Molecular Typing of Acanthamoeba Using Mitochondrial rDNA Spacers
Abstract
1. Introduction
2. Materials and Methods
3. Results
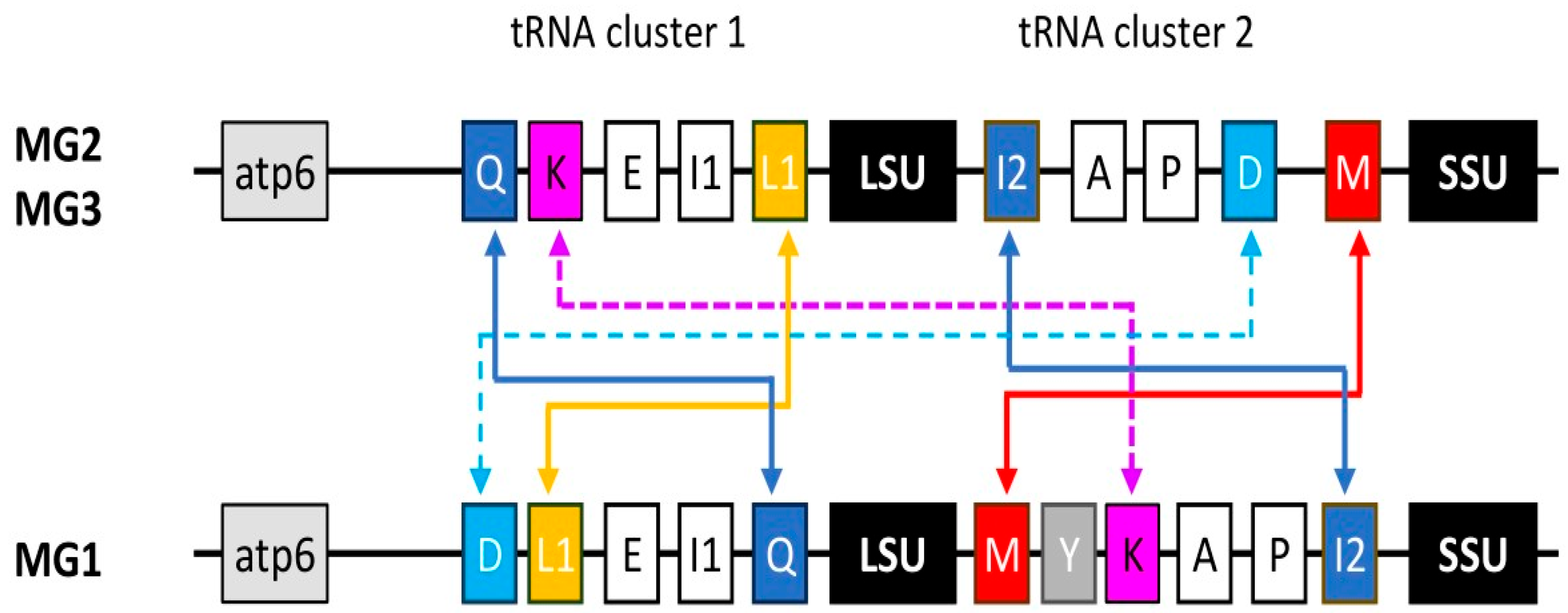
3.1. Acanthamoeba Species MG2/MG3 and MG1 Differ in the Arrangement of tRNA Gene Clusters
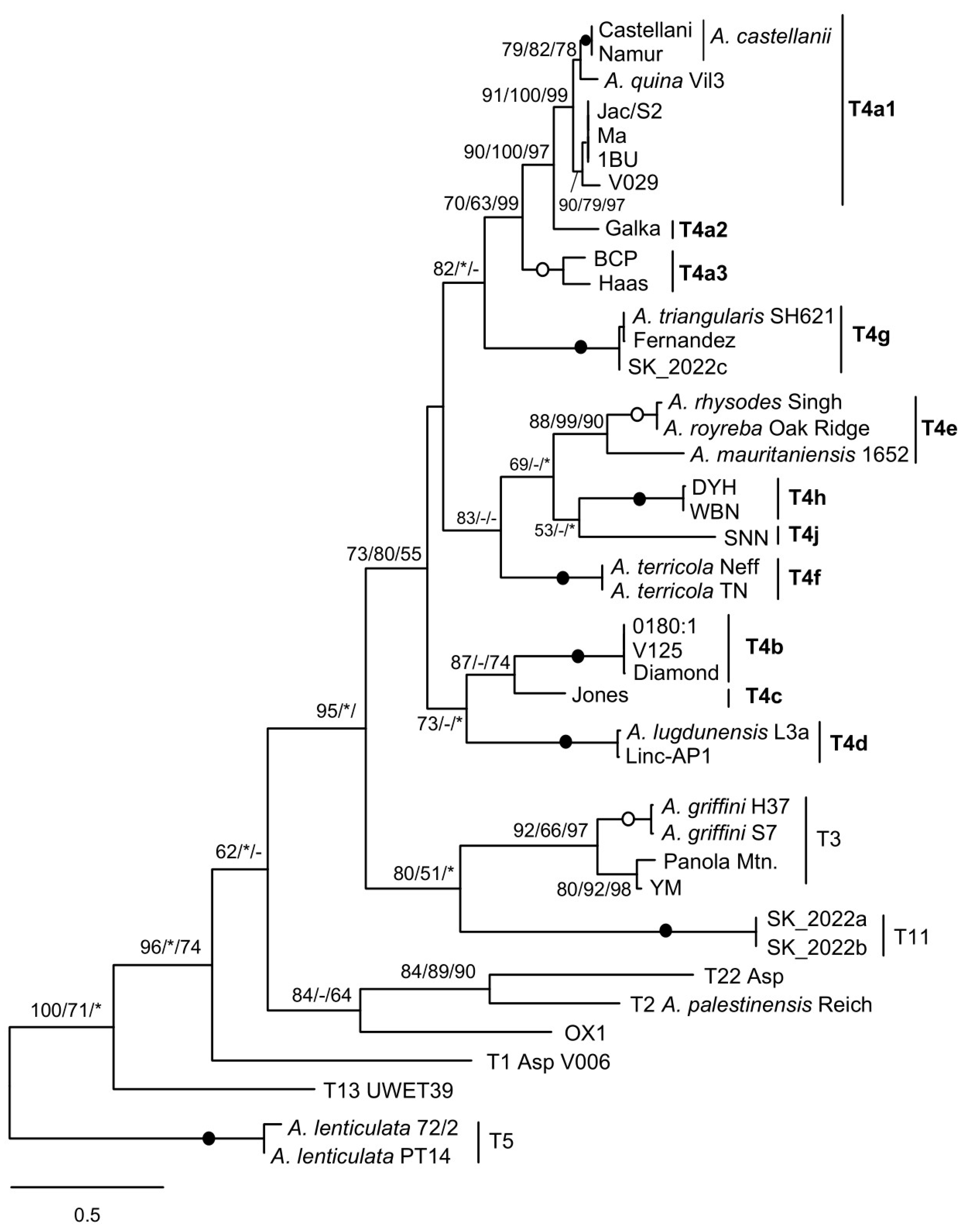
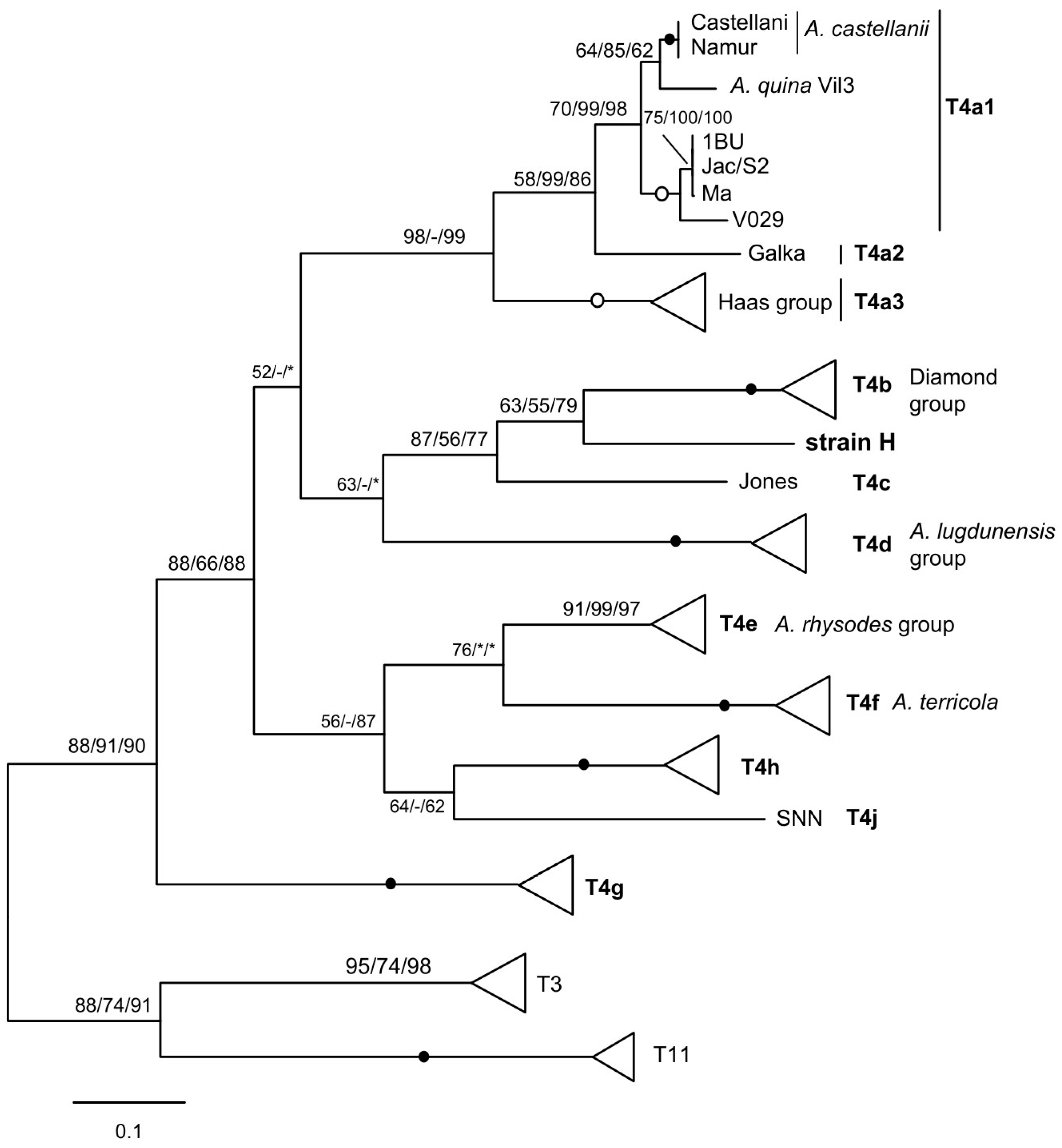
3.2. Mitochondrial Spacers of Acanthamoeba Species MG2/MG3 Correlate with Lineage-Specific Profiles
3.3. RNA Editing
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marciano-Cabral, F.; Cabral, G. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 2003, 16, 273–307. [Google Scholar] [CrossRef]
- Visvesvara, G.S.; Moura, H.; Schuster, F.L. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 2007, 50, 1–26. [Google Scholar] [CrossRef]
- Rowbotham, T.J. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 1980, 33, 1179–1183. [Google Scholar] [CrossRef]
- Pussard, M.; Pons, R. Morphologie de la paroi kystique et taxonomie du genre Acanthamoeba (Protozoa, Amoebida). Protistologica 1977, 13, 557–598. [Google Scholar]
- Stothard, D.R.; Schroeder-Diedrich, J.M.; Awwad, M.H.; Gast, R.J.; Ledee, D.R.; Rodriguez-Zaragoza, S.; Dean, C.L.; Fuerst, P.A.; Byers, T.J. The evolutionary history of the genus Acanthamoeba and the identification of eight new 18S rRNA gene sequence types. J. Eukaryot. Microbiol. 1998, 45, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, D. Update on Acanthamoeba phylogeny. Parasitol. Res. 2020, 119, 3327–3338, Erratum in Parasitol. Res. 2021, 120, 1927–1928. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, D. Molecular evidence for greater diversity within Acanthamoeba. Acta Parasitol. 2025, 70, 128. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, D.; Venditti, D. Molecular evidence for a new lineage within the Acanthamoeba T4 genotype. Parasitol. Res. 2023, 122, 1445–1450. [Google Scholar] [CrossRef]
