Remdesivir: Effectiveness and Safety in Hospitalized COVID-19 Patients—Analysis of Retrospectively Collected Data from Daily Practice in the Omicron Variant Era and Comparison with the Pre-Omicron Period
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Eligibility Criteria
2.2. Endpoints
2.3. Ethics
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
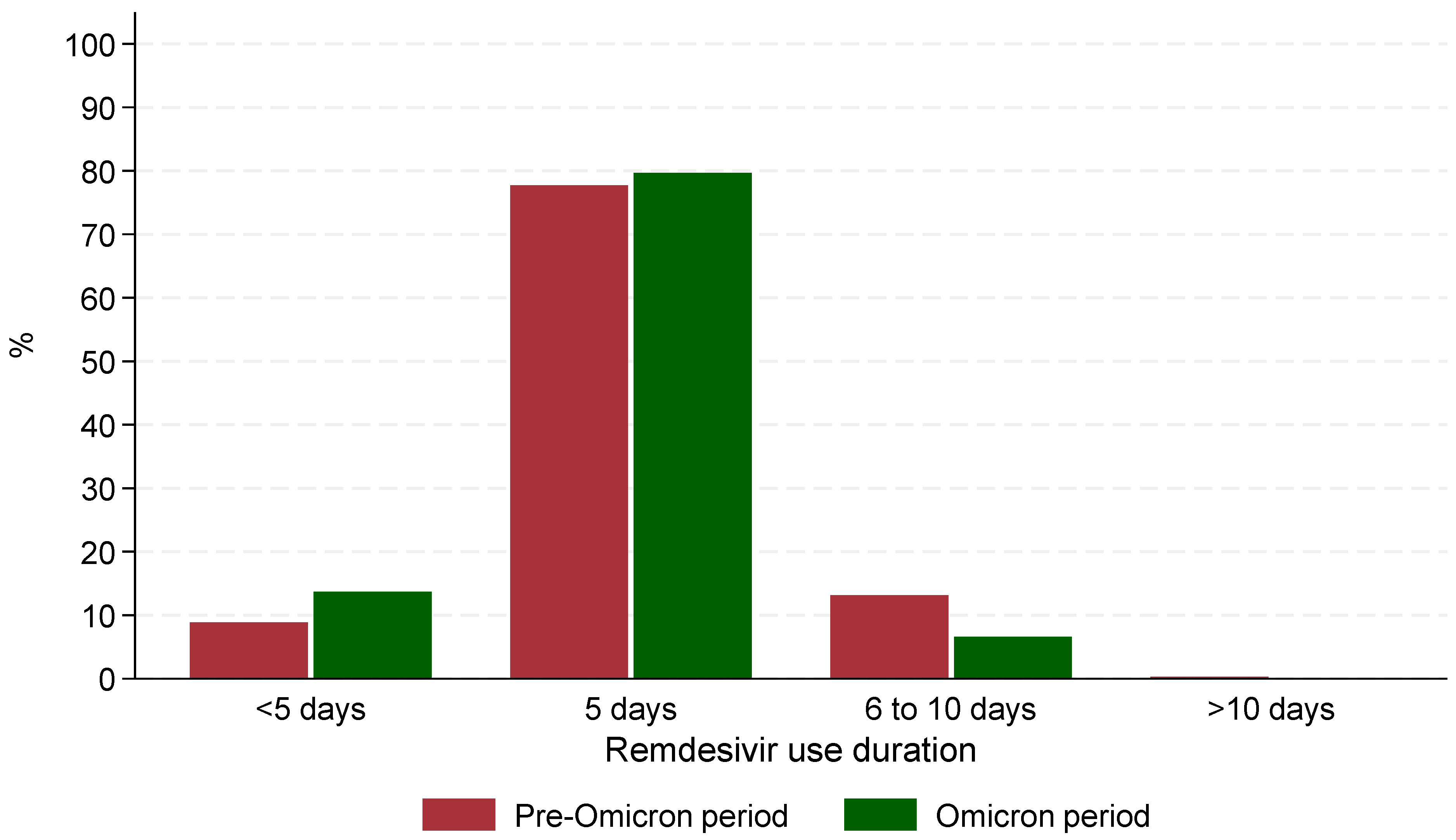
3.2. Remdesivir Patterns of Use
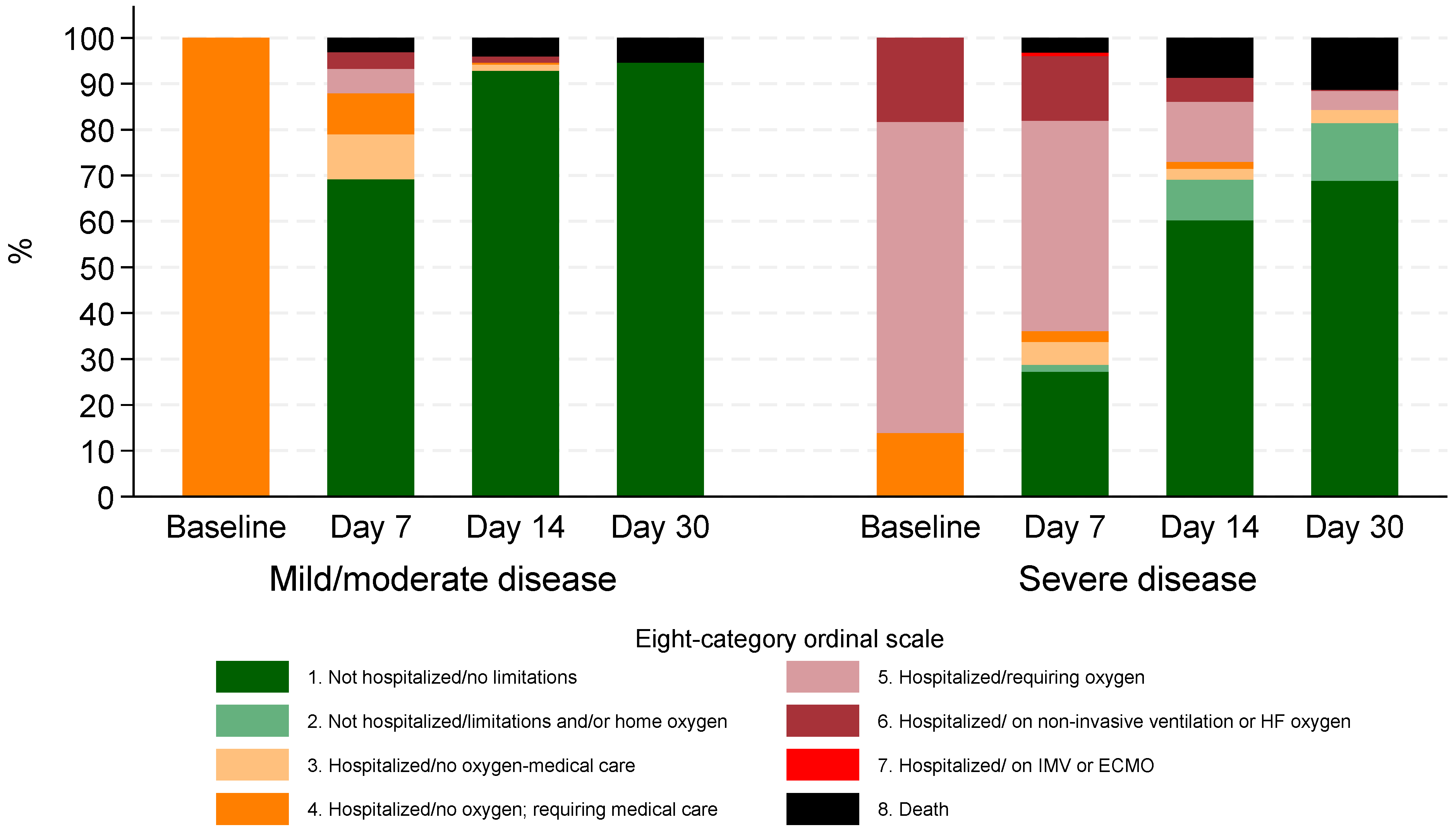
3.3. Clinical Progression and Mortality
3.4. Effectiveness of Remdesivir
3.5. Side Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Variable | Pre-Omicron Period | Omicron Period | Overall | p-Value |
|---|---|---|---|---|
| n = 1004 (62.4%) | n = 606 (37.6%) | n = 1610 (100%) | ||
| Arterial pH | 7.5 (7.4, 7.5) | 7.4 (7.4, 7.5) | 7.5 (7.4, 7.5) | <0.001 |
| Partial pressure of CO2 (PaCO2, mmHg) | 30.9 (28.3, 34.1) | 34.0 (30.0, 37.8) | 32.0 (28.7, 35.4) | <0.001 |
| Partial pressure of O2 (PaO2, mmHg) | 66.6 (59.3, 78.0) | 67.0 (58.0, 76.7) | 66.6 (58.9, 77.0) | 0.089 |
| Bicarbonate (HCO3−, mmol/L) | 24.2 (22.0, 25.9) | 24.0 (21.9, 26.2) | 24.0 (22.0, 26.0) | 0.913 |
| Oxygen saturation (SaO2, %) | 95.0 (92.6, 96.1) | 94.0 (92.0, 96.0) | 95.0 (92.0, 96.0) | 0.016 |
| Hematocrit (%, %) | 40.6 (37.4, 43.6) | 37.7 (33.7, 41.9) | 39.8 (36.2, 42.9) | <0.001 |
| Hemoglobin (g/dL) | 13.6 (12.5, 14.7) | 12.5 (11.1, 13.9) | 13.2 (12.0, 14.4) | <0.001 |
| White blood cell count (103/μL) | 5530.0 (4305.0, 7195.0) | 3330.0 (7.0, 7120.0) | 5300.0 (3450.0, 7170.0) | <0.001 |
| Neutrophil count (103/μL) | 3.9 (2.8, 5.6) | 1620.5 (5.1, 5140.0) | 5.1 (3.3, 14.1) | <0.001 |
| Neutrophils (% of WBCs) | 71.7 (64.2, 79.7) | 74.7 (65.6, 83.0) | 72.7 (64.8, 81.1) | <0.001 |
| Lymphocyte count (103/μL) | 1.1 (0.8, 1.6) | 300.0 (1.0, 1070.0) | 1.3 (0.8, 18.7) | <0.001 |
| Lymphocytes (% of WBCs) | 18.6 (12.6, 25.2) | 14.8 (9.5, 21.4) | 17.2 (11.2, 24.3) | <0.001 |
| Platelet count (103/μL) | 190.0 (154.5, 237.0) | 94,900.0 (194.0, 195,000.0) | 210.6 (164.0, 336.0) | <0.001 |
| C-reactive protein (CRP, mg/L) | 21.1 (6.7, 69.2) | 20.1 (6.9, 61.4) | 20.9 (6.8, 66.9) | 0.551 |
| Procalcitonin (PCT, ng/mL) | 0.1 (0.1, 0.2) | 0.1 (0.1, 0.2) | 0.1 (0.1, 0.2) | 0.153 |
| Ferritin (ng/mL) | 388.5 (192.5, 757.1) | 211.3 (105.0, 449.2) | 327.0 (154.0, 669.0) | <0.001 |
| International Normalized Ratio (INR) | 1.0 (1.0, 1.1) | 1.1 (1.0, 1.3) | 1.1 (1.0, 1.2) | <0.001 |
| Covariate | Odds Ratio | 95% C.I. | p-Value |
|---|---|---|---|
| Period | |||
| – Pre-Omicron (reference) | |||
| – Omicron | 2.54 | (1.66, 3.89) | <0.001 |
| Covariate | Odds Ratio | 95% C.I. | p-Value |
|---|---|---|---|
| Period | |||
| – Pre-Omicron (reference) | |||
| – Omicron | 1.23 | (0.76, 1.97) | 0.398 |
| Age (per 10 years) | 2.10 | (1.72, 2.57) | <0.001 |
| Charlson Comorbidity Index (without age contribution; per unit) | 1.07 | (0.89, 1.27) | 0.485 |
