In Vitro Determination of Antimicrobial, Antioxidant and Antiviral Properties of Greek Plant Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation and Extraction of the Samples
2.3. Antioxidant Properties
2.4. Total Phenolic Content—Folin–Ciocalteu Method
2.5. Determination of Antimicrobial Activity
2.5.1. Tested Pathogenic Bacteria
2.5.2. Evaluation of Antimicrobial Activity
2.6. Cell Culture and Viral Constructs
2.7. Cytotoxicity Assay
2.8. DENV Stocks and Cell Infection
2.9. Cell-Based Antiviral Assays
2.10. Luciferase Assay and Bradford Assays
2.11. Statistical Analysis
3. Results and Discussion
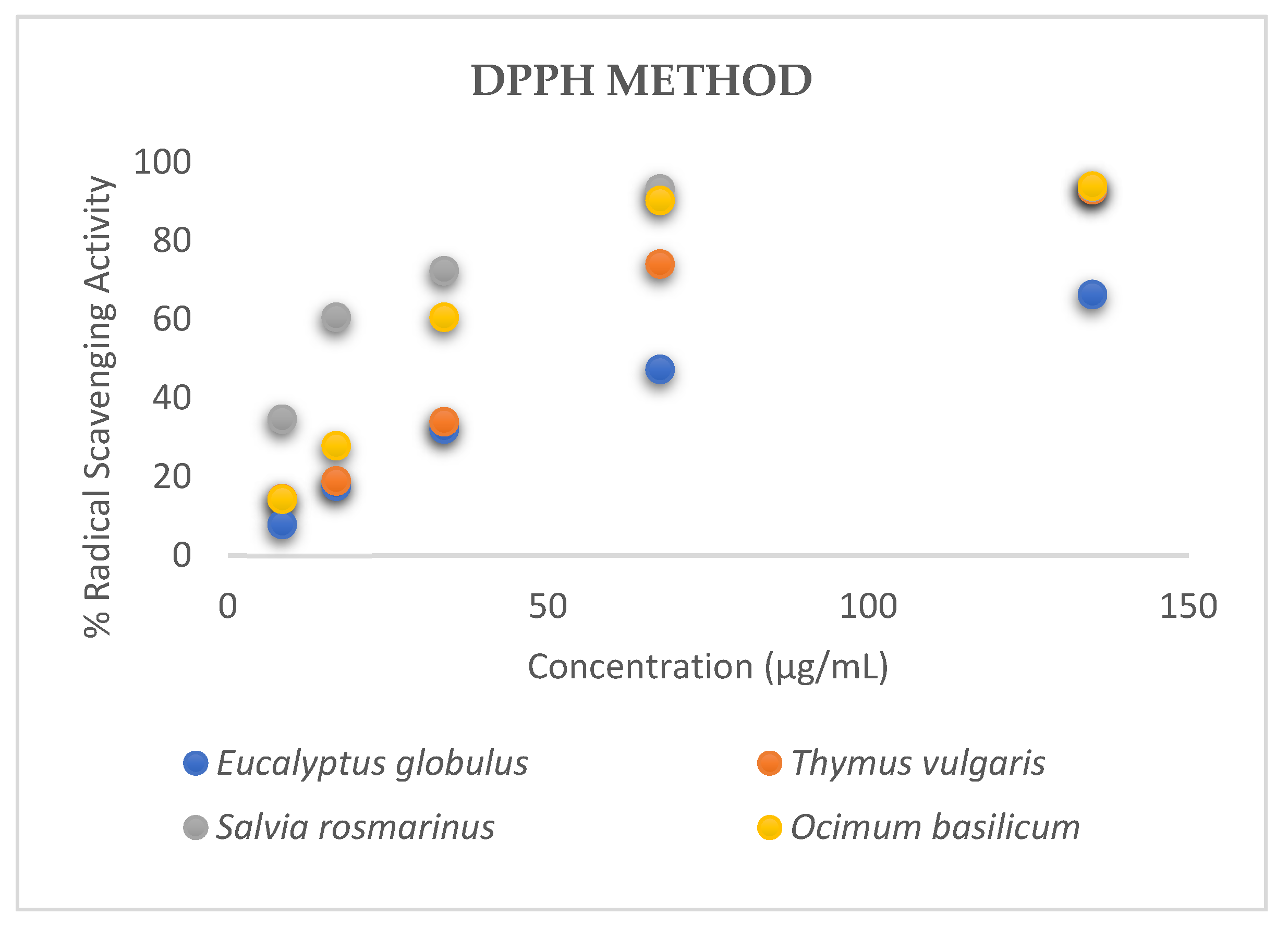
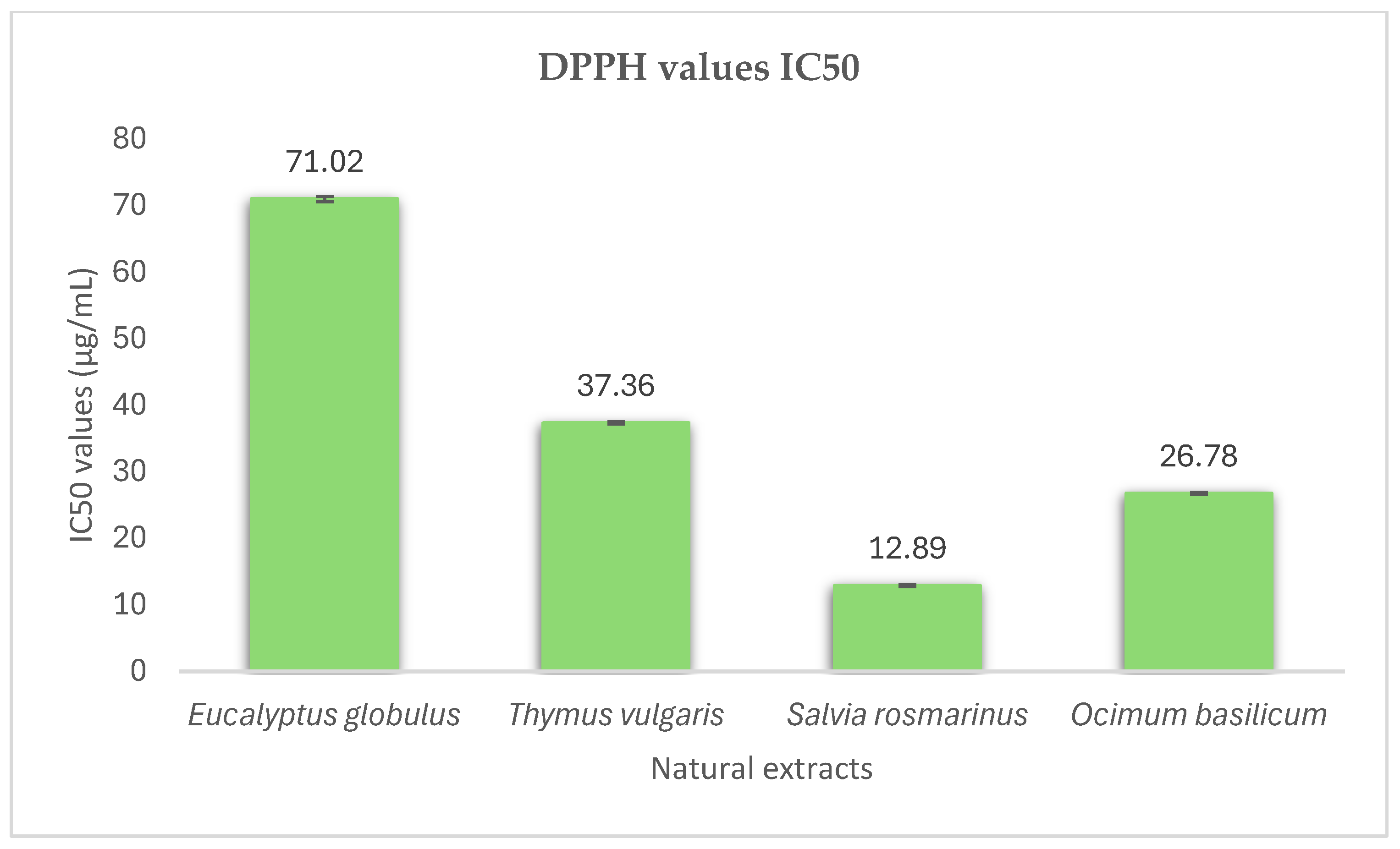
3.1. Antioxidant Activity
3.2. Total Phenolic Content—Folin–Ciocalteu Method
3.3. Antimicrobial Activity
3.4. Cytotoxicity Assay
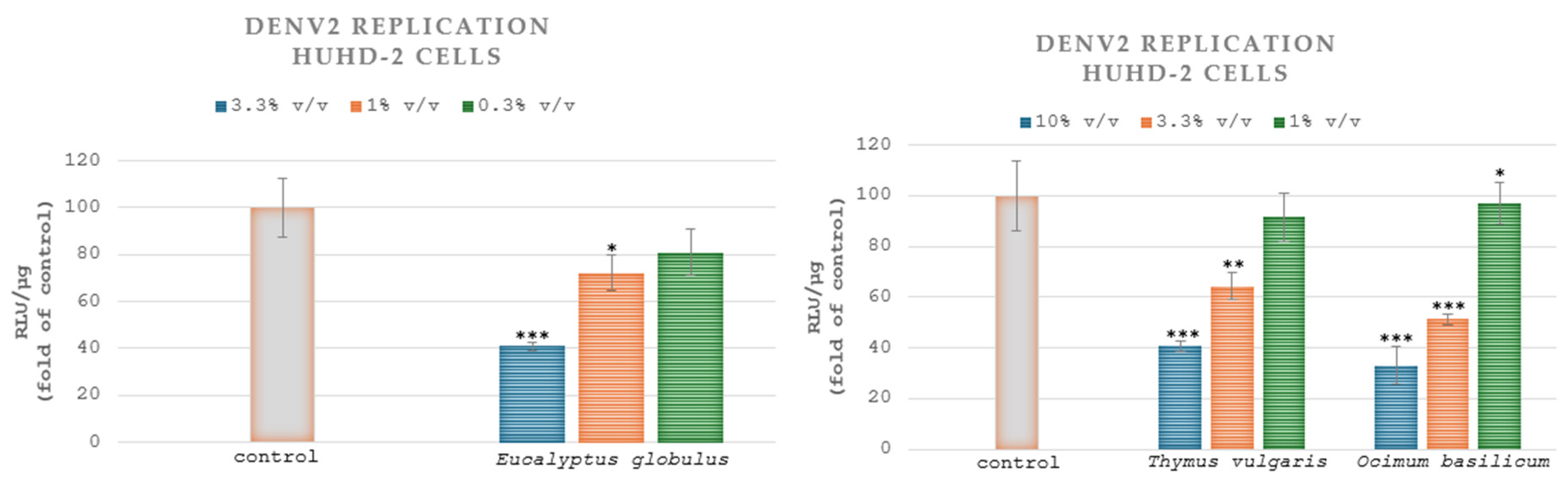
3.5. Cell-Based Antivirus Assays
Plant Extracts Positively Affect DENV Replication and Attenuate the Infection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solomou, A.D.; Martinos, K.; Skoufogianni, E.; Danalatos, N.G. Medicinal and aromatic plants diversity in Greece and their future prospects: A review. Agric. Sci. 2016, 4, 9–21. [Google Scholar] [CrossRef]
- Pombal, S.; Rodilla, J.; Gomes, A.; Silva, L.; Rocha, P. Evaluation of the antibacterial activity of the essential oil and antioxidant activity of aqueous extracts of the Eucalyptus globulus Labill. leaves. Glob. Adv. Res. J. Agric. Sci. 2014, 3, 356–366. [Google Scholar]
- Andreou, V.; Strati, I.F.; Fotakis, C.; Liouni, M.; Zoumpoulakis, P.; Sinanoglou, V.J. Herbal distillates: A new era of grape marc distillates with enriched antioxidant profile. Food Chem. 2018, 253, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Santoro, G.F.; das Graças Cardoso, M.; Guimarães, L.G.L.; Salgado, A.P.S.; Menna-Barreto, R.F.; Soares, M.J. Effect of oregano (Origanum vulgare L.) and thyme (Thymus vulgaris L.) essential oils on Trypanosoma cruzi (Protozoa: Kinetoplastida) growth and ultrastructure. Parasitol. Res. 2007, 100, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Oubannin, S.; Asbbane, A.; Goh, K.W.; Singh, J.; Zafar, I.; Bouyahya, A.; Gharby, S. Green enrichment of argan oil (Argania spinosa L.) with thyme (Thymus vulgaris L.) and oregano (Origanum vulgare L.) leaves: Evaluating quality and stability improvements. Food Chem. X 2024, 24, 101818. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Schwarz, K.; Alegre, L. Enhanced formation of α-tocopherol and highly oxidized abietane diterpenes in water-stressed rosemary plants. Plant Physiol. 1999, 121, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Nogués, I.; Muzzini, V.; Loreto, F.; Bustamante, M.A. Drought and soil amendment effects on monoterpene emission in rosemary plants. Sci. Total Environ. 2015, 538, 768–778. [Google Scholar] [CrossRef]