- Ledee, D.R.; Booton, G.C.; Awwad, M.H.; Sharma, S.; Aggarwal, R.K.; Niszl, I.A.; Markus, M.B.; Fuerst, P.A.; Byers, T.J. Advantages of using mitochondrial 16S rDNA sequences to classify clinical isolates of Acanthamoeba. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1142–1149. [Google Scholar] [CrossRef]
- Rahman, M.M.; Yagita, K.; Kobayashi, A.; Oikawa, Y.; Hussein, A.I.A.; Matsumura, T.; Tokoro, M. Genetic characterization of clinical Acanthamoeba isolates from Japan using nuclear and mitochondrial small subunit ribosomal RNA. Korean J. Parasitol. 2013, 51, 401–411. [Google Scholar] [CrossRef]
- Köhsler, M.; Leitner, B.; Blaschitz, M.; Michel, R.; Aspöck, H.; Walochnik, J. ITS1 sequence variabilities correlate with 18S rDNA sequence types in the genus Acanthamoeba (Protozoa: Amoebozoa). Parasitol. Res. 2006, 98, 86–93. [Google Scholar] [CrossRef]
- Corsaro, D. Exploring LSU and ITS rDNA sequences for Acanthamoeba identification and phylogeny. Microorganisms 2022, 10, 1776. [Google Scholar] [CrossRef]
- Corsaro, D. Learning from the rDNA operon: A reanalysis of the Acanthamoeba palestinensis group. Microorganisms 2024, 12, 2105. [Google Scholar] [CrossRef] [PubMed]
- De Jonckheere, J.F. What do we know by now about the genus Naegleria? Exp. Parasitol. 2014, 145, S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Coleman, A.W. Pan-eukaryote ITS2 homologies revealed by RNA secondary structure. Nucleic Acids Res. 2007, 35, 3322–3329. [Google Scholar] [CrossRef] [PubMed]
- Nazar, R.N. A 5.8 S rRNA-like sequence in prokaryotic 23 S rRNA. FEBS Lett. 1980, 119, 212–214. [Google Scholar] [CrossRef]
