Comparative Analysis of Bacterial Diversity and Functional Potential in Two Athalassohaline Lagoons in the Monegros Desert (NE Spain)
Abstract
1. Introduction
2. Materials and Methods
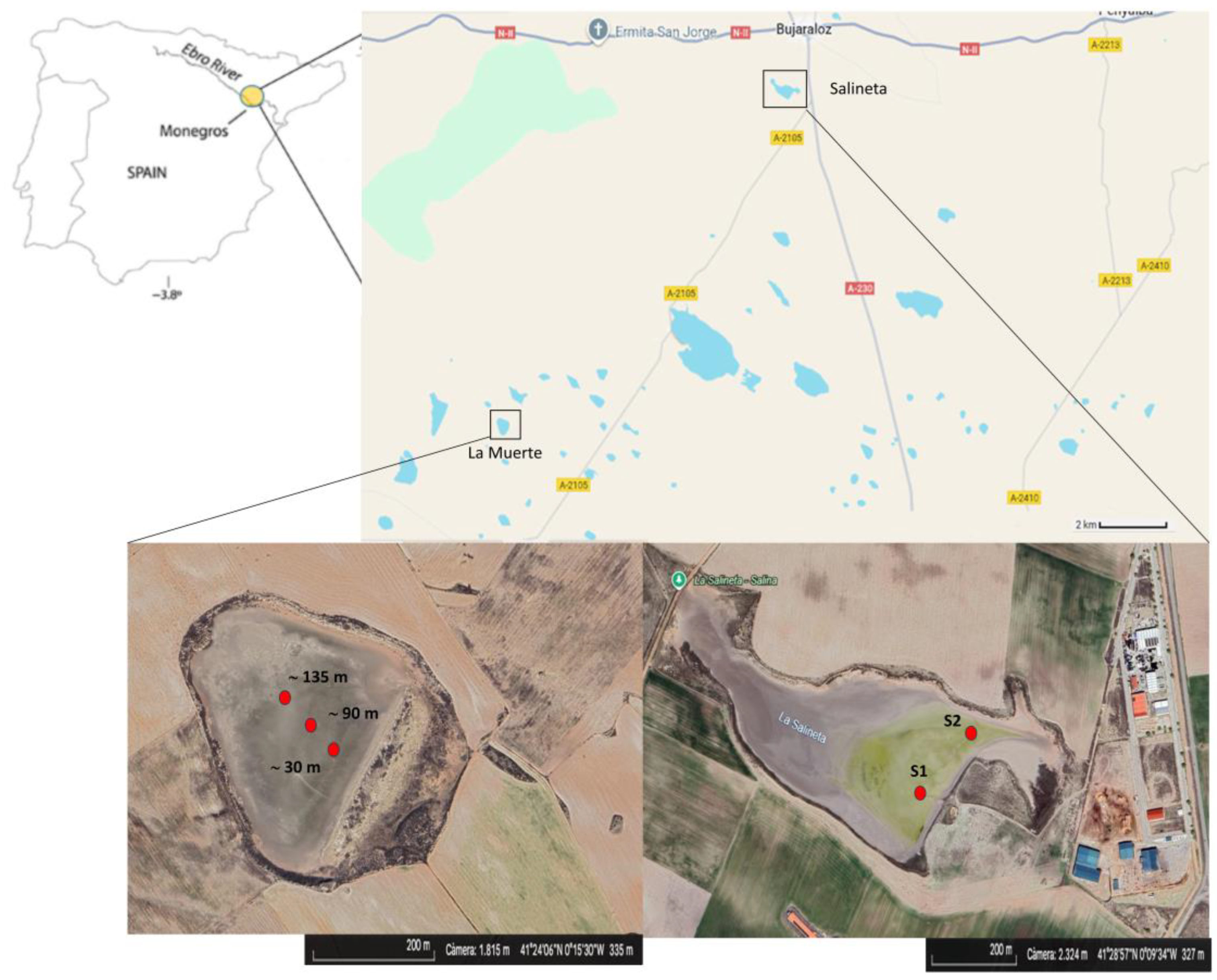
2.1. Sample Collection
2.2. DNA Extraction and Amplicon Sequencing
2.3. Bioinformatic Analyses
3. Results
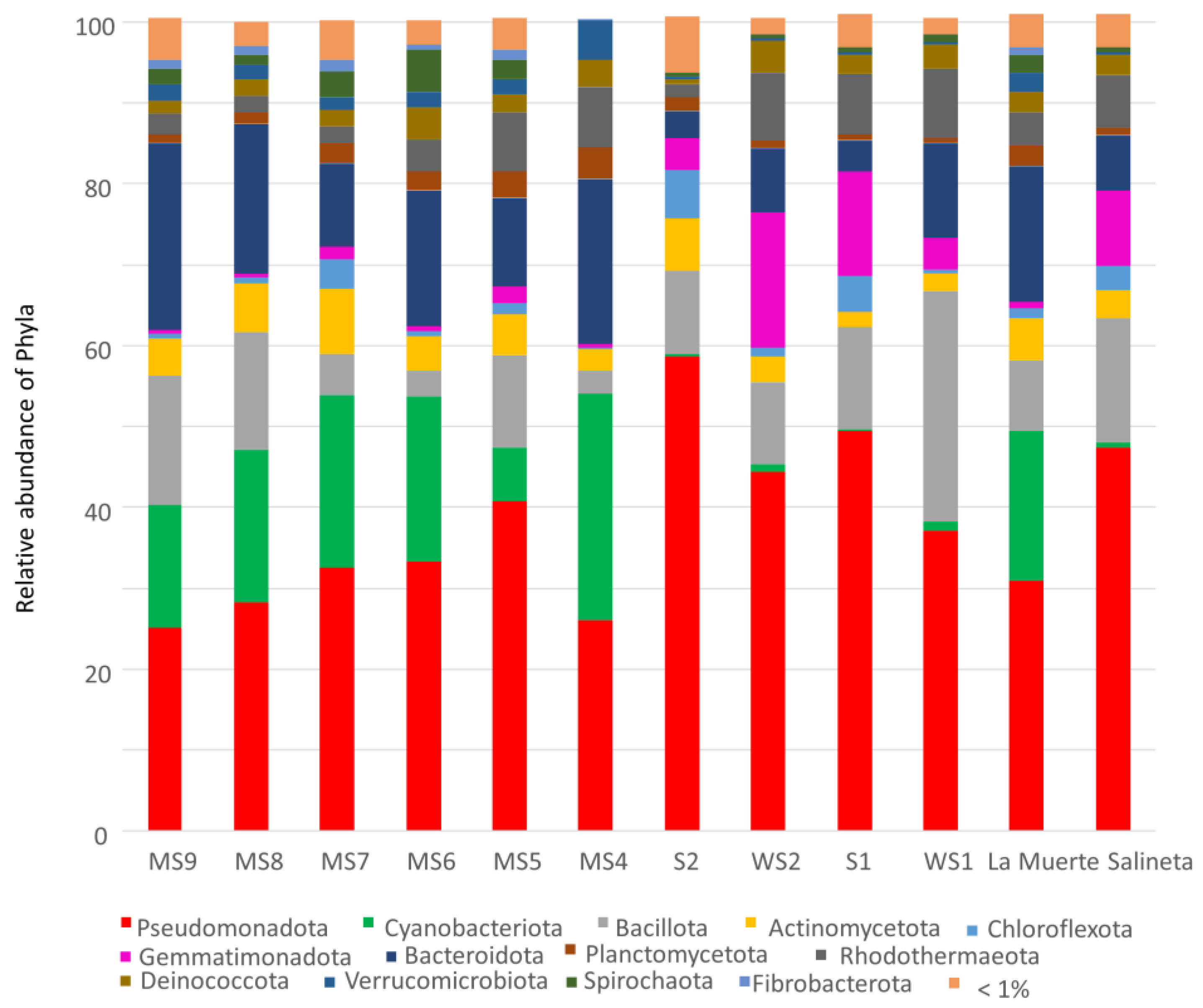
3.1. Bacterial Diversity in La Muerte and Salineta Lagoons
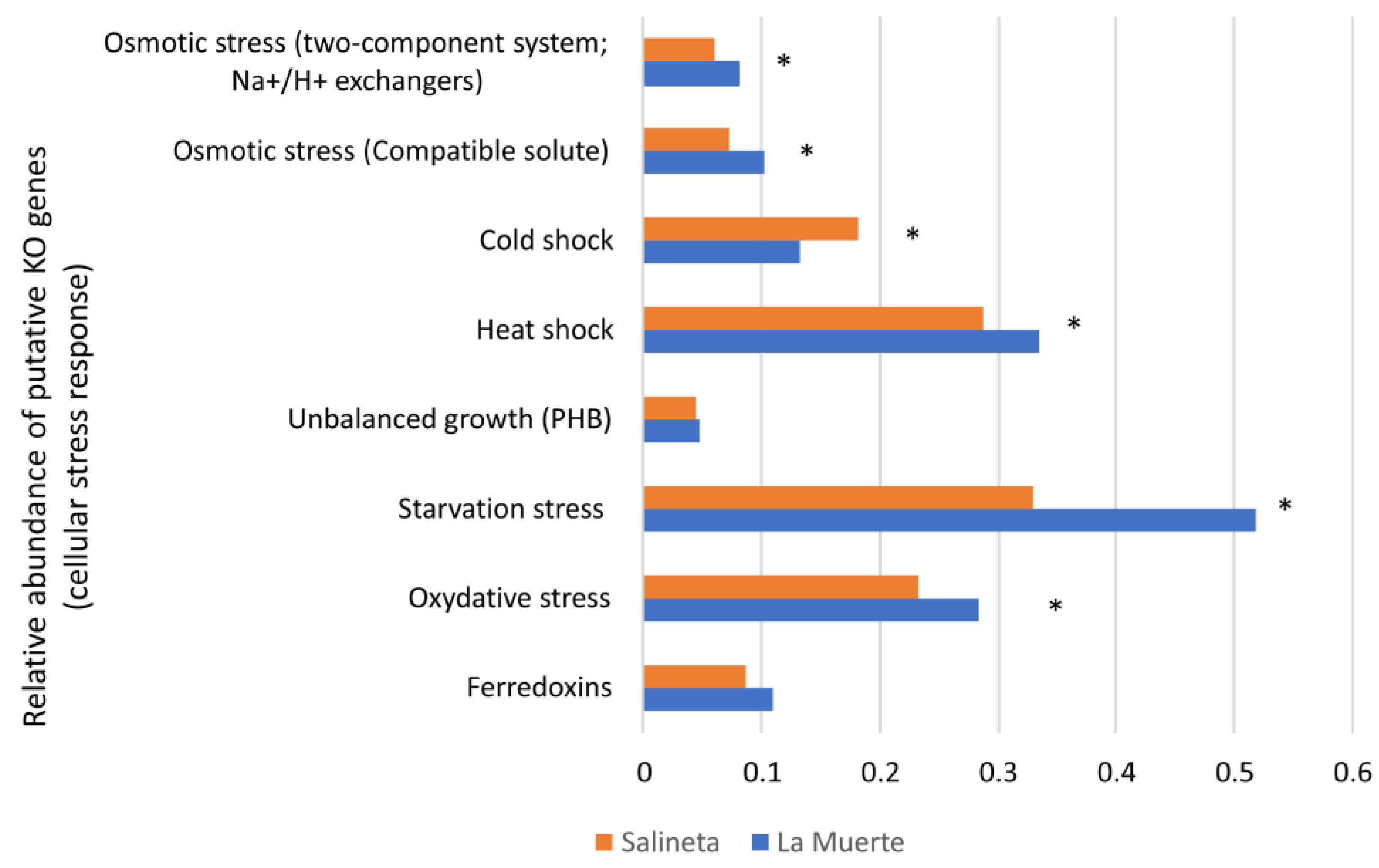
3.2. Putative Functionality in La Muerte and Salineta Lagoons
4. Discussion
4.1. Bacterial Diversity and Putative Functionality in La Muerte and Salineta Lagoons
4.2. Perspectives, Implications, and Future Research Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Song, C.; Reager, J.; Yao, F.; Famiglietti, J.; Sheng, Y.; MacDonald, G.; Brun, F.; Schmied, H.; Marston, R.; et al. Recent global decline in endorheic basin water storages. Nat. Geosci. 2018, 11, 926–932, Erratum in Nat. Geosci. 2019, 12, 220. [Google Scholar] [CrossRef]
- Gunde-Cimerman, N.; Plemenitaš, A.; Oren, A. Strategies of adaptation of microorganisms to high salt concentrations. FEMS Microbiol. Rev. 2018, 42, 353–375. [Google Scholar] [CrossRef] [PubMed]
- Casamayor, E.O.; Triadó-Margarit, X.; Castañeda, C. Microbial biodiversity in saline shallow lakes of the Monegros Desert, Spain. FEMS Microbiol. Ecol. 2013, 85, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Menendez-Serra, M.; Triadó-Margarit, X.; Casamayor, E.O. Ecological and metabolic thresholds in the bacterial, protist, and fungal microbiome of ephemeral saline lakes (Monegros Desert, Spain). Microb Ecol. 2021, 82, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, C.; García-Vera, M.A. Water balance in the playa-lakes and arid environament, Monegros, NE Spain. Hydrogeol. J. 2008, 16, 87–102. [Google Scholar] [CrossRef]
