White Mold: A Global Threat to Crops and Key Strategies for Its Sustainable Management
Abstract
1. Introduction
2. Significance and Negative Impacts of White Mold
3. Clinical Symptoms and Signs of White Mold
4. Historical Perspective of the White Mold Pathogen
5. Distinct Morphology and Pathogen Biology
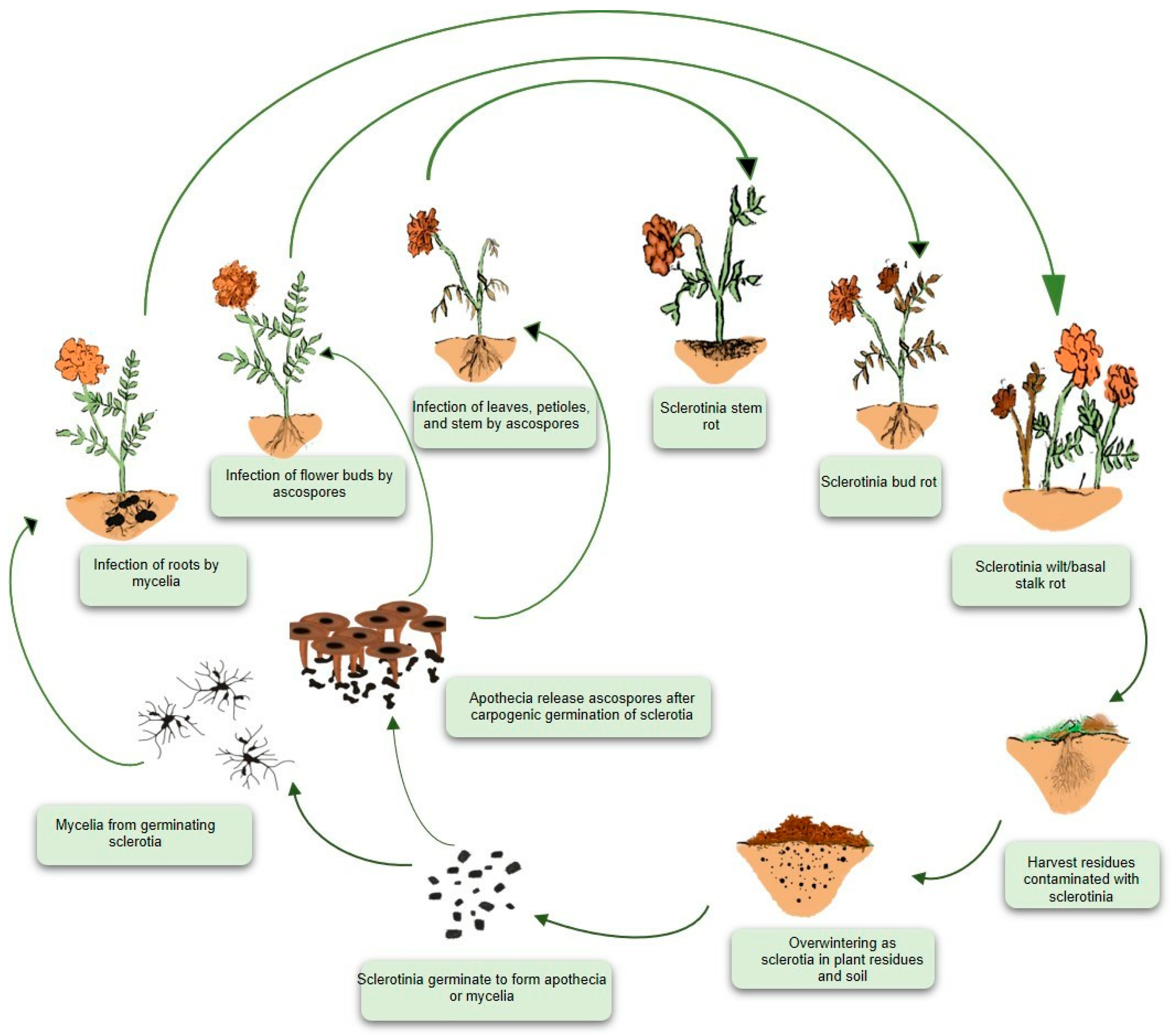
6. Monocyclic Disease Cycle
7. Epidemiological Conditions of the Disease
8. Infection Process and Mechanisms
9. Narrow Genetic Structures of the Pathogen
10. Early Detection of White Mold
10.1. Molecular Diagnosis of White Mold
| Methods | Application | Advantage | References |
|---|---|---|---|
| Restriction fragment length polymorphisms (RFLP) | Comparing relatedness and detecting intra- and inter-specific variants in the genus | Quick, simple, cost-effective, and highly reproducible | [29] |
| DNA barcoding | Species-level identifications based on DNA sequences from a signature region of the genome | Sequence availability and high accuracy | [38,72] |
| Simplex PCR | Single species identification based on the presence of marker gene | Higher sensitivity and accurate detection | [76] |
| Single-nucleotide polymorphisms (SNP) | Detect genetic variants (mutations) within Sclerotinia populations using PCR amplification and oligonucleotide hybridizations | Fast and robust and is amenable to the high-throughput screening of samples | [77] |
| qPCR | Identification and quantification of the pathogen in plant infections | A rapid, accurate, and reliable detection; and quantification of very low amounts of pathogen DNA | [78] |
| Multiplex qPCR | Multiple species identification in plant material, including seeds using species-specific primers | Cost-effective, time-saving, and higher throughput | [73] |
| Seed-soaking-based PCR | S. sclerotiorum detection in seeds or plant components without prior fungal DNA extraction | Precise detection and reduced assay time (about 9 h) | [79] |
| Loop-mediated isothermal amplification (LAMP) | Fungal, plant, and soil samples | Sensitive, specific, rapid, and suitable for field application | [9] |
10.2. Sensor-Based Detection of White Mold Disease
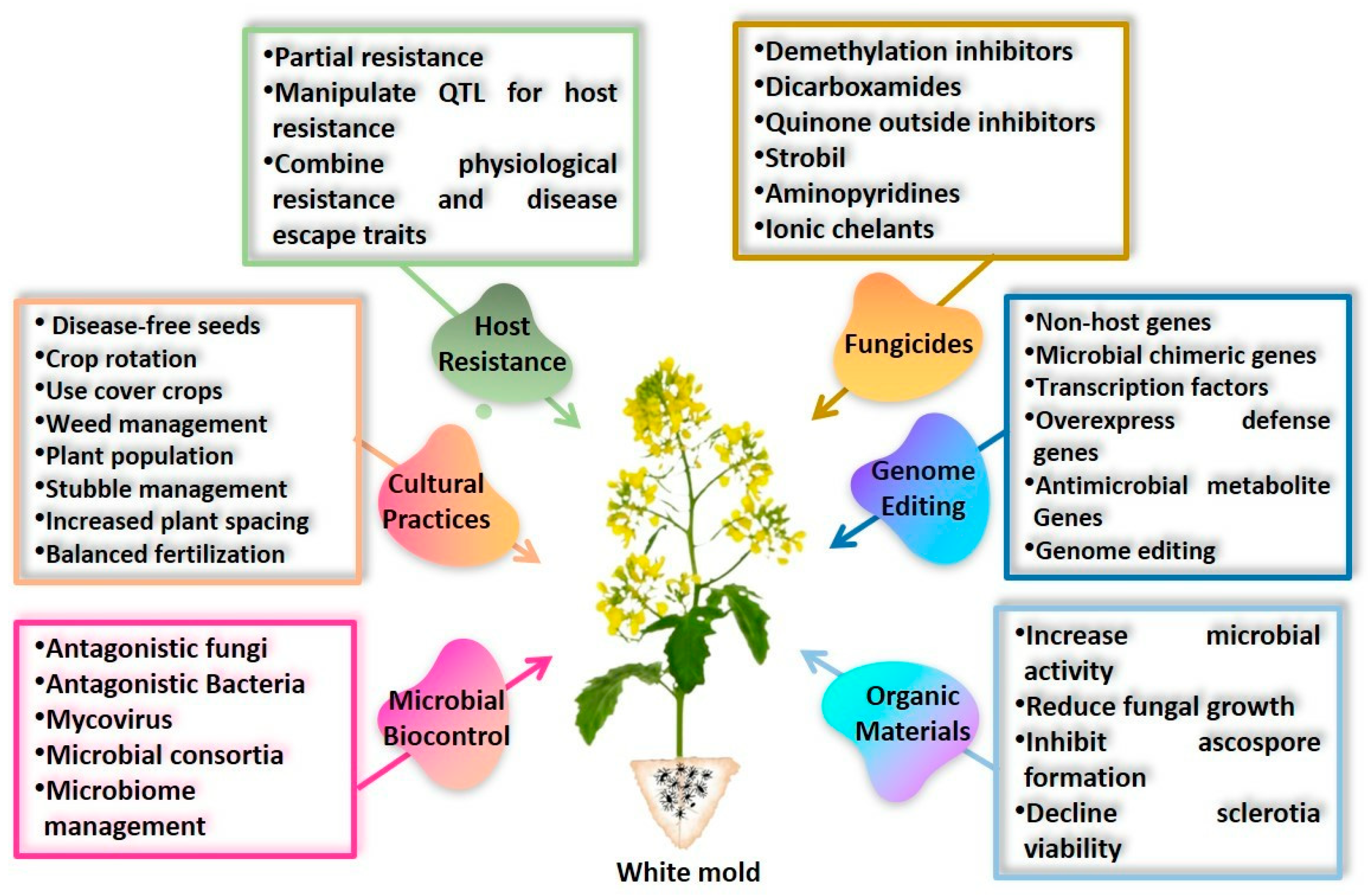
11. Conventional to Non-Conventional Disease Management
11.1. Modifying Crop Microclimate by Cultural Practices
11.2. Use of Natural Resistant Sources
11.3. Use of Transgenes
11.4. Genome Editing
11.5. Protection of Susceptible Plants by Fungicides
11.6. Eliminating the Pathogen by Biological Control Agents
11.6.1. Application of Solo Microbial Agent
| Species | Host Plants | Disease | References |
|---|---|---|---|
| Coniothyrium minitans | Dry bean | White mold | [133] |
| C. minitans | Lettuce | Sclerotinia disease | [137] |
| C. minitans | Carrot | Sclerotinia rot | [138] |
| C. minitans (Contans) | Lettuce | Lettuce drop | [139] |
| C. minitans CON/M/91-08 (Contans®WG) Streptomyces lydicus WYEC 108 (Actinovate®AG) Trichoderma harzianum T-22 (PlantShield®HC), | Soybean | Sclerotinia stem rot | [134] |
