Oenological Potential of Lachancea thermotolerans and Hanseniaspora uvarum from High-Sugar Musts: Impacts on Fermentation and Wine Volatilome
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Autochthonous Wine Yeasts
2.2. Culture Media and Enzymatic Screening Procedures
2.2.1. Wallerstein Laboratory Nutrient Agar (WLN Agar)
2.2.2. Lysine Medium
2.2.3. Determination of Yeast Killer Activity
2.2.4. Medium for Detecting β-Glucosidase Activity
2.2.5. Medium for Detecting Cellulase Activity
2.2.6. YPD Agar Medium
2.3. Yeast Identification
2.4. Alcoholic Fermentation Tests in Synthetic Media
2.4.1. Preparation of Pre-Inoculum
2.4.2. Fermentation Monitoring
2.5. Determination of Microbiological Parameters
2.6. Determination of Oenological Parameters
2.7. Analytical Determinations of Major Aroma Compounds
2.8. Statistical Analysis
3. Results and Discussion
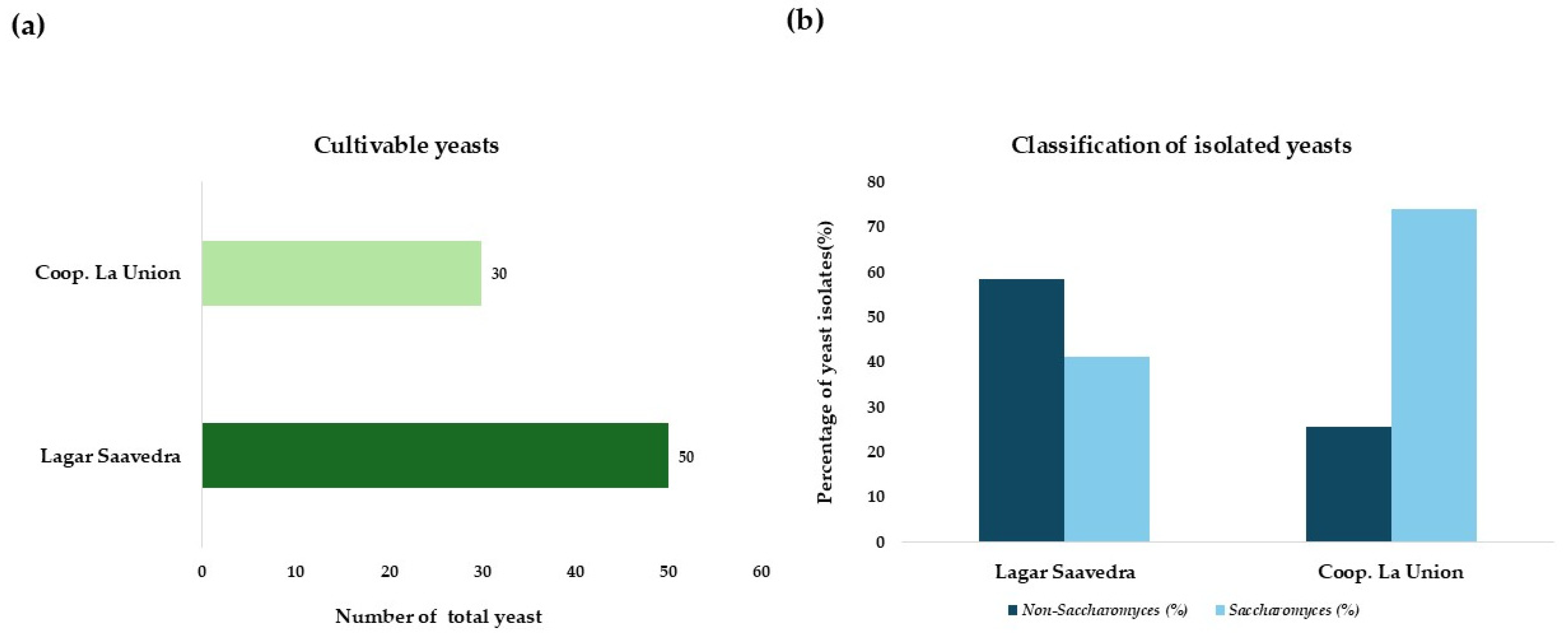
3.1. Microbiological Analysis
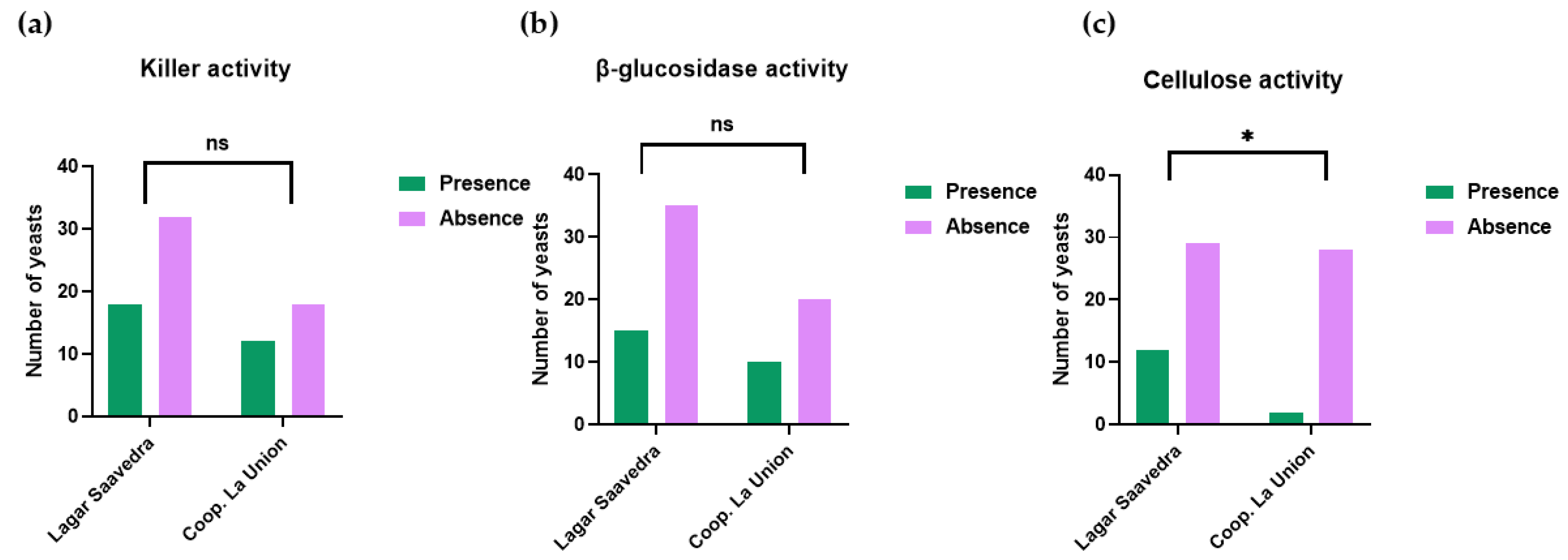
3.2. Yeast Identification and Selection
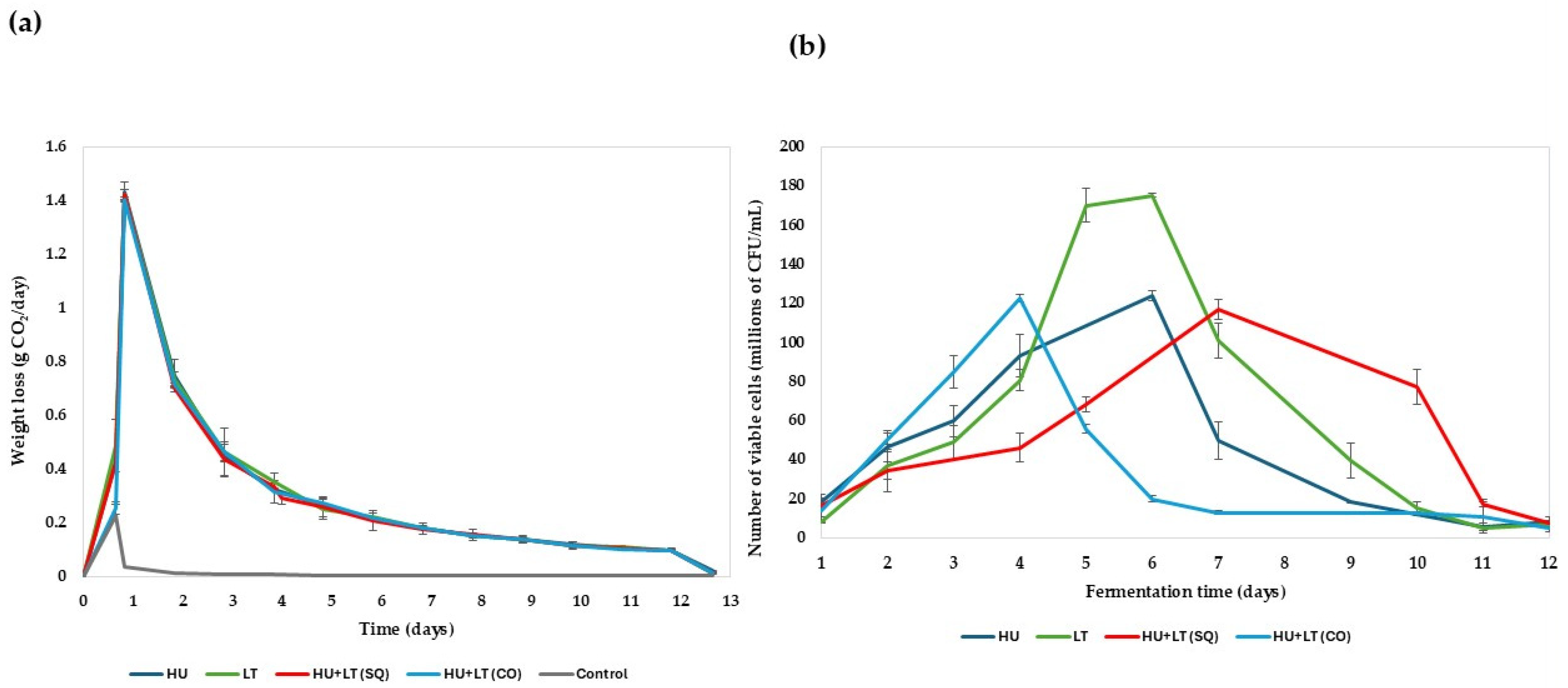
3.3. Fermentation Kinetics
3.4. Oenological Parameters
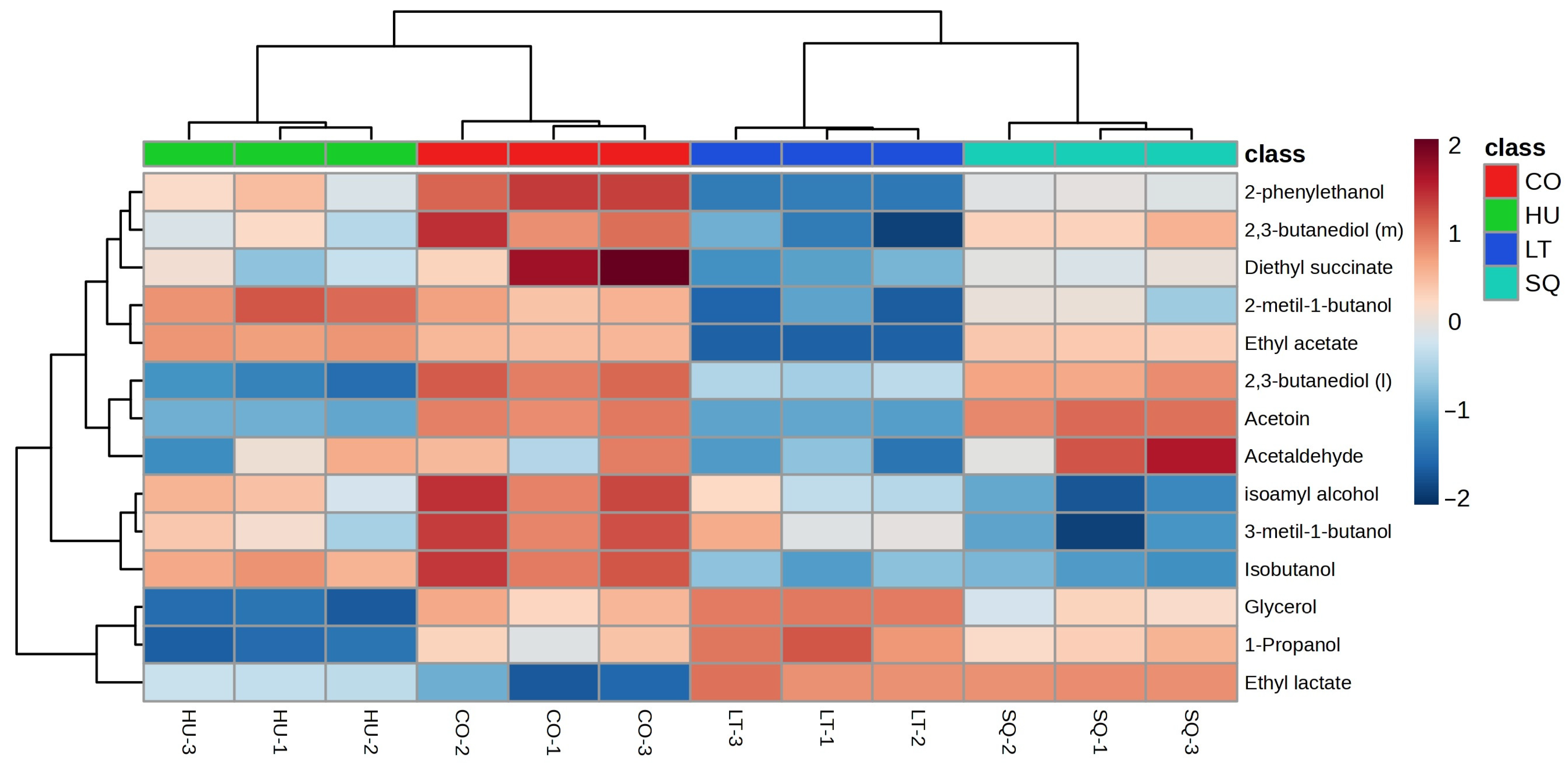
3.5. Major Aroma and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Testa, B.; Coppola, F.; Lombardi, S.J.; Iorizzo, M.; Letizia, F.; Di Renzo, M.; Succi, M.; Tremonte, P. Influence of Hanseniaspora uvarum AS27 on chemical and sensorial characteristics of Aglianico wine. Processes 2021, 9, 326. [Google Scholar] [CrossRef]
- Mancic, S.; Stamenković Stojanović, S.; Danilović, B.; Djordjević, N.; Malićanin, M.; Lazić, M.; Karabegović, I. Oenological characterization of native Hanseniaspora uvarum strains. Fermentation 2022, 8, 92. [Google Scholar] [CrossRef]
