LncRNA MEG3 Regulates Glaesserella parasuis-Induced Apoptosis of Porcine Alveolar Macrophages via Regulating ssc-miR-135/CASP8 Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Cell Line
2.2. Cell Transfection and Bacterial Infection
2.3. Cell Viability Assay
2.4. Annexin V-FITC/PI Staining Assay
2.5. Bioinformatics Analysis
2.6. Dual-Luciferase Reporter Assay
2.7. RNA Extraction and Quantitative Real-Time PCR (qPCR) Detection
2.8. Western Blot
2.9. Statistical Analysis
3. Results
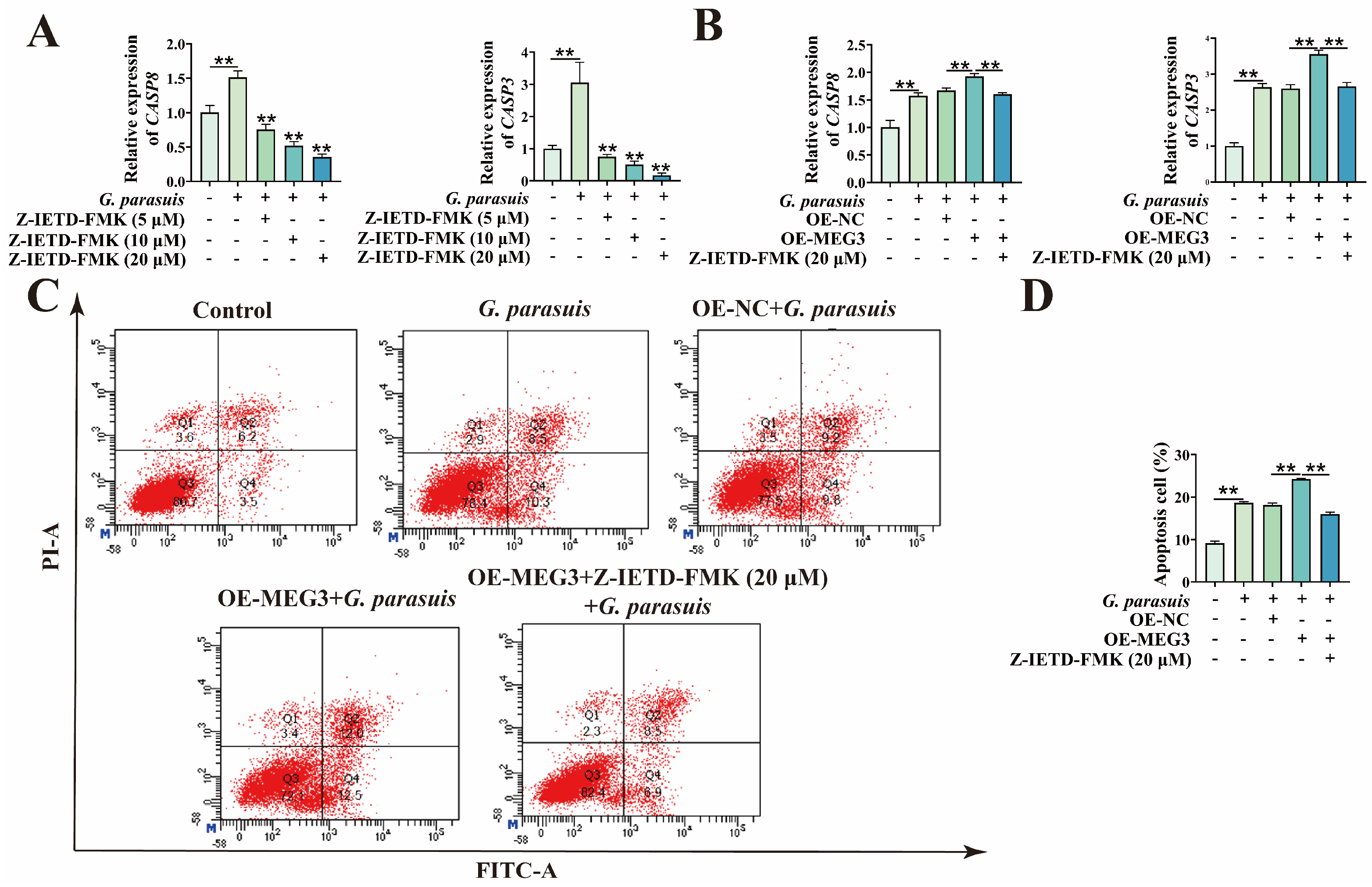
3.1. Overexpression of MEG3 Promotes G. parasuis-Induced Apoptosis of 3D4/21 Cells
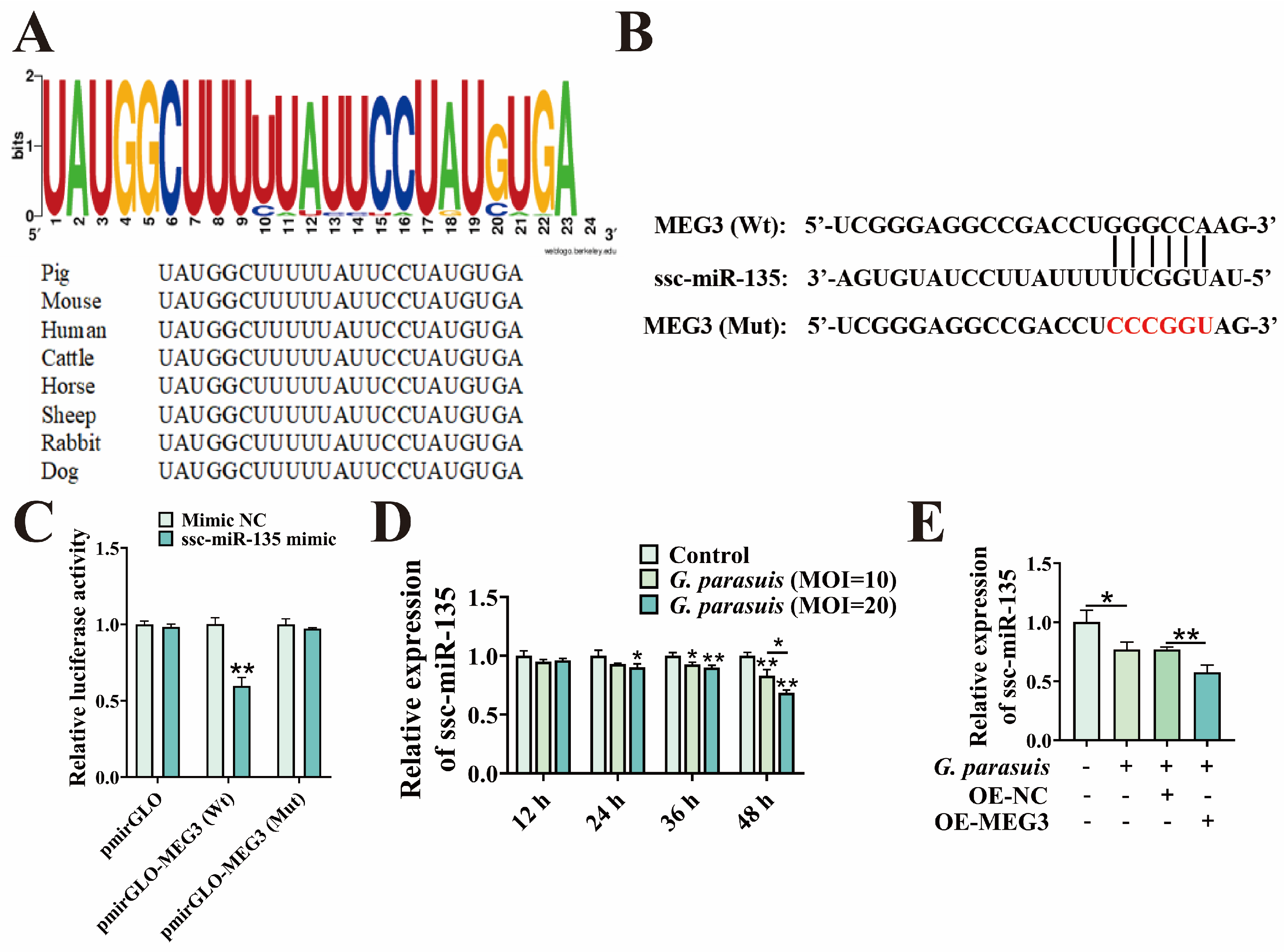
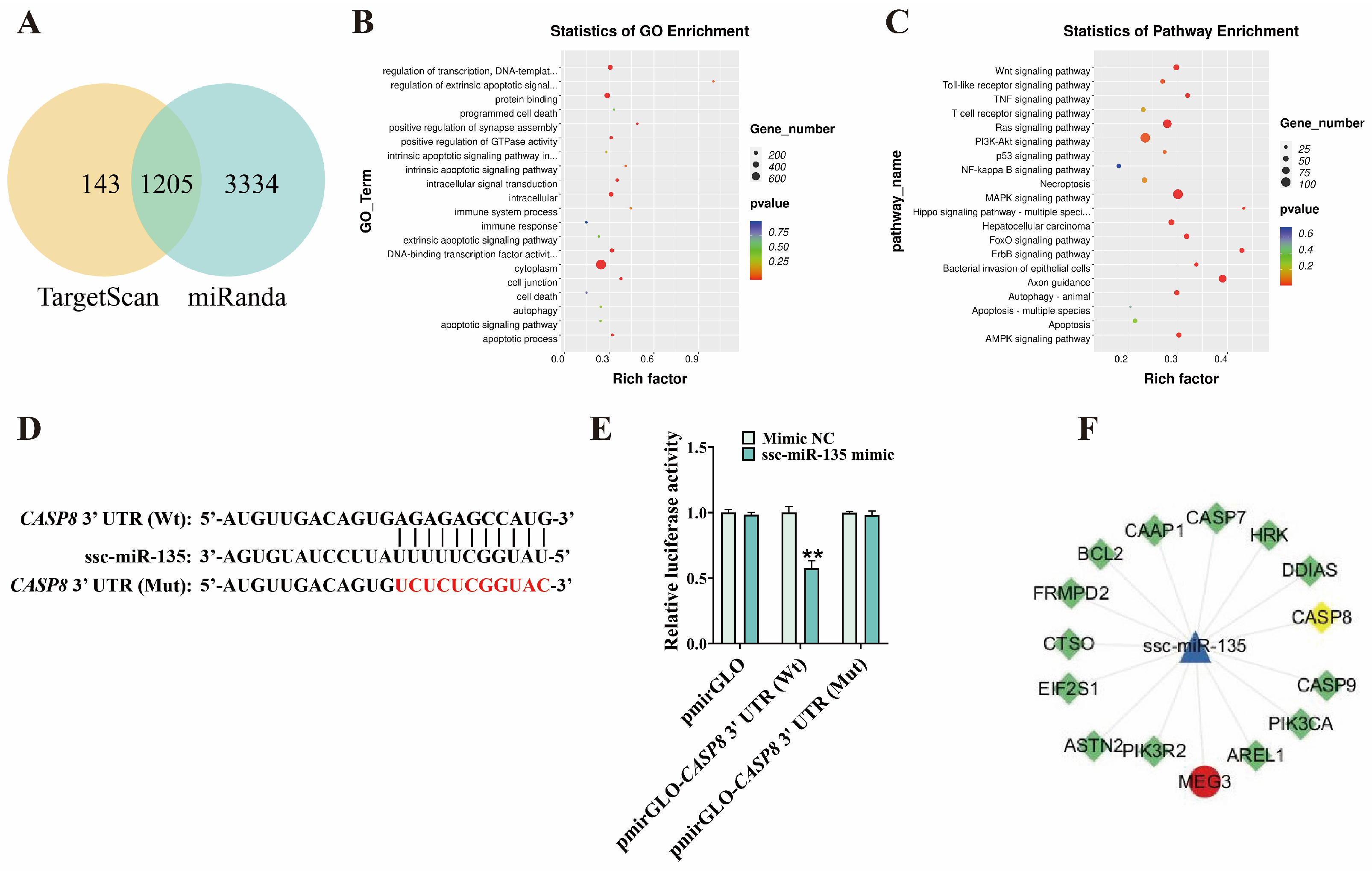
3.2. MEG3 as a Molecular Sponge for ssc-miR-135
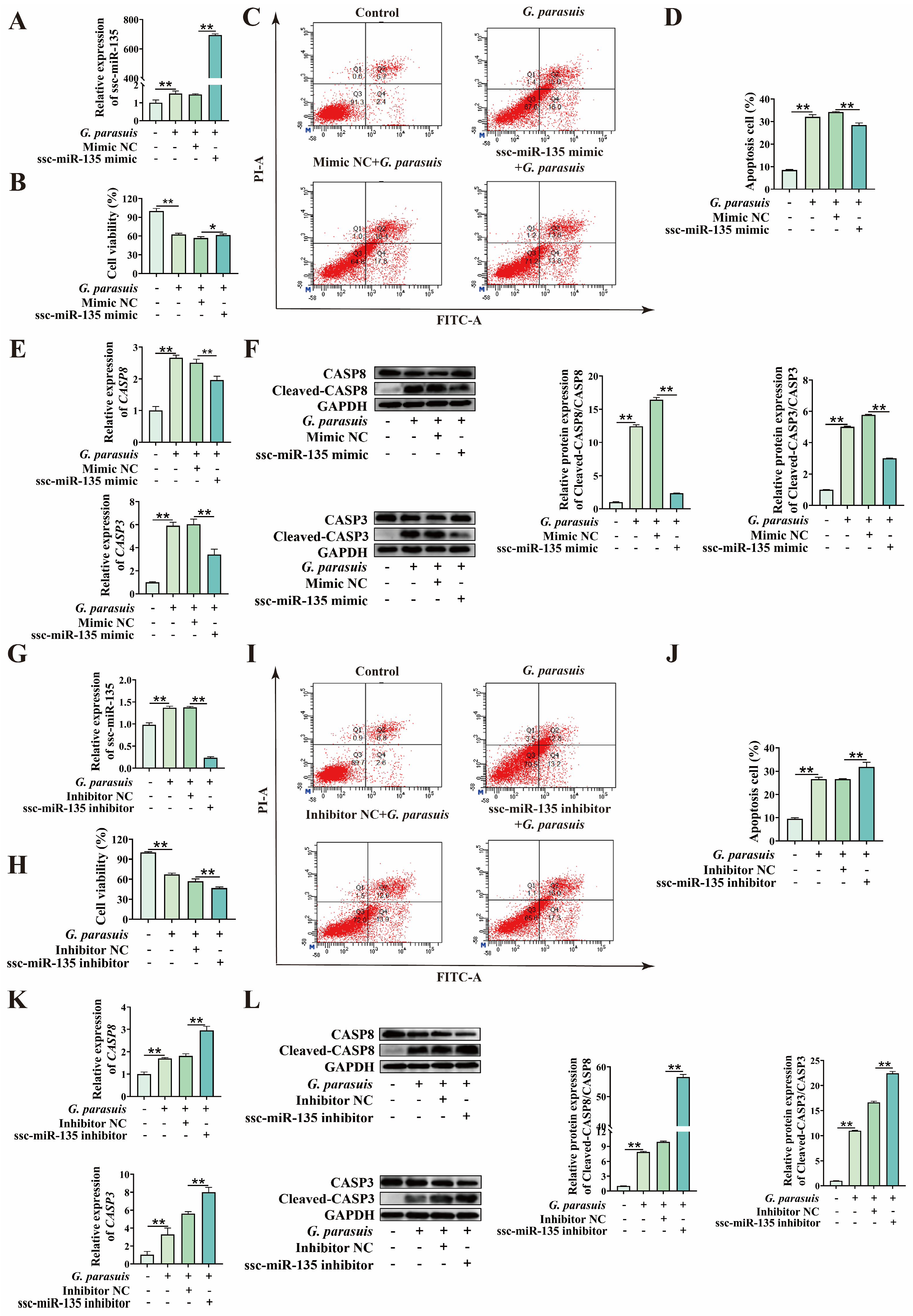
3.3. ssc-miR-135 Regulates G. parasuis-Induced Apoptosis in 3D4/21 Cells
3.4. ssc-miR-135 Directly Interacts with CASP8
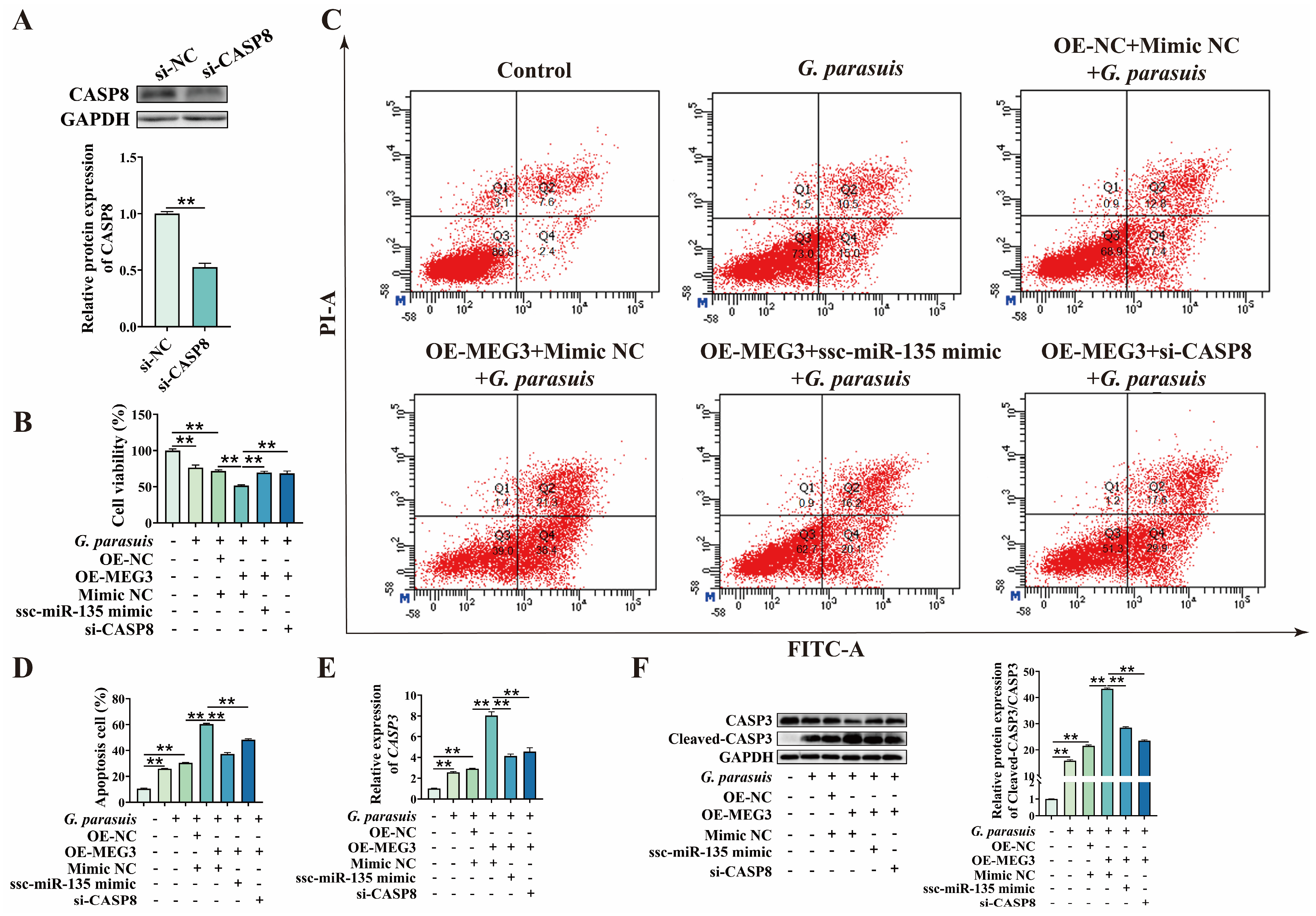
3.5. MEG3 Regulates G. parasuis-Induced Apoptosis in 3D4/21 Cells via ssc-miR-135/CASP8 Axis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCK-8 | Cell counting kit-8 |
| CDT | Cytolethal distending toxin |
| ceRNA | Competitive endogenous RNA |
| circRNA | circular RNA |
| ER | Endoplasmic reticulum |
| G. parasuis | Glaesserella parasuis |
| LncRNA | Long non-coding RNA |
| MEG3 | Maternally expressed gene 3 |
| miRNA | microRNA |
| MOI | Multiplicity of infection |
| NAD | Nicotinamide adenine dinucleotide |
| nt | Nucleotide |
| qPCR | Quantitative Real-Time PCR |
| SD | Standard deviation |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| snoRNA | small nucleolar RNA |