| C. minitans LU112 T. virens LU556 | Cabbage | Sclerotinia disease | [140] |
| Fusarium oxysporum (S6) | Soybean | Sclerotinia disease | [141] |
| Coniothyrium minitans Microsphaeropsis ochracea | NT | - | [142] |
| Sporidesmium sclerotivorum | Soybean | Sclerotinia stem rot | [143] |
| Trichoderma asperellum | Common bean | White mold | [144] |
| Trichoderma asperellum T2 Trichoderma hamatum T3 T. harzianum T6 | Arabidopsis | Root rot | [145] |
| C. minitans T. atroviride | Alfalfa | sclerotinia blossom blight | [132] |
| T. harzianum-8 T. atroviride PTCC5220 T. longibrachiatum PTCC5140 | Canola | Stem rot | [146] |
| Ulocladium atrum | Canola | Sclerotinia disease | [147] |
| Bacillus subtilis BY-2 Bacillus megaterium A6 | Oilseed rape | Sclerotinia disease | [148] |
| Bacillus cereus SC-1 | Canola | Stem rot | [149] |
| Bacillus subtilis SB01 Bacillus subtilis SB24 | Soybean | Sclerotinia stem rot | [150] |
| Bacillus cereus Bacillus amyloliquefaciens | Carnation | Sclerotinia root rot | [151] |
| Bacillus sp. B19 Bacillus sp. P12 Bacillus amyloliquefaciens B1 | Common bean | White mold | [152] |
| Streptomyces lydicus WYEC 108 | Brassica vegetables | Sclerotinia disease | [89] |
| Streptomyces exfoliates FT05W Streptomyces cyaneus ZEA17I | Lettuce drop | Lettuce | [129] |
| Serratia plymuthica IC14 | White mold | Cucumber | [126] |
11.6.2. Microbial Consortia and Microbiome Management
11.6.3. Organic Material Amendments
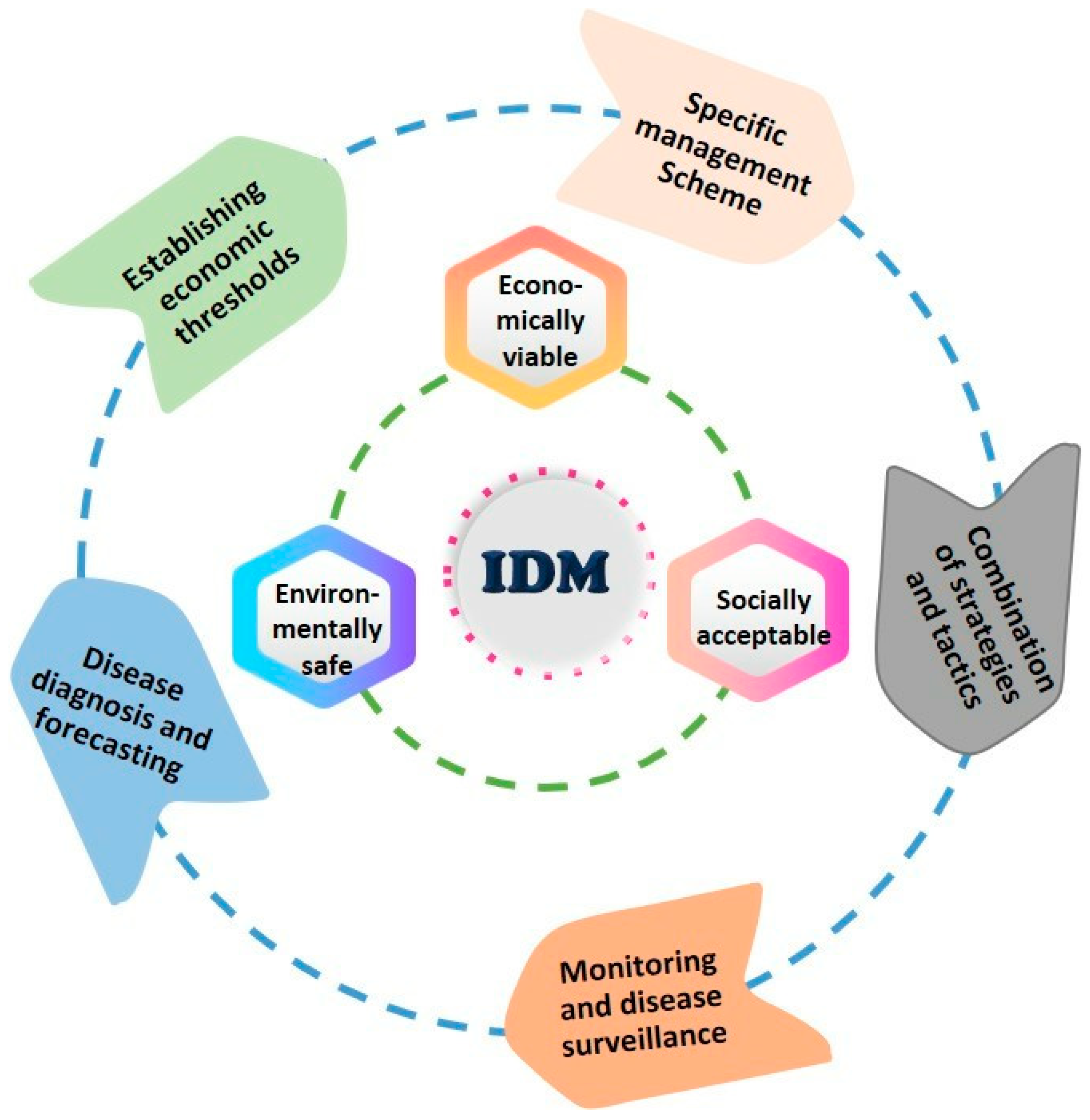
11.6.4. Integrated Disease Management
12. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehner, M.S.; De Paula Junior, T.J.; Del Ponte, E.M.; Mizubuti, E.S.G.; Pethybridge, S.J. Independently Founded Populations of Sclerotinia sclerotiorum from a Tropical and a Temperate Region Have Similar Genetic Structure. PLoS ONE 2017, 12, e0173915. [Google Scholar] [CrossRef] [PubMed]
- Jahan, R.; Siddique, S.S.; Jannat, R.; Hossain, M.M. Cosmos White Rot: First Characterization, Physiology, Host Range, Disease Resistance, and Chemical Control. J. Basic Microbiol. 2022, 62, 911–929. [Google Scholar] [CrossRef]
- Islam, M.R.; Akanda, A.M.; Hossain, M.M.; Hossain, M.M. First Characterization of A Newly Emerging Phytopathogen, Sclerotinia sclerotiorum Causing White Mold in Pea. J. Basic Microbiol. 2021, 61, 923–939. [Google Scholar] [CrossRef] [PubMed]
- Prova, A.; Akanda, A.M.; Islam, S.; Hossain, M.M. First report of Sclerotinia sclerotiorum causing pod rot disease on okra in Bangladesh. Can. J. Plant Pathol. 2017, 39, 72–76. [Google Scholar] [CrossRef]
- Pritchard, M.K.; Boese, D.E.; Rimmer, S.R. Rapid Cooling and Field-Applied Fungicides for Reducing Losses in Stored Carrots Caused by Cottony Soft Rot. Can. J. Plant Pathol. 1992, 14, 177–181. [Google Scholar] [CrossRef]
- Rader, W.E. Diseases of stored carrots in New York State. In New York Agricultural Experiment Station Geneva Bulletin; No. 889; 1952; Volume 899, pp. 10–14. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19540300488 (accessed on 24 January 2023).
