Ecological Imprint of Rare Earth Mining on Microbial Communities and Water Quality Across Depth and Distance Gradients in Ganzhou, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Water Analysis Tests
2.3. DNA Extraction and Metagenomic Analysis
3. Results and Discussion
3.1. Spatial Patterns of Water Chemistry and Environmental Parameters
3.2. Distribution Characteristics of Heavy Metals and Rare Earth Elements
3.3. Bacterial Community Responses Based on Gene Annotation
3.4. KEGG Functional Gene Response Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamzat, A.K.; Murad, M.S.; Subeshan, B.; Asmatulu, R.; Asmatulu, E. Rare earth element recycling: A review on sustainable solutions and impacts on semiconductor and chip industries. J. Mater. Cycles Waste Manag. 2025, 27, 3009–3032. [Google Scholar] [CrossRef]
- Chen, W.; Wang, P.; Meng, F.; Pehlken, A.; Wang, Q.-C.; Chen, W.-Q. Reshaping heavy rare earth supply chains amidst China’s stringent environmental regulations. Fundam. Res. 2025, 5, 505–513. [Google Scholar] [CrossRef]
- Xiao, Y.; Feng, Z.; Huang, X.; Huang, L.; Chen, Y.; Wang, L.; Long, Z. Recovery of rare earths from weathered crust elution-deposited rare earth ore without ammonia-nitrogen pollution: I. leaching with magnesium sulfate. Hydrometallurgy 2015, 153, 58–65. [Google Scholar] [CrossRef]
- Dinh, T.; Dobo, Z.; Kovacs, H. Phytomining of rare earth elements—A review. Chemosphere 2022, 297, 134259. [Google Scholar] [CrossRef]
- Long, Q.; Yan, H.; Wu, H.; Qiu, S.; Zhou, X.; Qiu, T. Influence mechanism of leaching agent anions on the leaching of aluminium impurities in ionic-type rare earth ores: A DFT simulation combined with experimental verification. Sep. Purif. Technol. 2025, 354, 128768. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, C.; Chen, M.; Du, W.; Xu, X. Geobiochemistry characteristics of rare earth elements in soil and ground water: A case study in Baotou, China. Sci. Rep. 2020, 10, 11740. [Google Scholar] [CrossRef]
- Stanić, I.; Kajan, K.; Selak, L.; Orlić, S. Environmental drivers of microbial assembly and stability in lakes across biogeographical regions. Ecol. Indic. 2025, 172, 113324. [Google Scholar] [CrossRef]
- Guo, L.; Chen, X.; Sheng, Y.; Yang, N.; Hou, E.; Fang, H. Impact of soil fissure status on microbial community in mining-disturbed area, the northern Shaanxi province. Front. Microbiol. 2024, 15, 1463665. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.V.S.R.; Tiedje, J.M. Ranking environmental and edaphic attributes driving soil microbial community structure and activity with special attention to spatial and temporal scales. mLife 2024, 3, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Dingnan County. County Meteorological Bureau: In August, the Weather Was Mainly Characterized by Intermittent High Temperatures. The Temperature Was Particularly Intense in the First Ten Days of the Month. Available online: https://www.dingnan.gov.cn/dnxzf/c103867/202408/04b84d03dbde491480d82a78f7883d70.shtml (accessed on 1 August 2025).