| Baseline severity | |||
| – Mild/moderate (reference) | |||
| – Severe | 2.43 | (1.32, 4.50) | 0.005 |
| Covariate | Odds Ratio | 95% C.I. | p-Value |
|---|---|---|---|
| Fully vaccinated | |||
| – No (reference) | |||
| – Yes | 0.45 | (0.25, 0.81) | 0.007 |
| Age (per 10 years) | 1.85 | (1.41, 2.42) | <0.001 |
| Charlson Comorbidity Index (without age contribution; per unit) | 0.91 | (0.71, 1.18) | 0.488 |
| Baseline severity | |||
| – Mild/moderate (reference) | |||
| – Severe | 1.66 | (0.84, 3.31) | 0.147 |
References
- Ader, F.; Bouscambert-Duchamp, M.; Hites, M.; Peiffer-Smadja, N.; Poissy, J.; Belhadi, D.; Diallo, A.; Lê, M.P.; Peytavin, G.; Descamps, D.; et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): A phase 3, randomised, controlled, open-label trial. Lancet Infect. Dis. 2022, 22, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, J.; Sobocinska, J.; Lewicki, M.; Lemanska, Z.; Rzymski, P. When science goes viral: The research response during three months of the COVID-19 outbreak. Biomed. Pharmacother. 2020, 129, 110451. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Tian, D. Bibliometric analysis of global scientific research on COVID-19. J. Biosaf. Biosecurity 2021, 3, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.G.; Carafoli, E. Remdesivir: From Ebola to COVID-19. Biochem. Biophys. Res. Commun. 2021, 538, 145–150. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G.; et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Eastman, R.T.; Roth, J.S.; Brimacombe, K.R.; Simeonov, A.; Shen, M.; Patnaik, S.; Hall, M.D.; Hooper, A.T.; Kleinschmidt, H.D.; McKnight, C.; et al. Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19. ACS Cent. Sci. 2020, 6, 672–683. [Google Scholar] [CrossRef]
- Holshue, M.L.; DeBolt, C.; Lindquist, S.; Lofy, K.H.; Wiesman, J.; Bruce, H.; Spitters, C.; Ericson, K.; Wilkerson, S.; Tural, A.; et al. First Case of 2019 Novel Coronavirus in the United States. N. Engl. J. Med. 2020, 382, 929–936. [Google Scholar] [CrossRef]
- Frediansyah, A.; Nainu, F.; Dhama, K.; Mudatsir, M.; Harapan, H. Remdesivir and its antiviral activity against COVID-19: A systematic review. Clin. Epidemiol. Glob. Health 2021, 9, 123–127. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, A.; Singh, R.; Misra, A. Remdesivir in COVID-19: A critical review of pharmacology, pre-clinical and clinical studies. Diabetes Metab. Syndr. 2020, 14, 641–648. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Goldman, J.D.; Lye, D.C.B.; Hui, D.S.; Marks, K.M.; Bruno, R.; Montejano, R.; Spinner, C.D.; Galli, M.; Ahn, M.Y.; Nahass, R.G.; et al. Remdesivir for 5 or 10 Days in Patients with Severe COVID-19. N. Engl. J. Med. 2020, 383, 1827–1837. [Google Scholar] [CrossRef]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; Arribas López, J.R.; Cattelan, A.M.; Soriano Viladomiu, A.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; Castagna, A.; et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients with Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 1048–1057. [Google Scholar] [CrossRef]