- Mateo, J.J.; Maicas, S. Application of non-Saccharomyces yeasts to wine-making process. Fermentation 2016, 2, 14. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Gao, H.; Bai, X.; Li, L.; Wei, R.; Dong, Z. Metabolomics and flavor diversity in Cabernet Sauvignon wines fermented by various origins of Hanseniaspora uvarum in the presence and absence of Saccharomyces cerevisiae. LWT 2024, 203, 116396. [Google Scholar] [CrossRef]
- Martin, V.; Valera, M.J.; Medina, K.; Boido, E.; Carrau, F. Oenological impact of the Hanseniaspora/Kloeckera yeast genus on wines—A review. Fermentation 2018, 4, 76. [Google Scholar] [CrossRef]
- Kurtzman, C.P. Phylogenetic circumscription of Saccharomyces, Kluyveromyces and other members of the Saccharomycetaceae, and the proposal of the new genera Lachancea, Nakaseomyces, Naumovia, Vanderwaltozyma and Zygotorulaspora. FEMS Yeast Res. 2003, 4, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Jin, G.J.; Mei, W.C.; Li, T.; Tao, Y.S. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar] [CrossRef]
- Borren, E.; Tian, B. The important contribution of non-Saccharomyces yeasts to the aroma complexity of wine: A review. Foods 2021, 10, 13. [Google Scholar] [CrossRef]
- Rementeria, A.; Rodriguez, J.A.; Cadaval, A.; Amenabar, R.; Muguruza, J.R.; Hernando, F.L.; Sevilla, M.J. Yeast associated with spontaneous fermentations of white wines from the “Txakoli de Bizkaia” region (Basque Country, North Spain). Int. J. Food Microbiol. 2003, 86, 201–207. [Google Scholar] [CrossRef]
- Ganter, P.F. Yeast and invertebrate associations. In Biodiversity and Ecophysiology of Yeasts; Springer: Berlin/Heidelberg, Germany, 2006; pp. 303–370. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Tesfaye, W.; Bañuelos, M.A.; González, C.; Suárez Lepe, J.A. Lachancea termotolerans applications in wine technology. Fermentation 2018, 4, 53. [Google Scholar] [CrossRef]
- Combina, M.; Elía, A.; Mercado, L.; Catania, C.; Ganga, A.; Martinez, C. Dynamics of indigenous yeast populations during spontaneous fermentation of wines from Mendoza, Argentina. Int. J. Food Microbiol. 2005, 99, 237–243. [Google Scholar] [CrossRef]