- Abbaszadeh, B.; Layeghhaghighi, M.; Azimi, R.; Hadi, N. Improving water use efficiency through drought stress and using salicylic acid for proper production of Rosmarinus officinalis L. Ind. Crops Prod. 2020, 144, 111893. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Changes in carotenoids, tocopherols and diterpenes during drought and recovery, and the biological significance of chlorophyll loss in Rosmarinus officinalis plants. Planta 2000, 210, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Nogues, S.; Baker, N.R. Effects of drought on photosynthesis in Mediterranean plants grown under enhanced UV-B radiation. J. Exp. Bot. 2000, 51, 1309–1317. [Google Scholar] [CrossRef]
- Sánchez-Blanco, M.J.; Ferrández, T.; Morales, M.A.; Morte, A.; Alarcón, J.J. Variations in water status, gas exchange, and growth in Rosmarinus officinalis plants infected with Glomus deserticola under drought conditions. J. Plant Physiol. 2004, 161, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Jené, L.; Massó-Rodríguez, M.; Munné-Bosch, S. Interactive effects of Orobanche latisquama parasitism and drought stress in Salvia rosmarinus plants growing under Mediterranean field conditions. Physiol. Plant. 2024, 176, e14652. [Google Scholar] [CrossRef] [PubMed]
- Keskin, C. Medicinal Plants and their Traditional Uses. J. Adv. Plant Biol. 2018, 1, 8–12. [Google Scholar] [CrossRef]
- Qasem, A.; Assaggaf, H.; Mrabti, H.N.; Minshawi, F.; Rajab, B.S.; Attar, A.A.; Alyamani, R.A.; Hamed, M.; Mrabti, N.N.; El Baaboua, A.; et al. Determination of Chemical Composition and Investigation of Biological Activities of Ocimum basilicum L. Molecules 2023, 28, 614. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, E.; Starowicz, M.; Ciska, E.; Topolska, J.; Farouk, A. Determination of volatiles, antioxidant activity, and polyphenol content in the postharvest waste of Ocimum basilicum L. Food Chem. 2022, 375, 131692. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, M.H.; Abdelaziz, A.M.; Kalaba, M.H.; Radwan, A.A.; Hashem, A.H. Antimicrobial, antioxidant, cytotoxic activities and phytochemical analysis of fungal endophytes isolated from Ocimum basilicum. Appl. Biochem. Biotechnol. 2021, 194, 1271–1289. [Google Scholar] [CrossRef] [PubMed]
- Dolghi, A.; Buzatu, R.; Dobrescu, A.; Olaru, F.; Popescu, G.A.; Marcovici, I.; Pinzaru, I.; Navolan, D.; Cretu, O.M.; Popescu, I.; et al. Phytochemical Analysis and In Vitro Cytotoxic Activity against Colorectal Adenocarcinoma Cells of Hippophae rhamnodies L., Cymbopogon citratus (D.C.) Stapf, and Ocimum basilicum L. Essential Oils. Plants 2021, 10, 2752. [Google Scholar] [CrossRef]
- Sestili, P.; Ismail, T.; Calcabrini, C.; Guescini, M.; Catanzaro, E.; Turrini, E.; Layla, A.; Akhtar, S.; Fimognari, C. The Potential Effects of Ocimum Basilicum on Health: A Review of Pharmacological and Toxicological Studies. Expert Opin. Drug Metab. Toxicol. 2018, 14, 679–692. [Google Scholar] [CrossRef]
- Azizah, N.S.; Irawan, B.; Kusmoro, J.; Safriansyah, W.; Farabi, K.; Oktavia, D.; Doni, F.; Miranti, M. Sweet Basil (Ocimum basilicum L.)—A Review of Its Botany, Phytochemistry, Pharmacological Activities, and Biotechnological Development. Plants 2023, 12, 4148. [Google Scholar] [CrossRef] [PubMed]
- Qaiser, A.; Ali, S.; Manzoor, S. Prospects of Carica papaya in the treatment of human viral infections: A comprehensive and a systematic review. Heliyon 2024, 10, e39635. [Google Scholar]
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef]
