Enhanced MICP for Soil Improvement and Heavy Metal Remediation: Insights from Landfill Leachate-Derived Ureolytic Bacterial Consortium
Abstract
1. Introduction
2. Materials and Methods
2.1. Landfill Leachate Sampling Collection Site
2.2. Chemicals and Reagents Utilized
2.3. Enrichment Culturing of Leachate Sample
2.4. Temperature and pH Impact on Enriched Ureolytic Culture
2.5. Preliminary Test on Heavy Metals Removal
2.6. Soil Biocementation Treatment
2.7. Measurement of Effluent, Surface Strength Test, and CaCO3 Content
2.8. SEM-EDS, XRD, and FTIR Analyses
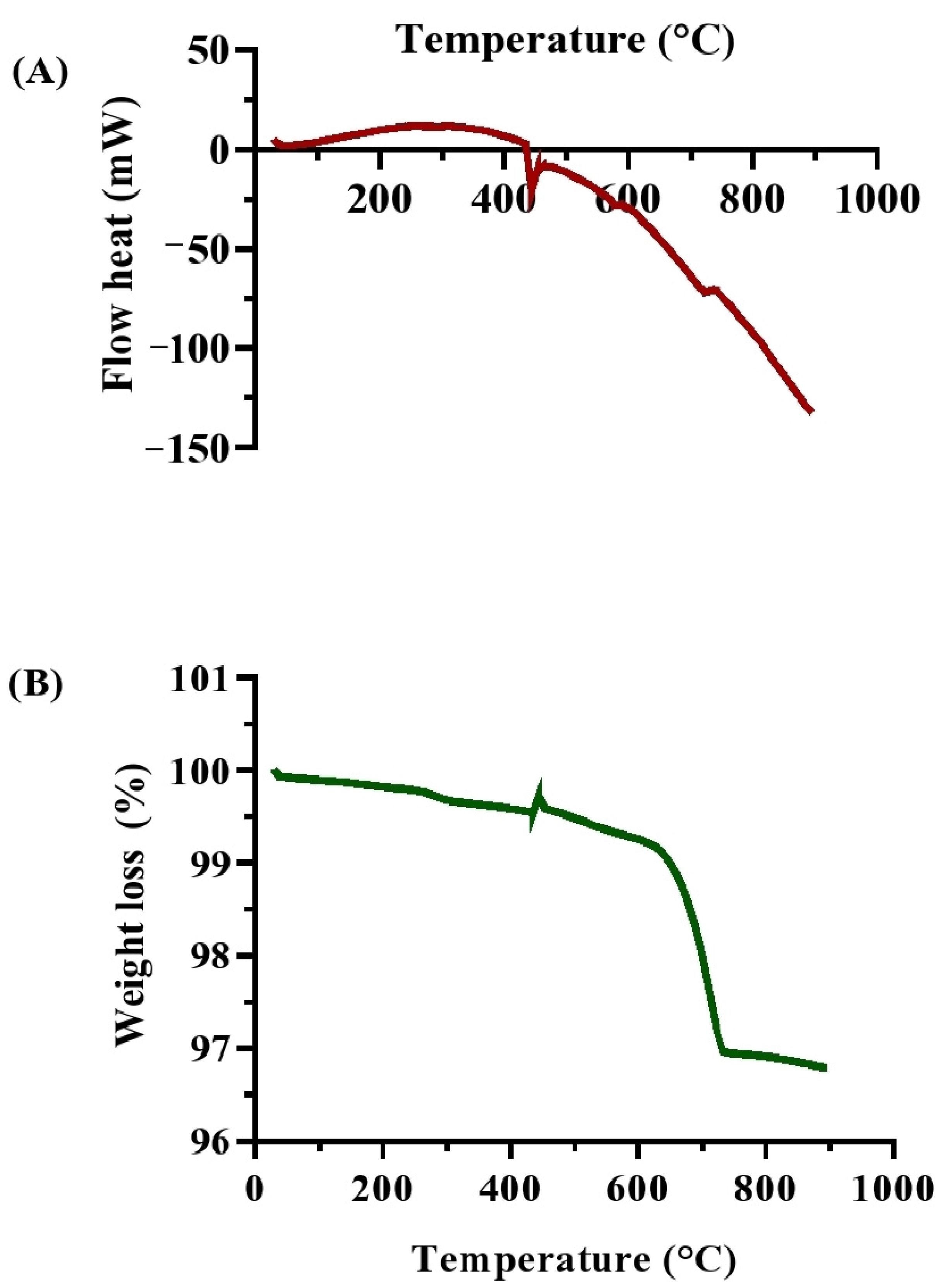
2.9. Thermal Analysis
2.10. Durability Test Using Enriched Cultures
2.11. Statistical Analysis
3. Results and Discussion