- Jiang, Y.-H.; Lin, L.-J.; Chen, L.-D.; Ni, H.-Y.; Ge, W.-Y.; Cheng, H.-X.; Zhai, G.-Y.; Wang, G.-L.; Ban, Y.-Z.; Li, Y.; et al. An overview of the resources and environment conditions and major geological problems in the Yangtze River economic zone, China. China Geol. 2018, 1, 435–449. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Y.; Cheng, Y.; Li, J.; Li, F.; Wang, C.; Shi, L.; Qin, G.; Zhan, W.; Cai, Y.; et al. PD-1 blockade plus COX inhibitors in dMMR metastatic colorectal cancer: Clinical, genomic, and immunologic analyses from the PCOX trial. Med 2024, 5, 998–1015.e1016. [Google Scholar] [CrossRef]
- Arumugam, K.; Bessarab, I.; Haryono, M.A.S.; Liu, X.; Zuniga–Montanez, R.E.; Roy, S.; Qiu, G.; Drautz–Moses, D.I.; Law, Y.Y.; Wuertz, S.; et al. Recovery of complete genomes and non-chromosomal replicons from activated sludge enrichment microbial communities with long read metagenome sequencing. NPJ Biofilms Microbiomes 2021, 7, 23. [Google Scholar] [CrossRef]
- Matebese, F.; Mosai, A.K.; Tutu, H.; Tshentu, Z.R. Mining wastewater treatment technologies and resource recovery techniques: A review. Heliyon 2024, 10, e24730. [Google Scholar] [CrossRef]
- Anh, N.T.; Can, L.D.; Nhan, N.T.; Schmalz, B.; Luu, T.L. Influences of key factors on river water quality in urban and rural areas: A review. Case Stud. Chem. Environ. Eng. 2023, 8, 100424. [Google Scholar] [CrossRef]
- Huang, Y.; An, S. Weak hypoxia enhanced denitrification in a dissimilatory nitrate reduction to ammonium (DNRA)-dominated shallow and eutrophic coastal waterbody, Jinhae Bay, South Korea. Front. Mar. Sci. 2022, 9, 897474. [Google Scholar] [CrossRef]
- Zhang, F.; Qu, Z.; Zhao, Q.; Xi, Z.; Liu, Z. Mechanisms of N2O emission in drip-irrigated saline soils: Unraveling the role of soil moisture variation in nitrification and denitrification. Agronomy 2025, 15, 10. [Google Scholar] [CrossRef]
- Bouranis, D.L.; Chorianopoulou, S.N. Foliar application of sulfur-containing compounds—Pros and cons. Plants 2023, 12, 3794. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.-L.; Zhou, G.-J.; Sun, Y.-Y.; Hu, Y.-R.; Hao, W.-D. Geochemistry of cherts from the northern Jiangxi region, South China: Implication for paleoenvironment. J. Palaeogeogr. 2024, 13, 823–838. [Google Scholar] [CrossRef]
- Zhu, Z.; Guo, F.; Qiu, A. Trace element geochemical characteristics of gypsum and its geologic significance from the Luotang depression in Xinjiang basin, Jiangxi. Geol. J. China Univ. 2016, 22, 598–607. [Google Scholar] [CrossRef]
- Zhang, Q.; Ren, F.; Li, F.; Chen, G.; Yang, G.; Wang, J.; Du, K.; Liu, S.; Li, Z. Ammonia nitrogen sources and pollution along soil profiles in an in-situ leaching rare earth ore. Environ. Pollut. 2020, 267, 115449. [Google Scholar] [CrossRef]
