne infection in immunosuppressed patients. J. Intern. Med. 2023, 293, 782–790. [Google Scholar] [CrossRef]
- Abe, H.; Ishii, N.; Kaneko, Y.; Maeda, K.; Miura, S.; Yoneda, M. A Pictorial Guide to the Mammals of Japan; Tokai University Press: Tokyo, Japan, 1994. (In Japanese) [Google Scholar]
- Ohdachi, S.; Kawahara, A. The first record of Laxmann’s shrew (Sorex caecutiens) with completely white pelage, captured in Hokkaido, Japan. Mamm. Sci. 2018, 58, 63–66. [Google Scholar]
- Zamoto-Niikura, A.; Saigo, A.; Sato, M.; Kobayashi, H.; Sasaki, M.; Nakao, M.; Suzuki, T.; Morikawa, S. The presence of Ixodes pavlovskyi and I. pavlovskyi-borne microorganisms in Rishiri Island: An ecological survey. mSphere 2023, 8, e0021323. [Google Scholar] [CrossRef]
- Zamoto-Niikura, A.; Morikawa, S.; Hanaki, K.I.; Holman, P.J.; Ishihara, C. Ixodes persulcatus ticks as vectors for the Babesia microti U.S. lineage in Japan. Appl. Environ. Microbiol. 2016, 82, 6624–6632. [Google Scholar] [CrossRef]
- Zamoto, A.; Tsuji, M.; Kawabuchi, T.; Wei, Q.; Asakawa, M.; Ishihara, C. U.S.-type Babesia microti isolated from small wild mammals in Eastern Hokkaido, Japan. J. Vet. Med. Sci. 2004, 66, 919–926. [Google Scholar] [CrossRef]
- Tabara, K.; Arai, S.; Kawabuchi, T.; Itagaki, A.; Ishihara, C.; Satoh, H.; Okabe, N.; Tsuji, M. A molecular survey of Babesia microti, Ehrlichia species, and Candidatus Neoehrlichia mikurensis in wild rodents from Shimane Prefecture, Japan. Microbiol. Immunol. 2007, 51, 359–367. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Ohdachi, S.; Dokuchaev, N.E.; Hasegawa, M.; Masuda, R. Intraspecific phylogeny and geographical variation of six species of northeastern Asiatic Sorex shrews based on the mitochondrial cytochrome b sequences. Mol. Ecol. 2001, 10, 2199–2213. [Google Scholar] [CrossRef]
- Taira, M.; Ando, S.; Kawabata, H.; Fujita, H.; Kadosaka, T.; Sato, H.; Monma, N.; Ohashi, N.; Saijo, M. Isolation and molecular detection of Ehrlichia species from ticks in western, central, and eastern Japan. Ticks Tick Borne Dis. 2019, 10, 344–351. [Google Scholar] [CrossRef]
- Inokuma, H.; Ohashi, M.; Jilintai Tanabe, S.; Miyahara, K. Prevalence of tick-borne Rickettsia and Ehrlichia in Ixodes persulcatus and Ixodes ovatus in Tokachi district, Eastern Hokkaido, Japan. J. Vet. Med. Sci. 2007, 69, 661–664. [Google Scholar] [CrossRef][Green Version]
- Goethert, H.K.; Lubelcyzk, C.; LaCombe, E.; Holman, M.; Rand, P.; Smith, R.P., Jr.; Telford, S.R., 3rd. Enzootic Babesia microti in Maine. J. Parasitol. 2003, 89, 1069–1071. [Google Scholar] [CrossRef]
- Goethert, H.K.; Cook, J.A.; Lance, E.W.; Telford, S.R. Fay and Rausch 1969 revisited: Babesia microti in Alaskan small mammals. J. Parasitol. 2006, 92, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, M.A.M.; Taylor, K.; Nakao, R.; Shimozuru, M.; Sashika, M.; Rosà, R.; Thu, M.J.; Rizzoli, A.; Tsubota, T. Dynamics, co-infections and characteristics of zoonotic tick-borne pathogens in Hokkaido small mammals, Japan. Ticks Tick Borne Dis. 2016, 7, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Rikihisa, Y.; Isogai, E.; Takahashi, M.; Misumi, H.; Suto, C.; Shibata, S.; Zhang, C.; Tsuji, M. Ultrastructure and phylogenetic analysis of ‘Candidatus Neoehrlichia mikurensis’ in the family Anaplasmataceae, isolated from wild rats and found in Ixodes ovatus ticks. Int. J. Syst. Evol. Microbiol. 2004, 54, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- McQuiston, J.H.; McCall, C.L.; Nicholson, W.L. Ehrlichiosis and related infections. J. Am. Vet. Med. Assoc. 2003, 223, 1750–1756. [Google Scholar] [CrossRef]