| TRAIL | TNF-related apoptosis-inducing ligand |
| TSA | Tryptic soy agar |
| TSB | Tryptic soy broth |
| UTR | Untranslated region |
References
- Xu, J.; Jin, X.; Li, X.; Yang, D. Epidemiology and pathogenicity of Haemophilus parasuis in eastern China. Front. Microbiol. 2025, 16, 1589975. [Google Scholar] [CrossRef]
- Guo, Z.; Zhou, Y.; Li, N.; Shen, A.; Jia, Y.; Yin, R.; Yang, J.; Yuan, J.; Yin, R. The Molecular Mechanism by Which miR-129a-3p Targets the TLR4/NF-κB Signaling Pathway to Regulate Inflammatory Damage in 3D4/21 Cells Infected with Glaesserella parasuis. Animals 2025, 15, 1355. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.W.; Sarlo Davila, K.M.; Brockmeier, S.L.; Hau, S.J. Transcriptional profile of Glaesserella parasuis in swine serosal and joint fluids. Front. Vet. Sci. 2025, 12, 1452973. [Google Scholar] [CrossRef]
- Dai, L.; Wan, J.; Zhang, R.; Xie, T.; Jia, Y.; Lu, Z.; Zhang, F.; Ke, W.; Liu, F.; Lei, L. Multi-epitope vaccines Xlc and Ddc against Glaesserella parasuis infection in mice. Vet. Microbiol. 2025, 304, 110491. [Google Scholar] [CrossRef]
- Fu, S.; Liu, H.; Xu, L.; Qiu, Y.; Liu, Y.; Wu, Z.; Ye, C.; Hou, Y.; Hu, C.A. Baicalin modulates NF-κB and NLRP3 inflammasome signaling in porcine aortic vascular endothelial cells Infected by Haemophilus parasuis Causing Glässer’s disease. Sci. Rep. 2018, 8, 807. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Jia, Y.C.; Zhang, X.L.; Zhou, Y.Y.; Guo, Y.; Yin, R.L.; Yuan, J.; Wang, L.X.; Guo, Z.B.; Wang, J.Y.; et al. Virulence assessment of four Glaesserella parasuis strains isolated in Liaoning province of China. Res. Vet. Sci. 2023, 158, 226–234. [Google Scholar] [CrossRef]