- Alkooranee, J.T.; Aledan, T.R.; Ali, A.K.; Lu, G.; Zhang, X.; Wu, J.; Fu, C.; Li, M. Detecting the Hormonal Pathways in Oilseed Rape Behind Induced Systemic Resistance by Trichoderma harzianum TH12 to Sclerotinia sclerotiorum. PLoS ONE 2017, 12, e0168850. [Google Scholar] [CrossRef]
- Kora, C.; McDonald, M.R.; Boland, G.J. Sclerotinia Rot of Carrot: An Example of Phenological Adaptation and Bicyclic Development by Sclerotinia sclerotiorum. Plant Dis. 2003, 87, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Grabicoski, E.M.G.; Jaccoud Filho, D.D.S.; Lee, D.; Henneberg, L.; Pileggi, M. Real-time Quantitative and Ion-Metal Indicator LAMP-Based Assays for Rapid Detection of Sclerotinia sclerotiorum. Plant Dis. 2020, 104, 1514–1526. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.C. Remote Sensing of Diseases. Annu. Rev. Phytopathol. 2020, 58, 225–252. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Liu, F.; Guo, H.; Kong, W.; Zhang, C.; He, Y. Fast Detection of Sclerotinia sclerotiorum on Oilseed Rape Leaves Using Low-Altitude Remote Sensing Technology. Sensors 2018, 18, 4464. [Google Scholar] [CrossRef]
- Perveen, K.; Haseeb, A.; Shukla, P.K. Effect of Sclerotinia sclerotiorum on Disease Development, Growth, Oil Yield, and Biochemical Changes in Plants of Mentha arvensis. Saudi J. Biol. Sci. 2010, 17, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Link, V.H.; Johnson, K.B. White Mold (Sclerotinia); The American Phytopathological Society: St. Paul, MN, USA, 2007. [Google Scholar]
- Antwi-Boasiako, A.; Wang, Y.; Dapaah, H.K.; Zhao, T. Mitigating Against Sclerotinia Diseases in Legume Crops: A Comprehensive Review. Agronomy 2022, 12, 3140. [Google Scholar] [CrossRef]
- Danielson, G.A.; Nelson, B.D.; Helms, T.C. Effect of Sclerotinia Stem Rot on Yield of Soybean Inoculated at Different Growth Stages. Plant Dis. 2004, 88, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Saharan, G.S.; Mehta, N. Sclerotinia Diseases of Crop Plants: Biology, Ecology and Disease Management; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Peltier, A.J.; Bradley, C.A.; Chilvers, M.I.; Malvick, D.K.; Mueller, D.S.; Wise, K.A.; Esker, P.D. Biology, Yield Loss and Control of Sclerotinia Stem Rot of Soybean. J. Integr. Pest Manag. 2012, 3, B1–B7. [Google Scholar] [CrossRef]
- Del Río, L.E.; Bradley, C.A.; Henson, R.A.; Endres, G.J.; Hanson, B.K.; McKay, K.; Halvorson, M.; Porter, P.M.; Le Gare, D.G.; Lamey, H.A. Impact of Sclerotinia Stem Rot on Yield of Canola. Plant Dis. 2007, 91, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Mathew, F.; Harveson, R.; Block, C.; Gulya, T.; Ryley, M.; Thompson, S.; Markell, S. Sclerotinia sclerotiorum Diseases of Sunflower (White Mold). Plant Health Instr. 2020, 20. [Google Scholar] [CrossRef]
- Derbyshire, M.C.; Denton-Giles, M. The Control of Sclerotinia Stem Rot on Oilseed Rape (Brassica napus): Current Practices and Future Opportunities. Plant Pathol. 2016, 65, 859–877. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Li, W.; Tran, L.P.; Mostofa, M.G. Sclerotinia sclerotiorum (Lib.) de Bary: Insights into the Pathogenomic Features of a Global Pathogen. Cells 2023, 12, 1063. [Google Scholar] [CrossRef] [PubMed]
- Whetzel, H.H. A Synopsis of the Genera and Species of the Sclerotiniaceae, a Family of Stromatic Inoperculate Discomycetes. Mycologia 1945, 37, 648–714. [Google Scholar] [CrossRef]
- Libert, M.A. Plante Crytogamicae Arduennae (Exsiccati); No. 326; Published by the Author; 1837. [Google Scholar]
- Fuckel, K.W.G.L. Symbolae Mycologicae. Beiträge zur Kenntniss der Rheinischen Pilze. Jahrbücher Des. Nassau. Ver. Für Naturkunde 1870, 23–24, 459. [Google Scholar]
- Purdy, L.H. Sclerotinia sclerotiorum: History, Diseases and Symptomatology, Host Range, Geographic Distribution, and Impact. Phytopathology 1979, 69, 875–880. [Google Scholar] [CrossRef]
- Wakefield, E.N. On the names Sclerotinia sclerotiorum (Lib.) Massee, and S. libertiana Fuckel. Phytopathology 1924, 14, 126–127. [Google Scholar]
- Korf, R.P.; Dumont, K.P. Whetzelinia, a New Generic Name of Sclerotinia sclerotiorum and S. tuberose. Mycologia 1972, 64, 248–251. [Google Scholar] [CrossRef]
- Kohn, L.M. A Monographic Revision of the Genus Sclerotinia. Mycotaxon 1979, 9, 365–444. [Google Scholar]
- Kohn, L.M.; Petsche, D.M.; Bailey, S.R.; Novak, L.A.; Anderson, J.B. Restriction Fragment Length Polymorphisms in Nuclear and Mitochondrial DNA of Sclerotinia species. Phytopathology 1988, 78, 1047–1051. [Google Scholar] [CrossRef]
- Rossman, A.Y.; Palm, M.E.; Spielman, L.J. A Literature Guide for the Identification of Plant Pathogenic Fungi; American Phytopathological Society Press: St Paul, MN, USA, 1987; pp. 202–203. [Google Scholar]
- Hoist-Jensen, A.; Vaage, M.; Schumacher, T. An Approximation to the Phylogeny of Sclerotinia and Related Genera. Nord. J. Bot. 1998, 18, 705–719. [Google Scholar] [CrossRef]
- Sanogo, S.; Puppala, N. Characterization of A Darkly Pigmented Mycelial Isolate of Sclerotinia sclerotiorum on Valencia Peanut in New Mexico. Plant Dis. 2007, 91, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, R.D.; Dow, R.L. Histopathology of Sclerotinia sclerotiorum Infection of Bean. Phytopathology 1973, 63, 708–715. [Google Scholar] [CrossRef]
- Rather, R.A.; Ahanger, F.A.; Ahanger, S.A.; Basu, U.; Wani, M.A.; Rashid, Z.; Sofi, P.A.; Singh, V.; Javeed, K.; Baazeem, A.; et al. Morphological and Pathogenic Variability of Sclerotinia sclerotiorum Causing White Mold of Common Beans in Temperate Climate. J. Fungi 2022, 8, 755. [Google Scholar] [CrossRef] [PubMed]
- Prova, A.; Akanda, M.A.M.; Islam, S.; Sultana, F.; Islam, M.T.; Hossain, M.M. First Report of Stem and Pod Blight of Hyacinth Bean Caused by Sclerotinia sclerotiorum. J. Plant Pathol. 2014, 96, 603–611. [Google Scholar]
- Lazarovits, G.; Starratt, A.; Huang, H. The Effect of Tricyclazole and Culture Medium on Production of the Melanin Precursor 1,8-Dihydroxynaphthalene by Sclerotinia sclerotiorum Isolate SS7. Pestic. Biochem. Physiol. 2000, 67, 54–62. [Google Scholar] [CrossRef]
- Bolton, M.D.; Thomma, B.P.H.J.; Nelson, B.D. Sclerotinia sclerotiorum (Lib.) de Bary: Biology and Molecular Traits of a Cosmopolitan Pathogen. Mol. Plant Pathol. 2006, 7, 1–16. [Google Scholar] [CrossRef]
- Prova, A.; Akanda, A.M.; Islam, S.; Hossain, M.M. Characterization of Sclerotinia sclerotiorum, an Emerging Fungal Pathogen Causing Blight in Hyacinth Bean (Lablab purpureus). Plant Pathol. J. 2018, 34, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Thomma, B.P.H.J. Alternaria spp. from General Saprophyte to Specific Parasite. Mol. Plant Pathol. 2003, 4, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C. Factors Affecting Myceliogenic Germination of Sclerotia of Sclerotinia sclerotiorum. Phytopathology 1985, 75, 433–437. [Google Scholar] [CrossRef]
