Modulating Chitinase in the QS Biosensor Strain CV026: Do Not Forget to Release Carbon Catabolite Repression. Comment on Deryabin et al. Quorum Sensing in Chromobacterium subtsugae ATCC 31532 (Formerly Chromobacterium violaceum ATCC 31532): Transcriptomic and Genomic Analyses. Microorganisms 2025, 13, 1021
Abstract
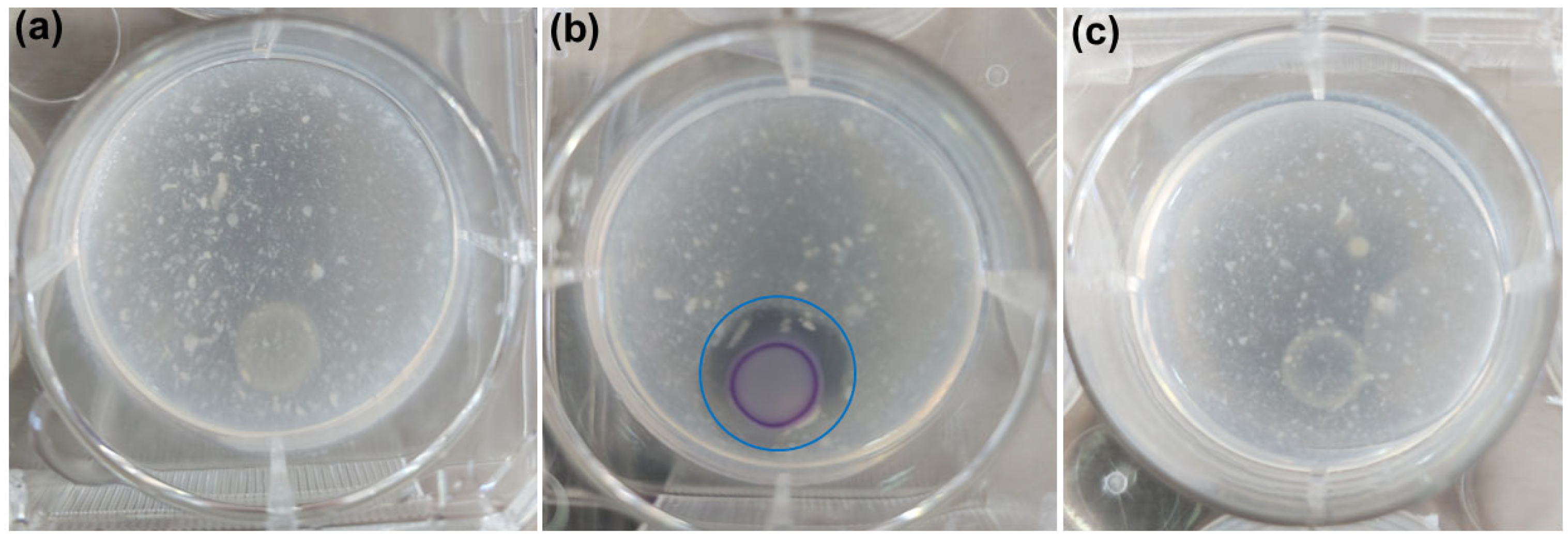
1. Introduction
2. Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| QS | Quorum Sensing |
| ATCC | American Type Culture Collection |
| C6-HSL | N-(hexanoyl)-L-homoserine lactone |
| LB | Luria broth medium |
| CCR | Carbon Catabolite Repression |
| GacA | global regulator A |
| cAMP | Cyclic Adenosine Monophosphate |
| PEP | Phosphoenolpyruvate |
| PTS | Phosphotransferase System |
| CRP | cAMP receptor protein |
| EIIA | Sugar-specific Permease Enzime IIA |
| CcpA | Catabolic Control Protein A |
| CviR | QS-modulated transcriptional activator in Chromobacterium spp. |
References
- Deryabin, D.G.; Inchagova, K.S.; Nikonorova, E.R.; Karimov, I.F.; Duskaev, G.K. Quorum Sensing in Chromobacterium subtsugae ATCC 31532 (Formerly Chromobacterium violaceum ATCC 31532): Transcriptomic and Genomic Analyses. Microorganisms 2025, 13, 1021. [Google Scholar] [CrossRef]
- Favero, F.; Tolentino, T.A.; Fernandes, V.; Treptow, W.; Pereira, A.L.; Machado, A.H.L. α-Alkylidene δ-lactones inhibit quorum sensing phenotypes in Chromobacterium strain CV026 showing interaction with the CviR receptor. RSC Adv. 2023, 26, 18045–18057. [Google Scholar] [CrossRef]
- McClean, K.H.; Winson, M.K.; Fish, L.; Taylor, A.; Chhabra, S.R.; Camara, M.; Daykin, M.; Lamb, J.H.; Swift, S.; Bycroft, B.W.; et al. Quorum sensing and Chromobacterium violaceum: Exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 1997, 143, 3703–3711. [Google Scholar] [CrossRef]
- Chernin, L.S.; Winson, M.K.; Thompson, J.M.; Haran, S.; Bycroft, B.W.; Chet, I.; Williams, P.; Stewart, G.S.A.B. Chitinolytic Activity in Chromobacterium violaceum: Substrate Analysis and Regulation by Quorum Sensing. J. Bacteriol. 1998, 180, 4435–4441. [Google Scholar] [CrossRef]
- Devescovi, G.; Kojic, M.; Covaceuszach, S.; Cámara, M.; Williams, P.; Bertani, I.; Subramoni, S.; Venturi, V. Negative regulation of violacein biosynthesis in Chromobacterium violaceum. Front. Microbiol. 2017, 8, 349. [Google Scholar] [CrossRef]
- Blosser, R.S.; Gray, K.M. Extraction of violacein from Chromobacterium violaceum provides a new quantitative bioassay for N-acyl homoserine lactone autoinducers. J. Microbiol. Methods 2000, 40, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Morohoshi, T.; Kato, M.; Fukamachi, K.; Kato, N.; Ikeda, T. N-Acylhomoserine lactone regulates violacein production in Chromobacterium violaceum type strain ATCC 12472. FEMS Microbiol. Lett. 2008, 279, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Vasavi, H.S.; Arun, A.B.; Rekha, P.-D. Anti-quorum sensing activity of Psidium guajava L. flavonoids against Chromobacterium violaceum and Pseudomonas aeruginosa PAO1. Microbiol. Immunol. 2014, 58, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Görke, B.; Stülke, J. Carbon catabolite repression in bacteria: Many ways to make the most out of nutrients. Nat. Rev. Microbiol. 2008, 6, 613–624. [Google Scholar] [CrossRef]