- Escribano, R.; González-Arenzana, L.; Garijo, P.; Berlanas, C.; López-Alfaro, I.; López, R.; Gutiérrez, A.R.; Santamaría, P. Screening of enzymatic activities within different enological non-Saccharomyces yeasts. J. Food Sci. Technol. 2017, 54, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Aponte, M.; Blaiotta, G. Potential role of yeast strains isolated from grapes in the production of Taurasi DOCG. Front. Microbiol. 2016, 7, 809. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, C.; Escott, C.; Heras, J.M.; Carrau, F.; Morata, A. Co-inoculations of Lachancea thermotolerans with different Hanseniaspora spp.: Acidification, aroma, biocompatibility, and effects of nutrients in wine. Food Res. Int. 2022, 161, 111891. [Google Scholar] [CrossRef]
- Muñoz-Castells, R.; Moreno, J.; García-Martínez, T.; Mauricio, J.C.; Moreno-García, J. Assessing the impact of commercial Lachancea thermotolerans immobilized in biocapsules on wine quality: Odor active compounds and organoleptic properties. Fermentation 2024, 10, 303. [Google Scholar] [CrossRef]
- Alcalá-Jiménez, M.T.; García-Martínez, T.; Mauricio, J.C.; Moreno, J.; Peinado, R.A. Influence of terroir on microbial diversity and wine volatilome. Appl. Sci. 2025, 15, 3237. [Google Scholar] [CrossRef]
- Ramírez, M.; Vinagre, A.; Ambrona, J.; Molina, F.; Maqueda, M.; Rebollo, J.E. Genetic instability of heterozygous hybrid populations of natural wine yeasts. Appl. Environ. Microbiol. 2004, 70, 4686–4691. [Google Scholar] [CrossRef]
- Kaiser, C.; Michaelis, S.; Mitchell, A. Methods in Yeast Genetics; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1994. [Google Scholar]
- Lodder, J.; Kreger-van Rij, N.J.W. The Yeasts: A Taxonomic Study, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1952; pp. 84–85. [Google Scholar]
