Characterization of Gut Bacteria in Natural Populations of Sand Flies (Diptera: Psychodidae) from Endemic and Non-Endemic Areas of Leishmaniasis in Morocco
Abstract
1. Introduction
2. Materials and Methods
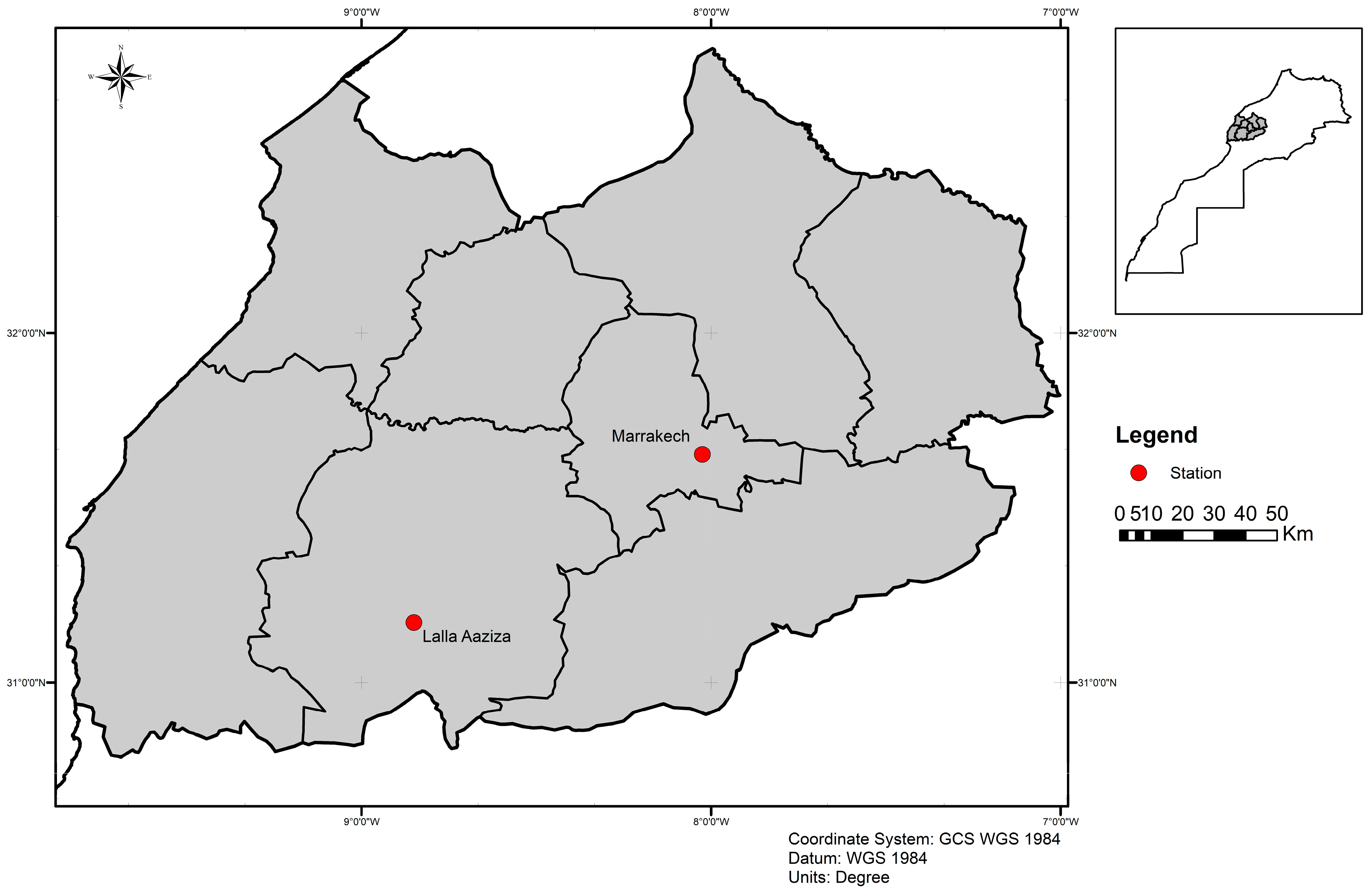
2.1. Sand Fly Collection, Species Identification, and Gut Dissection
2.2. Bacterial Isolation and Identification
2.3. MALDI-TOF Assay
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MALDI-TOF | Matrix-Assisted Laser Desorption Ionization—Time of Flight |
| P | Phlebotomus |
| S | Sergentomyia |
| L | Leishmania |
| DGGE | Denaturing Gradient Gel Electrophoresis |
| CFUs | Colony-Forming Units |
| LA | Lala Laaziza |
| MA | Marrakech |
| PBS | Phosphate-Buffered Saline |
References
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef] [PubMed]
- El Idrissi Saik, I.; Benlabsir, C.; Fellah, H. Transmission patterns of Leishmania tropica around the Mediterranean basin: Could Morocco be impacted by a zoonotic spillover? PLoS Negl. Trop. Dis. 2022, 16, e0010009. [Google Scholar] [CrossRef]
- Hakkour, M.; Badaoui, B.; El Hamiani Khatat, S.; Sahibi, H.; Fellah, H.; Sadak, A.; Sebti, F. Genetic diversity in Leishmania infantum and Leishmania tropica isolates from human and canine hosts in northern Morocco. Gene 2024, 921, 148484. [Google Scholar] [CrossRef]
- Hakkour, M.; El Alem, M.M.; Hmamouch, A.; Rhalem, A.; Delouane, B.; Habbari, K.; Fellah, H.; Sadak, A.; Sebti, F. Leishmaniasis in Northern Morocco: Predominance of Leishmania infantum Compared to Leishmania tropica. Biomed. Res. Int. 2019, 2019, 5327287. [Google Scholar] [CrossRef] [PubMed]
- Mouttaki, T.; Maksouri, H.; El Mabrouki, J.; Merino-Espinosa, G.; Fellah, H.; Itri, M.; Martin-Sanchez, J.; Soussi-Abdallaoui, M.; Chiheb, S.; Riyad, M. Concomitant visceral and localized cutaneous leishmaniasis in two Moroccan infants. Infect. Dis. Poverty 2018, 7, 32. [Google Scholar] [CrossRef]
- Bennis, I.; Thys, S.; Filali, H.; De Brouwere, V.; Sahibi, H.; Boelaert, M. Psychosocial impact of scars due to cutaneous leishmaniasis on high school students in Errachidia Province, Morocco. Infect. Dis. Poverty 2017, 6, 46. [Google Scholar] [CrossRef]
- McCarthy, C.B.; Diambra, L.A.; Rivera Pomar, R.V. Metagenomic analysis of taxa associated with Lutzomyia longipalpis, vector of visceral leishmaniasis, using an unbiased high-throughput approach. PLoS Negl. Trop. Dis. 2011, 5, e103. [Google Scholar] [CrossRef]