- Ekins, M.G.; Aitken, E.A.B.; Goulter, K.C. Carpogenic Germination of Sclerotinia minor and Potential Distribution in Australia. Australas. Plant Pathol. 2002, 31, 259–265. [Google Scholar] [CrossRef]
- Smolińska, U.; Kowalska, B.; Kowalczyk, W.; Szczech, M.; Murgrabia, A. Eradication of Sclerotinia sclerotiorum Sclerotia from Soil Using Organic Waste Materials as Trichoderma Fungi Carriers. J. Hortic. Res. 2016, 24, 101–110. [Google Scholar] [CrossRef]
- Clarkson, J.P.; Fawcett, L.; Anthony, S.G.; Young, C. A Model for Sclerotinia sclerotiorum Infection and Disease Development in Lettuce, Based on the Effects of Temperature, Relative Humidity, and Ascospore Density. PLoS ONE 2014, 9, e94049. [Google Scholar] [CrossRef] [PubMed]
- Willbur, J.; Mccaghey, M.; Kabbage, M.; Smith, D.L. An Overview of the Sclerotinia sclerotiorum Pathosystem in Soybean: Impact, Fungal Biology, and Current Management Strategies. Trop. Plant Pathol. 2019, 44, 3–11. [Google Scholar] [CrossRef]
- Uloth, M.B.; You, M.P.; Cawthray, G.; Barbetti, M.J. Temperature adaptation in isolates of Sclerotinia sclerotiorum affects their ability to infect Brassica carinata. Plant Pathol. 2015, 64, 1140–1148. [Google Scholar] [CrossRef]
- Heller, A.; Witt-Geiges, T. Oxalic Acid has an Additional, Detoxifying Function in Sclerotinia sclerotiorum Pathogenesis. PLoS ONE 2013, 8, e72292. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhu, W.; Wei, W.; Zhao, X.; Wang, Q.; Zeng, W.; Zheng, Y.; Chen, P.; Zhang, S. De Novo Assembly and Transcriptome Analysis of Sclerotial Development in Wolfiporia cocos. Gene 2016, 588, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Seifbarghi, S.; Borhan, M.H.; Wei, Y. Changes in the Sclerotinia sclerotiorum Transcriptome During Infection of Brassica napus. BMC Genom. 2017, 18, 266. [Google Scholar] [CrossRef] [PubMed]
- Chittem, K.; Yajima, W.R.; Goswami, R.S.; del Río Mendoza, L.E. Transcriptome Analysis of the Plant Pathogen Sclerotinia sclerotiorum Interaction with Resistant and Susceptible Canola (Brassica napus) Lines. PLoS ONE 2020, 15, e0229844. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Xue, M.; Shen, Z.; Jia, X.; Hou, X.; Lai, D.; Zhou, L. Phytotoxic Secondary Metabolites from Fungi. Toxins 2021, 13, 261. [Google Scholar] [CrossRef] [PubMed]
- Bashi, Z.D.; Rimmer, S.R.; Khachatourians, G.G.; Hegedus, D.D. Brassica napus Polygalacturonase Inhibitor Proteins Inhibit Sclerotinia sclerotiorum Polygalacturonase Enzymatic and Necrotizing Activities and Delay Symptoms in Transgenic Plants. Can. J. Microbiol. 2012, 59, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Kull, L.S.; Pedersen, W.L.; Palmquist, D.; Hartman, G.L. Mycelial Compatibility Grouping and Aggressiveness of Sclerotinia sclerotiorum. Plant Dis. 2004, 88, 325–332. [Google Scholar] [CrossRef]
- Faraghati, M.; Abrinbana, M.; Ghosta, Y. Genetic Structure of Sclerotinia sclerotiorum Populations from Sunflower and Cabbage in West Azarbaijan Province of Iran. Sci. Rep. 2022, 12, 9263. [Google Scholar] [CrossRef]
- Sun, J.; Irzykowski, W.; Jedryczka, M.; Han, F. Analysis of the Genetic Structure of Sclerotinia sclerotiorum Populations from Different Regions and Host Plants by RAPD Markers. J. Integr. Plant Biol. 2005, 47, 385–395. [Google Scholar] [CrossRef]
- Kohli, Y.; Kohn, L.M. Random Association among Alleles in Clonal Populations of Sclerotinia sclerotiorum. Fungal Genet. Biol. 1998, 23, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, Q.; Zhao, L.H.; Zhang, S.M.; Wang, Y.X.; Zhao, X.Y. Purification and Characterization of a Novel Antifungal Protein from Bacillus subtilis Strain B29. J. Zhejiang Univ. Sci. B 2009, 10, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y. Population Structure and Aggressiveness of Sclerotinia sclerotiorum from Rapeseed (Brassica napus) in Chongqing City. Plant Dis. 2020, 104, 1201–1206. [Google Scholar] [CrossRef]
- Buchwaldt, L.; Garg, H.; Puri, K.D.; Durkin, J.; Adam, J.; Harrington, M.; Liabeuf, D.; Davies, A.; Hegedus, D.D.; Sharpe, A.G.; et al. Sources of Genomic Diversity in the Self-Fertile Plant Pathogen, Sclerotinia sclerotiorum, and Consequences for Resistance Breeding. PLoS ONE 2022, 17, e0262891. [Google Scholar] [CrossRef] [PubMed]
- Abán, C.L.; Taboada, G.; Spedaletti, Y.; Aparicio, M.; Curti, R.N.; Casalderrey, N.B.; Maggio, M.E.; Chocobar, M.O.; Salgado, M.; Galván, M.Z. Molecular, Morphological and Pathogenic Diversity of Sclerotinia sclerotiorum Isolates from Common Bean (Phaseolus vulgaris) Fields in Argentina. Plant Pathol. 2018, 67, 1740–1748. [Google Scholar] [CrossRef]
- Carbone, I.; Anderson, J.B.; Kohn, L.M. Patterns of Descent in Clonal Lineages and Their Multilocus Fingerprints Are Resolved with Combined Gene Genealogies. Evolution 1999, 53, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Hambleton, S.; Walker, C.; Kohn, L.M. Clonal lineages of Sclerotinia sclerotiorum previously known from other crops predominate in 1999–2000 samples from Ontario and Quebec soybean. Can. J. Plant Pathol. 2002, 24, 309–315. [Google Scholar] [CrossRef]
- Kull, L.S.; Vuong, T.D.; Powers, K.S.; Eskridge, K.M.; Steadman, J.R.; Hartman, G.L. Evaluation of Three Resistance Screening Methods Using Six Sclerotinia sclerotiorum Isolates and Three Entries of Each Soybean and Dry Bean. Plant Dis. 2003, 87, 1471–1476. [Google Scholar] [CrossRef]
- Malvárez, G.; Carbone, I.; Grünwald, N.J.; Subbarao, K.V.; Schafer, M.; Kohn, L.M. New Populations of Sclerotinia sclerotiorum from Lettuce in California and Peas and Lentils in Washington. Phytopathology 2007, 97, 470–483. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.W.; Yun, S.H.; Lee, T.; Turgeon, B.G. Altering Sexual Reproductive Mode by Interspecific Exchange of MAT Loci. Fungal Genet. Biol. 2011, 48, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, B.; Yoder, O. Proposed Nomenclature for Mating Type Genes of Filamentous Ascomycetes. Fungal Genet. Biol. 2000, 31, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Amselem, J.; Cuomo, J.C.A.; van Kan, J.A.L.; Viaud, M.; Benito, E.P.; Couloux, A.; Coutinho, P.M.; de Vries, R.P.; Dyer, P.S.; Fillinger, S.; et al. Genomic Analysis of the Necrotrophic Fungal Pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet. 2011, 7, e1002230. [Google Scholar] [CrossRef]
- Samuels, G.J.; Lodge, D.J. Three Species of Hypocrea with Stipitate Stromata and Trichoderma Anamorphs. Mycologia 1996, 88, 302. [Google Scholar] [CrossRef]
- Uhm, J.Y.; Fujii, H. Heterothallism and Mating Type Mutation in Sclerotinia trifoliorum. Phytopathology 1983, 73, 569–572. [Google Scholar] [CrossRef]
- Woudenberg, J.H.C.; De Gruyter, J.; Crous, P.W.; Zwiers, L.H. Analysis of the Mating-Type Loci of Co-occurring and Phylogenetically Related Species of Ascochyta and Phoma. Mol. Plant Pathol. 2012, 13, 350–362. [Google Scholar] [CrossRef]
- Chitrampalam, P.; Inderbitzin, P.; Maruthachalam, K.; Wu, B.M.; Subbarao, K.V. The Sclerotinia sclerotiorum Mating Type Locus (MAT) Contains a 3.6-kb Region That Is Inverted in Every Meiotic Generation. PLoS ONE 2013, 8, e56895. [Google Scholar] [CrossRef]