- Green, V.E.; Klancher, C.A.; Yamamoto, S.; Dalia, A.B. The molecular mechanism for carbon catabolite repression of the chitin response in Vibrio cholerae. PLoS Genet. 2023, 19, e1010767. [Google Scholar] [CrossRef]
- Laville, J.; Voisard, C.; Keel, C.; Maurhofer, M.; Défago, G.; Haas, D. Global control in Pseudomonas fluorescens mediating antibiotic synthesis and suppression of black root rot of tobacco. Proc. Natl. Acad. Sci. USA 1992, 89, 1562–1566. [Google Scholar] [CrossRef]
- Gaffney, T.D.; Lam, S.T.; Ligon, J.; Gates, K.; Frazelle, A.; Di Maio, J.; Hill, S.; Goodwin, S.; Torkewitz, N.; Allshouse, A.M.; et al. Global regulation of expression of antifungal factors by a Pseudomonas fluorescens biological control strain. Mol. Plant Microbe Interact. 1994, 7, 455–463. [Google Scholar] [CrossRef]
- Kay, E.; Humair, B.; Dénervaud, V.; Riedel, K.; Spahr, S.; Eberl, L.; Valverde, C.; Haas, D. Two GacA-Dependent Small RNAs Modulate the Quorum-Sensing Response in Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 6026–6033. [Google Scholar] [CrossRef]
- Winson, M.K.; Camara, M.; Latifi, A.; Foglino, M.; Chhabra, S.R.; Daykin, M.; Bally, M.; Chapon, V.; Salmond, G.P.C.; Bycroft, B.W. Multiple N-acyl-L-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1995, 92, 9427–9431. [Google Scholar] [CrossRef] [PubMed]
- Reimmann, C.; Beyeler, M.; Latifi, A.; Winteler, H.; Foglino, M.; Lazdunski, A.; Haas, D. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol. Microbiol. 1997, 24, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Brückner, R.; Titgemeyer, F. Carbon catabolite repression in bacteria: Choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol. Lett. 2002, 209, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kalivoda, E.J.; Stella, N.A.; O’Dee, D.M.; Nau, G.J.; Shanks, R.M.Q. The Cyclic AMP-Dependent Catabolite Repression System of Serratia marcescens Mediates Biofilm Formation through Regulation of Type 1 Fimbriae. Appl. Environ. Microbiol. 2008, 74, 3461–3470. [Google Scholar] [CrossRef]
- Uchiyama, T.; Kaneko, R.; Yamaguchi, J.; Inoue, A.; Yanagida, T.; Nikaidou, N.; Regue, M.; Watanabe, T. Uptake of N,N′-Diacetylchitobiose [(GlcNAc)_2] via the Phosphotransferase System Is Essential for Chitinase Production by Serratia marcescens 2170. J. Bacteriol. 2003, 185, 1776–1782. [Google Scholar] [CrossRef]
- Shanks, R.M.Q.; Stella, N.A.; Arena, K.E.; Fender, J.E. Mutation of crp mediates Serratia marcescens serralysin and global secreted protein production. Res. Microbiol. 2013, 164, 38–45. [Google Scholar] [CrossRef]
- Martínez-Zavala, S.A.; Barboza-Pérez, U.E.; Hernández-Guzmán, G.; Bideshi, D.K.; Barboza-Corona, J.E. Chitinases of Bacillus thuringiensis: Phylogeny, Modular Structure, and Applied Potentials. Front. Microbiol. 2020, 10, 3032. [Google Scholar] [CrossRef]
- Stauff, D.L.; Bassler, B.L. Quorum Sensing in Chromobacterium violaceum: DNA Recognition and Gene Regulation by the CviR Receptor. J. Bacteriol. 2011, 193, 3871–3878. [Google Scholar] [CrossRef] [PubMed]
- Lima-Bittencourt, C.I.; Costa, P.S.; Hollatz, C.; Raposeiras, R.; Santos, F.R.; Chartone-Souza, E.; Nascimento, A.M.A. Comparative biogeography of Chromobacterium from the neotropics. Antonie Van. Leeuwenhoek 2011, 99, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, W.; Zhu, Y.; Gong, Q.; Yu, W.; Lu, X. Antibiotics at subinhibitory concentrations improve the quorum sensing behavior of Chromobacterium violaceum. FEMS Microbiol. Lett. 2013, 341, 37–44. [Google Scholar] [CrossRef] [PubMed]