- Delgado, A.; Stewart, S.; Urroz, M.; Rodriguez, A.; Borobia, A.M.; Akatbach-Bousaid, I.; Garcia, M.; Jimenez, D.; Duran, J.C.; Dominguez, L.; et al. Characterisation of Drug-Induced Liver Injury in Patients with COVID-19 Detected by a Proactive Pharmacovigilance Program from Laboratory Signals. J. Clin. Med. 2021, 10, 4432. [Google Scholar] [CrossRef] [PubMed]
- US Food & Drug Administration. FDA Approves First Treatment for COVID-19; US Food & Drug Administration: Silver Spring, MD, USA, 2020.
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.X.; et al. Compassionate Use of Remdesivir for Patients with Severe COVID-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Hellenic Society of Infectious Diseases. Therapeutic Algorithm of Adult Hospitalized Patients with COVID-19; Hellenic Society of Infectious Diseases: Athens, Greece, 2022. [Google Scholar]
- Peck, K.M.; Lauring, A.S. Complexities of Viral Mutation Rates. J. Virol. 2018, 92, e01031-17. [Google Scholar] [CrossRef]
- World Health Organization. Tracking SARS-CoV-2 Variants; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Andre, M.; Lau, L.S.; Pokharel, M.D.; Ramelow, J.; Owens, F.; Souchak, J.; Salom, J.; Harkins, E.; Hennekens, C.; Cormier, J.; et al. From Alpha to Omicron: How Different Variants of Concern of the SARS-Coronavirus-2 Impacted the World. Biology 2023, 12, 1267. [Google Scholar] [CrossRef]
- Dryden-Peterson, S.; Kim, A.; Kim, A.Y.; Caniglia, E.C.; Lennes, I.T.; Patel, R.; Jacobson, J.; Reynolds, G.; Johnson, K.; Miller, D.; et al. Nirmatrelvir Plus Ritonavir for Early COVID-19 in a Large U.S. Health System: A Population-Based Cohort Study. Ann. Intern. Med. 2023, 176, 77–84. [Google Scholar] [CrossRef]
- Focosi, D.; Maggi, F.; McConnell, S.; Casadevall, A. Very low levels of remdesivir resistance in SARS-CoV-2 genomes after 18 months of massive usage during the COVID19 pandemic: A GISAID exploratory analysis. Antivir. Res. 2022, 198, 105247. [Google Scholar] [CrossRef]
- Wiegand, T.; Nemudryi, A.; Nemudraia, A.; McVey, A.; Little, A.; Taylor, D.N.; Phan, M.V.T.; Green, C.; Pipas, J.M.; Koelle, K.; et al. The Rise and Fall of SARS-CoV-2 Variants and Ongoing Diversification of Omicron. Viruses 2022, 14, 2009. [Google Scholar] [CrossRef]
- Hakmaoui, A.; Khan, F.; Liacini, A.; Kaur, A.; Berka, Y.; Machraoui, S.; Benali, M.; Naciri, S.; Lahlou, O.; El Kari, K.; et al. Relevant SARS-CoV-2 Genome Variation through Six Months of Worldwide Monitoring. Biomed. Res. Int. 2021, 2021, 5553173. [Google Scholar] [CrossRef] [PubMed]
- Eskier, D.; Karakulah, G.; Suner, A.; Oktay, Y. RdRp mutations are associated with SARS-CoV-2 genome evolution. PeerJ 2020, 8, e9587. [Google Scholar] [CrossRef]