- Vleugels, T.; De Riek, J.; Heungens, K.; Van Bockstaele, E.; Baert, J. Genetic diversity and population structure of Sclerotinia species from European red clover crops. J. Plant Pathol. 2012, 94, 493–503. [Google Scholar]
- Baturo-Ciesniewska, A.; Groves, C.L.; Albrecht, K.A.; Grau, C.R.; Willis, D.K.; Smith, D.L. Molecular Identification of Sclerotinia trifoliorum and Sclerotinia sclerotiorum Isolates from the United States and Poland. Plant Dis. 2017, 101, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Ciampi-Guillardi, M.; Ramiro, J.; Moraes, M.H.D.; Barbieri, M.C.G.; Massola, J.N.S. Multiplex qPCR Assay for Direct Detection and Quantification of Colletotrichum truncatum, Corynespora cassiicola and Sclerotinia sclerotiorum in Soybean Seeds. Plant Dis. 2020, 104, 3002–3009. [Google Scholar] [CrossRef]
- Freeman, J.; Ward, E.; Calderon, C.; McCartney, A. A polymerase chain reaction (PCR) assay for the detection of inoculum of Sclerotinia sclerotiorum. Eur. J. Plant Pathol. 2002, 108, 877–886. [Google Scholar] [CrossRef]
- Hirschhäuser, S.; Fröhlich, J. Multiplex PCR for Species Discrimination of Sclerotiniaceae by Novel Laccase Introns. Int. J. Food Microbiol. 2007, 118, 151–157. [Google Scholar] [CrossRef]
- Abd-Elmagid, A.; Garrido, P.A.; Hunger, R.; Lyles, J.L.; Mansfield, M.A.; Gugino, B.K.; Smith, D.L.; Melouk, H.A.; Garzon, C.D. Discriminatory Simplex and Multiplex PCR for Four Species of the Genus Sclerotinia. J. Microbiol. Methods 2013, 92, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.; Kohn, L.M. Single Nucleotide Polymorphism-Based Diagnostic System for Crop-Associated Sclerotinia Species. Appl. Environ. Microbiol. 2009, 75, 5600–5606. [Google Scholar] [CrossRef]
- Ramiro, J.; Ciampi-Guillardi, M.; Caldas, D.G.G.; Moraes, M.H.D.; Barbieri, M.C.G.; Pereira, W.V.; Massola, N.S., Jr. Quick and Accurate Detection of Sclerotinia sclerotiorum and Phomopsis spp. in Soybean Seeds Using qPCR and Seed-Soaking Method. J. Phytopathol. 2019, 167, 273–282. [Google Scholar] [CrossRef]
- Grabicoski, E.M.G.; Jaccoud Filho, D.D.S.; Pileggi, M.; Henneberg, L.; Pierre, M.L.C.; Vrisman, C.M.; Dabul, A.N.G. Rapid PCR-Based Assay for Sclerotinia sclerotiorum Detection on Soybean Seeds. Sci. Agric. 2015, 72, 69–74. [Google Scholar] [CrossRef][Green Version]
- Schena, L.; Abdelfattah, A.; Mosca, S.; Li Destri Nicosia, M.G.; Agosteo, G.E.; Cacciola, S.O. Quantitative Detection of Colletotrichum godetiae and C. acutatum Sensu Stricto in the Phyllosphere and Carposphere of Olive During Four Phenological Phases. Eur. J. Plant Pathol. 2017, 149, 337–347. [Google Scholar] [CrossRef]
- Oerke, E.C.; Mahlein, A.K.; Steiner, U. Proximal Sensing of Plant Diseases. In Detection and Diagnostics of Plant Pathogens; Springer: Dordrecht, The Netherlands, 2014; pp. 55–58. [Google Scholar]
- Kong, W.; Zhang, C.; Cao, F.; Liu, F.; Luo, S.; Tang, Y.; He, Y. Detection of Sclerotinia Stem Rot on Oilseed Rape (Brassica napus L.) Leaves using Hyperspectral Imaging. Sensors 2018, 18, 1764. [Google Scholar] [CrossRef]
- Kong, W.W.; Zhang, C.; Huang, W.H.; Liu, F.; He, Y. Application of Hyperspectral Imaging to Detect Sclerotinia sclerotiorum on Oilseed Rape Stems. Sensors 2018, 18, 123. [Google Scholar] [CrossRef] [PubMed]
- Shoute, L.C.T.; Anwar, A.; MacKay, S.; Abdelrasoul, G.N.; Lin, D.; Yan, Z.; Nguyen, A.H.; McDermott, M.T.; Shah, M.A.; Yang, J.; et al. Immuno-impedimetric biosensor for onsite monitoring of ascospores and forecasting of Sclerotinia stem rot of canola. Sci. Rep. 2018, 8, 12396. [Google Scholar] [CrossRef]
- Wang, A.; Gao, B.; Cao, H.; Wang, P.; Zhang, T.; Wei, X. Early Detection of Sclerotinia sclerotiorum on Oilseed Rape Leaves Based on Optical Properties. Biosyst. Eng. 2022, 224, 80–91. [Google Scholar] [CrossRef]
- Rousseau, G.; Rioux, S.; Dostaler, D. Effect of Crop Rotation and Soil Amendments on Sclerotinia stem rot on Soybean in Two Soils. Can. J. Plant Sci. 2007, 87, 605–614. [Google Scholar] [CrossRef]
- Tian, B.; Xie, J.; Fu, Y.; Cheng, J.; Li, B.; Chen, T.; Zhao, Y.; Gao, Z.; Yang, P.; Barbetti, M.J.; et al. A Cosmopolitan Fungal Pathogen of Dicots Adopts an Endophytic Lifestyle on Cereal Crops and Protects Them from Major Fungal Diseases. ISME J. 2020, 14, 3120–3135. [Google Scholar] [CrossRef]
- McDonald, M.R.; Gossen, B.D.; Kora, C.; Parker, M.; Boland, G. Using Crop Canopy Modification to Manage Plant Diseases. Eur. J. Plant Pathol. 2013, 135, 581–593. [Google Scholar] [CrossRef]
- O’Sullivan, C.A.; Belt, K.; Thatcher, L.F. Tackling Control of a Cosmopolitan Phytopathogen: Sclerotinia. Front. Plant Sci. 2021, 12, 707509. [Google Scholar] [CrossRef]
- Simpfendorfer, S.; Heenan, D.P.; Kirkegaard, J.A.; Lindbeck, K.D.; Murray, G.M. Impact of Tillage on Lupin Growth and The Incidence of Pathogenic Fungi in Southern New South Wales. Aust. J. Exp. Agric. 2004, 44, 53–56. [Google Scholar] [CrossRef]
- Adams, P.B.; Ayers, W.A. Ecology of Sclerotinia Species. Phytopathology 1979, 69, 897–899. [Google Scholar] [CrossRef]
- Tu, J.C. The Role of White Mold-Infected White Bean (Phaseolus vulgaris L.) Seeds in the Dissemination of Sclerotinia sclerotiorum (Lib.) de Bary. J. Phytopathol. 1988, 121, 40–50. [Google Scholar] [CrossRef]
- Bennett, R.S.; Chamberlin, K.D.; Damicone, J.P. Sclerotinia Blight Resistance in the US Peanut Mini-Core Collection. Crop Sci. 2018, 58, 1306–1317. [Google Scholar] [CrossRef]
- McCaghey, M.; Willbur, J.; Smith, D.L.; Kabbage, M. The Complexity of the Sclerotinia sclerotiorum Pathosystem in Soybean: Virulence Factors, Resistance Mechanisms, and Their Exploitation to Control Sclerotinia stem rot. Trop. Plant Pathol. 2019, 44, 12–22. [Google Scholar] [CrossRef]
- Denton-Giles, M.; Derbyshire, M.C.; Khentry, Y.; Buchwaldt, L.; Kamphuis, L.G. Partial Stem Resistance in Brassica napus to Highly Aggressive and Genetically Diverse Sclerotinia sclerotiorum Isolates from Australia. Can. J. Plant Pathol. 2018, 40, 551–561. [Google Scholar] [CrossRef]
- Cruickshank, A.W.; Cooper, M.; Ryley, M.J. Peanut Resistance to Sclerotinia minor and S. sclerotiorum. Aust. J. Agric. Res. 2002, 53, 1105–1110. [Google Scholar] [CrossRef]
- Lithourgidis, A.S.; Roupakias, D.G.; Damalas, C.A. Inheritance of Resistance to Sclerotinia Stem Rot (Sclerotinia trifoliorum) in Faba Beans (Vicia faba L.). Field Crops Res. 2005, 91, 125–130. [Google Scholar] [CrossRef]
- Mikaliuniene, J.; Lemeziene, N.; Danyte, V.; Suproniene, S. Evaluation of Red Clover (Trifolium pratense L.) Resistance to Sclerotinia Crown and Root Rot (Sclerotinia trifoliorum) in Laboratory and Field Conditions. Zemdirbyste-Agriculture 2015, 102, 167–176. [Google Scholar] [CrossRef][Green Version]
- Mwape, V.W.; Khentry, Y.; Newman, T.E.; Denton-Giles, M.; Derbyshire, M.; Chen, K.; Berger, J.; Kamphuis, L.G. Identification of Sources of Sclerotinia sclerotiorum Resistance in a Collection of Wild Cicer Germplasm. Plant Dis. 2021, 105, 2314–2324. [Google Scholar] [CrossRef]