| Findings | Experimental Approach | Tested Strain | Ref. |
|---|---|---|---|
| 1. Chitinase activity was induced by QS autoinducer C6-HSL, chitinase activity was blocked in presence of glucose or sucrose, and the expression of chitinase enzymes was induced by C6-HSL | Endogenous expression of chitinase in minimal medium supplemented with chitin, chitinase control under QS autoinducers, chitinase control under carbon catabolite repression, and enzyme detection after protein resolution on SDS-PAGE | ATCC 31532 CV026 | [4] |
| 2. Chitinase activity was abolished in cviR knockout strain, and chitinase down regulation by QS repressor protein VioS | Endogenous expression of chitinase in minimal medium supplemented with chitin, knockout and complementation strains | ATCC 31532 cviR::Gm knockout strain vioS::Km knockout strain | [5] |
| 3. Chitinase gene (CV_4240) was activated by QS regulator CviR | Heterologous expression of cviR and PCV_4240::luxCDABE reporter system in E. coli | ATCC 12472 | [21] |
| 4. Chitinase activity induced by QS autoinducer C6-HSL, C6-HSL-induced chitinase blocked by QS antagonist chlorolactone | Endogenous expression of chitinase in minimal medium supplemented with chitin, and QS modulation by autoinducers and antagonists | CV026 | [2] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.L.; Favero, F.; Machado, A.H.L. Modulating Chitinase in the QS Biosensor Strain CV026: Do Not Forget to Release Carbon Catabolite Repression. Comment on Deryabin et al. Quorum Sensing in Chromobacterium subtsugae ATCC 31532 (Formerly Chromobacterium violaceum ATCC 31532): Transcriptomic and Genomic Analyses. Microorganisms 2025, 13, 1021. Microorganisms 2025, 13, 2235. https://doi.org/10.3390/microorganisms13102235
Pereira AL, Favero F, Machado AHL. Modulating Chitinase in the QS Biosensor Strain CV026: Do Not Forget to Release Carbon Catabolite Repression. Comment on Deryabin et al. Quorum Sensing in Chromobacterium subtsugae ATCC 31532 (Formerly Chromobacterium violaceum ATCC 31532): Transcriptomic and Genomic Analyses. Microorganisms 2025, 13, 1021. Microorganisms. 2025; 13(10):2235. https://doi.org/10.3390/microorganisms13102235
Chicago/Turabian StylePereira, Alex Leite, Fernanda Favero, and Angelo Henrique Lira Machado. 2025. "Modulating Chitinase in the QS Biosensor Strain CV026: Do Not Forget to Release Carbon Catabolite Repression. Comment on Deryabin et al. Quorum Sensing in Chromobacterium subtsugae ATCC 31532 (Formerly Chromobacterium violaceum ATCC 31532): Transcriptomic and Genomic Analyses. Microorganisms 2025, 13, 1021" Microorganisms 13, no. 10: 2235. https://doi.org/10.3390/microorganisms13102235
APA StylePereira, A. L., Favero, F., & Machado, A. H. L. (2025). Modulating Chitinase in the QS Biosensor Strain CV026: Do Not Forget to Release Carbon Catabolite Repression. Comment on Deryabin et al. Quorum Sensing in Chromobacterium subtsugae ATCC 31532 (Formerly Chromobacterium violaceum ATCC 31532): Transcriptomic and Genomic Analyses. Microorganisms 2025, 13, 1021. Microorganisms, 13(10), 2235. https://doi.org/10.3390/microorganisms13102235