- Stevens, L.J.; Pruijssers, A.J.; Lee, H.W.; Gordon, C.J.; Tchesnokov, E.P.; Gribble, J.; George, A.; Hill, C.; Barton, J.; Cihlar, T.; et al. Mutations in the SARS-CoV-2 RNA-dependent RNA polymerase confer resistance to remdesivir by distinct mechanisms. Sci. Transl. Med. 2022, 14, eabo0718. [Google Scholar] [CrossRef]
- Dobrowolska, K.; Zarebska-Michaluk, D.; Brzdek, M.; Rzymski, P.; Rogalska, M.; Moniuszko-Malinowska, A.; Wasniewski, T.; Szymanek-Pasternak, A.; Glowacka, A.; Wroblewska, A.; et al. Retrospective Analysis of the Effectiveness of Remdesivir in COVID-19 Treatment during Periods Dominated by Delta and Omicron SARS-CoV-2 Variants in Clinical Settings. J. Clin. Med. 2023, 12, 2371. [Google Scholar] [CrossRef]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, H.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [CrossRef]
- Pantazis, N.; Pechlivanidou, E.; Antoniadou, A.; Akinosoglou, K.; Kalomenidis, I.; Poulakou, G.; Rapti, V.; Kavatha, D.; Anagnostopoulos, A.; Kazakou, P.; et al. Remdesivir: Effectiveness and Safety in Hospitalized Patients with COVID-19 (ReEs-COVID-19)-Analysis of Data from Daily Practice. Microorganisms 2023, 11, 1998. [Google Scholar] [CrossRef]
- Wong, C.K.H.; Lau, K.T.K.; Au, I.C.H.; Xiong, X.; Lau, E.H.Y.; Cowling, B.J. Clinical Improvement, Outcomes, Antiviral Activity, and Costs Associated with Early Treatment with Remdesivir for Patients with Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2022, 74, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Pitts, J.; Li, J.; Perry, J.K.; Du Pont, V.; Riola, N.; Rodriguez, L.; Simmons, G.; Granato, M.; Li, M.; Hu, J.; et al. Remdesivir and GS-441524 Retain Antiviral Activity against Delta, Omicron, and Other Emergent SARS-CoV-2 Variants. Antimicrob. Agents Chemother. 2022, 66, e0022222. [Google Scholar] [CrossRef] [PubMed]
- Vangeel, L.; Chiu, W.; De Jonghe, S.; Maes, P.; Slechten, B.; Raymenants, J.; Andre, E.; Leyssen, P.; Neyts, J.; Jochmans, D.; et al. Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antivir. Res. 2022, 198, 105252. [Google Scholar] [CrossRef]
- Knight, S.R.; Ho, A.; Pius, R.; Buchan, I.; Carson, G.; Drake, T.M.; Dunning, J.; Fairfield, C.J.; Hardwick, H.E.; Holden, K.A.; et al. Risk stratification of patients admitted to hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. bmj 2020, 370, m3339. [Google Scholar] [CrossRef]
- National Public Health Organization. Therapeutic Algorithm for Adult Hospitalized Patients with COVID-19; National Public Health Organization: Marousi, Greece, 2021. (In Greek)
- Kidney Disease: Improving Global Outcomes Acute Kidney Injury Work, Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2 (Suppl. S1), 1–138. [Google Scholar]
- Mercado, M.G.; Smith, D.K.; Guard, E.L. Acute Kidney Injury: Diagnosis and Management. Am. Fam. Physician 2019, 100, 687–694. [Google Scholar] [PubMed]