- Johnson, E.S.; Bilbao, J.M. Flaviviruses 2: West Nile, St. Louis encephalitis, Murray valley encephalitis, yellow fever, and dengue. In Infections of the Central Nervous System Pathology and Genetics, 1st ed.; Chrétien, F., Wong, K.T., Sharer, L.R., Keohane, C., Gray, F., Eds.; WILEY Blackwell: Hoboken, NJ, USA, 2020; pp. 147–162. [Google Scholar]
- Harapan, H.; Michie, A.; Sasmono, R.T.; Imrie, A. Dengue: A minireview. Viruses 2020, 12, 829. [Google Scholar] [CrossRef]
- Papa, A.; Papadopoulou, E.; Paliwal, R.; Kalaitzopoulou, S.; Mourelatos, S.; Niedrig, M. Insect-specific flaviviruses in Aedes mosquitoes in Greece. Arch. Virol. 2016, 161, 2183–2188. [Google Scholar] [CrossRef]
- Anand, U.; Carpena, M.; Kowalska-Góralska, M.; Garcia-Perez, P.; Sunita, K.; Bontempi, E.; Dey, A.; Prieto, M.A.; Proćków, J.; Simal-Gandara, J. Safer plant-based nanoparticles for combating antibiotic resistance in bacteria: A comprehensive review on its potential applications, recent advances, and future perspective. Sci. Total Environ. 2022, 821, 153472. [Google Scholar] [CrossRef]
- Arip, M.; Selvaraja, M.; Tan, L.F.; Leong, M.Y.; Tan, P.L.; Yap, V.L.; Chinnapan, S.; Tat, N.C.; Abdullah, M.; Kumar, D.; et al. Review on plant-based management in combating antimicrobial resistance-mechanistic perspective. Front. Pharmacol. 2022, 13, 879495. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, K.; Martvall, A.; Lima, M.G.B.; Franca, C.S. Herbal medicine promotion for a restorative bioeconomy in tropical forests: A reality check on the Brazilian Amazon. For. Policy Econ. 2023, 155, 103058. [Google Scholar] [CrossRef]
- Ćujić, N.; Šavikin, K.; Janković, T.; Pljevljakušić, D.; Zdunić, G.; Ibrić, S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef]
- Veličković, D.T.; Nikolova, M.T.; Ivancheva, S.V.; Stojanović, J.B.; Veljković, V.B. Extraction of flavonoids from garden (Salvia officinalis L.) and glutinous (Salvia glutinosa L.) sage by ultrasonic and classical maceration. J. Serbian Chem. Soc. 2007, 72, 73–80. [Google Scholar] [CrossRef]
- Thakur, L.; Ghodasra, U.; Patel, N.; Dabhi, M. Novel approaches for stability improvement in natural medicines. Pharmacogn. Rev. 2011, 5, 48. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.G.; Hah, D.S.; Kim, C.H.; Kim, Y.H.; Kim, E.; Kim, J.S. Evaluation of antimicrobial activity of the methanol extracts from 8 traditional medicinal plants. Toxicol. Res. 2011, 27, 31–36. [Google Scholar] [CrossRef]
- Steglińska, A.; Bekhter, A.; Wawrzyniak, P.; Kunicka-Styczyńska, A.; Jastrząbek, K.; Fidler, M.; Śmigielski, K.; Gutarowska, B. Antimicrobial activities of plant extracts against Solanum tuberosum L. phytopathogens. Molecules 2022, 27, 1579. [Google Scholar] [CrossRef] [PubMed]
- Dufner-Beattie, J.; O’Guin, A.; O’Guin, S.; Briley, A.; Wang, B.; Balsarotti, J.; Roth, R.; Starkey, G.; Slomczynska, U.; Noueiry, A.; et al. Identification of AP80978, a novel small-molecule inhibitor of hepatitis C virus replication that targets NS4B. Antimicrob. Agents Chemother. 2014, 58, 3399–3410. [Google Scholar] [CrossRef]
- Scaturro, P.; Cortese, M.; Chatel-Chaix, L.; Fischl, W.; Bartenschlager, R. Dengue virus non-structural protein 1 modulates infectious particle production via interaction with the structural proteins. PLoS Pathog. 2015, 11, e1005277. [Google Scholar] [CrossRef]