- Hillesland, H.; Read, A.; Subhadra, B.; Hurwitz, I.; McKelvey, R.; Ghosh, K.; Das, P.; Durvasula, R. Identification of aerobic gut bacteria from the kala azar vector, Phlebotomus argentipes: A platform for potential paratransgenic manipulation of sand flies. Am. J. Trop. Med. Hyg. 2008, 79, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, J.; Braig, H.R.; Rowton, E.D.; Ghosh, K. Naturally occurring culturable aerobic gut flora of adult Phlebotomus papatasi, vector of Leishmania major in the Old World. PLoS ONE 2012, 7, e35748. [Google Scholar] [CrossRef]
- Vivero, R.J.; Villegas-Plazas, M.; Cadavid-Restrepo, G.E.; Herrera, C.X.M.; Uribe, S.I.; Junca, H. Wild specimens of sand fly phlebotomine Lutzomyia evansi, vector of leishmaniasis, show high abundance of Methylobacterium and natural carriage of Wolbachia and Cardinium types in the midgut microbiome. Sci. Rep. 2019, 9, 17746. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, J.; Vavre, F. Bacterial symbionts in insects or the story of communities affecting communities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.; Bharathiraja, C.; Pandiarajan, J.; Prasanna, V.A.; Rajendhran, J.; Gunasekaran, P. Insect gut microbiome—An unexploited reserve for biotechnological application. Asian Pac. J. Trop. Biomed. 2014, 4 (Suppl. S1), S16–S21. [Google Scholar] [CrossRef]
- Wang, H.; Wu, N.; Liu, Y.; Kundu, J.K.; Liu, W.; Wang, X. Higher bacterial diversity of gut microbiota in different natural populations of leafhopper vector does not influence WDV transmission. Front. Microbiol. 2019, 10, 1144. [Google Scholar] [CrossRef]
- Dennison, N.J.; Jupatanakul, N.; Dimopoulos, G. The mosquito microbiota influences vector competence for human pathogens. Curr. Opin. Insect Sci. 2014, 3, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Akorli, J.; Namaali, P.A.; Ametsi, G.W.; Egyirifa, R.K.; Pels, N.A.P. Generational conservation of composition and diversity of field-acquired midgut microbiota in Anopheles gambiae (sensu lato) during colonization in the laboratory. Parasit. Vectors 2019, 12, 27. [Google Scholar] [CrossRef]