- Öztekin, S.; Karbancioglu-Guler, F. Biological control of Green Mould on Mandarin fruit through the combined use of antagonistic yeasts. Biol. Control 2023, 180, 105186. [Google Scholar] [CrossRef]
- Alcalá-Jiménez, M.T.; García-García, J.C.; Mauricio, J.C.; Moreno, J.; Peinado, R.; García-Martínez, T. Selection of non-Saccharomyces yeasts from extreme oenological environments for potential use in winemaking. Microorganisms 2025, 13, 1260. [Google Scholar] [CrossRef]
- Spedding, G. Alcohol and its measurement. In Brewing Materials and Processes; Academic Press: Cambridge, MA, USA, 2016; pp. 123–149. [Google Scholar] [CrossRef]
- OIV. International Code of Oenological Practices; International Organisation of Vine and Wine: Dijon, France, 2023. [Google Scholar]
- Pilone, G.J. Determination of ethanol in wine by titrimetric and spectrophotometric dichromate methods: Collaborative study. J. AOAC Int. 1985, 68, 188–190. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.A.; Muñoz, D.; Medina, M.; Moreno, J. Gas chromatographic quantification of major volatile compounds and polyols in wine by direct injection. J. Agric. Food Chem. 2004, 52, 6389–6393. [Google Scholar] [CrossRef]
- Miranda, A.; Pereira, V.; Jardim, H.; Malfeito-Ferreira, M.; Marques, J.C. Impact of non-Saccharomyces yeast fermentation in madeira wine chemical composition. Processes 2023, 11, 482. [Google Scholar] [CrossRef]
- Ruiz-Muñoz, M.; Hernández-Fernández, M.; Cordero-Bueso, G.; Martínez-Verdugo, S.; Pérez, F.; Cantoral, J.M. Non-Saccharomyces are also forming the veil of flor insherry wines. Fermentation 2022, 8, 456. [Google Scholar] [CrossRef]
- Michlmayr, H.; Kneifel, W. β-Glucosidase activities of lactic acid bacteria: Mechanisms, impact on fermented food and human health. FEMS Microbiol. Lett. 2014, 352, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Fang, Z.; Pai, A.; Luo, J.; Gan, R.; Gao, Y.; Lu, J.; Zhang, P. Glycosidically bound aroma precursors in fruits: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 215–243. [Google Scholar] [CrossRef]