- Jacq, B. Sequence homologies between eukaryotic 5.8S rRNA and the 5′ end of prokaryotic 23S rRNA: Evidences for a common evolutionary origin. Nucleic Acids Res. 1981, 9, 2913–2932. [Google Scholar] [CrossRef]
- Gürtler, V.; Stanisich, V.A. New approaches to typing and identification of bacteria using the 16S-23S rDNA spacer region. Microbiology 1996, 142, 3–16. [Google Scholar] [CrossRef]
- Asthana, V.; Nieves, E.M.; Bugga, P.; Smith, C.; Dunn, T.; Narayanasamy, S.; Dickson, R.P.; VanEpps, J.S. Development of a rapid, culture-free, universal microbial identification system using Internal Transcribed Spacer targeting primers. J. Infect. Dis. 2025, 231, 1155–1164. [Google Scholar] [CrossRef]
- Corsaro, D.; Mrva, M.; Colson, P.; Walochnik, J. Validation and redescription of Acanthamoeba terricola Pussard, 1964 (Amoebozoa: Acanthamoebidae). Eur. J. Protistol. 2024, 94, 126091. [Google Scholar] [CrossRef]
- Burger, G.; Plante, I.; Lonergan, K.M.; Gray, M.W. The mitochondrial DNA of the amoeboid protozoon, Acanthamoeba castellanii: Complete sequence, gene content and genome organization. J. Mol. Biol. 1995, 245, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Ledee, D.R.; Byers, T.J. Length and sequence heterogeneity in the mitochondrial internal transcribed spacer of Acanthamoeba spp. J. Eukaryot. Microbiol. 2009, 56, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, D.; Venditti, D. Phylogenetic evidence for a new genotype of Acanthamoeba (Amoebozoa, Acanthamoebida). Parasitol. Res. 2010, 107, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, D.; Walochnik, J.; Köhsler, M.; Rott, M.B. Acanthamoeba misidentification and multiple labels: Redefining genotypes T16, T19 and T20, and proposal for Acanthamoeba micheli sp. nov. (genotype T19). Parasitol. Res. 2015, 114, 2481–2490. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, K.M.; Gray, M.W. The ribosomal RNA gene region in Acanthamoeba castellanii mitochondrial DNA. A case of evolutionary transfer of introns between mitochondria and plastids? J. Mol. Biol. 1994, 239, 476–499. [Google Scholar] [CrossRef]