- Bourhane, Z.; Cagnon, C.; Castañeda, C.; Rodriguez-Ochoa, R.; Alvaro-Fuentes, J.; Cravo-Laureau, C.; Duran, R. Vertical organization of microbial communities in Salineta hypersaline wetland, Spain. Front. Microbiol. 2023, 14, 1146747. [Google Scholar] [CrossRef]
- Berlanga, M.; Picart, P.; Blasco, A.; Benaiges-Fernandez, R.; Guerrero, R.; Butturini, A.; Urmeneta, J. Biodiversity and potential functionality of biofilm-sediment biotope in La Muerte lagoon, Monegros Desert, Spain. Front. Ecol. Evol. 2024, 12, 1412124. [Google Scholar] [CrossRef]
- Menéndez-Serra, M.; Cáliz, J.; Triadó-Margarit, X.; Alonso, D.; Casamayor, E.O. Selective pressure influences inter-biome dispersal in the assembly of saline microbial communities. Environ. Microbiol. 2024, 26, e70019. [Google Scholar] [CrossRef]
- Varliero, G.; Lebre, P.H.; Stevens, M.I.; Czchowski, C.; Makhalanyane, T.; Cowan, D.A. The use of different 16S rRNA gene variable regions in biogeographical studies. Environ. Microbiol. Rep. 2023, 15, 216–228. [Google Scholar] [CrossRef]
- Chalita, M.; Kim, Y.O.; Oh, H.-S.; Cho, J.H.; Moon, J.; Baek, N.; Moon, C.; Lee, K.; Yang, J.; Nam, G.G.; et al. EzBioCloud: A genome-driven database and platform for microbiome identification and discovery. Int J. Syst. Evol. Microbiol. 2024, 74, 006421. [Google Scholar] [CrossRef] [PubMed]