- Li, G.; Niu, H.; Zhang, Y.; Li, Y.; Xie, F.; Langford, P.R.; Liu, S.; Wang, C. Haemophilus parasuis cytolethal distending toxin induces cell cycle arrest and p53-dependent apoptosis. PLoS ONE 2017, 12, e0177199. [Google Scholar] [CrossRef]
- Feng, W.; Han, Y.; Zhang, W.; Wu, L.; Zhou, A.; Shi, L.; Zhang, J. Glaesserella parasuis infection triggers endoplasmic reticulum stress-mediated pyroptosis via PERK/eIF2α/ATF4 axis and metabolic reprogramming in porcine alveolar macrophages. Vet. Res. 2025, 56, 150. [Google Scholar] [CrossRef]
- Meng, J.; Ding, T.; Chen, Y.; Long, T.; Xu, Q.; Lian, W.; Liu, W. LncRNA-Meg3 promotes Nlrp3-mediated microglial inflammation by targeting miR-7a-5p. Int. Immunopharmacol. 2021, 90, 107141. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Cai, L.; Wang, C.; Deng, X.; Yi, S.; Lei, Z.; Xiao, Q.; Xu, H.; Luo, H.; Sun, J. CASC2/miR-24/miR-221 modulates the TRAIL resistance of hepatocellular carcinoma cell through caspase-8/caspase-3. Cell Death Dis. 2018, 9, 318. [Google Scholar] [CrossRef]
- Mu, Q.; Wang, X.; Huang, K.; Xia, B.; Bi, S.; Kong, Y. THUMPD3-AS1 inhibits ovarian cancer cell apoptosis through the miR-320d/ARF1 axis. FASEB J. 2024, 38, e23772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shi, S.; Li, J.; Costa, M. Long Non-Coding RNA MEG3 in Metal Carcinogenesis. Toxics 2023, 11, 157. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.; Peng, F.; Cao, X.; Xie, X.; Peng, C. The multifaceted biology of lncR-Meg3 in cardio-cerebrovascular diseases. Front. Genet. 2023, 14, 1132884. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Zhang, Q.; Liu, H.; Xu, J. The Mechanism Underlying the Regulation of Long Non-coding RNA MEG3 in Cerebral Ischemic Stroke. Cell Mol. Neurobiol. 2023, 43, 69–78. [Google Scholar] [CrossRef]