- Smith, D.L.; Garrison, M.C.; Hollowell, J.E.; Isleib, T.G.; Shew, B.B. Evaluation of Application Timing and Efficacy of The Fungicides Fluazinam and Boscalid for Control of Sclerotinia Blight of Peanut. Crop Prot. 2008, 27, 823–833. [Google Scholar] [CrossRef]
- Wang, Z.; Bao, L.L.; Zhao, F.Y.; Tang, M.Q.; Chen, T.; Li, Y.; Wang, B.-X.; Fu, B.; Fang, H.; Li, G.-Y.; et al. BnaMPK3 is a Key Regulator of Defense Responses to The Devastating Plant Pathogen Sclerotinia sclerotiorum in Oilseed Rape. Front. Plant Sci. 2019, 10, 91. [Google Scholar] [CrossRef]
- Gyawali, S.; Harrington, M.; Durkin, J.; Horner, K.; Parkin, I.A.P.; Hegedus, D.D.; Bekkaoui, D.; Buchwaldt, L. Microsatellite Markers Used for Genome-Wide Association Mapping of Partial Resistance to Sclerotinia Sclerotiorum in a World Collection of Brassica napus. Mol. Breed. 2016, 36, 72. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Zhao, Z.K.; Hayward, A.; Cheng, H.Y.; Fu, D.H. Integration Analysis of Quantitative Trait Loci for Resistance to Sclerotinia sclerotiorum in Brassica napus. Euphytica 2015, 205, 483–489. [Google Scholar] [CrossRef]
- Wen, Z.X.; Tan, R.J.; Zhang, S.C.; Collins, P.J.; Yuan, J.Z.; Du, W.Y.; Gu, C.; Ou, S.; Song, Q.; An, Y.-Q.C.; et al. Integrating GWAS and Gene Expression Data for Functional Characterization of Resistance to White Mould in Soybean. Plant Biotechnol. J. 2018, 16, 1825–1835. [Google Scholar] [CrossRef]
- Ding, L.N.; Li, T.; Guo, X.J.; Li, M.; Liu, X.Y.; Cao, J.; Tan, X.L. Sclerotinia Stem Rot Resistance in Rapeseed: Recent Progress and Future Prospects. J. Agric. Food Chem. 2021, 69, 2965–2978. [Google Scholar] [CrossRef] [PubMed]
- Grau, C.R.; Dorrance, A.E.; Bond, J.; Russin, J.S. Fungal Diseases. In Soybeans: Improvement, Production, and Uses; Boerma, H.R., Specht, J.E., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2004; pp. 679–763. [Google Scholar]
- Zarinpanjeh, N.; Motallebi, M.; Zamani, M.R.; Ziaei, M. Enhanced Resistance to Sclerotinia Sclerotiorum in Brassica Napus by Co-Expression of Defensin and Chimeric Chitinase Genes. J. Appl. Genet. 2016, 57, 417–425. [Google Scholar] [CrossRef]
- Guimaraes, P.M.; Quintana, A.C.; Mota, A.P.Z.; Berbert, P.S.; Ferreira, D.D.S.; De Aguiar, M.N.; Pereira, B.M.; De Araújo, A.C.G.; Brasileiro, A.C.M. Engineering Resistance Against Sclerotinia sclerotiorum Using a Truncated NLR (TNx) and a Defense-Priming Gene. Plants 2022, 11, 3483. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, J.; Wang, Y.; He, H.; Niu, L.; Guo, D.; Xing, G.; Zhao, Q.; Zhong, X.; Sui, L.; et al. Enhanced Resistance to Sclerotinia Stem Rot in Transgenic Soybean That Overexpresses A Wheat Oxalate Oxidase. Transgen. Res. 2019, 28, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Van Esse, H.P.; Reuber, T.L.; Van Der Does, D. Genetic Modification to Improve Disease Resistance in Crops. New Phytol. 2020, 225, 70–86. [Google Scholar] [CrossRef]
- Amorim, L.; da Fonseca dos Santos, R.; Neto, J.P.B.; Guida-Santos, M.; Crovella, S.; Benko-Iseppon, A.M. Transcription Factors Involved in Plant Resistance to Pathogens. Curr. Protein Pept. Sci. 2017, 18, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, X.; Wang, M.; Wen, J.; Yi, B.; Shen, J.; Ma, C.; Fu, T.; Tu, J. Interactions of WRKY15 and WRKY33 Transcription Factors and Their Roles in the Resistance of Oilseed Rape to Sclerotinia Infection. Plant Biotechnol. J. 2018, 16, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, L.-Y.; Cao, J.; Li, Y.-L.; Ding, L.-N.; Zhu, K.-M.; Yang, Y.H.; Tan, X.L. Recent Advances in Mechanisms of Plant Defense to Sclerotinia sclerotiorum. Front. Plant Sci. 2019, 10, 1314. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Li, H.; Niu, L.; Xing, G.; Zhang, Y.; Xu, W.; Zhao, Q.; Li, Q.; Dong, Y. Overexpression of the Chitinase Gene CmCH1 from Coniothyrium minitans Renders Enhanced Resistance to Sclerotinia sclerotiorum in Soybean. Transgen. Res. 2020, 29, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Westrick, N.M.; Jain, S.; Piotrowski, J.S.; Ranjan, M.; Kessens, R.; Stiegman, L.; Grau, C.R.; Conley, S.P.; Smith, D.L.; et al. Resistance Against Sclerotinia sclerotiorum in Soybean Involves A Reprogramming of The Phenylpropanoid Pathway and Up-Regulation of Antifungal Activity Targeting Ergosterol Biosynthesis. Plant Biotechnol. J. 2019, 17, 1567–1581. [Google Scholar] [CrossRef]
- Langner, T.; Kamoun, S.; Belhaj, K. CRISPR Crops: Plant Genome Editing Toward Disease Resistance. Annu. Rev. Phytopathol. 2018, 56, 479–512. [Google Scholar] [CrossRef]
- Thompson, D.B.; Aboulhouda, S.; Hysolli, E.; Smith, C.J.; Wang, S.; Castanon, O.; Church, G.M. The Future of Multiplexed Eukaryotic Genome Engineering. ACS Chem. Biol. 2018, 13, 313–325. [Google Scholar] [CrossRef]
- Paul, N.C.; Park, S.-W.; Liu, H.; Choi, S.; Ma, J.; MacCready, J.S.; Chilvers, M.I.; Sang, H. Plant and Fungal Genome Editing to Enhance Plant Disease Resistance Using the Crispr/Cas9 System. Front. Plant Sci. 2021, 12, 700925. [Google Scholar] [CrossRef] [PubMed]
- Boubakri, H. Recent Progress in CRISPR/Cas9-Based Genome Editing for Enhancing Plant Disease Resistance. Gene 2023, 866, 147334. [Google Scholar] [CrossRef]
- Lyu, X.; Shen, C.; Fu, Y.; Xie, J.; Jiang, D.; Li, G.; Cheng, J. A Small Secreted Virulence-Related Protein Is Essential for the Necrotrophic Interactions of Sclerotinia sclerotiorum with Its Host Plants. PLoS Pathog. 2016, 12, e1005435. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, J.; Lin, Y.; Fu, Y.; Xie, J.; Li, B.; Bian, X.; Feng, Y.; Liang, W.; Tang, Q.; et al. Editing Homologous Copies of An Essential Gene Affords Crop Resistance Against Two Cosmopolitan Necrotrophic Pathogens. Plant Biotechnol. J. 2021, 19, 13667. [Google Scholar] [CrossRef] [PubMed]
- Paulitz, T.; Schroeder, K.; Beard, T.L. Sclerotinia Stem Rot or White Mold of Canola; Washington State University Extension: Pullman, WA, USA, 2015. [Google Scholar]
- Vallalta, O.; Porter, I.J. Development of Biological Controls for Sclerotinia Diseases of Horticultural Crops in Australasia; Horticultural Australia Ltd.: Sydney, Australia, 2004. [Google Scholar]
- Aldrich-Wolfe, L.; Travers, S.; Nelson, B.D., Jr. Genetic Variation of Sclerotinia sclerotiorum from Multiple Crops in the North Central United States. PLoS ONE 2015, 10, e0139188. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fu, L.; Chen, J.; Wang, S.; Liu, J.; Jiang, J.; Che, Z.; Tian, Y.; Chen, G. Baseline Sensitivity of Sclerotinia sclerotiorum to Metconazole and the Analysis of Cross-Resistance with Carbendazim, Dimethachlone, Boscalid, Fluazinam, and Fludioxonil. Phytoparasitica 2021, 49, 123–130. [Google Scholar] [CrossRef]
- Kamensky, M.; Ovadis, M.; Chet, I.; Chernin, L. Soil-Borne Strain Ic14 of Serratia Plymuthica with Multiple Mechanisms of Antifungal Activity Provides Biocontrol of Botrytis Cinerea and Sclerotinia Sclerotiorum Diseases. Soil Biol. Biochem. 2003, 35, 323–331. [Google Scholar] [CrossRef]