- Fischl, W.; Bartenschlager, R. High-throughput screening using dengue virus reporter genomes. In Antiviral Methods and Protocols, 2nd ed.; Gong, E.Y., Ed.; Humana Press: Totowa, NJ, USA, 2013; Volume 1030, pp. 205–219. [Google Scholar]
- Vassilaki, N.; Friebe, P.; Meuleman, P.; Kallis, S.; Kaul, A.; Paranhos-Baccala, G.; Leroux-Roels, G.; Mavromara, P.; Bartenschlager, R. Role of the hepatitis C virus core+ 1 open reading frame and core cis-acting RNA elements in viral RNA translation and replication. J. Virol. 2008, 82, 11503–11515. [Google Scholar] [CrossRef] [PubMed]
- Byrd, C.M.; Dai, D.; Grosenbach, D.W.; Berhanu, A.; Jones, K.F.; Cardwell, K.B.; Schneider, C.; Wineinger, K.A.; Page, J.M.; Harver, C.; et al. A novel inhibitor of dengue virus replication that targets the capsid protein. Antimicrob. Agents Chemother. 2013, 57, 15–25. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018, 9, 360203. [Google Scholar] [CrossRef]
- Ácsová, A.; Martiniaková, S.; Hojerová, J. Selected methods to determine antioxidant activity of hydrophilic/lipophilic substances. Acta Chim. Slovaca 2019, 12, 200–211. [Google Scholar] [CrossRef]
- Akar, Z.; Küçük, M.; Doğan, H. A new colorimetric DPPH• scavenging activity method with no need for a spectrophotometer applied on synthetic and natural antioxidants and medicinal herbs. J. Enzym. Inhib. Med. Chem. 2017, 32, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Jadid, N.; Hidayati, D.; Hartanti, S.R.; Arraniry, B.A.; Rachman, R.Y.; Wikanta, W. Antioxidant activities of different solvent extracts of Piper retrofractum Vahl. using DPPH assay. In Proceedings of the International Biology Conference, Dresden, Germany, 26 June 2017. [Google Scholar]
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep. 2021, 11, 10041. [Google Scholar] [CrossRef]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative stress: The role of antioxidant phytochemicals in the prevention and treatment of diseases. Int. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef]
- Bencheikh, D.; Gueddah, A.; Soualat, K.; Ben-aissi, H.; Benslama, A.; Harrar, A.; Khennouf, S. Polyphenolic contents, antioxidant and antibacterial activities of aqueous extracts of Eucalyptus globulus L. and Trigonella foenum-greacum L. J. Appl. Biol. Sci. 2021, 15, 53–63. [Google Scholar]
- Mokhtari, R.; Kazemi Fard, M.; Rezaei, M.; Moftakharzadeh, S.A.; Mohseni, A. Antioxidant, Antimicrobial Activities, and Characterization of Phenolic Compounds of Thyme (Thymus vulgaris L.), Sage (Salvia officinalis L.), and Thyme–Sage Mixture Extracts. J. Food Qual. 2023, 2023, 2602454. [Google Scholar] [CrossRef]
- Sharma, Y.; Fagan, J.; Schaefer, J. In vitro screening for acetylcholinesterase inhibition and antioxidant potential in different extracts of sage (Salvia officinalis L.) and rosemary (Rosmarinus officinalis L.). J. Biol. Act. Prod. Nat. 2020, 10, 59–69. [Google Scholar]
- Naidu, J.R.; Ismail, R.B.; Sasidharan, S. Chemical profiling and antioxidant activity of Thai basil (Ocimum basilicum). J. Essent. Oil Bear. Plants 2016, 19, 750–755. [Google Scholar] [CrossRef]