- Li, Z.; Gao, J.; Sun, D.; Jiao, Q.; Ma, J.; Cui, W.; Lou, Y.; Xu, F.; Li, S.; Li, H. LncRNA MEG3: Potential stock for precision treatment of cardiovascular diseases. Front. Pharmacol. 2022, 13, 1045501. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, X.; Zhu, C.; Wang, K. A review of current evidence about lncRNA MEG3: A tumor suppressor in multiple cancers. Front. Cell Dev. Biol. 2022, 10, 997633. [Google Scholar] [CrossRef]
- Jiang, X. Long noncoding RNA MEG3: An active player in fibrosis. Pharmacol. Rep. 2025, 77, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Puri, B.; Majumder, S.; Gaikwad, A.B. The multifaceted role of lncRNA MEG3 in kidney disease: A focus on mechanisms, therapeutic and diagnostic potential. Drug Discov. Today 2025, 30, 104437. [Google Scholar] [CrossRef]
- Jia, H.Y.; Zhang, K.; Lu, W.J.; Xu, G.W.; Zhang, J.F.; Tang, Z.L. LncRNA MEG3 influences the proliferation and apoptosis of psoriasis epidermal cells by targeting miR-21/caspase-8. BMC Mol. Cell Biol. 2019, 20, 46. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Yang, Z. Silence of MEG3 intensifies lipopolysaccharide-stimulated damage of human lung cells through modulating miR-4262. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2369–2378. [Google Scholar] [CrossRef]
- Yin, R.H.; Guo, Z.B.; Zhou, Y.Y.; Wang, C.; Yin, R.L.; Bai, W.L. LncRNA-MEG3 Regulates the Inflammatory Responses and Apoptosis in Porcine Alveolar Macrophages Infected with Haemophilus parasuis Through Modulating the miR-210/TLR4 Axis. Curr. Microbiol. 2021, 78, 3152–3164. [Google Scholar] [CrossRef]