- Dillon, R.J.; El Kordy, E.; Lane, R.P. The prevalence of microbiota in the digestive tract of Phlebotomus papatasi. Ann. Trop. Med. Parasitol. 1996, 90, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Volf, P.; Kiewegova, A.; Nemec, A. Bacterial colonization in the gut of Phlebotomus duboscqi: Transtadial passage and the role of female diet. Folia Parasitol. 2002, 49, 73–77. [Google Scholar] [CrossRef]
- Sant’Anna, M.R.; Darby, A.C.; Brazil, R.P.; Montoya-Lerma, J.; Dillon, V.M.; Bates, P.A.; Dillon, R.J. Investigation of the bacterial communities associated with females of Lutzomyia sand fly species from South America. PLoS ONE 2012, 7, e42531. [Google Scholar] [CrossRef]
- Maleki-Ravasan, N.; Oshaghi, M.A.; Afshar, D.; Arandian, M.H.; Hajikhani, S.; Akhavan, A.A.; Yakhchali, B.; Shirazi, M.H.; Rassi, Y.; Jafari, R.; et al. Aerobic bacterial flora of biotic and abiotic compartments of a hyperendemic zoonotic cutaneous leishmaniasis (ZCL) focus. Parasit. Vectors 2015, 8, 63. [Google Scholar] [CrossRef]
- Dong, Y.; Manfredini, F.; Dimopoulos, G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009, 5, e1000423. [Google Scholar] [CrossRef]
- Kelly, P.H.; Bahr, S.M.; Serafim, T.D.; Ajami, N.J.; Petrosino, J.F.; Meneses, C.; Kirby, J.R.; Valenzuela, J.G.; Kamhawi, S.; Wilson, M.E. The gut microbiome of the vector Lutzomyia longipalpis is essential for survival of Leishmania infantum. mBio 2017, 8, e01121-16. [Google Scholar] [CrossRef] [PubMed]
- Schlein, Y.; Polacheck, I.; Yuva, B. Mycoses, bacterial infections and antibacterial activity in sand flies (Psychodidae) and their possible role in the transmission of leishmaniasis. Parasitology 1985, 90, 57–66. [Google Scholar] [CrossRef]
- Rajendran, P.; Modi, G.B. Bacterial flora of sand fly gut (Diptera: Psychodidae). Indian J. Public Health 1982, 26, 49–52. [Google Scholar]
- Fraihi, W.; Fares, W.; Perrin, P.; Dorkeld, F.; Sereno, D.; Barhoumi, W.; Sbissi, I.; Cherni, S.; Chelbi, I.; Durvasula, R.; et al. An integrated overview of the midgut bacterial flora composition of Phlebotomus perniciosus, a vector of zoonotic visceral leishmaniasis in the Western Mediterranean Basin. PLoS Negl. Trop. Dis. 2017, 11, e0005484. [Google Scholar] [CrossRef]
- Louradour, I.; Monteiro, C.C.; Inbar, E.; Ghosh, K.; Merkhofer, R.; Lawyer, P.; Paun, A.; Smelkinson, M.; Secundino, N.; Lewis, M.; et al. The midgut microbiota plays an essential role in sand fly vector competence for Leishmania major. Cell. Microbiol. 2017, 19, e12755. [Google Scholar] [CrossRef] [PubMed]