- Lamuela-Raventós, R.M. Folin–Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. Meas. Antioxid. Act. Capacit. Recent Trends Appl. 2018, 6, 107–115. [Google Scholar]
- De la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-Garcia, J.; Alvarez-Parrilla, E. Phenolic Compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables, 1st ed.; Yahia, E.M., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 253–271. [Google Scholar]
- Cai, Y.Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Hafeez, E.Y.; Karamova, N.S.; Ilinskaya, O.N. Antioxidant activity and total phenolic compound content of certain medicinal plants. Int. J. Biosci. 2014, 5, 213–222. [Google Scholar]
- Stagos, D.; Portesis, N.; Spanou, C.; Mossialos, D.; Aligiannis, N.; Chaita, E.; Panagoulis, C.; Reri, E.; Skaltsounis, E.; Tsatsakis, A.M.; et al. Correlation of total polyphenolic content with antioxidant and antibacterial activity of 24 extracts from Greek domestic Lamiaceae species. Food Chem. Toxicol. 2012, 50, 4115–4124. [Google Scholar] [CrossRef] [PubMed]
- Zargoosh, Z.; Ghavam, M.; Bacchetta, G.; Tavili, A. Effects of ecological factors on the antioxidant potential and total phenol content of Scrophularia striata Boiss. Sci. Rep. 2019, 9, 16021. [Google Scholar] [CrossRef]
- Tsakni, A.; Chatzilazarou, A.; Tsakali, E.; Tsantes, A.G.; Van Impe, J.; Houhoula, D. Identification of Bioactive Compounds in Plant Extracts of Greek Flora and Their Antimicrobial and Antioxidant Activity. Separations 2023, 10, 373. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef]
- Hemeg, H.A.; Moussa, I.M.; Ibrahim, S.; Dawoud, T.M.; Alhaji, J.H.; Mubarak, A.S.; Kabli, S.A.; Alsubki, R.A.; Tawfik, A.M.; Marouf, S.A. Antimicrobial effect of different herbal plant extracts against different microbial population. Saudi J. Biol. Sci. 2020, 27, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Rohde, M. The Gram-positive bacterial cell wall. Microbiol. Spectr. 2019, 7, 10–1128. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Concepción Alvarez, A.; Arias-Santé, M.F.; Oyarzún, J.E.; Andia, M.E.; Uribe, S.; Pizarro, P.N.; Bustos, S.M.; Schwember, A.R.; Shahidi, F.; et al. Soluble free, esterified and insoluble-bound phenolic antioxidants from chickpeas prevent cytotoxicity in human hepatoma HuH-7 cells induced by peroxyl radicals. Antioxidants 2022, 11, 1139. [Google Scholar] [CrossRef]
- Hamzawy, M.A.; El-Denshary, E.S.; Abdel-Wahhab, M.A. Effects of natural compounds in treatment and prevention of hepatotoxicity and hepatocellular carcinoma. Hepatoma Res. 2015, 1, 111–118. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism (s) of action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Li, S.; Yin, S.; Ding, H.; Shao, Y.; Zhou, S.; Pu, W.; Han, L.; Wang, T.; Yu, H. Polyphenols as potential metabolism mechanisms regulators in liver protection and liver cancer prevention. Cell Prolif. 2023, 56, e13346. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.F.; Miranda, R.G.; Dorta, D.J.; Rolo, A.P.; Palmeira, C.M. Targeting oxidative stress with polyphenols to fight liver diseases. Antioxidants 2023, 12, 1212. [Google Scholar] [CrossRef]