- Lonergan, K.M.; Gray, M.W. Editing of transfer RNAs in Acanthamoeba castellanii mitochondria. Science 1993, 259, 812–816. [Google Scholar] [CrossRef]
- Lonergan, K.M.; Gray, M.W. Predicted editing of additional transfer RNAs in Acanthamoeba castellanii mitochondria. Nucleic Acids Res. 1993, 21, 4402. [Google Scholar] [CrossRef]
- Schroeder, J.M.; Booton, G.C.; Hay, J.; Niszl, I.A.; Seal, D.V.; Markus, M.B.; Fuerst, P.A.; Byers, T.J. Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of acanthamoebae from humans with keratitis and from sewage sludge. J. Clin. Microbiol. 2001, 39, 1903–1911. [Google Scholar] [CrossRef]
- Malavin, S.; Shmakova, L. Isolates from ancient permafrost help to elucidate species boundaries in Acanthamoeba castellanii complex (Amoebozoa: Discosea). Eur. J. Protistol. 2020, 73, 125671. [Google Scholar] [CrossRef]
- Nassonova, E.; Smirnov, A.; Fahrni, J.; Pawlowski, J. Barcoding amoebae: Comparison of SSU, ITS and COI genes as tools for molecular identification of naked lobose amoebae. Protist 2010, 161, 102–115. [Google Scholar] [CrossRef]
- Heger, T.J.; Pawlowski, J.; Lara, E.; Leander, B.S.; Todorov, M.; Golemansky, V.; Mitchell, E.A. Comparing potential COI and SSU rDNA barcodes for assessing the diversity and phylogenetic relationships of cyphoderiid testate amoebae (Rhizaria: Euglyphida). Protist 2011, 162, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Kosakyan, A.; Heger, T.J.; Leander, B.S.; Todorov, M.; Mitchell, E.A.; Lara, E. COI barcoding of Nebelid testate amoebae (Amoebozoa: Arcellinida): Extensive cryptic diversity and redefinition of the Hyalospheniidae Schultze. Protist 2012, 163, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Stoeckle, M. Taxonomy, DNA, and the Bar Code of Life. BioScience 2003, 53, 796–797. [Google Scholar] [CrossRef]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Hebert, P.D.; Ratnasingham, S.; deWaard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, S96–S99. [Google Scholar] [CrossRef]
- Greninger, A.L.; Messacar, K.; Dunnebacke, T.; Naccache, S.N.; Federman, S.; Bouquet, J.; Mirsky, D.; Nomura, Y.; Yagi, S.; Glaser, C.; et al. Clinical metagenomic identification of Balamuthia mandrillaris encephalitis and assembly of the draft genome: The continuing case for reference genome sequencing. Genome Med. 2015, 7, 113, Erratum in Genome Med. 2016, 8, 1. [Google Scholar] [CrossRef]
- Bondarenko, N.; Smirnov, A.; Nassonova, E.; Glotova, A.; Fiore-Donno, A.M. Mitochondrial genomes of Amoebozoa. Protistology 2019, 13, 179–191. [Google Scholar] [CrossRef]
- Byers, T.J. Molecular biology of DNA in Acanthamoeba, Amoeba, Entamoeba, and Naegleria. Int. Rev. Cytol. 1986, 99, 311–341. [Google Scholar] [CrossRef]
- Yang, Q.; Zwick, M.G.; Paule, M.R. Sequence organization of the Acanthamoeba rRNA intergenic spacer: Identification of transcriptional enhancers. Nucleic Acids Res. 1994, 22, 4798–4805. [Google Scholar] [CrossRef]
| SSU GT1 | Species/strain | Culture collection | Spacer length | LSU-I2 | I2-A | A-P | P-D | D-M | M-SSU | |
|---|---|---|---|---|---|---|---|---|---|---|
| mt | nucl | |||||||||
| T4a1 | T4A | A. castellanii Castellani2 | ATCC 50374 | 812 | 15 | 6 | 11 | 3 | 349 | 68 |
| T4a1 | T4A | A. castellanii Namur | na | 815 | 15 | 6 | 11 | 3 | 352 | 68 |
| T4a1 | T4A | A. quina Vil3 | ATCC 50241 | 799 | 19 | 6 | 9 | 2 | 343 | 60 |