- Huang, Y.; Dou, Z.; Zhang, T.-A.; Liu, J. Leaching kinetics of rare earth elements and fluoride from mixed rare earth concentrate after roasting with calcium hydroxide and sodium hydroxide. Hydrometallurgy 2017, 173, 15–21. [Google Scholar] [CrossRef]
- Rambabu, K.; Banat, F.; Pham, Q.M.; Ho, S.-H.; Ren, N.-Q.; Show, P.L. Biological remediation of acid mine drainage: Review of past trends and current outlook. Environ. Sci. Ecotechnol. 2020, 2, 100024. [Google Scholar] [CrossRef]
- Wang, P.; Sun, Z.; Hu, Y.; Cheng, H. Leaching of heavy metals from abandoned mine tailings brought by precipitation and the associated environmental impact. Sci. Total Environ. 2019, 695, 133893. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Wu, W.; Huang, X.; Ou, P.; Lin, Z.; Zhiling, W.; Song, Y.; Lang, T.; Huangfu, W.; Zhang, Y.; et al. Mining and restoration monitoring of rare earth element (REE) exploitation by new remote sensing indicators in Southern Jiangxi, China. Remote Sens. 2020, 12, 3558. [Google Scholar] [CrossRef]
- Bai, X.; Li, Y.; Jing, X.; Zhao, X.; Zhao, P. Response mechanisms of bacterial communities and nitrogen cycle functional genes in millet rhizosphere soil to chromium stress. Front. Microbiol. 2023, 14, 1116535. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, J.; Li, C.; Liu, W.; Wu, W. Redistribution and enhanced bioavailability of rare earth elements speciation induced by mining-driven transformation in ionic rare earth mining areas. Sci. Rep. 2025, 15, 27345. [Google Scholar] [CrossRef] [PubMed]
- Brouziotis, A.A.; Giarra, A.; Libralato, G.; Pagano, G.; Guida, M.; Trifuoggi, M. Toxicity of rare earth elements: An overview on human health impact. Front. Environ. Sci. 2022, 10, 948041. [Google Scholar] [CrossRef]
- Wang, X.; Liu, T.-C.; Wang, X.-W.; Dang, C.-C.; Tan, X.; Lu, Y.; Liu, B.-F.; Xing, D.-F.; Ren, N.-Q.; Xie, G.-J. Microbial manganese redox cycling drives co-removal of nitrate and ammonium. J. Environ. Manag. 2025, 375, 124095. [Google Scholar] [CrossRef]
- Jiang, Z.; Huang, X.; Wang, S.; Xiong, J.; Xie, C.; Chen, Y. Divalent manganese stimulates the removal of nitrate by anaerobic sludge. RSC Adv. 2024, 14, 2447–2452. [Google Scholar] [CrossRef]
- Opande, T.; Kong, M.; Feng, D.; Wen, Y.; Okoth, N.; Yatoo, A.M.; Khalil, F.M.A.; Elrys, A.S.; Meng, L.; Zhang, J. Edaphic factors mediate the response of nitrogen cycling and related enzymatic activities and functional genes to heavy metals: A review. Ecotoxicol. Environ. Saf. 2025, 290, 117766. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, H. Aberrance of zinc metalloenzymes-induced human diseases and its potential mechanisms. Nutrients 2021, 13, 4456. [Google Scholar] [CrossRef] [PubMed]
- Eben, S.S.; Imlay, J.A. Excess copper catalyzes protein disulfide bond formation in the bacterial periplasm but not in the cytoplasm. Mol. Microbiol. 2023, 119, 423–438. [Google Scholar] [CrossRef]