- Liu, M.; Li, B.; Peng, W.; Ma, Y.; Huang, Y.; Lan, X.; Lei, C.; Qi, X.; Liu, G.E.; Chen, H. LncRNA-MEG3 promotes bovine myoblast differentiation by sponging miR-135. J. Cell. Physiol. 2019, 234, 18361–18370. [Google Scholar] [CrossRef]
- Gong, X.; Cui, Q.; Zhang, W.; Shi, Y.; Zhang, P.; Zhang, C.; Hu, G.; Sahin, O.; Wang, L.; Shen, Z.; et al. Genomic insight into the diversity of Glaesserella parasuis isolates from 19 countries. mSphere 2024, 9, e0023124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Yuan, J.; Jia, Y.C.; Guo, Y.; Yin, R.L.; Guo, Z.B.; Wang, J.Y.; Wang, C.; Yin, R.H. Transcriptomics analysis reveals key lncRNAs and genes related to the infection of porcine lung macrophages by Glaesserella parasuis. Microb. Pathog. 2022, 169, 105617. [Google Scholar] [CrossRef]
- Cheng, Y.; Liang, Y.; Tan, X.; Liu, L. Host long noncoding RNAs in bacterial infections. Front. Immunol. 2024, 15, 1419782. [Google Scholar] [CrossRef] [PubMed]
- Aznaourova, M.; Schmerer, N.; Schmeck, B.; Schulte, L.N. Disease-Causing Mutations and Rearrangements in Long Non-coding RNA Gene Loci. Front. Genet. 2020, 11, 527484. [Google Scholar] [CrossRef]
- Wang, B.; Liu, X.; Li, C.; Yang, N. LncRNA (BCO1-AS) regulate inflammatory responses in bacterial infection through caspase-1 in turbot (Scophthalmus maximus). Int. J. Biol. Macromol. 2024, 279, 135131. [Google Scholar] [CrossRef]