- Humeniuk, R.; Mathias, A.; Cao, H.; Osinusi, A.; Shen, G.; Chng, E.; Ling, J.; German, P.; Mo, H.; McColl, D.; et al. Safety, Tolerability, and Pharmacokinetics of Remdesivir, An Antiviral for Treatment of COVID-19, in Healthy Subjects. Clin. Transl. Sci. 2020, 13, 896–906. [Google Scholar] [CrossRef]
- Mozaffari, E.; Chandak, A.; Gottlieb, R.L.; Chima-Melton, C.; Read, S.H.; Jiang, H.; Sarkar, P.; Wan, H.; Thrun, M.; Chokkalingam, A.P.; et al. Remdesivir Reduced Mortality in Immunocompromised Patients Hospitalized for COVID-19 Across Variant Waves: Findings from Routine Clinical Practice. Clin. Infect. Dis. 2023, 77, 1626–1634. [Google Scholar] [CrossRef]
- Rapti, V.; Papanikolopoulou, A.; Kokkotis, G.; Livanou, M.E.; Alexiou, P.; Pechlivanidou, E.; Rovina, N.; Karagiannidis, N.; Syrigos, K.; Antoniadou, A.; et al. The Burden of COVID-19 in Adult Patients with Hematological Malignancies: A Single-center Experience After the Implementation of Mass-vaccination Programs Against SARS-CoV-2. In Vivo 2023, 37, 2743–2754. [Google Scholar] [CrossRef]
- Godwin, P.O.; Polsonetti, B.; Caron, M.F.; Oppelt, T.F. Remdesivir for the Treatment of COVID-19: A Narrative Review. Infect. Dis. Ther. 2024, 13, 1–19. [Google Scholar] [CrossRef]
- Ryu, B.H.; Lee, J.Y.; Lee, S.H. The effect of early versus late remdesivir treatment in hospitalized mild to moderate COVID-19 patients in the Omicron era: A retrospective study. Medicine 2024, 103, e39035. [Google Scholar] [CrossRef]
- Molina, K.C.; Webb, B.J.; Kennerley, V.; Beaty, L.E.; Bennett, T.D.; Carlson, N.E.; Christensen, E.; Hohmann, S.; Zeng, C.; Donnelly, J.P.; et al. Real-world evaluation of early remdesivir in high-risk COVID-19 outpatients during Omicron including BQ.1/BQ.1.1/XBB.1.5. BMC Infect. Dis. 2024, 24, 802. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.C.; Chan, P.K.; Hung, C.T.; Wong, C.K.; Xiong, X.; Wei, Y.; Lee, J.; Leung, C.; Xu, W.; Chen, C.; et al. Changes in all-cause and cause-specific excess mortality before and after the Omicron outbreak of COVID-19 in Hong Kong. J. Glob. Health 2023, 13, 06017. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Xiao, J.; Jin, Y.; Jian, R.; Hu, Y.; Liang, X.; Li, L.; Sun, J.; Huang, R.; Wang, Y.; et al. Multilayer factors associated with excess all-cause mortality during the omicron and non-omicron waves of the COVID-19 pandemic: Time series analysis in 29 countries. BMC Public Health 2024, 24, 350. [Google Scholar] [CrossRef] [PubMed]
- Faust, J.S.; Du, C.; Liang, C.; Mayes, K.D.; Renton, B.; Panthagani, K.; Jin, G.; Das, A.; Li, Z.; Yoo, E.; et al. Excess Mortality in Massachusetts During the Delta and Omicron Waves of COVID-19. JAMA 2022, 328, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Basoulis, D.; Logioti, K.; Papaodyssea, I.; Chatzopoulos, M.; Alexopoulou, P.; Mavroudis, P.; Frouda, S.; Vlachogiannis, N.; Tziolos, N.; Karapanou, A.; et al. Deaths “due to” COVID-19 and deaths “with” COVID-19 during the Omicron variant surge, among hospitalized patients in seven tertiary-care hospitals, Athens, Greece. Sci. Rep. 2025, 15, 13728. [Google Scholar] [CrossRef]