- Luo, Y.; Yuan, H.; Zhao, J.; Qi, Y.; Cao, W.-W.; Liu, J.-M.; Guo, W.; Bao, Z.-H. Multiple factors influence bacterial community diversity and composition in soils with rare earth element and heavy metal co-contamination. Ecotoxicol. Environ. Saf. 2021, 225, 112749. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, D.; Guo, Y.; Guo, Y.; Nishizawa, T.; Liu, J.-M.; Bai, H.-G.; Su, R.; Zhang, S.-H.; Qi, Y.; et al. Microbe-mediated denitrification promotes nitrogen loss in mining soils contaminated with rare earth elements. Appl. Soil Ecol. 2025, 213, 106306. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, Z.; Liu, F.; Qian, L.; Yu, X.; Huang, F.; Hu, R.; Su, H.; Gu, H.; Yan, Q.; et al. The vertical partitioning between denitrification and dissimilatory nitrate reduction to ammonium of coastal mangrove sediment microbiomes. Water Res. 2024, 262, 122113. [Google Scholar] [CrossRef]
- Li, B.; Feng, L.; Chouari, R.; Samoili, S.; Giannakis, S. Trace metals induce microbial risk and antimicrobial resistance in biofilm in drinking water. NPJ Clean Water 2025, 8, 8. [Google Scholar] [CrossRef]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef]
- Long, Y.; Ma, Y.; Wan, J.; Wang, Y.; Tang, M.; Fu, H.; Cao, J. Denitrification efficiency, microbial communities and metabolic mechanisms of corn cob hydrolysate as denitrifying carbon source. Environ. Res. 2023, 221, 115315. [Google Scholar] [CrossRef]
- Phan, H.V.; Yasuda, S.; Oba, K.; Tsukamoto, H.; Hori, T.; Kuroiwa, M.; Terada, A. Active bacteria driving N2O mitigation and dissimilatory nitrate reduction to ammonium in ammonia recovery bioreactors. ISME J. 2025, 19, wraf021. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Sun, R.-Z.; Wang, Y.; Chen, G.-L.; Fu, Y.-Y.; Yu, H.-Q. Carbon source shaped microbial ecology, metabolism and performance in denitrification systems. Water Res. 2023, 243, 120330. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Wang, J. Various electron donors for biological nitrate removal: A review. Sci. Total Environ. 2021, 794, 148699. [Google Scholar] [CrossRef]
- Große, C.; Grau, J.; Herzberg, M.; Nies, D.H. Antisense transcription is associated with expression of metal resistance determinants in Cupriavidus metallidurans CH34. Metallomics 2024, 16, mfae057. [Google Scholar] [CrossRef]
- Zuo, Y.; Li, Y.; Chen, H.; Ran, G.; Liu, X. Effects of multi-heavy metal composite pollution on microorganisms around a lead-zinc mine in typical karst areas, southwest China. Ecotoxicol. Environ. Saf. 2023, 262, 115190. [Google Scholar] [CrossRef]
- Pol, A.; Barends, T.R.M.; Dietl, A.; Khadem, A.F.; Eygensteyn, J.; Jetten, M.S.M.; Op den Camp, H.J.M. Rare earth metals are essential for methanotrophic life in volcanic mudpots. Environ. Microbiol. 2014, 16, 255–264. [Google Scholar] [CrossRef]