- Chu, S.; Zhao, T.; Li, M.; Sun, Y.; Yang, Y.; Yang, Z. Long non-coding RNA (CMR) involved in autoprotection in S. aureus mastitis in dairy cows by regulating miR-877/FOXM1. Ecotoxicol. Environ. Saf. 2024, 278, 116456. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Huang, Z.; Jing, H.; Yin, B.; Guo, S.; Deng, G.; Guo, M. Exosomal lnc-AFTR as a novel translation regulator of FAS ameliorates Staphylococcus aureus-induced mastitis. Biofactors 2022, 48, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Wang, Z.; Wen, S.; Lin, Y.; Gu, J.; Sun, J.; Wang, H.; Cao, Q.; Xu, Y.; Xu, X.; et al. LRRC8A promotes Glaesserella parasuis cytolethal distending toxin-induced p53-dependent apoptosis in NPTr cells. Virulence 2023, 14, 2287339. [Google Scholar] [CrossRef]
- Zhang, B.; He, Y.; Xu, C.; Xu, L.; Feng, S.; Liao, M.; Ren, T. Cytolethal distending toxin (CDT) of the Haemophilus parasuis SC096 strain contributes to serum resistance and adherence to and invasion of PK-15 and PUVEC cells. Vet. Microbiol. 2012, 157, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yan, P.; Jia, Y.; Guo, Z.; Guo, Y.; Yin, R.; Wang, L.; Fan, Z.; Zhou, Y.; Yuan, J.; et al. Expression profiles of miRNAs in the lung tissue of piglets infected with Glaesserella parasuis and the roles of ssc-miR-135 and ssc-miR-155-3p in the regulation of inflammation. Comp. Immunol. Microbiol. Infect. Dis. 2024, 111, 102214. [Google Scholar] [CrossRef]
- Wang, J.Y.; Yang, Y.; Ma, Y.; Wang, F.; Xue, A.; Zhu, J.; Yang, H.; Chen, Q.; Chen, M.; Ye, L.; et al. Potential regulatory role of lncRNA-miRNA-mRNA axis in osteosarcoma. Biomed. Pharmacother. 2020, 121, 109627. [Google Scholar] [CrossRef]
- Yu, Y.; Fan, Z.; Han, Y.; Sun, X.; Dong, C.; Liu, G.; Yin, X.; Liu, L.; Bai, Y.; Yang, B. miR-135 protects against atrial fibrillation by suppressing intracellular calcium-mediated NLRP3 inflammasome activation. J. Cell Commun. Signal. 2023, 17, 813–825. [Google Scholar] [CrossRef]