- Teixeira, A.; DaCunha, D.C.; Barros, L.; Caires, H.R.; Xavier, C.P.; Ferreira, I.C.; Vasconcelos, M.H. Eucalyptus globulus Labill. decoction extract inhibits the growth of NCI-H460 cells by increasing the p53 levels and altering the cell cycle profile. Food Funct. 2019, 10, 3188–3197. [Google Scholar] [CrossRef]
- Martins-Gomes, C.; Nunes, F.M.; Silva, A.M. Modulation of cell death pathways for cellular protection and anti-tumoral activity: The role of Thymus spp. extracts and their bioactive molecules. Int. J. Mol. Sci. 2023, 24, 1691. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Eldeeb, H.M.; Khan, R.A.; Al-Omar, M.S.; Mohammed, S.A.; Sajid, M.S.; Aly, M.S.A.; Ahmad, A.M.; Abdellatif, A.A.H.; Eid, S.Y.; et al. Sage, Salvia officinalis L., constituents, hepatoprotective activity, and cytotoxicity evaluations of the essential oils obtained from fresh and differently timed dried herbs: A comparative analysis. Molecules 2021, 26, 5757. [Google Scholar] [CrossRef] [PubMed]
- Palanichamy, P.; Krishnamoorthy, G.; Kannan, S.; Marudhamuthu, M. Bioactive potential of secondary metabolites derived from medicinal plant endophytes. Egypt. J. Basic Appl. Sci. 2018, 5, 303–312. [Google Scholar] [CrossRef]
- Hernández-Castro, C.; Diaz-Castillo, F.; Martínez-Gutierrez, M. Ethanol extracts of Cassia grandis and Tabernaemontana cymosa inhibit the in vitro replication of dengue virus serotype 2. Asian Pac. J. Trop. Dis. 2015, 5, 98–106. [Google Scholar] [CrossRef]
- Talarico, L.B.; Pujol, C.A.; Zibetti, R.G.M.; Faría, P.C.S.; Noseda, M.D.; Duarte, M.E.R.; Damonte, E.B. The antiviral activity of sulfated polysaccharides against dengue virus is dependent on virus serotype and host cell. Antivir. Res. 2005, 66, 103–110. [Google Scholar] [CrossRef]
- Loaiza-Cano, V.; Monsalve-Escudero, L.M.; Filho, C.D.S.M.B.; Martinez-Gutierrez, M.; Sousa, D.P.D. Antiviral role of phenolic compounds against dengue virus: A review. Biomolecules 2020, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Polyphenols and their potential role to fight viral diseases: An overview. Sci. Total Environ. 2021, 801, 149719. [Google Scholar] [CrossRef]
- Songprakhon, P.; Panya, A.; Choomee, K.; Limjindaporn, T.; Noisakran, S.; Tarasuk, M.; Yenchitsomanus, P.T. Cordycepin exhibits both antiviral and anti-inflammatory effects against dengue virus infection. iScience 2024, 27, 110711. [Google Scholar] [CrossRef]
- Liao, Y.C.; Yeh, C.C.; Chueh, Y.F.; Huang, M.S.; Wu, J.S.; Wen, Y.X.; Chang, Y.T.; Lai, Y.R.; Chen, J.J.; Chang, T.H. Effects of the oxoaporphine alkaloid hernandonine on dengue virus. Evidence for its mechanisms of action. Phytomedicine 2024, 134, 155986. [Google Scholar] [CrossRef]
- Fomitcheva, V.; Strauch, C.J.; Bonse, S.; Bauer, P.; Kühne, T.; Niehl, A. Bio-control of soil-borne virus infection by seed application of Glycyrrhiza glabra extract and the rhamnolipid Rhapynal. Planta 2024, 260, 94. [Google Scholar] [CrossRef]
- Lee, J.K.; Choi, J.W.; Park, I.; Kim, N.E.; Kwon, H.C.; Kwon, J.; Song, Y.J. Roseoside Is a Bioactive Compound in Kirengeshoma koreana Nakai Extract with Potent In Vitro Antiviral Activity Against Hepatitis C Virus. Molecules 2024, 29, 5130. [Google Scholar] [CrossRef]