- Gorgen, C.A.; Da Silveira, A.N.; Carneiro, L.C.; Ragagnin, V.; Lobo, M. White Mold Control with Mulch and Trichoderma harzianum 1306 on Soybean. Pesqui. Agropecu. Bras. 2009, 44, 1583–1590. [Google Scholar]
- Luduena, L.M.; Taurian, T.; Tonelli, M.L.; Angelini, J.G.; Anzuay, M.S.; Valetti, L.; Muñoz, V.; Fabra, A.I. Biocontrol Bacterial Communities Associated with Diseased Peanut (Arachis hypogaea L.) Plants. Eur. J. Soil Biol. 2012, 53, 48–55. [Google Scholar] [CrossRef]
- Chen, X.; Pizzatti, C.; Bonaldi, M.; Saracchi, M.; Erlacher, A.; Kunova, A.; Berg, G.; Cortesi, P. Biological Control of Lettuce Drop and Host Plant Colonization by Rhizospheric and Endophytic Streptomycetes. Front. Microbiol. 2016, 7, 714. [Google Scholar] [CrossRef]
- De Vrije, T.; Antoine, N.; Buitelaar, R.M.; Bruckner, S.; Dissevelt, M.; Durand, A.; Gerlagh, M.; Jones, E.; Lüth, P.; Oostra, J.; et al. The Fungal Biocontrol Agent Coniothyrium minitans: Production by Solid-State Fermentation, Application, and Marketing. Appl. Microbiol. Biotechnol. 2001, 56, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Elsheshtawi, M.; Elkhaky, M.T.; Sayed, S.R.; Bahkali, A.H.; Mohammed, A.A.; Gambhir, D.; Mansour, A.S.; Elgorban, A.M. Integrated Control of White Rot Disease on Beans Caused By Sclerotinia Sclerotiorum Using Contans (R) and Reduced Fungicides Application. Saudi J. Biol. Sci. 2017, 24, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Q.; Huang, H.C.; Acharya, S.N.; Ericson, R.S. Effectiveness of Coniothyrium minitans and Trichoderma atroviride in Suppression of Sclerotinia Blossom Blight of Alfalfa. Plant Pathol. 2005, 54, 204–211. [Google Scholar] [CrossRef]
- Huang, H.C.; Bremer, E.; Hynes, R.K.; Ericson, R.S. Foliar Application of Fungal Biocontrol Agents for The Control of White Mold of Dry Bean Caused by Sclerotinia sclerotiorum. Biol. Control 2000, 18, 270–276. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, D.; Kirk, W.; Hao, J. Use of Coniothyrium minitans and Other Microorganisms for Reducing Sclerotinia sclerotiorum. Biol. Control 2012, 60, 225–232. [Google Scholar] [CrossRef]
- Nicot, P.C.; Avril, F.; Duffaud, M.; Leyronas, C.; Troulet, C.; Villeneuve, F.; Bardin, M. Differential Susceptibility to The mycoparasite Paraphaeosphaeria minitans among Sclerotinia sclerotiorum Isolates. Trop. Plant Pathol. 2019, 44, 82–93. [Google Scholar] [CrossRef]
- Kamal, M.M.; Savocchia, S.; Lindbeck, K.D.; Ash, G.J. Biology and Biocontrol of Sclerotinia sclerotiorum (Lib.) de Bary in Oilseed Brassicas. Aust. Plant Pathol. 2016, 45, 1–14. [Google Scholar] [CrossRef]
- Jones, E.E.; Whipps, J.M. Effect of Inoculum Rates and Sources of Coniothyrium minitans on Control of Sclerotinia sclerotiorum Disease in Glasshouse Lettuce. Eur. J. Plant Pathol. 2002, 108, 527–538. [Google Scholar] [CrossRef]
- McQuilken, M.P.; Chalton, D. Potential of Biocontrol of Sclerotinia Rot of Carrot with Foliar Sprays of Contans WG (Coniothyrium minitans). Biocontrol Sci. Technol. 2009, 19, 229–235. [Google Scholar] [CrossRef]
- Chitrampalam, P.; Figuli, P.J.; Matheron, M.E.; Subbarao, K.V.; Pryor, B.M. Biocontrol of Lettuce Drop Caused by Sclerotinia sclerotiorum and S. minor in Desert Agroecosystems. Plant Dis. 2008, 92, 1625–1634. [Google Scholar] [CrossRef]
- Jones, S.J.; Pilkington, S.J.; Gent, D.H.; Hay, F.S.; Pethybridge, S.J. A Polymerase Chain Reaction Assay for Ascosporic Inoculum of Sclerotinia species. N. Z. J. Crop Hortic. Sci. 2015, 43, 233–240. [Google Scholar] [CrossRef]
- Rodriguez, M.; Cabrera, G.; Godeas, A. Cyclosporine A from A Nonpathogenic Fusarium oxysporum Suppressing Sclerotinia sclerotiorum. J. Appl. Microbiol. 2006, 100, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Bitsadze, N.; Siebold, M.; Koopmann, B.; von Tiedemann, A. Single and Combined Colonization of Sclerotinia sclerotiorum Sclerotia by the Fungal Mycoparasites Coniothyrium minitans and Microsphaeropsis ochracea. Plant Pathol. 2015, 64, 690–699. [Google Scholar] [CrossRef]
- Del Río, L.E.; Martinson, C.A.; Yang, X.B. Biological Control of Sclerotinia Stem Rot of Soybean with Speridesmium sclerotivorum. Plant Dis. 2002, 86, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Geraldine, A.M.; Lopes, F.A.C.; Carvalho, D.D.C.; Barbosa, E.T.; Rodrigues, A.R.; Brandão, R.S.; Ulhoa, C.J.; Junior, M.L. Cell wall-degrading Enzymes and Parasitism of Sclerotia Are Key Factors on Field Biocontrol of White Mold by Trichoderma spp. Biol. Control 2013, 67, 308–316. [Google Scholar] [CrossRef]
- Aleandri, M.P.; Chilosi, G.; Bruni, N.; Tomassini, A.; Vettraino, A.M.; Vannini, A. Use of Nursery Potting Mixes Amended with Local Trichoderma Strains with Multiple Complementary Mechanisms to Control Soil-Borne Disease. Crop Prot. 2015, 67, 269–278. [Google Scholar] [CrossRef]
- Matroudi, S.; Zamani, M.R.; Motallebi, M. Antagonistic Effects of Three Species of Trichoderma sp. on Sclerotinia sclerotiorum, The Causal Agent of Canola Stem Rot. Egypt. J. Biol. 2009, 11, 37–44. [Google Scholar]
- Li, G.Q.; Huang, H.C.; Acharya, S.N. Antagonism and Biocontrol Potential of Ulocladium atrum on Sclerotinia sclerotiorum. Biol. Control 2003, 28, 11–18. [Google Scholar] [CrossRef]
- Hu, X.; Roberts, D.P.; Xie, L.; Maul, J.E.; Yu, C.; Li, Y.; Jiang, M.; Liao, X.; Che, Z.; Liao, X. Formulation of Bacillus subtilis BY-2 Suppresses Sclerotinia sclerotiorum on Oilseed Rape in The Field. Biol. Control 2014, 70, 54–64. [Google Scholar] [CrossRef]
- Kamal, M.M.; Lindbeck, K.D.; Savocchia, S.; Ash, G.J. Biological Control of Sclerotinia Stem Rot of Canola Using Antagonistic Bacteria. Plant Pathol. 2015, 64, 1375–1384. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, A.G.; Morrison, M.J.; Meng, Y. Impact of Time Between Field Application of Bacillus subtilis Strains SB01 and SB24 and Inoculation With Sclerotinia sclerotiorum on The Suppression of Sclerotinia Stem Rot in Soybean. Eur. J. Plant Pathol. 2011, 131, 95–102. [Google Scholar] [CrossRef]
- Vinodkumar, S.; Nakkeeran, S.; Renukadevi, P.; Malathi, V.G. Biocontrol Potentials of Antimicrobial Peptide-Producing Bacillus species: Multifaceted Antagonists for The Management of Stem Rot of Carnation Caused by Sclerotinia sclerotiorum. Front. Microbiol. 2017, 8, 446. [Google Scholar] [CrossRef] [PubMed]
- Sabate, D.C.; Brandan, C.P.; Petroselli, G.; Erra-Balsells, R.; Audisio, M.C. Biocontrol of Sclerotinia sclerotiorum (Lib.) de Bary on Common Bean by Native Lipopeptide-Producer Bacillus strains. Microbiol. Res. 2018, 211, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based Products and Their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef]
- Jones, E.E.; Rabeendran, N.; Stewart, A. Biocontrol of Sclerotinia sclerotiorum Infection of Cabbage by Coniothyrium minitans and Trichoderma spp. Biocontrol Sci. Technol. 2014, 24, 1363–1382. [Google Scholar] [CrossRef]