| T4a1 | T4B | Asp Ma2 | ATCC 50370 | 765 | 15 | 6 | 11 | 2 | 305 | 66 |
| T4a1 | T4B | Asp Jac/S22 | ATCC 50372 | 766 | 15 | 6 | 11 | 2 | 306 | 66 |
| T4a1 | T4B | Asp 1BU | ATCC PRA-105 | 766 | 15 | 6 | 11 | 2 | 307 | 66 |
| T4a1 | T4B | Asp CDC V0292 | ATCC 50495 | 736 | 14 | 6 | 11 | 2 | 281 | 62 |
| T4a2 | T4A | Asp Galka2 | ATCC 50496 | 847 | 15 | 7 | 11 | 2 | 378 | 74 |
| T4a3 | T4A | Asp Haas2 | ATCC 50368 | 823 | 19 | 6 | 12 | 2 | 361 | 64 |
| T4a3 | T4A | Asp BCP | na | 841 | 19 | 7 | 12 | 2 | 372 | 69 |
| T4b | T4B | Asp Diamond2 | ATCC 50724 | 635 | 14 | 6 | 17 | 2 | 161 | 75 |
| T4b | T4B | Asp CDC V1252 | ATCC 50498 | 636 | 14 | 6 | 19 | 2 | 160 | 75 |
| T4b | T4B | Asp CDC 0180:12 | ATCC 50491 | 636 | 14 | 6 | 19 | 2 | 160 | 75 |
| T4c | T4A | Asp Jones | ATCC 30461 | 597 | 14 | 9 | 14 | 2 | 125 | 73 |
| T4d | T4A | A. lugdunensis L3a | ATCC 50240 | 885 | 15 | 8 | 11 | 3 | 427 | 61 |
| T4d | T4A | Asp Linc-AP1 | CCAP 1501/18 | 888 | 15 | 8 | 14 | 3 | 427 | 61 |
| T4e | T4D | A. royreba Oak Ridge2 | ATCC 30884 | 772 | 11 | 48 | 11 | 2 | 293 | 47 |
| T4e | T4D | A. rhysodes Singh | ATCC 30973 | 773 | 11 | 48 | 11 | 2 | 295 | 47 |
| T4e | T4D | A. mauritaniensis 1652 | ATCC 50253 | 676 | 14 | 57 | 14 | 3 | 179 | 49 |
| T4g | T4F | A. triangularis SH621 | ATCC 50254 | 659 | 17 | 20 | 11 | 2 | 210 | 39 |
| T4g | T4C | Asp SK_2022c | na | 659 | 17 | 20 | 11 | 2 | 211 | 38 |
| T4g | T4C | Asp Fernandez2 | ATCC 50369 | 663 | 17 | 20 | 11 | 2 | 214 | 39 |
| T4f | T4G | A. terricola Neff3 | ATCC 30010 | 635 | 33 | 30 | 7 | 3 | 136 | 65 |
| T4f | T4G | A. terricola TN | na | 635 | 34 | 30 | 7 | 3 | 136 | 65 |
| T4j | T4H | Asp SNN | na | 547 | 36 | 42 | 9 | 3 | 42 | 55 |
| T4h | T4E | Asp WBN | na | 588 | 11 | 34 | 6 | 5 | 117 | 55 |