- Fayez, D.; Youssif, A.; Sabry, S.; Ghozlan, H.; El-Sayed, F. Some novel bioactivities of Virgibacillus halodenitrificans carotenoids, isolated from Wadi El-Natrun lakes. Saudi J. Biol. Sci. 2023, 30, 103825. [Google Scholar] [CrossRef]
- Joshi, R.K.; Agarwal, S.; Patil, P.; Alagarasu, K.; Panda, K.; Cherian, S.; Parashar, D.; Roy, S. Anti-dengue activity of lipophilic fraction of Ocimum basilicum L. Stem. Molecules 2023, 28, 1446. [Google Scholar] [CrossRef] [PubMed]
- González-Burgos, E.; Liaudanskas, M.; Viškelis, J.; Žvikas, V.; Janulis, V.; Gómez-Serranillos, M.P. Antioxidant activity, neuroprotective properties and bioactive constituents analysis of varying polarity extracts from Eucalyptus globulus leaves. J. Food Drug Anal. 2018, 26, 1293–1302. [Google Scholar] [CrossRef]
- Szilvássy, B.; Rak, G.; Sárosi, S.; Novák, I.; Pluhár, Z.; Abrankó, L. Polyphenols in the aqueous extracts of garden thyme (Thymus vulgaris) chemotypes cultivated in Hungary. Nat. Prod. Commun. 2013, 8, 605–608. [Google Scholar] [CrossRef]
- Pereira, E.; Barros, L.; Antonio, A.L.; Verde, S.C.; Santos-Buelga, C.; Ferreira, I.C. Infusions from Thymus vulgaris L. treated at different gamma radiation doses: Effects on antioxidant activity and phenolic composition. LWT 2016, 74, 34–39. [Google Scholar] [CrossRef]
- Roberto, P.M.; Anunciação, P.C.; Lucia, C.M.D.; Pinheiro, S.S.; Souza, E.C.G.; Pinheiro-Sant’ana, H.M. Macronutrients, vitamins, minerals and bioactive compounds in fresh and dehydrated basil (Ocimum basilicum) and its hot and cold infusion. Acta Sci. 2020, 43, e55423. [Google Scholar] [CrossRef]
- Silva, P.G.D.; Chaves, E.J.F.; Silva, T.M.S.; Rocha, G.B.; Dantas, W.M.; Oliveira, R.N.D.; Pena, L.J. Antiviral activity of flavonoids from Geopropolis of the Brazilian Jandaira bee against zika and dengue viruses. Pharmaceutics 2023, 15, 2494. [Google Scholar] [CrossRef] [PubMed]
- Frabasile, S.; Koishi, A.C.; Kuczera, D.; Silveira, G.F.; Verri, W.A., Jr.; Duarte dos Santos, C.N.; Bordignon, J. The citrus flavanone naringenin impairs dengue virus replication in human cells. Sci. Rep. 2017, 7, 41864. [Google Scholar] [CrossRef]
- Bondhon, T.A.; Hasan, A.; Jannat, K.; Paul, A.; Jahan, R.; Mahboob, T.; Nissapatorn, V.; Dolma, K.G.; Pereira, M.L.; Wiart, C.; et al. Molecular docking study of Lens culinaris L. phytochemicals to NS3-NS2B protease of dengue virus serotype 2. Ger. J. Microbiol. 2021, 1, 26–37. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Johargy, A.; Gammoh, S.; Ereifej, K.; Alhamad, M.N.; Brewer, M.S.; Saati, A.A.; Kubow, S.; Rawshdeh, M. Extraction, optimisation and characterisation of phenolics from Thymus vulgaris L.: Phenolic content and profiles in relation to antioxidant, antidiabetic and antihypertensive properties. Int. J. Food Sci. Technol. 2016, 51, 720–730. [Google Scholar] [CrossRef]
- De Sousa, L.R.F.; Wu, H.; Nebo, L.; Fernandes, J.B.; das Graças Fernandes da Silva, M.F.; Kiefer, W.; Kanitz, M.; Bodem, J.; Diederich, W.E.; Schirmeister, T.; et al. Flavonoids as noncompetitive inhibitors of Dengue virus NS2B-NS3 protease: Inhibition kinetics and docking studies. Bioorg. Med. Chem. 2015, 23, 466–470. [Google Scholar] [CrossRef]