- Elad, Y. Biological Control of Foliar Pathogens by Means of Trichoderma harzianum and Potential Modes of Action. Crop Prot. 2000, 19, 709–714. [Google Scholar] [CrossRef]
- Lopes, K.B.D.; Carpentieri-Pipolo, V.; Fira, D.; Balatti, P.A.; Lopez, S.M.Y.; Oro, T.H.; Pagliosa, E.S.; Degrassi, G. Screening of Bacterial Endophytes as Potential Biocontrol Agents Against Soybean Diseases. J. Appl. Microbiol. 2018, 125, 1466–1481. [Google Scholar] [CrossRef] [PubMed]
- Palaniyandi, S.A.; Yang, S.H.; Zhang, L.X.; Suh, J.W. Effects of Actinobacteria on Plant Disease Suppression and Growth Promotion. Appl. Microbiol. Biotechnol. 2013, 97, 9621–9636. [Google Scholar] [CrossRef]
- Rey, T.; Dumas, B. Plenty is no plague: Streptomyces Symbiosis with Crops. Trends Plant Sci. 2017, 22, 30–37. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, D. New Insights into Mycoviruses and Exploration for The Biological Control of Crop Fungal Diseases. Annu. Rev. Phytopathol. 2014, 52, 45–68. [Google Scholar] [CrossRef]
- Yu, X.; Li, B.; Fu, Y.; Xie, J.; Cheng, J.; Ghabrial, S.A.; Li, G.; Yi, X.; Jiang, D. Extracellular Transmission of A DNA Mycovirus and Its Use as A Natural Fungicide. Proc. Natl. Acad. Sci. USA 2013, 110, 1452–1457. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, J.; Fu, Y.; Cheng, J.; Qu, Z.; Zhao, Z.; Cheng, S.; Chen, T.; Li, B.; Wang, Q.; et al. A 2-kb Mycovirus Converts A Pathogenic Fungus into A Beneficial Endophyte for Brassica Protection and Yield Enhancement. Mol. Plant 2020, 13, 1420–1433. [Google Scholar] [CrossRef]
- Czajkowski, R.; Maciag, T.; Krzyzanowska, D.M.; Jafra, S. Biological Control Based on Microbial Consortia—From Theory to Commercial Products. In How Research Can Stimulate the Development of Commercial Biological Control Against Plant Diseases; De Cal, A., Melgarejo, P., Magan, N., Eds.; Springer: Cham, Switzerland, 2020; pp. 183–202. [Google Scholar] [CrossRef]
- Jain, A.; Singh, A.; Singh, S.; Singh, H.B. Biological Management of Sclerotinia sclerotiorum in Pea Using Plant Growth-Promoting Microbial Consortium. J. Basic Microbiol. 2015, 55, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Bahkali, A.H.; Elsheshtawi, M.; Mousa, R.A.; Elgorban, A.M.; Alzarqaa, A.A. Biological Control of Sclerotinia sclerotiorum in Beans with Antagonistic Microorganisms Under Greenhouse Conditions. Res. Crops 2014, 15, 884. [Google Scholar] [CrossRef]
- French, E.; Kaplan, I.; Iyer-Pascuzzi, A.; Nakatsu, C.H.; Enders, L. Emerging Strategies for Precision Microbiome Management in Diverse Agroecosystems. Nat. Plants 2021, 7, 256–267. [Google Scholar] [CrossRef]
- Emmerling, C.; Schloter, M.; Hartmann, A.; Kandeler, E. Functional Diversity of Soil Organisms—A Review of Recent Research Activities in Germany. J. Plant Nutr. Soil Sci. 2002, 165, 408. [Google Scholar] [CrossRef]
- Borneman, J.; Becker, J.O. Identifying Microorganisms Involved in Specific Pathogen Suppression in Soil. Annu. Rev. Phys. Chem. 2007, 45, 153–172. [Google Scholar]
- Huang, H.C.; Erickson, R.S. Overwintering of Coniothyrium minkans, a Mycoparasite of Sclerotinia sclerotiorum, on the Canadian Prairies. Australas. Plant Pathol. 2002, 31, 291–293. [Google Scholar] [CrossRef]
- Bonanomi, G.; Antignani, V.; Pane, C.; Scala, F. Suppression of Soilborne Fungal Diseases with Organic Amendments. J. Plant Pathol. 2007, 89, 311–324. [Google Scholar]
- Ferraz, L.C.L.; Café Filho, A.C.; Nasser, L.C.B.; Azevedo, J. Effects of Soil Moisture, Organic Matter, and Grass Mulching on The Carpogenic Germination of Sclerotia and Infection of Bean by Sclerotinia sclerotiorum. Plant Pathol. 1999, 48, 77–82. [Google Scholar] [CrossRef]
- Gomaa, N.A.; Mahdy, A.M.M.; Fawzy, R.N.; Ahmed, G.A. Integrated Management of Tomato White Mold Disease Caused by Sclerotinia Sclerotiorum Using Combined Treatments of Compost, Chemical Inducers, and Fungicides. Middle East J. Agric. 2016, 5, 479–486. [Google Scholar]
- Faruk, M.I. Integrated Management of Sclerotinia sclerotiorum, an Emerging Fungal Pathogen Causing White Mold Disease. J. Plant Cell Dev. 2022, 1, 1–14. [Google Scholar] [CrossRef]
- Roy, J.; Shaikh, T.M.; del Río Mendoza, L.; Davis, J.B.; McKay, K.; Chen, C.; Thakur, A.; Rahman, H. Genome-wide Association Mapping and Genomic Prediction for Adult Stage Sclerotinia Stem Rot Resistance in Brassica napus (L.) under field environments. Sci. Rep. 2021, 11, 21773. [Google Scholar] [CrossRef]

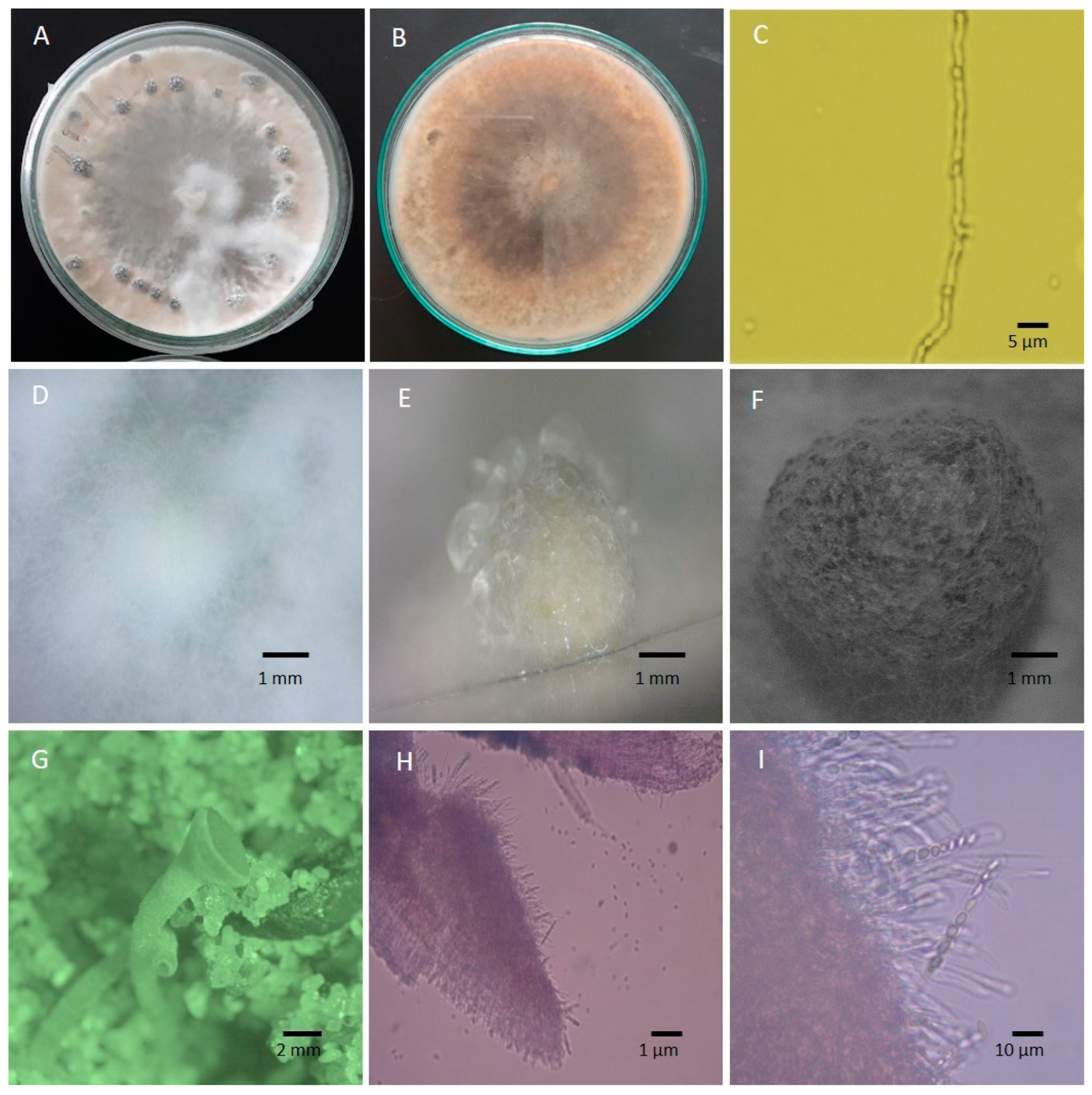


| Host Plant | Symptoms | Signs |
|---|---|---|
| General (All Plants) |
|
|
| Sunflowers |
|
|
| Canola |
|
|
| Salvia |
|
|
| Marigolds |
|
|
| Cosmos |
|
|
| Fungicide Group | Important Fungicide | Types of Fungicides | Mode of Action |
|---|---|---|---|
| Anilinopyrimidines | Cyprodinil, mepanipyrim, and pyrimethanil | Contact | Inhibit methionine biosynthesis |
| Methyl benzimidazole carbamates (MBCs) | Benomyl, carbendazim, thiophanate-methyl, thiabendazole, and fuberidazole | Systemic | Inhibit cell division by disrupting microtubule formation |
| Dicarboxamides | Vinclozolin, iprodione, and procymidone | Contact | Inhibit osmotic signal transduction |
| Demethylation inhibitors (DMIs) | Imidazole, and propiconazole | Systemic | Inhibit membrane sterol biosynthesis and the development of functional cell walls) |
| Quinone outside inhibitors (QoIs) or strobil | Azoxystrobin, kresoxim-methyl, picoxystrobin, pyraclostrobin, and trifloxystrobin | Systemic | Block the transfer of electrons at the Quinone “outside” site of the bc1 complex (complex III in the electron transport chain) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.M.; Sultana, F.; Rubayet, M.T.; Khan, S.; Mostafa, M.; Mishu, N.J.; Sabbir, M.A.A.; Akter, N.; Kabir, A.; Mostofa, M.G. White Mold: A Global Threat to Crops and Key Strategies for Its Sustainable Management. Microorganisms 2025, 13, 4. https://doi.org/10.3390/microorganisms13010004
Hossain MM, Sultana F, Rubayet MT, Khan S, Mostafa M, Mishu NJ, Sabbir MAA, Akter N, Kabir A, Mostofa MG. White Mold: A Global Threat to Crops and Key Strategies for Its Sustainable Management. Microorganisms. 2025; 13(1):4. https://doi.org/10.3390/microorganisms13010004
Chicago/Turabian StyleHossain, Md. Motaher, Farjana Sultana, Md. Tanbir Rubayet, Sabia Khan, Mahabuba Mostafa, Nusrat Jahan Mishu, Md. Abdullah Al Sabbir, Nabela Akter, Ahmad Kabir, and Mohammad Golam Mostofa. 2025. "White Mold: A Global Threat to Crops and Key Strategies for Its Sustainable Management" Microorganisms 13, no. 1: 4. https://doi.org/10.3390/microorganisms13010004
APA StyleHossain, M. M., Sultana, F., Rubayet, M. T., Khan, S., Mostafa, M., Mishu, N. J., Sabbir, M. A. A., Akter, N., Kabir, A., & Mostofa, M. G. (2025). White Mold: A Global Threat to Crops and Key Strategies for Its Sustainable Management. Microorganisms, 13(1), 4. https://doi.org/10.3390/microorganisms13010004