- Gupta, S.; Lopez, M.A.; Ektesabi, A.M.; Tsoporis, J.N.; Vaswani, C.M.; Gandhi, S.Y.; Fairn, G.D.; Dos Santos, C.C.; Marshall, J.C. Caspase-8: Arbitrating Life and Death in the Innate Immune System. Cells 2025, 14, 240. [Google Scholar] [CrossRef] [PubMed]
- Samidurai, A.; Olex, A.L.; Ockaili, R.; Kraskauskas, D.; Roh, S.K.; Kukreja, R.C.; Das, A. Integrated Analysis of lncRNA-miRNA-mRNA Regulatory Network in Rapamycin-Induced Cardioprotection against Ischemia/Reperfusion Injury in Diabetic Rabbits. Cells 2023, 12, 2820. [Google Scholar] [CrossRef] [PubMed]



| Genes | Primer Sequences (5′–3′) | Annealing Temperature (°C) | Product Length (bp) |
|---|---|---|---|
| MEG3 | F: CAGGACGACCAAGGAGGAGGAC | 60 | 135 |
| R: TCAGAGCAACAAGGCAGAAGCATAG | |||
| FASLG | F: CCTGTGTCTCCTTGTGATGT | 57 | 165 |
| R: TTGGGGTGACCTATTTGCTT | |||
| TNF | F: GCACTGAGAGCATGATCCG | 57 | 161 |
| R: AACCTCGAAGTGCAGTAGG | |||
| BAX | F: TGAGCAGATCATGAAGACAGGG | 58 | 142 |
| R: GAGACACTCGCTCAACTTCTT | |||
| BCL2 | F: GGCCTTCTTTGAGTTCGGT | 58 | 163 |
| R: ATACAGCTCCACAAAGGCATC | |||
| CASP8 | F: TCTGAGCAAGACCTTTAGTG | 55 | 107 |
| R: TATGGTCCAAGTTTCGGTAG | |||
| CASP9 | F: AAGCAAATGGTCCAGGCTTT | 57 | 164 |
| R: ACAATTTTCTCCACGGACAC | |||
| CASP3 | F: ACAGCACCTGGTTACTATTC | 55 | 145 |
| R: ATTCTACTGCTACCTTTCGG | |||
| CYCS | F: GATTCACTTACACAGATGCCA | 56 | 102 |
| R: TTGTTCCAGGGATGTACTTCTT | |||
| TP53 | F: GATGAAAATCCAGATGACGC | 55 | 150 |
| R: TAGACGGAAATCATAGCTGC | |||
| AIFM1 | F: TAGAACTCCAGATGACAAGAC | 54 | 100 |
| R: CCTATTGTTGATAAGCCCAC | |||