- Cecilio, P.; Rogerio, L.A.; Serafim, T.D.; Tang, K.; Willen, L.; Iniguez, E.; Meneses, C.; Chaves, L.F.; Zhang, Y.; Dos Santos Felix, L.; et al. Leishmania sand fly-transmission is disrupted by Delftia tsuruhatensis TC1 bacteria. Nat. Commun. 2025, 16, 3571. [Google Scholar] [CrossRef] [PubMed]
- Guernaoui, S.; Boumezzough, A.; Pesson, B.; Pichon, G. Entomological investigations in Chichaoua: An emerging epidemic focus of cutaneous leishmaniasis in Morocco. J. Med. Entomol. 2005, 42, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Akhoundi, M.; Bakhtiari, R.; Guillard, T.; Baghaei, A.; Tolouei, R.; Sereno, D.; Toubas, D.; Depaquit, J.; Abyaneh, M.R. Diversity of the bacterial and fungal microflora from the midgut and cuticle of phlebotomine sand flies collected in North-Western Iran. PLoS ONE 2012, 7, e50259. [Google Scholar] [CrossRef]
- Boussaa, S. Epidémiologie des Leishmanioses dans la Région de Marrakech, Maroc: Effet de L’urbanisation sur la Répartition Spatio-Temporelle des Phlébotomes et Caractérisation Moléculaire de Leurs Populations. Ph.D. Thesis, Louis Pasteur University, Strasbourg, France, 2008; 217p. [Google Scholar]
- Seng, P.; Rolain, J.M.; Fournier, P.E.; La Scola, B.; Drancourt, M.; Raoult, D. MALDI-TOF-mass spectrometry applications in clinical microbiology. Future Microbiol. 2010, 5, 1733–1754. [Google Scholar] [CrossRef]
- Kahime, K.; Boussaa, S.; Bounoua, L.; Fouad, O.; Messouli, M.; Boumezzough, A. Leishmaniasis in Morocco: Diseases and vectors. Asian Pac. J. Trop. Dis. 2014, 4, S530–S534. [Google Scholar] [CrossRef]
- Daoudi, M.; Boussaa, S.; Hafidi, M.; Boumezzough, A. Potential distributions of phlebotomine sandfly vectors of human visceral leishmaniasis caused by Leishmania infantum in Morocco. Med. Vet. Entomol. 2020, 34, 385–393. [Google Scholar] [CrossRef]
- Boussaa, S.; Kahime, K.; Samy, A.M.; Salem, A.B.; Boumezzough, A. Species composition of sand flies and bionomics of Phlebotomus papatasi and P. sergenti (Diptera: Psychodidae) in cutaneous leishmaniasis endemic foci, Morocco. Parasit. Vectors 2016, 9, 60. [Google Scholar] [CrossRef]
- Mhaidi, I.; El Kacem, S.; Ait Kbaich, M.; El Hamouchi, A.; Sarih, M.; Akarid, K.; Lemrani, M. Molecular identification of Leishmania infection in the most relevant sand fly species and in patient skin samples from a cutaneous leishmaniasis focus, in Morocco. PLoS Negl. Trop. Dis. 2018, 12, e0006315. [Google Scholar] [CrossRef]