- Zhuang, J.-L.; Sun, X.; Zhao, W.-Q.; Zhang, X.; Zhou, J.-J.; Ni, B.-J.; Liu, Y.-D.; Shapleigh, J.P.; Li, W. The anammox coupled partial-denitrification process in an integrated granular sludge and fixed-biofilm reactor developed for mainstream wastewater treatment: Performance and community structure. Water Res. 2022, 210, 117964. [Google Scholar] [CrossRef]
- Nakagawa, S.; Yagi, H.; Suyama, T.; Shimamura, S.; Yanaka, S.; Yagi-Utsumi, M.; Kato, S.; Ohkuma, M.; Kato, K.; Takai, K. Exploring protein N-glycosylation in ammonia-oxidizing Nitrososphaerota archaea through glycoproteomic analysis. mBio 2025, 16, e03859-24. [Google Scholar] [CrossRef]
- Zhang, F.; Du, Z.; Wang, J.; Du, Y.; Peng, Y. Acidophilic partial nitrification (pH < 6) facilitates ultra-efficient short-flow nitrogen transformation: Experimental validation and genomic insights. Water Res. 2024, 260, 121921. [Google Scholar] [CrossRef] [PubMed]
- Mehrani, M.-J.; Sobotka, D.; Kowal, P.; Ciesielski, S.; Makinia, J. The occurrence and role of Nitrospira in nitrogen removal systems. Bioresour. Technol. 2020, 303, 122936. [Google Scholar] [CrossRef]
- Mosley, O.E.; Gios, E.; Handley, K.M. Implications for nitrogen and sulphur cycles: Phylogeny and niche-range of Nitrospirota in terrestrial aquifers. ISME Commun. 2024, 4, ycae047. [Google Scholar] [CrossRef] [PubMed]
- Hay Mele, B.; Monticelli, M.; Leone, S.; Bastoni, D.; Barosa, B.; Cascone, M.; Migliaccio, F.; Montemagno, F.; Ricciardelli, A.; Tonietti, L.; et al. Oxidoreductases and metal cofactors in the functioning of the earth. Essays Biochem. 2023, 67, 653–670. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, V.; Li, X.; Elk, M.; Chandran, K.; Impellitteri, C.A.; Santo Domingo, J.W. Impact of heavy metals on transcriptional and physiological activity of nitrifying bacteria. Environ. Sci. Technol. 2015, 49, 13454–13462. [Google Scholar] [CrossRef]
- Hao, X.; Zhu, J.; Rensing, C.; Liu, Y.; Gao, S.; Chen, W.; Huang, Q.; Liu, Y.-R. Recent advances in exploring the heavy metal(loid) resistant microbiome. Comput. Struct. Biotechnol. J. 2021, 19, 94–109. [Google Scholar] [CrossRef]
- Hou, L.; Bai, X.; Sima, Z.; Zhang, J.; Yan, L.; Li, D.; Jiang, Y. Biological and chemical processes of nitrate reduction and ferrous oxidation mediated by Shewanella oneidensis MR-1. Microorganisms 2024, 12, 2454. [Google Scholar] [CrossRef] [PubMed]
- Quach, N.T.; Dam, H.T.; Tran, D.M.; Vu, T.H.N.; Nguyen, Q.V.; Nguyen, K.T.; Nguyen, Q.H.; Phi, C.B.; Le, T.H.; Chu, H.H.; et al. Diversity of microbial community and its metabolic potential for nitrogen and sulfur cycling in sediments of Phu Quoc island, Gulf of Thailand. Braz. J. Microbiol. 2021, 52, 1385–1395. [Google Scholar] [CrossRef]