- Seghezzo, G.; Allen, H.; Griffiths, C.; Pooley, J.; Beardsmore, L.; Caul, S.; Jones, C.; Oliver, D.; Price, S.; Watterson, T.; et al. Comparison of two COVID-19 mortality measures used during the pandemic response in England. Int. J. Epidemiol. 2024, 53, dyad116. [Google Scholar] [CrossRef]
- Chokkalingam, A.P.; Hayden, J.; Goldman, J.D.; Li, H.; Asubonteng, J.; Mozaffari, E.; Kost, R.G.; Batech, M.; He, X.; Wei, X.; et al. Association of Remdesivir Treatment with Mortality Among Hospitalized Adults with COVID-19 in the United States. JAMA Netw. Open 2022, 5, e2244505. [Google Scholar] [CrossRef]
- Takashita, E.; Fujisaki, S.; Morita, H.; Nagata, S.; Miura, H.; Nagashima, M.; Saito, T.; Yamayoshi, S.; Ito, M.; Iwatsuki-Horimoto, K.; et al. Assessment of the frequency of SARS-CoV-2 Omicron variant escape from RNA-dependent RNA polymerase inhibitors and 3C-like protease inhibitors. Antivir. Res. 2023, 216, 105671. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.W.; Sung, H.K.; Jeong, J.Y.; Lim, D.H.; Choi, J.; Kwon, H.C.; Kim, H.J.; Park, S.; Shin, J.; Lee, S.; et al. Changing Features of Liver Injury in COVID-19 Patients: Impact of Infection with the SARS-CoV-2 Delta (B.1.617.2) Variants. Infect. Chemother. 2022, 54, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Fang, J.; Chen, S.; Rajaofera, M.J.N.; Li, X.; Wang, B.; Liu, Y.; Zhao, J.; Yu, H.; Zhou, L.; et al. The efficacy and safety of remdesivir alone and in combination with other drugs for the treatment of COVID-19: A systematic review and meta-analysis. BMC Infect. Dis. 2023, 23, 672. [Google Scholar] [CrossRef]
- Sise, M.E.; Santos, J.R.; Goldman, J.D.; Tuttle, K.R.; Teixeira, J.P.; Seibert, A.F.; Ma, J.; Magner, W.; Lang, J.; Dogra, S.; et al. Efficacy and Safety of Remdesivir in People with Impaired Kidney Function Hospitalized for COVID-19 Pneumonia: A Randomized Clinical Trial. Clin. Infect. Dis. 2024, 79, 1172–1181. [Google Scholar] [CrossRef]
- Pieralli, F.; Pomero, F.; Dentali, F.; Norbiato, C.; Attardo, T.; Vicari, S.; Falsetti, L.; Agostinelli, E.; Spasiano, A.; Monaco, S.; et al. Real-world use of remdesivir for the treatment of patients admitted to Italian hospitals with COVID-19: The nationwide retrospective FADOI-RECOVER study. BMC Infect. Dis. 2023, 23, 454. [Google Scholar] [CrossRef]
- Burhan, E.; Syahruddin, E.; Isbaniah, F.; Desianti, G.A.; Fachrucha, F.; Sari, C.Y.I.; Setiawaty, V.; Soedarsono, S.; Susanto, A.D.; Wibowo, A.; et al. Evaluation of safety and effectiveness of remdesivir in treating COVID-19 patients after emergency use authorization study. Front. Pharmacol. 2023, 14, 1205238. [Google Scholar] [CrossRef]