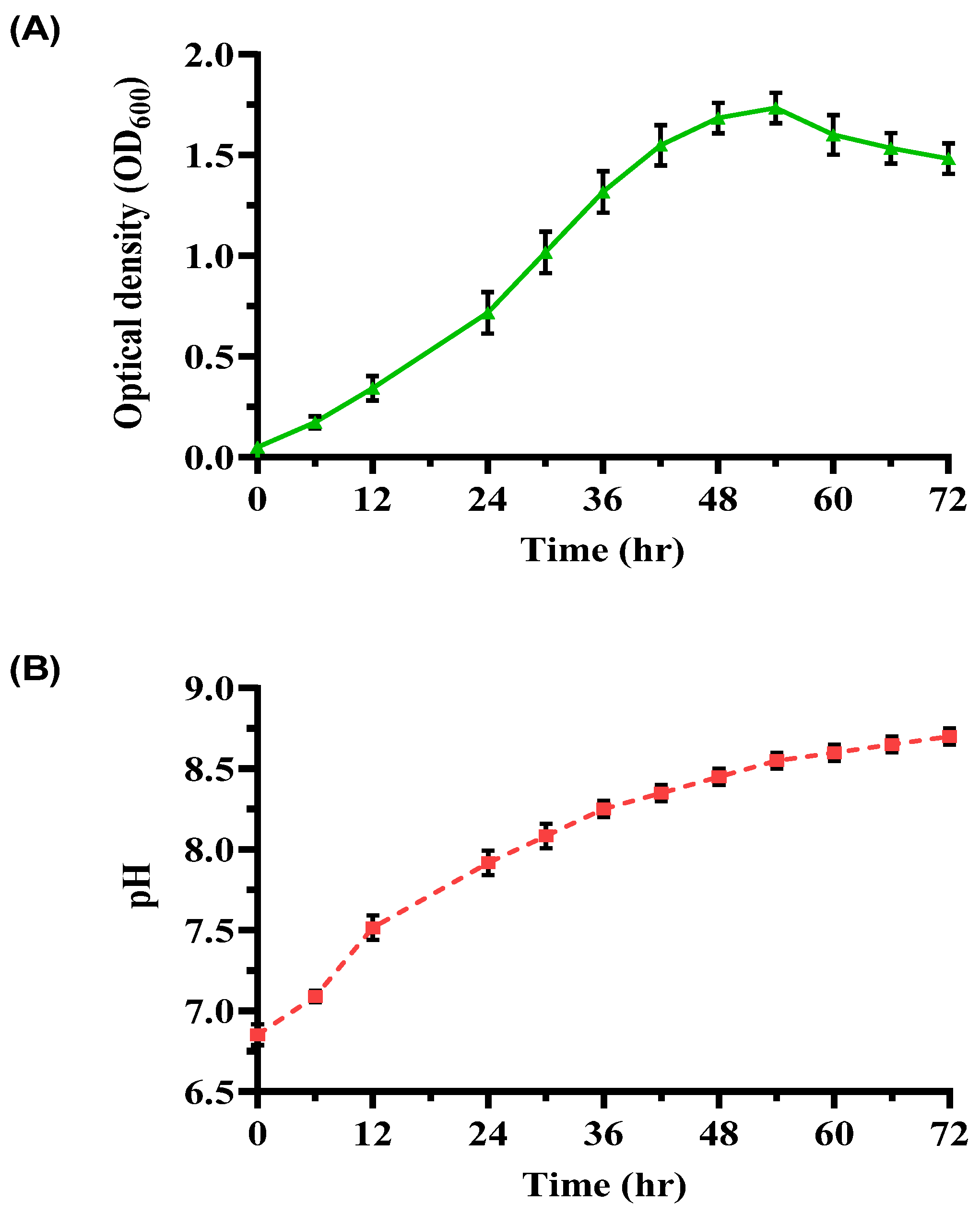
3.1. Biostimulation of Native Ureolytic Microbes
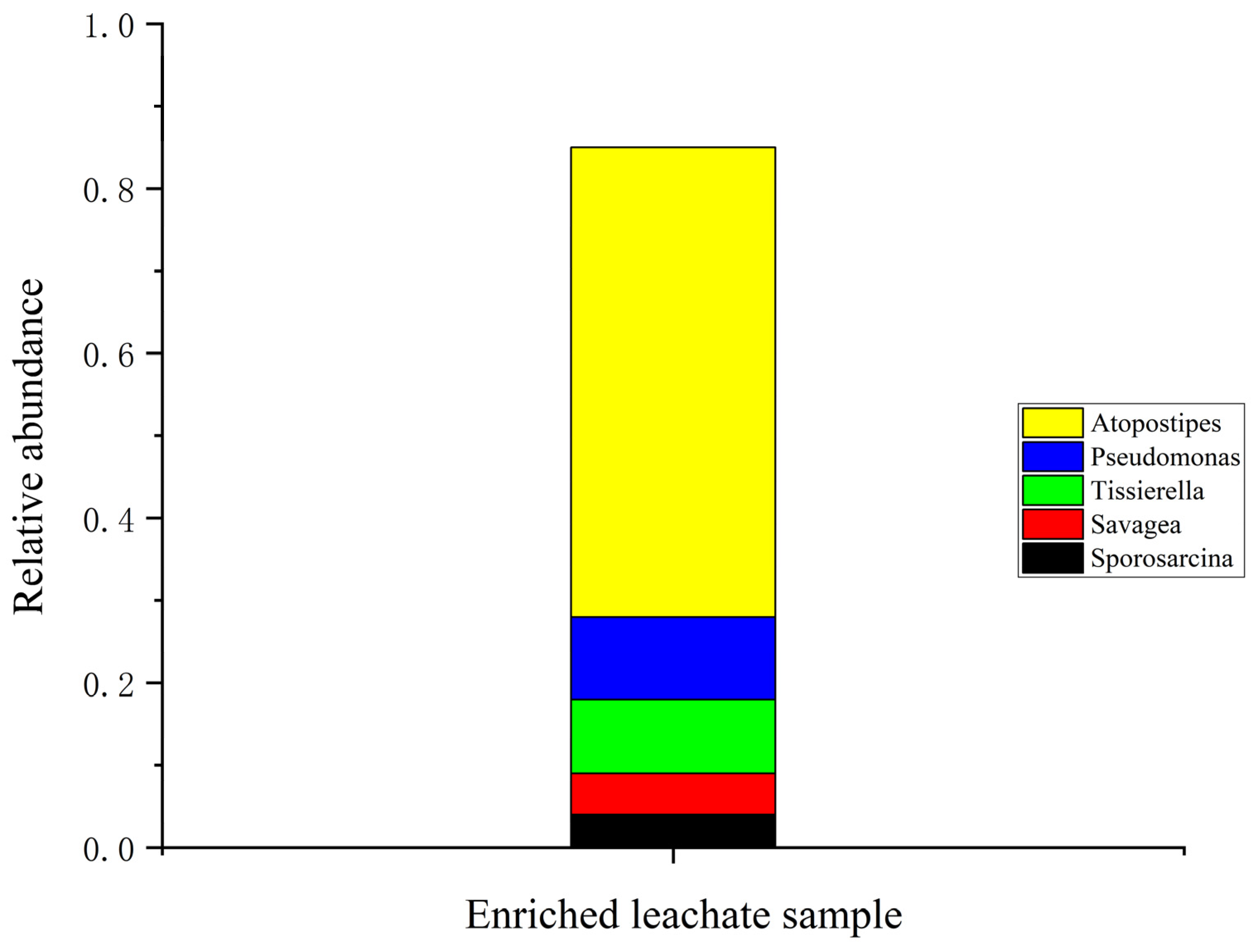
3.2. Ureolytic Bacterial Community in Biostimulated Leachate