- Miao, X.; Xu, J.; Yang, B.; Luo, J.; Zhi, Y.; Li, W.; He, Q.; Li, H. Indigenous mixotrophic aerobic denitrifiers stimulated by oxygen micro/nanobubble-loaded microporous biochar. Bioresour. Technol. 2024, 391, 129997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Peng, Y.; Yan, P.; Huang, J.-C.; He, S.; Sun, S.; Bai, X.; Tian, Y. Molecular analysis of microbial nitrogen transformation and removal potential in the plant rhizosphere of artificial tidal wetlands across salinity gradients. Environ. Res. 2022, 215, 114235. [Google Scholar] [CrossRef]
- Qu, M.; Liu, Y.; Hao, M.; Wang, M.; Chen, R.; Wang, X.C.; Zheng, Y.; Dzakpasu, M. Microbial community and carbon–nitrogen metabolism pathways in integrated vertical flow constructed wetlands treating wastewater containing antibiotics. Bioresour. Technol. 2022, 354, 127217. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, M.; Yang, S.; He, D.; Fang, F.; Yang, L. The response of structure and nitrogen removal function of the biofilm on submerged macrophytes to high ammonium in constructed wetlands. J. Environ. Sci. 2024, 142, 129–141. [Google Scholar] [CrossRef]
- Anderson, E.L.; Jang, J.; Venterea, R.T.; Feyereisen, G.W.; Ishii, S. Isolation and characterization of denitrifiers from woodchip bioreactors for bioaugmentation application. J. Appl. Microbiol. 2020, 129, 590–600. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, X.; Fan, Y.; Qiu, T.; Gao, M.; Wang, X. Heterotrophic nitrification and aerobic denitrification by Diaphorobacter polyhydroxybutyrativorans SL-205 using poly(3-hydroxybutyrate-co-3-hydroxyvalerate) as the sole carbon source. Bioresour. Technol. 2017, 241, 500–507. [Google Scholar] [CrossRef]
- Wang, F.; Wang, H.; Sun, C.; Yan, Z. Conventional bioretention column with Fe-hydrochar for stormwater treatment: Nitrogen removal, nitrogen behaviour and microbial community analysis. Bioresour. Technol. 2021, 334, 125252. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zheng, H.; Liao, Y.; Feng, L.; Jiang, L.; Liu, C.; Mao, Y.; Shen, Q.; Zhang, Q.; Ji, F. Effects of iron-based substrate on coupling of nitrification, aerobic denitrification and Fe(II) autotrophic denitrification in tidal flow constructed wetlands. Bioresour. Technol. 2022, 361, 127657. [Google Scholar] [CrossRef] [PubMed]
- Nagata, A.; Clough, T.; Uchida, Y. Impact of lead contamination on denitrification after nitrate addition to soil: An interaction between nitrogen substrates, denitrification genes, and microbial community structures. Soil Sediment Contam. Int. J. 2025; online. [Google Scholar] [CrossRef]
- Visser, A.-N.; Wankel, S.D.; Frey, C.; Kappler, A.; Lehmann, M.F. Unchanged nitrate and nitrite isotope fractionation during heterotrophic and Fe(II)-mixotrophic denitrification suggest a non-enzymatic link between denitrification and Fe(II) oxidation. Front. Microbiol. 2022, 13, 927475. [Google Scholar] [CrossRef]
- Wang, R.; Yang, S.; Zhao, W. Microbial community responses and nitrogen cycling in the nitrogen-polluted urban Shi river revealed by metagenomics. Microorganisms 2025, 13, 1007. [Google Scholar] [CrossRef]