- Romo-Sánchez, S.; Arévalo-Villena, M.; García Romero, E.; Ramirez, H.L.; Briones Pérez, A. Immobilization of β-glucosidase and its application for enhancement of aroma precursors in muscat wine. FABT 2014, 7, 1381–1392. [Google Scholar] [CrossRef]
- Maturano, Y.P.; Toro, M.E.; Castellanos, L.I.; Vazquez, F. Determinación de actividades celulolítica y xilanolítica en levaduras no-Saccharomyces de origen enológico. Rev. Enol. 2007, 2, 1–9. [Google Scholar]
- Maturano, Y.P.; Assof, M.; Fabani, M.P.; Nally, M.C.; Jofré, V.; Rodríguez Assaf, L.A.; Toro, M.E.; Castellanos de Figueroa, L.I.; Vazquez, F. Enzymatic activities produced by mixed Saccharomyces and non-Saccharomyces cultures: Relationship with wine volatile composition. Antonie Van Leeuwenhoek 2015, 108, 1239–1256. [Google Scholar] [CrossRef]
- González Alonso, I. Aislamiento y caracterización de levaduras asociadas a la uva y a la fermentación espontánea de la variedad Negro Saurí. Ph.D. Thesis, Universidad de León, León, Spain, 2021. [Google Scholar]
- Muradova, M.; Proskura, A.; Canon, F.; Aleksandrova, I.; Schwartz, M.; Heydel, J.-M.; Baranenko, D.; Nadtochii, L.; Neiers, F. Unlocking flavor potential using microbial β-glucosidases in food processing. Foods 2023, 12, 4484. [Google Scholar] [CrossRef]
- Sánchez-Suárez, F.; de Lerma, N.L.; del Valle Palenzuela, M.; Rosal, A.; Moreno, J.; Peinado, R.A. Empleo de levaduras no-Saccharomyces como estrategia para aumentar la acidez de vinos dulces en un contexto de cambio climático. BIO Web Conf. 2023, 68, 02042. [Google Scholar] [CrossRef]
- Moreno, J.; Peinado, R. Enological Chemistry; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Gómez-Míguez, M.J.; Cacho, J.F.; Ferreira, V.; Vicario, I.M.; Heredia, F.J. Volatile components of Zalema white wines. Food Chem. 2007, 100, 1464–1473. [Google Scholar] [CrossRef]