- Es-Sette, N.; Ajaoud, M.; Bichaud, L.; Hamdi, S.; Mellouki, F.; Charrel, R.N.; Lemrani, M. Phlebotomus sergenti, a common vector of Leishmania tropica and Toscana virus in Morocco. J. Vector Borne Dis. 2014, 51, 86–90. [Google Scholar] [CrossRef]
- Es-Sette, N.; Ajaoud, M.; Anga, L.; Mellouki, F.; Lemrani, M. Toscana virus isolated from sandflies, Morocco. Parasit. Vectors 2015, 8, 205. [Google Scholar] [CrossRef]
- Rhajaoui, M.; Sebti, F.; Fellah, H.; Alam, M.Z.; Nasereddin, A.; Abbasi, I.; Schönian, G. Identification of the causative agent of cutaneous leishmaniasis in Chichaoua province, Morocco. Parasite 2012, 19, 81–84. [Google Scholar] [CrossRef]
- Boussaa, S.; Kasbari, M.; Mzabi, A.E.; Boumezzough, A. Epidemiological investigation of canine leishmaniasis in Southern Morocco. Adv. Epidemiol. 2014, 2014, 104697. [Google Scholar] [CrossRef]
- MMH. Bulletin of Epidemiology and Public Health; Directorate of Epidemiology and Disease Control, Ministry of Health: Rabat, Morocco, 2017. [Google Scholar]
- Killick-Kendrick, R. The biology and control of phlebotomine sand flies. Clin Dermatol. 1999, 17, 279–289. [Google Scholar] [CrossRef]
- Pumpuni, C.B.; Beier, M.S.; Nataro, J.P.; Guers, L.D.; Davis, J.R. Plasmodium falciparum: Inhibition of sporogonic development in Anopheles stephensi by gram-negative bacteria. Exp. Parasitol. 1993, 77, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, C.; Asensi, M.D.; Zahner, V.; Rangel, E.F.; Oliveira, S.M. Study on the bacterial midgut microbiota associated to different Brazilian populations of Lutzomyia longipalpis (Lutz & Neiva) (Diptera, Psychodidae). Neotrop. Entomol. 2008, 37, 597–601. [Google Scholar] [PubMed]
- Weiss, B.; Aksoy, S. Microbiome influences on insect host vector competence. Trends Parasitol. 2011, 27, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Omondi, Z.N.; Demir, S. Bacteria composition and diversity in the gut of sand fly: Impact on Leishmania and sand fly development. Int. J. Trop. Insect. Sci. 2020, 41, 25–32. [Google Scholar] [CrossRef]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef]