3.3. Impact of pH and Temperature on Enriched Ureolytic Culture
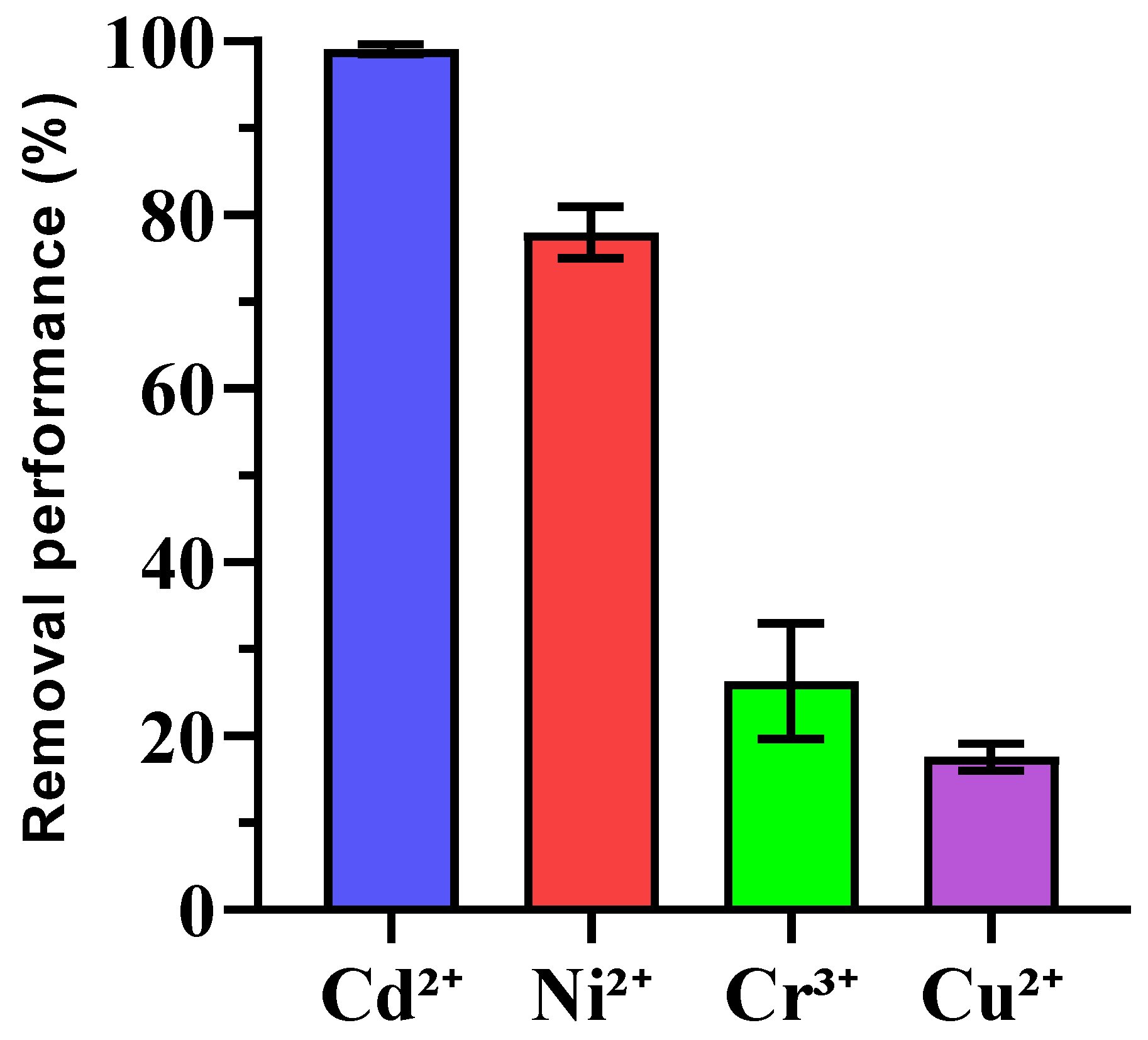
3.4. Preliminary Findings on Heavy Metal Immobilization