- Ahmed, A.; Munoz, F.M.; Muller, W.J.; Agwu, A.; Kimberlin, D.W.; Galli, L.; Bamford, A.; Rath, B.; Luck, S.; Pahud, B.A.; et al. Remdesivir for COVID-19 in Hospitalized Children: A Phase 2/3 Study. Pediatrics 2024, 153, e2023063775. [Google Scholar] [CrossRef] [PubMed]

| Variable | Pre-Omicron Variant n = 1004 (62.4%) | Omicron Variant n = 606 (37.6%) | Overall n = 1610 (100%) | p-Value 1 |
|---|---|---|---|---|
| Demographics | ||||
| Sex, male | 608 (60.6%) | 319 (52.6%) | 927 (57.6%) | 0.002 |
| Age (years) | 61 (51, 73) | 76 (66, 85) | 67 (54, 78) | <0.001 |
| Age (in groups; years) | <0.001 | |||
| – 19–49 | 215 (21.4%) | 52 (8.6%) | 267 (16.6%) | |
| – 50–59 | 237 (23.6%) | 45 (7.4%) | 282 (17.5%) | |
| – 60–69 | 244 (24.3%) | 99 (16.3%) | 343 (21.3%) | |
| – 70–79 | 186 (18.5%) | 166 (27.4%) | 352 (21.9%) | |
| – 80+ | 122 (12.2%) | 244 (40.3%) | 366 (22.7%) | |
| Area of residence | <0.001 | |||
| – Rural area | 74 (7.4%) | 15 (2.5%) | 89 (5.5%) | |
| – Semi-urban area | 830 (82.7%) | 27 (4.5%) | 857 (53.2%) | |
| – Urban area | 100 (10.0%) | 564 (93.1%) | 664 (41.2%) | |
| Region of residence | <0.001 | |||
| – Rest of Greece | 578 (57.6%) | 269 (44.4%) | 847 (52.6%) | |
| – Attika | 426 (42.4%) | 337 (55.6%) | 763 (47.4%) | |
| Greek nationality | 919 (91.5%) | 576 (95.0%) | 1495 (92.9%) | 0.009 |
| Baseline severity | ||||
| Disease severity | <0.001 | |||
| – Severe 2 | 745 (74.2%) | 382 (63.0%) | 1127 (70.0%) | |
| – Mild/moderate | 259 (25.8%) | 224 (37.0%) | 483 (30.0%) | |
| Baseline oxygen need | 0.001 | |||
| – Not requiring O2 | 394 (39.2%) | 277 (45.7%) | 671 (41.7%) | |
| – Requiring low flow O2 | 524 (52.2%) | 259 (42.7%) | 783 (48.6%) | |
| – Requiring high flow O2 or mechanical ventilation | 86 (8.6%) | 70 (11.6%) | 156 (9.7%) | |
| Comorbidities and scores | ||||
| Chronic Obstructive Pulmonary Disease | 62 (6.2%) | 93 (15.3%) | 155 (9.6%) | <0.001 |
| Diabetes mellitus | 236 (23.5%) | 165 (27.2%) | 401 (24.9%) | 0.096 |
| Cardiovascular disease | 101 (10.1%) | 354 (58.4%) | 455 (28.3%) | <0.001 |
| Coronary artery disease | 91 (9.1%) | 85 (14.0%) | 176 (10.9%) | 0.002 |
| Immunosuppression | 27 (2.7%) | 30 (5.0%) | 57 (3.5%) | 0.025 |
| Cancer | 49 (4.9%) | 107 (17.7%) | 156 (9.7%) | <0.001 |
| Chronic kidney disease | 17 (1.7%) | 36 (5.9%) | 53 (3.3%) | <0.001 |
| Chronic liver disease | 10 (1.0%) | 1 (0.2%) | 11 (0.7%) | 0.061 |
| Number of comorbidities | <0.001 | |||
| – None | 292 (29.1%) | 88 (14.5%) | 380 (23.6%) | |
| – One | 229 (22.8%) | 125 (20.6%) | 354 (22.0%) | |
| – Two or more | 483 (48.1%) | 393 (64.9%) | 876 (54.4%) | |
| Charlson Comorbidity Index | 2 (1, 4) | 4 (3, 5) | 3 (1, 4) | <0.001 |
| 4C Mortality Score 3 | 7 (5, 9) | 10 (7, 12) | 8 (5, 11) | <0.001 |
| Life-style factors | ||||
| Alcohol abuse | 9 (0.9%) | 10 (1.7%) | 19 (1.2%) | 0.233 |
| Smoking status | <0.001 | |||
| – Unknown | 309 (30.8%) | 281 (46.4%) | 590 (36.6%) | |
| – Active smoker | 68 (6.8%) | 64 (10.6%) | 132 (8.2%) | |