- Telleria, E.L.; Martins-da-Silva, A.; Tempone, A.J.; Traub-Csekö, Y.M. Leishmania, microbiota and sand fly immunity. Parasitology 2018, 145, 1336–1353. [Google Scholar] [CrossRef]
- Guernaoui, S.; Garcia, D.; Gazanion, E.; Ouhdouch, Y.; Boumezzough, A.; Pesson, B.; Fontenille, D.; Sereno, D. Bacterial flora as indicated by PCR-temperature gradient gel electrophoresis (TGGE) of 16S rDNA gene fragments from isolated guts of phlebotomine sand flies (Diptera: Psychodidae). J. Vector Ecol. 2011, 36 (Suppl. S1), S144–S147. [Google Scholar] [CrossRef]
- Azambuja, P.; Garcia, E.S.; Ratcliffe, N.A. Gut microbiota and parasite transmission by insect vectors. Trends Parasitol. 2005, 21, 568–572. [Google Scholar] [CrossRef]
- Pires, A.C.A.M.; Villegas, L.E.M.; Campolina, T.B.; Orfanó, A.S.; Pimenta, P.F.P.; Secundino, N.F.C. Bacterial diversity of wild-caught Lutzomyia longipalpis (a vector of zoonotic visceral leishmaniasis in Brazil) under distinct physiological conditions by metagenomics analysis. Parasit. Vectors 2017, 10, 627. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Karakuş, M.; Karabey, B.; Kalkan, Ş.O.; Özdemir, G.; Oğuz, G.; Kasap, Ö.E.; Alten, B.; Töz, S.; Özbel, Y. Midgut Bacterial Diversity of Wild Populations of Phlebotomus (P.) papatasi, the Vector of Zoonotic Cutaneous Leishmaniasis (ZCL) in Turkey. Sci. Rep. 2017, 7, 14812. [Google Scholar] [CrossRef] [PubMed]
- Kempf, M.J.; Chen, F.; Kern, R.; Venkateswaran, K. Recurrent isolation of hydrogen peroxide-resistant spores of Bacillus pumilus from a spacecraft assembly facility. Astrobiology 2005, 5, 391–405. [Google Scholar] [CrossRef]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef]
- Bochow, H.; Gantcheva, K. Soil introductions of Bacillus subtilis as biocontrol agent and its population and activity dynamic. Acta Hortic. 1995, 382, 164–172. [Google Scholar] [CrossRef]
- Williams, P. Bacillus subtilis: A shocking message from a probiotic. Cell Host Microbe 2007, 1, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, I.; Hillesland, H.; Fieck, A.; Das, P.; Durvasula, R. The paratransgenic sand fly: A platform for control of Leishmania transmission. Parasit. Vectors 2011, 4, 82. [Google Scholar] [CrossRef] [PubMed]
- Daoudi, M.; Boussaa, S.; Boumezzough, A. Modeling spatial distribution of Sergentomyia minuta (Diptera: Psychodidae) and its potential implication in leishmaniasis transmission in Morocco. J. Arthropods Borne Dis. 2020, 14, 17–28. [Google Scholar] [CrossRef]