- Wu, M.; Lai, C.-Y.; Wang, Y.; Yuan, Z.; Guo, J. Microbial nitrate reduction in propane- or butane-based membrane biofilm reactors under oxygen-limiting conditions. Water Res. 2023, 235, 119887. [Google Scholar] [CrossRef]
- Rojas-Rojas, F.U.; Gómez-Vázquez, I.M.; Estrada-de los Santos, P.; Shimada-Beltrán, H.; Vega-Arreguín, J.C. The potential of Paraburkholderia species to enhance crop growth. World J. Microbiol. Biotechnol. 2025, 41, 62. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, H.; Zeng, Q.; Xu, M.; Li, Y.; Wang, W.; Zhong, Y. Isolation and appraisal of a non-fermentative bacterium, Delftia tsuruhatensis, as denitrifying phosphate-accumulating organism and optimal growth conditions. J. Water Process Eng. 2020, 36, 101296. [Google Scholar] [CrossRef]
- Green Stefan, J.; Prakash, O.; Jasrotia, P.; Overholt Will, A.; Cardenas, E.; Hubbard, D.; Tiedje James, M.; Watson David, B.; Schadt Christopher, W.; Brooks Scott, C.; et al. Denitrifying bacteria from the genus Rhodanobacter dominate bacterial communities in the highly contaminated subsurface of a nuclear legacy waste site. Appl. Environ. Microbiol. 2012, 78, 1039–1047. [Google Scholar] [CrossRef]
- Tang, Y.; Yu, G.; Zhang, X.; Wang, Q.; Ge, J.; Liu, S. Changes in nitrogen-cycling microbial communities with depth in temperate and subtropical forest soils. Appl. Soil Ecol. 2018, 124, 218–228. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, W.; Kou, J.; Li, Q.; Liu, J.; Chi, L.; Zhang, Y.; Liu, Q.; Yu, Y. Impacts of phosphate-solubilizing bacterium strain MWP-1 on vegetation growth, soil characteristics, and microbial communities in the Muli coal mining area, China. Front. Microbiol. 2024, 15, 1500070. [Google Scholar] [CrossRef]
- Campeciño, J.; Lagishetty, S.; Wawrzak, Z.; Sosa Alfaro, V.; Lehnert, N.; Reguera, G.; Hu, J.; Hegg, E.L. Cytochrome c nitrite reductase from the bacterium Geobacter lovleyi represents a new NrfA subclass. J. Biol. Chem. 2020, 295, 11455–11465. [Google Scholar] [CrossRef] [PubMed]
- Sparacino-Watkins, C.; Stolz, J.F.; Basu, P. Nitrate and periplasmic nitrate reductases. Chem. Soc. Rev. 2014, 43, 676–706. [Google Scholar] [CrossRef] [PubMed]
- Bru, D.; Sarr, A.; Philippot, L. Relative abundances of proteobacterial membrane-bound and periplasmic nitrate reductases in selected environments. Appl. Environ. Microbiol. 2007, 73, 5971–5974. [Google Scholar] [CrossRef]
- McGarry, J.; Mintmier, B.; Metzger, M.C.; Giri, N.C.; Britt, N.; Basu, P.; Wilcoxen, J. Insights into periplasmic nitrate reductase function under single turnover. J. Biol. Inorg. Chem. 2024, 29, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Sanford, R.A.; Wagner, D.D.; Wu, Q.; Chee-Sanford, J.C.; Thomas, S.H.; Cruz-García, C.; Rodríguez, G.; Massol-Deyá, A.; Krishnani, K.K.; Ritalahti, K.M.; et al. Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils. Proc. Natl. Acad. Sci. USA 2012, 109, 19709–19714. [Google Scholar] [CrossRef]