- Van Nostrand, J.D.; Zhou, A.; Zhou, J. StressChip for monitoring microbial stress response in the environment. In Stress and Environmental Regulation of Gene Expression and Adaptation in Bacteria; De Brujin, F.J., Ed.; John Wiley: Hoboken, NJ, USA, 2016; pp. 9–22. [Google Scholar]
- Gao, L.; Rao, M.P.N.; Liu, Y.-H.; Wang, P.-D.; Lian, Z.-H.; Abdugheni, R.; Jiang, H.-C.; Jiao, J.-Y.; Shurigin, V.; Fang, B.-Z.; et al. Salinity-induced changes in diversity, stability, and functional profiles of microbial communities in different saline lakes in arid areas. Microb. Ecol. 2024, 87, 135. [Google Scholar] [CrossRef] [PubMed]
- Fanf, Y.; Liu, J.; Yang, J.; Wu, G.; Hua, Z.; Dong, H.; Hedlund, B.P.; Baker, B.J.; Jiang, H.; Mackelprang, R. Compositional metabolic responses of autotrophic microbial community to salinity in lacustrine environments. mSystems 2022, 7, e0033522. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, M.; Palau, M.; Guerrero, R. Functional stability and community Dynamics during spring and autumn seasons over 3 years in Camargue microbial mats. Front. Microbiol. 2017, 8, 2619. [Google Scholar] [CrossRef]
- Berlanga, M.; Palau, M.; Guerrero, R. Community homeostasis of coastal microbial mats from the Camargue during winter (cold) and summer (hot) seasons. Ecosphere 2022, 13, e3922. [Google Scholar] [CrossRef]
- Pastor, A.; Freixa, A.; Skovsholt, L.J.; Wu, N.; Romaní, A.M.; Riis, T. Microbial organic matter utilization in high-Artic streams: Key enzymatic controls. Microb. Ecol. 2019, 78, 539–554. [Google Scholar] [CrossRef]
- Castañeda, C.; Herrero, J.; Conesa, J.A. Distribution, morphology and habitats of saline wetlands: A case study from Monegros, Spain. Geologica Acta 2013, 11, 371–388. [Google Scholar] [CrossRef]