- Valera, M.J.; Boido, E.; Dellacassa, E.; Carrau, F. Comparison of the glycolytic and alcoholic fermentation pathways of Hanseniaspora vineae with Saccharomyces cerevisiae wine yeasts. Fermentation 2020, 6, 78. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J.; Zhu, B.; Liu, J.; Che, Y.; Li, C.; Song, J.; Zheng, Y.; Wang, M. Improving the sensory quality of Xinhui Citrus wine by mixed yeasts fermentation of Saccharomyces cerevisiae and non-Saccharomyces. Food Bioeng. 2025, 4, 53–66. [Google Scholar] [CrossRef]
- Bartolomé, R.; Alonso, E.; Morata, A.; López, C. Production of Lambic-like fruit sour beer with Lachancea thermotolerans. Antioxidants 2025, 14, 826. [Google Scholar] [CrossRef] [PubMed]
- Delač Salopek, D.; Vrhovsek, U.; Carlin, S.; Radeka, S.; Lukić, I. In-depth characterization of the volatile aroma profile and other characteristics of white wine produced by sequential inoculation with a Lachancea thermotolerans starter yeast strain. Fermentation 2024, 10, 515. [Google Scholar] [CrossRef]
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Acetate ester formation in wine by mixed cultures in laboratory fermentations. Int. J. Food Microbiol. 2003, 86, 181–188. [Google Scholar] [CrossRef]
- Benito-Castellanos, A.; Larreina, B.; Banda, M.T.C.d.L.; Santamaría, P.; González-Arenzana, L.; Gutiérrez, A.R. Biodiversity of yeast species isolated during spontaneous fermentation: Influence of grape origin, vinification conditions, and year of study. Microorganisms 2025, 13, 1707. [Google Scholar] [CrossRef]
- del Fresno, J.M.; Rodríguez, Y.; Soler, M.; Escott, C.; Palomero, F.; Cuerda, R.; Morata, A. Use of non-Saccharomyces yeasts to modulate oenological parameters in Albillo Mayor white wines. Eur. Food Res. Technol. 2025, 1–13. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, S.; Zhang, X. Effects of mixed adding crude extracts of β-glucosidases from three different non-Saccharomyces yeast strains on the quality of Cabernet Sauvignon wines. J. Fungi 2022, 8, 710. [Google Scholar] [CrossRef]