- Alomair, L.; Almsned, F.; Ullah, A.; Jafri, M.S. In silico prediction of the phosphorylation of NS3 as an essential mechanism for dengue virus replication and the antiviral activity of quercetin. Biology 2021, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, S.; Madushanka, A.; Wijegunawardana, N.A.D.; Mylvaganam, H.; Rathnayake, A.; Perera, E.G.; Jayamanna, I.; Chandrasena, P.; Ranaweera, A.; Jayasooriya, P. In silico study of 5, 7-dimethoxycoumarin and p-coumaric acid in Carica papaya leaves as dengue virus type 2 protease inhibitors. Proceedings 2020, 79, 11. [Google Scholar]
- Peng, M.; Watanabe, S.; Chan, K.W.K.; He, Q.; Zhao, Y.; Zhang, Z.; Lai, X.; Luo, D.; Vasudevan, S.G.; Li, G. Luteolin restricts dengue virus replication through inhibition of the proprotein convertase furin. Antivir. Res. 2017, 143, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Panchal, R.; Bapat, S.; Mukherjee, S.; Chowdhary, A. In silico binding analysis of lutein and rosmarinic acid against envelope domain III protein of dengue virus. Indian J. Pharmacol. 2021, 53, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Panchal, R.; Ghosh, S.; Mehla, R.; Ramalingam, J.; Gairola, S.; Mukherjee, S.; Chowdhary, A. Antiviral activity of rosmarinic acid against four serotypes of dengue virus. Curr. Microbiol. 2022, 79, 203. [Google Scholar] [CrossRef]


| MIC (Minimum Inhibitory Concentration) μg·mL−1 | ||||||
|---|---|---|---|---|---|---|
| Aqueοus Νatural Extracts | S. aureus | E. faecalis | L. monocytogenes | S. enterica | E. coli | K. pneumoniae |
| Eucalyptus globulus L. | 135 | 120 | 30 | 120 | 135 | 900 |
| Thymus vulgaris L. | 135 | 200 | 135 | 1000 | 1000 | nd |
| Salvia rosmarinus L. | 40 | 80 | 40 | 1000 | 1000 | 500 |
| Ocimum basilicum L. | 800 | 800 | 1000 | 1000 | 3000 | 3000 |
| Plant Extract | CC50 (% v/v) |
|---|---|
| Eucalyptus globulus L. | 5.94 ± 0.04 |
| Thymus vulgaris L. | >10 |
| Salvia rosmarinus L. | >10 |
| Ocimum basilicum L. | >10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsakni, A.; Kyriakopoulou, E.; Letsiou, S.; Halvatsiotis, P.; Rigopoulos, H.; Vassilaki, N.; Houhoula, D. In Vitro Determination of Antimicrobial, Antioxidant and Antiviral Properties of Greek Plant Extracts. Microorganisms 2025, 13, 177. https://doi.org/10.3390/microorganisms13010177
Tsakni A, Kyriakopoulou E, Letsiou S, Halvatsiotis P, Rigopoulos H, Vassilaki N, Houhoula D. In Vitro Determination of Antimicrobial, Antioxidant and Antiviral Properties of Greek Plant Extracts. Microorganisms. 2025; 13(1):177. https://doi.org/10.3390/microorganisms13010177
Chicago/Turabian StyleTsakni, Aliki, Eirini Kyriakopoulou, Sophia Letsiou, Panagiotis Halvatsiotis, Haralambos Rigopoulos, Niki Vassilaki, and Dimitra Houhoula. 2025. "In Vitro Determination of Antimicrobial, Antioxidant and Antiviral Properties of Greek Plant Extracts" Microorganisms 13, no. 1: 177. https://doi.org/10.3390/microorganisms13010177
APA StyleTsakni, A., Kyriakopoulou, E., Letsiou, S., Halvatsiotis, P., Rigopoulos, H., Vassilaki, N., & Houhoula, D. (2025). In Vitro Determination of Antimicrobial, Antioxidant and Antiviral Properties of Greek Plant Extracts. Microorganisms, 13(1), 177. https://doi.org/10.3390/microorganisms13010177