- Bertos-Fortis, M.; Farnelid, H.M.; Lindh, M.V.; Casini, M.; Andersson, A.; Pinhassi, J.; Legrand, C. Unscrambling Cyanobacteria community dynamics related to environmental factors. Front. Microbiol. 2016, 7, 625. [Google Scholar] [CrossRef]
- Wasmund, K.; Mussmann, M.; Loy, A. The life sulfuric: Microbial ecology of sulfur cycling in marine sediments. Environ. Microbiol. Rep. 2017, 9, 323–344. [Google Scholar] [CrossRef]
- Jørgensen, B.; Findlay, A.; Pellerin, A. The biogeochemical sulfur cycle of marine sediments. Front. Microbiol. 2019, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, W.; Jiang, L.; Li, E.; Yang, X.; Yang, J. Salinity affects microbial function genes related to nutrient cycling in arid regions. Front. Microbiol. 2024, 15, 1407760. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Chen, Q.; Qin, X.; Zhou, S.; Nethmini, R.T.; Jiang, G.; Hou, Q.; Li, X.; Huang, L.; Dong, K.; et al. Dissolved oxygen and nitrates gradient influence marine microbial complexity and stability in Beibu Gulf. Front. Microbiol. 2025, 16, 1622150. [Google Scholar] [CrossRef] [PubMed]
- Grzesiak, J.; Rogala, M.M.; Gawor, J.; Kouřilová, X.; Obruča, S. Polyhydroxyalkanoate involvement in stress-survival of two psychrophilic bacterial strains from the High Arctic. Appl. Microbiol. Biotechnol. 2024, 108, 273. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, G.; Pire, C.; Martínez-Espinosa, R.M. Hypersaline environments as natural sources of microbes with potential Applications in Biotechnology: The case of solar evaporation systems to produce sant in Alicante County (Spain). Curr. Res. Microbiol. 2022, 3, 100136. [Google Scholar] [CrossRef]