- Gallo, A.; Roman, T.; Paolini, M.; Cappello, N.; Guzzon, R.; Carrau, F.; Schneider, R.; Larcher, R. Características aromáticas de vinos con Hanseniaspora vineae Hv205 en estrategias secuenciales y de coinoculación. Fermentation 2024, 10, 191. [Google Scholar] [CrossRef]
- Zhang, M.; Zhong, T.; Heygi, F.; Wang, Z.; Du, M. Effects of inoculation protocols on aroma profiles and quality of plum wine in mixed culture fermentation of Metschnikowia pulcherrima with Saccharomyces cerevisiae. LWT 2022, 161, 113338. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, X.; Mao, Y.; Zhang, X.; Xu, B.; Yang, X. Effect of different inoculation strategies of mixed culture Saccharomyces cerevisiae/Oenococcus oeni on the aroma quality of Chardonnay wine. Food Res. Int. 2024, 190, 114636. [Google Scholar] [CrossRef] [PubMed]

| Oenological Parameters | H. uvarum | L. thermotolerans | Co-Inoculation | Sequential |
|---|---|---|---|---|
| Volatile acidity (g/L) | 0.176 ± 0.01 a | 0.349 ± 0.04 a | 0.332 ± 0.01 a | 0.343 ± 0.08 b |
| Reducing sugar(g/L) | 95.7 ± 1.6 c | 70.83 ± 3.4 a | 83.2 ± 2.5 b | 100.5 ± 0.00 d |
| Ethanol (%, v/v) | 5.20 ± 0.02 a | 7.00 ± 0.02 c | 6.20 ± 0.01 b | 5.30 ± 0.02 a |
| Compounds (mg/L) | H. uvarum | L. thermotolerans | Co-Inoculation | Sequential (SQ) |
|---|---|---|---|---|
| Alcohols | ||||
| 1-Propanol | 7.0 ± 0.3 a | 16.4 ± 1.2 c | 12.6 ±1.1 b | 13.3 ± 0.8 b |
| Isobutanol | 21.3 ± 0.6 b | 15.0 ± 0.7 a | 24.0 ± 1.2 c | 14.4 ± 0.6 a |
| Isoamyl alcohols | 46.3 ± 3.1 b | 42.8 ± 2.7 b | 54.5 ± 2.5 c | 35.2 ± 2.4 a |
| 2-phenylethanol | 11.5 ± 1.9 b | 4.8 ± 0.1 a | 21.0 ± 1.7 c | 9.9 ± 0.2 b |
| Carbonyl Compounds | ||||
| Acetaldehyde | 174.8 ± 15.3 a | 160.1 ± 5.6 ab | 183.1 ±12.1 ab | 193.7 ± 15.6 b |
| Acetoin | 145.8 ± 5.9 a | 132.7 ± 5.5 a | 155.0 ± 2.0 b | 156.0 ± 1.0 b |
| Esters | ||||