| – Never smoker | 455 (45.3%) | 154 (25.4%) | 609 (37.8%) | |
| – Ex-smoker | 172 (17.1%) | 107 (17.7%) | 279 (17.3%) |
| Covariate | Haz. Ratio | 95% C.I. | p-Value 1 |
|---|---|---|---|
| Variant period | |||
| – Omicron vs. Pre-Omicron (mild or moderate disease 2) | 1.91 | (1.57, 2.34) | <0.001 |
| – Omicron vs. Pre-Omicron (severe disease 2) | 1.11 | (0.96, 1.28) | 0.176 |
| Age (years) | |||
| – 50–59 vs. 19–49 | 0.92 | (0.78, 1.10) | 0.370 |
| – 60–69 vs. 19–49 | 0.72 | (0.61, 0.85) | <0.001 |
| – 70–79 vs. 19–49 | 0.65 | (0.55, 0.78) | <0.001 |
| – 80+ vs. 19–49 | 0.48 | (0.40, 0.57) | <0.001 |
| Charlson Comorbidity Index 3 (per unit) | 0.95 | (0.90, 1.00) | 0.047 |
| Remdesivir initiation | |||
| – 0–1 vs. 2+ days from admission | 1.32 | (1.16, 1.52) | <0.001 |
| Area | |||
| – Rural vs. Urban or semi-urban | 0.70 | (0.55, 0.88) | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantazis, N.; Pechlivanidou, E.; Rapti, V.; Kavatha, D.; Milionis, H.; Kalomenidis, I.; Akinosoglou, K.; Panagopoulos, P.; Metallidis, S.; Kofteridis, D.; et al. Remdesivir: Effectiveness and Safety in Hospitalized COVID-19 Patients—Analysis of Retrospectively Collected Data from Daily Practice in the Omicron Variant Era and Comparison with the Pre-Omicron Period. Microorganisms 2025, 13, 2242. https://doi.org/10.3390/microorganisms13102242
Pantazis N, Pechlivanidou E, Rapti V, Kavatha D, Milionis H, Kalomenidis I, Akinosoglou K, Panagopoulos P, Metallidis S, Kofteridis D, et al. Remdesivir: Effectiveness and Safety in Hospitalized COVID-19 Patients—Analysis of Retrospectively Collected Data from Daily Practice in the Omicron Variant Era and Comparison with the Pre-Omicron Period. Microorganisms. 2025; 13(10):2242. https://doi.org/10.3390/microorganisms13102242
Chicago/Turabian StylePantazis, Nikos, Evmorfia Pechlivanidou, Vassiliki Rapti, Dimitra Kavatha, Haralampos Milionis, Ioannis Kalomenidis, Karolina Akinosoglou, Periklis Panagopoulos, Symeon Metallidis, Diamantis Kofteridis, and et al. 2025. "Remdesivir: Effectiveness and Safety in Hospitalized COVID-19 Patients—Analysis of Retrospectively Collected Data from Daily Practice in the Omicron Variant Era and Comparison with the Pre-Omicron Period" Microorganisms 13, no. 10: 2242. https://doi.org/10.3390/microorganisms13102242
APA StylePantazis, N., Pechlivanidou, E., Rapti, V., Kavatha, D., Milionis, H., Kalomenidis, I., Akinosoglou, K., Panagopoulos, P., Metallidis, S., Kofteridis, D., Sipsas, N. V., Katsarolis, I., Poulakou, G., Antoniadou, A., Christaki, E., Rimpa, T., Marangos, M., Petrakis, V., Tsachouridou, O., ... Touloumi, G. (2025). Remdesivir: Effectiveness and Safety in Hospitalized COVID-19 Patients—Analysis of Retrospectively Collected Data from Daily Practice in the Omicron Variant Era and Comparison with the Pre-Omicron Period. Microorganisms, 13(10), 2242. https://doi.org/10.3390/microorganisms13102242