- De Miera, L.; Gutierrez-Gonzalez, J.; Arroyo, P.; Falagán, J.; Ansola, G. Prokaryotic community diversity in the sediments of saline lagoons and its resistance to seasonal disturbances by water level cycles. J. Soils Sed. 2021, 21, 3169–3184. [Google Scholar] [CrossRef]


| GLU:PHEN | GLU:PHOS | XYL:GLU | (GLU + XYL): CEL | |
|---|---|---|---|---|
| La Muerte | 4.8 | 1.05:1 | 0.1588 | 5.77 |
| Salineta | 1.02 | 2.2:1 | 0.0134 | 53.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berlanga, M.; Blasco, A.; Guerrero, R.; Butturini, A.; Urmeneta, J. Comparative Analysis of Bacterial Diversity and Functional Potential in Two Athalassohaline Lagoons in the Monegros Desert (NE Spain). Microorganisms 2025, 13, 2224. https://doi.org/10.3390/microorganisms13102224
Berlanga M, Blasco A, Guerrero R, Butturini A, Urmeneta J. Comparative Analysis of Bacterial Diversity and Functional Potential in Two Athalassohaline Lagoons in the Monegros Desert (NE Spain). Microorganisms. 2025; 13(10):2224. https://doi.org/10.3390/microorganisms13102224
Chicago/Turabian StyleBerlanga, Mercedes, Arnau Blasco, Ricardo Guerrero, Andrea Butturini, and Jordi Urmeneta. 2025. "Comparative Analysis of Bacterial Diversity and Functional Potential in Two Athalassohaline Lagoons in the Monegros Desert (NE Spain)" Microorganisms 13, no. 10: 2224. https://doi.org/10.3390/microorganisms13102224
APA StyleBerlanga, M., Blasco, A., Guerrero, R., Butturini, A., & Urmeneta, J. (2025). Comparative Analysis of Bacterial Diversity and Functional Potential in Two Athalassohaline Lagoons in the Monegros Desert (NE Spain). Microorganisms, 13(10), 2224. https://doi.org/10.3390/microorganisms13102224