| Ethyl lactate | 9.8 ± 0.5 a | 70.4 ± 7.0 b | 64.7 ± 2.0 b | 14.0 ± 0.1 a |
| Diethyl succinate | 126.0± 1.0 a | 134.2± 2.0 b | 133.2± 3.0 b | 128.0± 3.0 a |
| Ethyl acetate | 8.9 ± 0.8 d | 0.0± 0.0 a | 5.3 ± 0.3 c | 3.8 ± 0.3 b |
| Polyols | ||||
| 2,3-Butanediol (levo) | 47.4± 4 a | 69.3 ± 2.7 b | 137.9 ± 7 d | 117.8 ± 6 c |
| 2,3-Butanediol (meso) | 47.1 ± 3.9 b | 34.4 ± 4.4 a | 64.0 ± 5.0 c | 53.5 ± 2.0 b |
| Glycerol (g/L) | 1.90 ± 0.06 a | 3.9 ± 0.01 d | 3.20 ± 0.1 c | 2.90 ± 0.16 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcalá-Jiménez, M.T.; García-García, J.C.; Mauricio, J.C.; Moreno, J.; Peinado, R.A.; García-Martínez, T. Oenological Potential of Lachancea thermotolerans and Hanseniaspora uvarum from High-Sugar Musts: Impacts on Fermentation and Wine Volatilome. Microorganisms 2025, 13, 2260. https://doi.org/10.3390/microorganisms13102260
Alcalá-Jiménez MT, García-García JC, Mauricio JC, Moreno J, Peinado RA, García-Martínez T. Oenological Potential of Lachancea thermotolerans and Hanseniaspora uvarum from High-Sugar Musts: Impacts on Fermentation and Wine Volatilome. Microorganisms. 2025; 13(10):2260. https://doi.org/10.3390/microorganisms13102260
Chicago/Turabian StyleAlcalá-Jiménez, María Trinidad, Juan Carlos García-García, Juan Carlos Mauricio, Juan Moreno, Rafael A. Peinado, and Teresa García-Martínez. 2025. "Oenological Potential of Lachancea thermotolerans and Hanseniaspora uvarum from High-Sugar Musts: Impacts on Fermentation and Wine Volatilome" Microorganisms 13, no. 10: 2260. https://doi.org/10.3390/microorganisms13102260
APA StyleAlcalá-Jiménez, M. T., García-García, J. C., Mauricio, J. C., Moreno, J., Peinado, R. A., & García-Martínez, T. (2025). Oenological Potential of Lachancea thermotolerans and Hanseniaspora uvarum from High-Sugar Musts: Impacts on Fermentation and Wine Volatilome. Microorganisms, 13(10), 2260. https://doi.org/10.3390/microorganisms13102260