- Shu, W.; Zhang, Q.; Audet, J.; Li, Z.; Leng, P.; Qiao, Y.; Tian, C.; Chen, G.; Zhao, J.; Cheng, H.; et al. Non-negligible N2O emission hotspots: Rivers impacted by ion-adsorption rare earth mining. Water Res. 2024, 251, 121124. [Google Scholar] [CrossRef]
- Mise, K.; Masuda, Y.; Senoo, K.; Itoh, H. Undervalued pseudo-nifH sequences in public databases distort metagenomic insights into biological nitrogen fixers. mSphere 2021, 6, e00785-21. [Google Scholar] [CrossRef]
- Hatzenpichler, R. Diversity, physiology, and niche differentiation of ammonia-oxidizing archaea. Appl. Environ. Microbiol. 2012, 78, 7501–7510. [Google Scholar] [CrossRef]
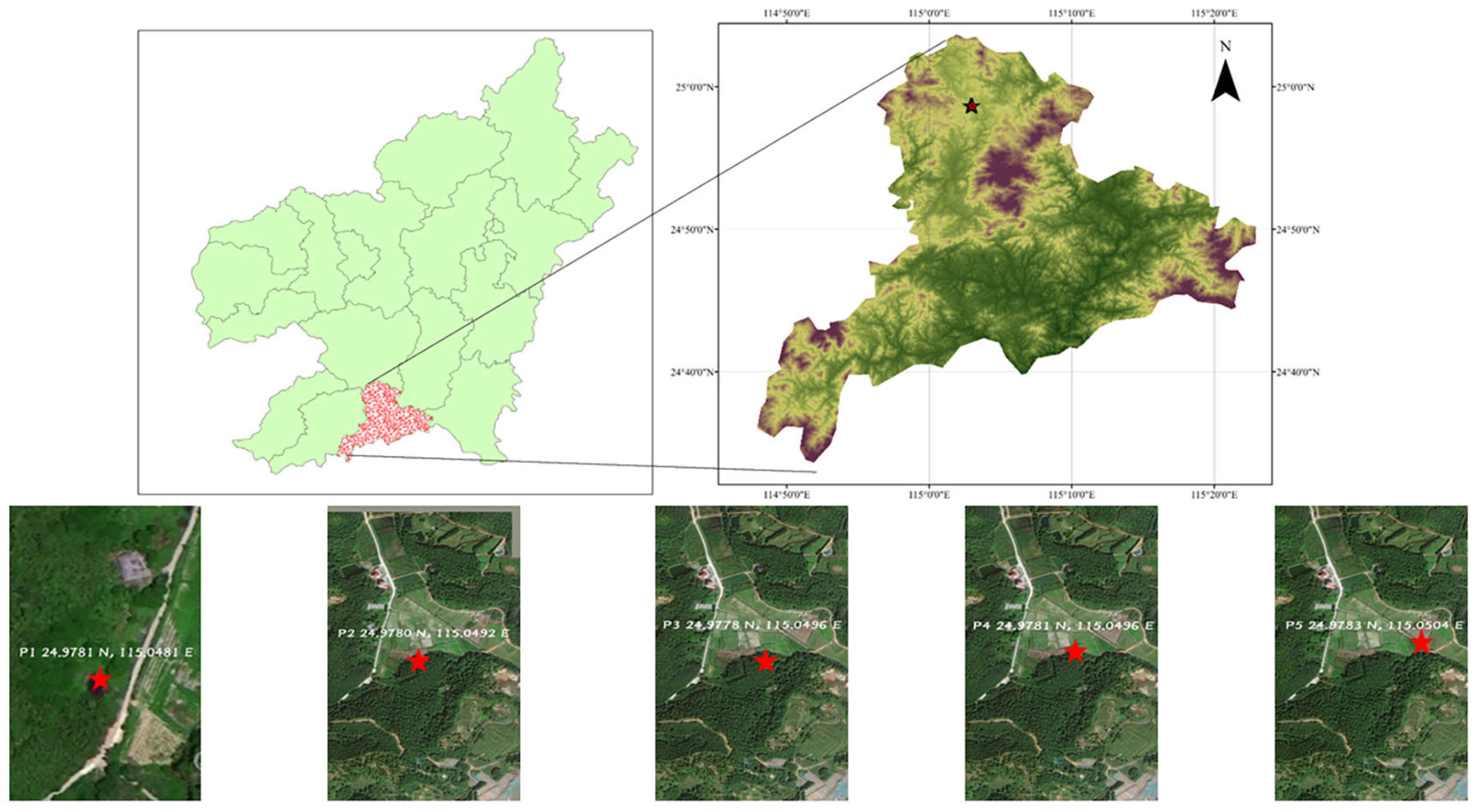
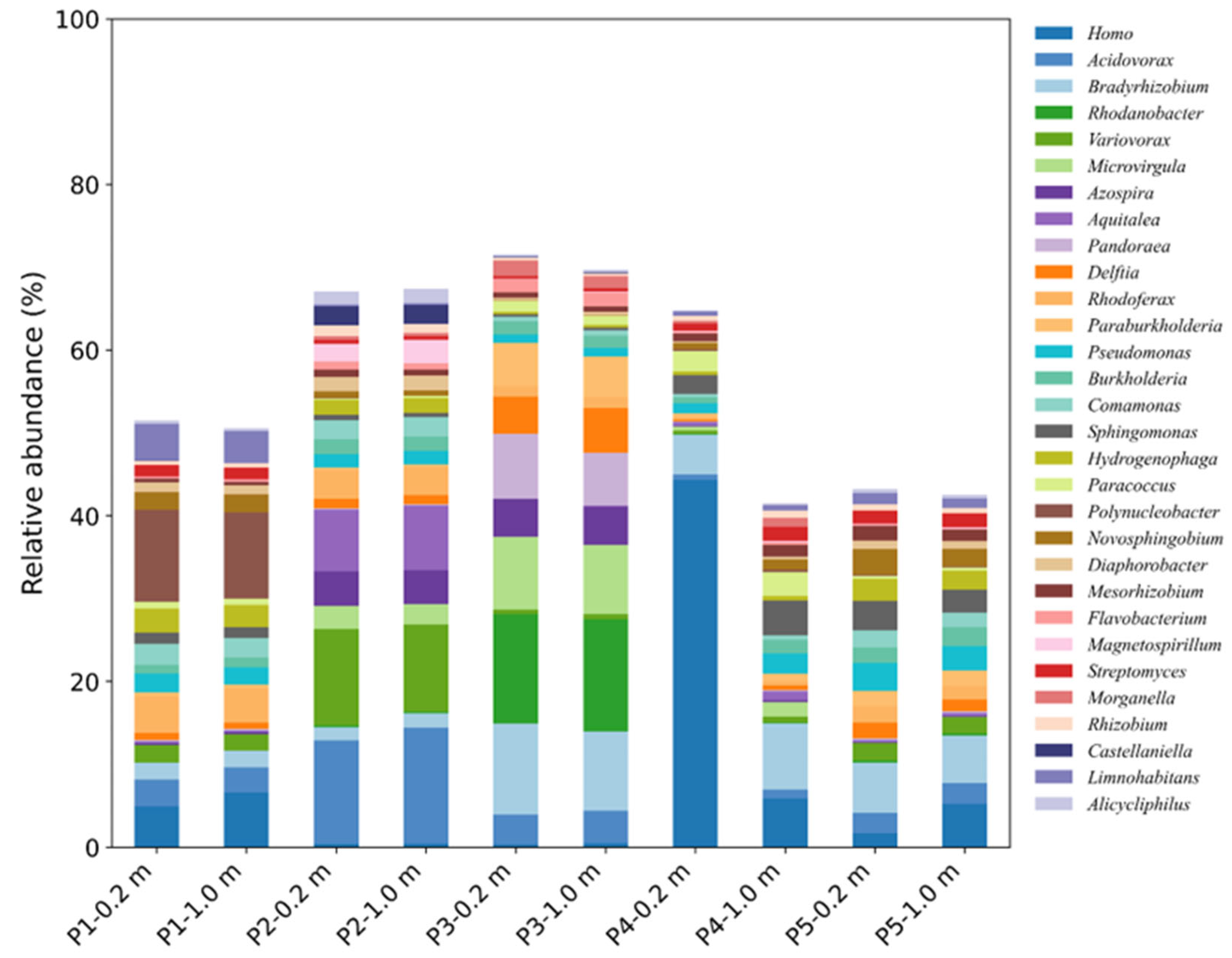
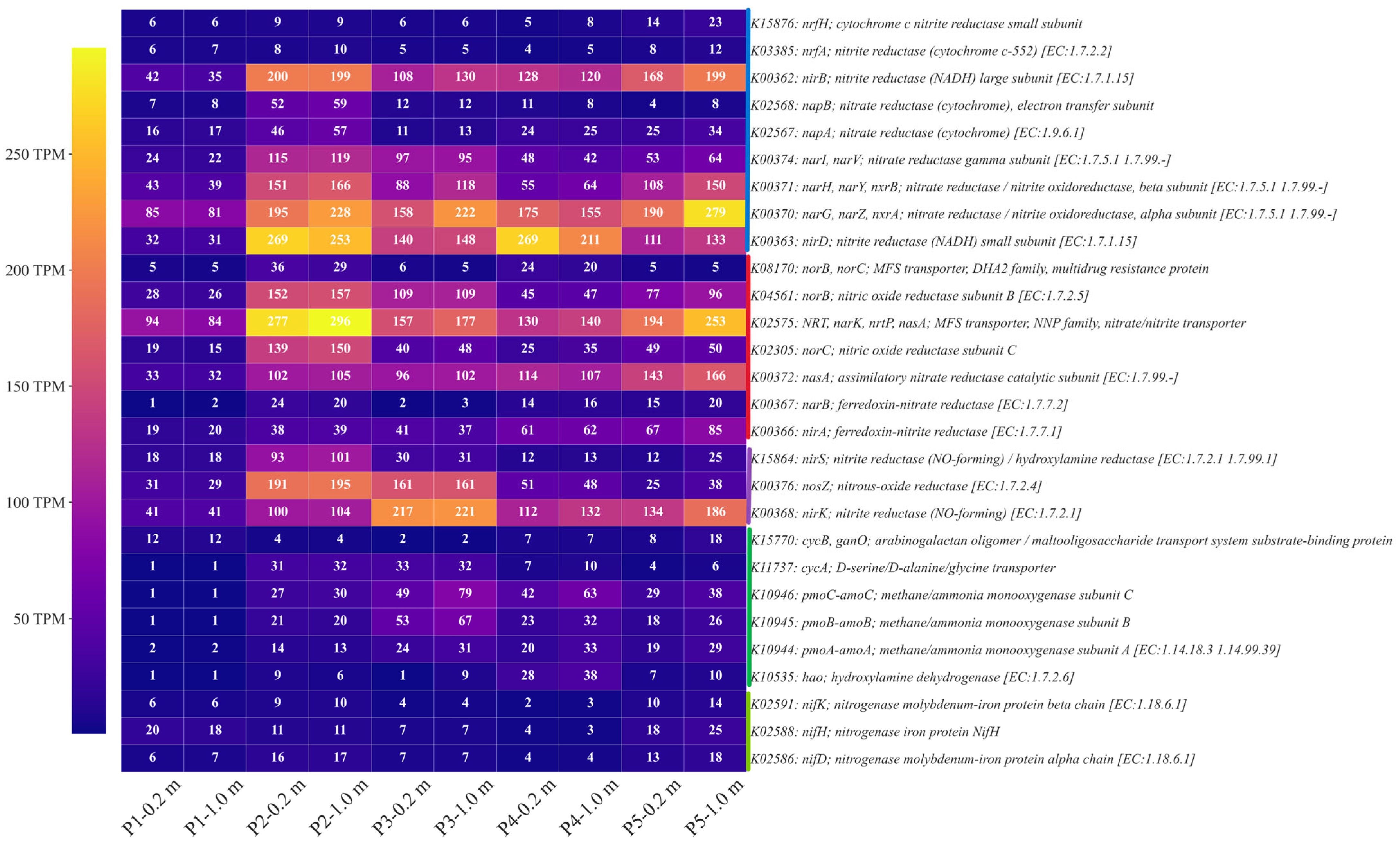
| TN Content (mg/L) | NH4+−N (mg/L) | NO3−−N (mg/L) | SO42− (mg/L) | |||||
|---|---|---|---|---|---|---|---|---|
| Depths | 0.2 m | 1.0 m | 0.2 m | 1.0 m | 0.2 m | 1.0 m | 0.2 m | 1.0 m |
| P1 | 5.0 ± 0.19 | 5.2 ± 0.30 | 0.4 ± 0.01 | 0.4 ± 0.01 | 6.1 ± 0.05 | 6.5 ± 0.11 | 232.0 ± 3.81 | 249.7 ± 1.89 |
| P2 | 88.2 ± 4.13 | 90.7 ± 2.26 | 4.9 ± 0.06 | 5.1 ± 0.02 | 89.7 ± 0.54 | 100.7 ± 0.37 | 62.6 ± 0.56 | 63.3 ± 0.63 |
| P3 | 103.4 ± 2.75 | 102.6 ± 3.96 | 4.2 ± 0.01 | 4.3 ± 0.01 | 104.1 ± 0.20 | 107.7 ± 0.56 | 123.4 ± 2.17 | 124.7 ± 1.46 |
| P4 | 22.4 ± 1.97 | 28.0 ± 1.77 | 4.2 ± 0.02 | 4.1 ± 0.02 | 29.4 ± 0.52 | 36.6 ± 0.55 | 96.7 ± 1.10 | 122.6 ± 1.92 |
| P5 | 1.9 ± 0.08 | 8.2 ± 0.68 | 0.2 ± 0.01 | 0.1 ± 0.00 | 2.3 ± 0.04 | 13.0 ± 0.19 | 17.0 ± 0.62 | 17.4 ± 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Shi, F.; Lang, F.; Wang, G.; Mao, Y.; Xiao, Y.; Yin, L.; He, G.; Liao, Y. Ecological Imprint of Rare Earth Mining on Microbial Communities and Water Quality Across Depth and Distance Gradients in Ganzhou, China. Microorganisms 2025, 13, 2236. https://doi.org/10.3390/microorganisms13102236
Wang Y, Shi F, Lang F, Wang G, Mao Y, Xiao Y, Yin L, He G, Liao Y. Ecological Imprint of Rare Earth Mining on Microbial Communities and Water Quality Across Depth and Distance Gradients in Ganzhou, China. Microorganisms. 2025; 13(10):2236. https://doi.org/10.3390/microorganisms13102236
Chicago/Turabian StyleWang, Yian, Fei Shi, Fengxiang Lang, Guohua Wang, Yan Mao, Yingjie Xiao, Li Yin, Genhe He, and Yonghui Liao. 2025. "Ecological Imprint of Rare Earth Mining on Microbial Communities and Water Quality Across Depth and Distance Gradients in Ganzhou, China" Microorganisms 13, no. 10: 2236. https://doi.org/10.3390/microorganisms13102236
APA StyleWang, Y., Shi, F., Lang, F., Wang, G., Mao, Y., Xiao, Y., Yin, L., He, G., & Liao, Y. (2025). Ecological Imprint of Rare Earth Mining on Microbial Communities and Water Quality Across Depth and Distance Gradients in Ganzhou, China. Microorganisms, 13(10), 2236. https://doi.org/10.3390/microorganisms13102236