- Wang, S.; Jacobs-Lorena, M. Genetic approaches to interfere with malaria transmission by vector mosquitoes. Trends Biotechnol. 2013, 31, 185–193. [Google Scholar] [CrossRef]

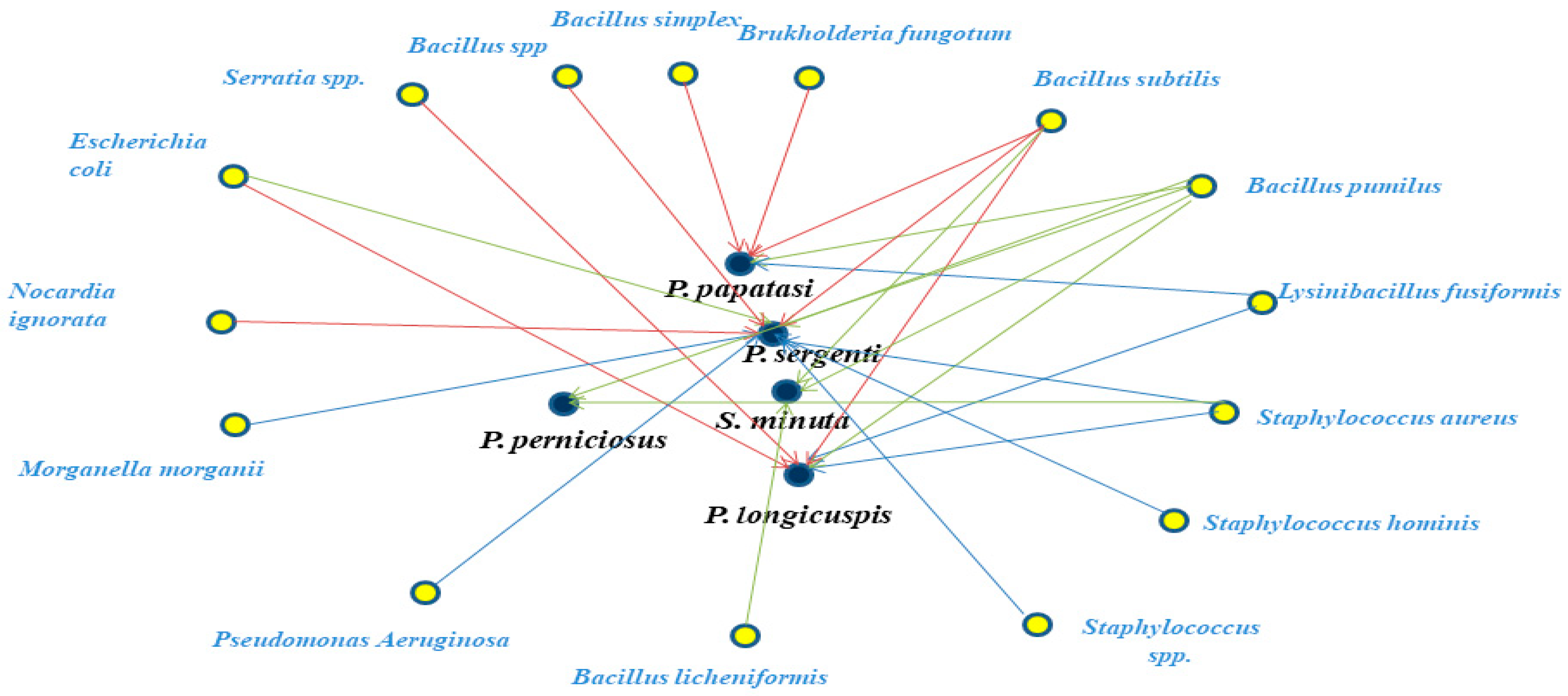
| Bacterial Strains | Gram-Stain | Identifying NCBI | Phylum | Sand Fly Species |
|---|---|---|---|---|
| Bacillus pumilus | Gram+ | 130148166 | Firmicutes | P. sergenti P. papatasi S. minuta P. longicuspis P. perniciosus |
| Bacillus simplex | Gram− | 133055080 | P. papatasi | |
| Bacillus subtilis | Gram+ | 133993714 | S. minuta P. papatasi P. sergenti P. longicuspis | |
| Bacillus licheniformis | Gram+ | 1402 | S. minuta | |
| Bacillus sp. | Gram+ | 1409 | P. sergenti | |
| Lysinibacillus fusiformis | Gram+ | 28031 | P. papatasi P. longicuspis | |
| Staphylococcus aureus | Gram+ | 703339 | P. sergenti P. perniciosus P. longicuspis | |
| Staphylococcus hominis | Gram− | 1290 | P. sergenti P. perniciosus | |
| Staphylococcus spp. | Gram+ | 29387 | P. sergenti | |
| Brukholderia fungorum | Gram− | 1218077 | Proteobacteria | P. papatasi |
| Morganella morganii | Gram− | 582 | P. sergenti | |
| Pseudomonas aeruginosa | Gram− | 287 | P. sergenti | |
| Escherichia coli | Gram− | 562 | P. longicuspis | |
| P. sergenti | ||||
| Serratia spp. | Gram− | 616 | P. longicuspis | |
| Nocardia ignorata | Gram+ | 145285 | Actinobacteria | P. sergenti |
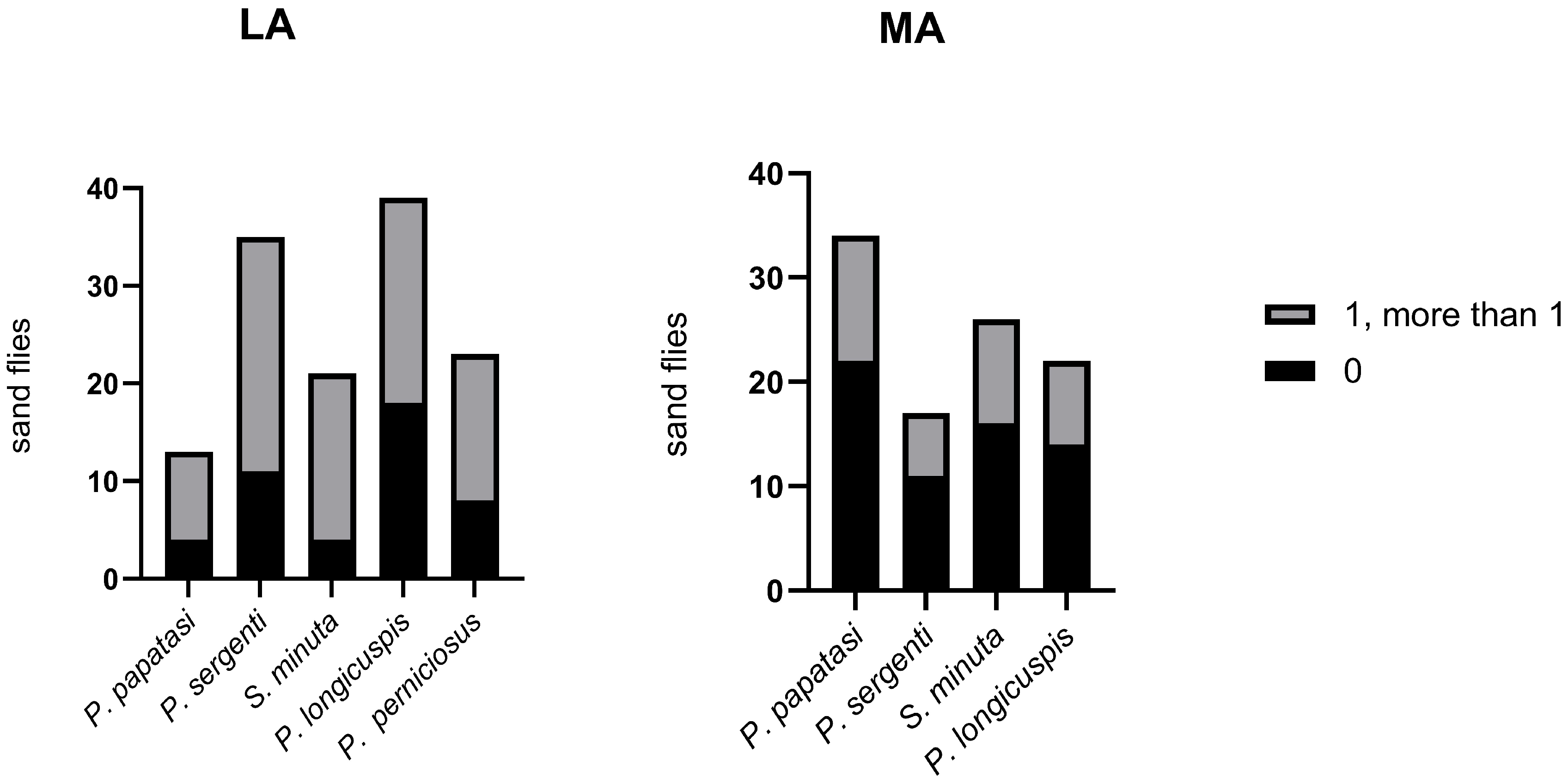
| Location | P. papatasi | P. longicuspis | P. sergenti | P. perniciosus | S. minuta | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | bspp | n | bspr | n | bspp | n | bspp | n | bspp | n | |
| Lalla Aaziza | 9 | 2 | 21 | 3 | 24 | 7 | 15 | 3 | 17 | 2 | 89 |
| Marrakech | 22 | 4 | 11 | 4 | 14 | 5 | * | * | 16 | 2 | 71 |
| Total | 31 | 32 | 38 | 15 | 33 | 160 | |||||
| Sand Fly Species | Locality | Bacterial Species | Relative Abundance (%) |
|---|---|---|---|
| P. sergenti | Lalla Aaziza | Bacillus pumilus | 38.5 |
| Staphylococcus hominis | 7.7 | ||
| Staphylococcus spp. | 7.7 | ||
| Staphylococcus aureus | 15.4 | ||
| Escherichia coli | 15.4 | ||
| Morganella morganii | 7.7 | ||
| Pseudomonas aeruginosa | 7.7 | ||
| Marrakech | Bacillus pumilus | 33 | |
| Bacillus subtilis | 16.6 | ||
| Bacillus sp. | 16.6 | ||
| Escherichia coli | 16.6 | ||