- Jackson, J.A.; Hall, A.J.; Friberg, I.M.; Ralli, C.; Lowe, A.; Zawadzka, M.; Turner, A.K.; Stewart, A.; Birtles, R.J.; Paterson, S.; et al. An immunological marker of tolerance to infection in wild rodents. PLoS Biol. 2014, 12, e1001901. [Google Scholar] [CrossRef]
- Taylor, K.R.; Takano, A.; Konnai, S.; Shimozuru, M.; Kawabata, H.; Tsubota, T. Differential tick burdens may explain differential Borrelia afzelii and Borrelia garinii infection rates among four, wild, rodent species in Hokkaido, Japan. J. Vet. Med. Sci. 2013, 75, 785–790. [Google Scholar] [CrossRef]

| Sorex spp. | Ixodes ovatus | I. persulcautus | |||||
|---|---|---|---|---|---|---|---|
| Larva | Nymph | Larva | Nymph | Total | Tick/Shrew | ||
| 2023 | S. unguiculatus (n = 10) | 11 | 8 | 11 | 18 | 48 | 4.8 |
| S. gracillimus (n = 1) | 0 | 1 | 0 | 0 | 1 | 1 | |
| S. caecutiens (n = 1) | 3 | 5 | 3 | 4 | 15 | 15 | |
| 2020 | S. unguiculatus (n = 10) | 83 | 8 | 41 | 0 | 132 | 13.2 |
| S. gracillimus (n = 5) | 8 | 2 | 6 | 0 | 16 | 3.2 | |
| Sorex spp. 1 | Resulted Nymph | |||
|---|---|---|---|---|
| I. ovatus | I. persulcatus | |||
| No. Examined | Microorganism Detected | No. Examined | Microorganism Detected | |
| S. unguiculatus | 1 | - 2 | 0 | - |
| S. unguiculatus | 7 | - | 5 | Babesia divergens Asia lineage |
| S. unguiculatus | 1 | - | 0 | - |
| S. unguiculatus | 0 | - | 1 | - |
| S. caecutiens | 2 | Neoehrlichia mikurensis | 3 | - |
| S. caecutiens | 1 | - | 0 | - |
| Sorex spp. | No. Examined | B. microti | N. mikurensis | E. japonica | E. muris | ||||
|---|---|---|---|---|---|---|---|---|---|
| S. unguiculatus | 53 | 3 1 | (5.7%) | 16 1,2 | (30.2%) | 11 2 | (20.8%) | 0 | (0%) |
| S. gracillimus | 34 | 3 | (8.8%) | 4 3 | (11.8%) | 3 3 | (8.8%) | 1 | (2.9%) |
| S. caecutiens | 16 | 4 4 | (25.0%) | 7 4 | (43.8%) | 0 | (0%) | 0 | (0%) |
| Microorganisms Co-Infected | S. unguiculatus | S. gracillimus | S. caecutiens | Total |
|---|---|---|---|---|
| Neoehrlichia mikurensis and Ehrlichia japonica | 3 | 1 | 0 | 4 |
| N. mikurensis and Babesia microti | 1 | 0 | 2 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamoto-Niikura, A.; Terui, S.; Sasaki, M.; Nakao, M.; Taira, M.; Hanaki, K.-I. Detection of Tick-Borne Microorganisms, Anaplasmataceae and Piroplasmida, in Sorex spp. in Hokkaido, Japan. Microorganisms 2025, 13, 2288. https://doi.org/10.3390/microorganisms13102288
Zamoto-Niikura A, Terui S, Sasaki M, Nakao M, Taira M, Hanaki K-I. Detection of Tick-Borne Microorganisms, Anaplasmataceae and Piroplasmida, in Sorex spp. in Hokkaido, Japan. Microorganisms. 2025; 13(10):2288. https://doi.org/10.3390/microorganisms13102288
Chicago/Turabian StyleZamoto-Niikura, Aya, Shigeharu Terui, Mizuki Sasaki, Minoru Nakao, Masakatsu Taira, and Ken-Ichi Hanaki. 2025. "Detection of Tick-Borne Microorganisms, Anaplasmataceae and Piroplasmida, in Sorex spp. in Hokkaido, Japan" Microorganisms 13, no. 10: 2288. https://doi.org/10.3390/microorganisms13102288
APA StyleZamoto-Niikura, A., Terui, S., Sasaki, M., Nakao, M., Taira, M., & Hanaki, K.-I. (2025). Detection of Tick-Borne Microorganisms, Anaplasmataceae and Piroplasmida, in Sorex spp. in Hokkaido, Japan. Microorganisms, 13(10), 2288. https://doi.org/10.3390/microorganisms13102288