| ENDOG | F: GAGCCGCGAGTCTTATGT | 57 | 121 |
| R: ATGGAAGTCACAAGAGCGG | |||
| ssc-miR-135 | F: TGCGGCGTATGGCTTTTTATTCCTATG | 60 | - |
| R: AGTGCAGGGTCCGAGGTATT | |||
| U6 | F: CTCGCTTCGGCAGCACA | 60 | - |
| R: AACGCTTCACGAATTTGCGT | |||
| GAPDH | F: CACAGTCAAGGCGGAGAAC | 58 | 106 |
| R: CGTAGCACCAGCATCACC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Qian, M.; Sun, X.; Yin, R.; Li, N.; Shen, A.; Wang, H.; Zeng, F.; Zhou, Y.; Yin, R. LncRNA MEG3 Regulates Glaesserella parasuis-Induced Apoptosis of Porcine Alveolar Macrophages via Regulating ssc-miR-135/CASP8 Axis. Microorganisms 2025, 13, 2287. https://doi.org/10.3390/microorganisms13102287
Jia Y, Qian M, Sun X, Yin R, Li N, Shen A, Wang H, Zeng F, Zhou Y, Yin R. LncRNA MEG3 Regulates Glaesserella parasuis-Induced Apoptosis of Porcine Alveolar Macrophages via Regulating ssc-miR-135/CASP8 Axis. Microorganisms. 2025; 13(10):2287. https://doi.org/10.3390/microorganisms13102287
Chicago/Turabian StyleJia, Yongchao, Meiling Qian, Xinlu Sun, Ronglan Yin, Na Li, Aobo Shen, Haoran Wang, Fanhua Zeng, Yuanyuan Zhou, and Ronghuan Yin. 2025. "LncRNA MEG3 Regulates Glaesserella parasuis-Induced Apoptosis of Porcine Alveolar Macrophages via Regulating ssc-miR-135/CASP8 Axis" Microorganisms 13, no. 10: 2287. https://doi.org/10.3390/microorganisms13102287
APA StyleJia, Y., Qian, M., Sun, X., Yin, R., Li, N., Shen, A., Wang, H., Zeng, F., Zhou, Y., & Yin, R. (2025). LncRNA MEG3 Regulates Glaesserella parasuis-Induced Apoptosis of Porcine Alveolar Macrophages via Regulating ssc-miR-135/CASP8 Axis. Microorganisms, 13(10), 2287. https://doi.org/10.3390/microorganisms13102287