| Nocardia ignorata | 16.6 | ||
| P. longicuspis | Lalla Aaziza | Bacillus pumilus | 71.4 |
| Staphylococcus aureus | 14.6 | ||
| Lysinibacillus fusiformis | 14 | ||
| Marrakech | Bacillus pumilus | 40 | |
| Bacillus subtilis | 20 | ||
| Escherichia coli | 20 | ||
| Serratia spp. | 20 | ||
| P. papatasi | Lalla Aaziza | Bacillus pumilus | 50 |
| Lysinibacillus fusiformis | 50 | ||
| Marrakech | Bacillus pumilus | 47.4 | |
| Bacillus subtilis | 14.2 | ||
| Bacillus simplex | 14.2 | ||
| Brukholderia fungorum | 14.2 | ||
| S. minuta | Lalla Aaziza | Bacillus pumilus | 50 |
| Bacillus licheniformis | 50 | ||
| Marrakech | Bacillus pumilus | 75 | |
| Bacillus subtilis | 25 | ||
| P. perniciosus | Lalla Aaziza | Bacillus subtilis | 60 |
| Staphylococcus hominis | 20 | ||
| Staphylococcus aureus | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daoudi, M.; Outammassine, A.; Redouane, E.M.; Loqman, S.; Hafidi, M.; Boumezzough, A.; Olivier, M.; Boussaa, S.; Ndao, M. Characterization of Gut Bacteria in Natural Populations of Sand Flies (Diptera: Psychodidae) from Endemic and Non-Endemic Areas of Leishmaniasis in Morocco. Microorganisms 2025, 13, 2279. https://doi.org/10.3390/microorganisms13102279
Daoudi M, Outammassine A, Redouane EM, Loqman S, Hafidi M, Boumezzough A, Olivier M, Boussaa S, Ndao M. Characterization of Gut Bacteria in Natural Populations of Sand Flies (Diptera: Psychodidae) from Endemic and Non-Endemic Areas of Leishmaniasis in Morocco. Microorganisms. 2025; 13(10):2279. https://doi.org/10.3390/microorganisms13102279
Chicago/Turabian StyleDaoudi, Mohamed, Abdelkrim Outammassine, El Mahdi Redouane, Souad Loqman, Mohamed Hafidi, Ali Boumezzough, Martin Olivier, Samia Boussaa, and Momar Ndao. 2025. "Characterization of Gut Bacteria in Natural Populations of Sand Flies (Diptera: Psychodidae) from Endemic and Non-Endemic Areas of Leishmaniasis in Morocco" Microorganisms 13, no. 10: 2279. https://doi.org/10.3390/microorganisms13102279
APA StyleDaoudi, M., Outammassine, A., Redouane, E. M., Loqman, S., Hafidi, M., Boumezzough, A., Olivier, M., Boussaa, S., & Ndao, M. (2025). Characterization of Gut Bacteria in Natural Populations of Sand Flies (Diptera: Psychodidae) from Endemic and Non-Endemic Areas of Leishmaniasis in Morocco. Microorganisms, 13(10), 2279. https://doi.org/10.3390/microorganisms13102279