| T4h | T4E | Asp DYH | na | 587 | 11 | 34 | 6 | 5 | 116 | 55 |
| T3 | T3 | A. griffini S72 | ATCC 30731 | 559 | 16 | 15 | 0 | 19 | 109 | 41 |
| T3 | T3 | A. griffini H37 | ATCC 50702 | 562 | 15 | 15 | 0 | 19 | 110 | 44 |
| T3 | T3 | Asp YM | na | 549 | 13 | 14 | 0 | 10 | 109 | 44 |
| T3 | T3 | Asp Panola Mtn.2 | ATCC 30487 | 611 | 14 | 14 | 0 | 10 | 143 | 71 |
| T11 | T11 | Asp SK_2022a | na | 1098 | 31 | 30 | 12 | 26 | 598 | 41 |
| T11 | T11 | Asp SK_2022b | na | 1099 | 31 | 30 | 12 | 26 | 599 | 41 |
| T2 | T2 | A. palestinensis Reich2 | ATCC 30870 | 1360 | 11 | 29 | 7 | 17 | 877 4 | 59 |
| OX1 | OX1 | Asp Sawyer OX12 | CCAP 1501/3c | 1190 | 16 | 44 | 9 | 19 | 684 | 58 |
| T1 | T1 | Asp CDC V0062 | ATCC 50494 | 1288 | 47 | 39 | –1 | 21 | 807 | 52 |
| T13 | T13 | Asp UWET39 | ATCC PRA-9 | 560 | 11 | 13 | –3 | 8 | 116 | 56 |
| T22 | T22 | Asp | na | 645 | 35 | 11 | 3 | 13 | 165 | 59 |
| T5 | T5 | A. lenticulata 72/2 | ATCC 50704 | 484 | 15 | 5 | 0 | 20 | 8 | 77 |
| T5 | T5 | A. lenticulata PT14 | na | 478 | 14 | 5 | 0 | 21 | 9 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corsaro, D. Molecular Typing of Acanthamoeba Using Mitochondrial rDNA Spacers. Microorganisms 2025, 13, 2285. https://doi.org/10.3390/microorganisms13102285
Corsaro D. Molecular Typing of Acanthamoeba Using Mitochondrial rDNA Spacers. Microorganisms. 2025; 13(10):2285. https://doi.org/10.3390/microorganisms13102285
Chicago/Turabian StyleCorsaro, Daniele. 2025. "Molecular Typing of Acanthamoeba Using Mitochondrial rDNA Spacers" Microorganisms 13, no. 10: 2285. https://doi.org/10.3390/microorganisms13102285
APA StyleCorsaro, D. (2025). Molecular Typing of Acanthamoeba Using Mitochondrial rDNA Spacers. Microorganisms, 13(10), 2285. https://doi.org/10.3390/microorganisms13102285