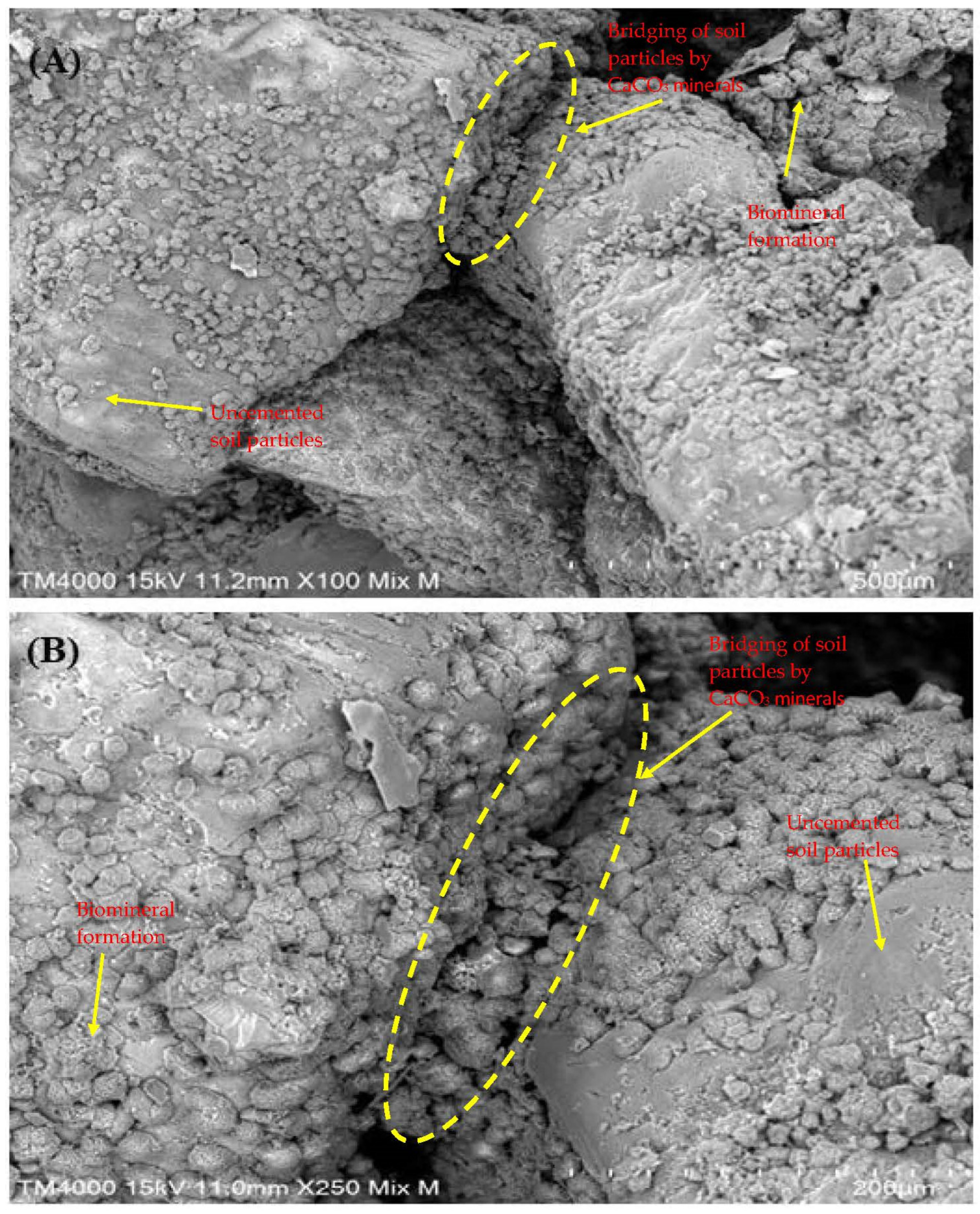
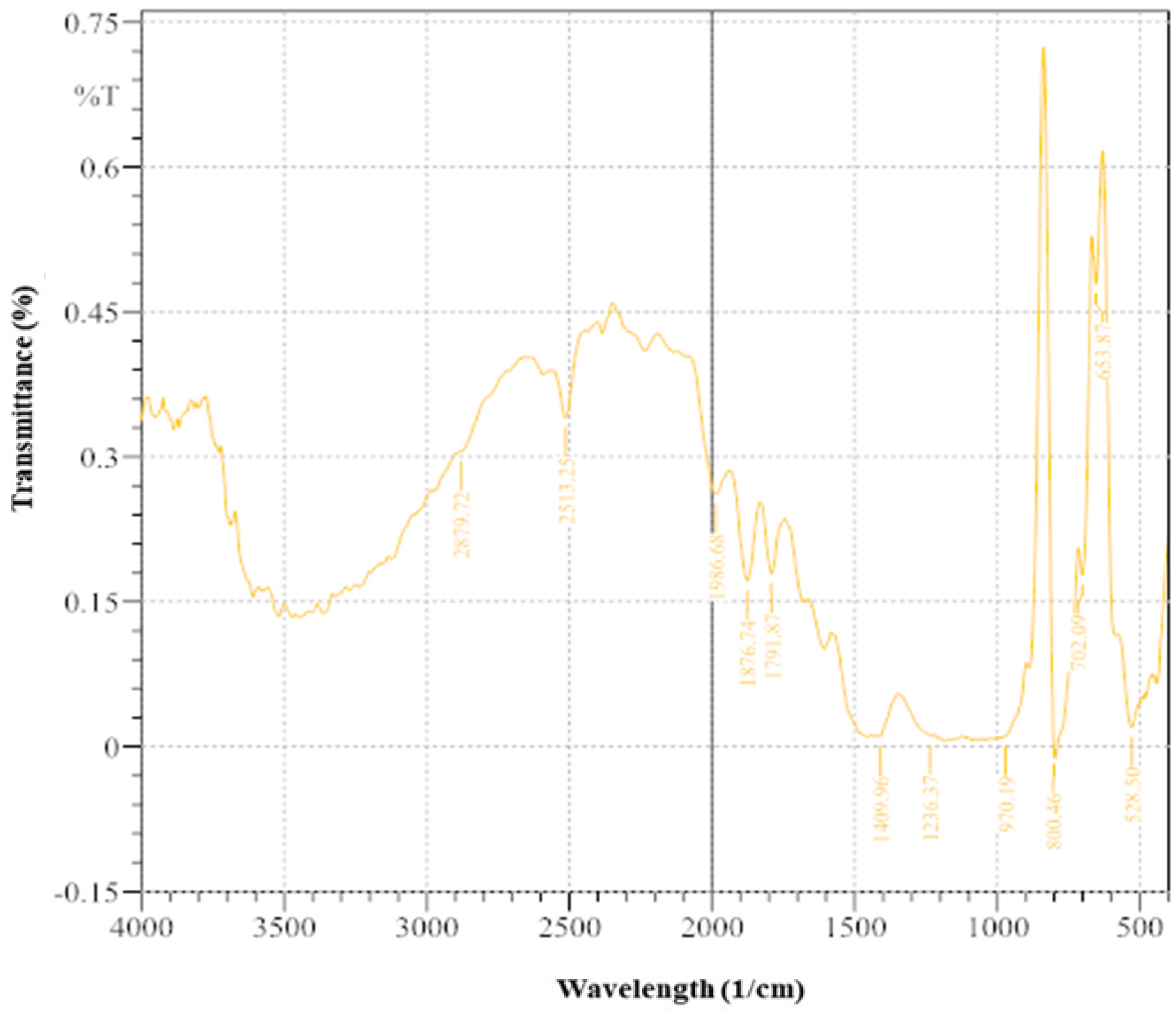
3.5. Crystal Structure and Mineralogical Composition of Treated Soil
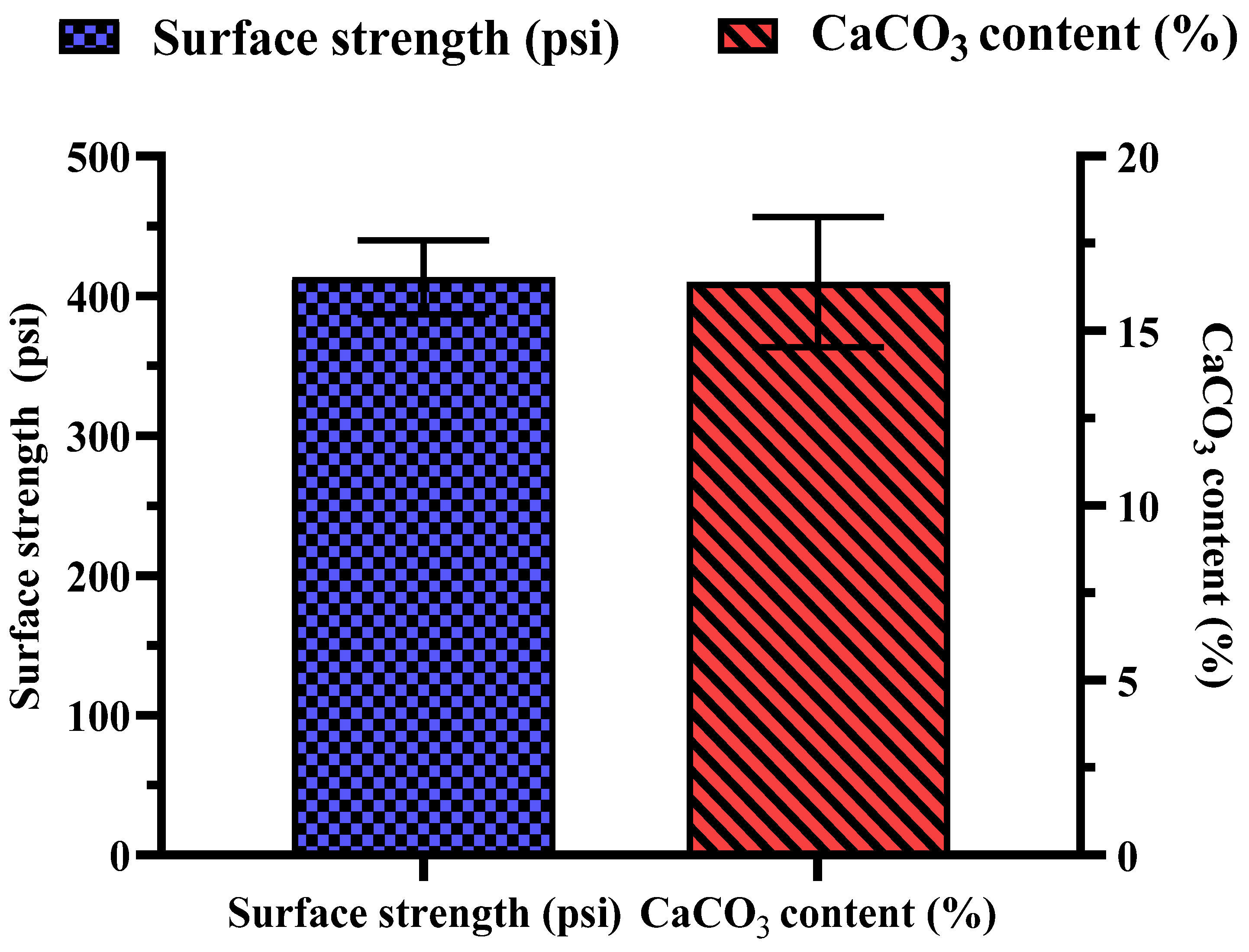
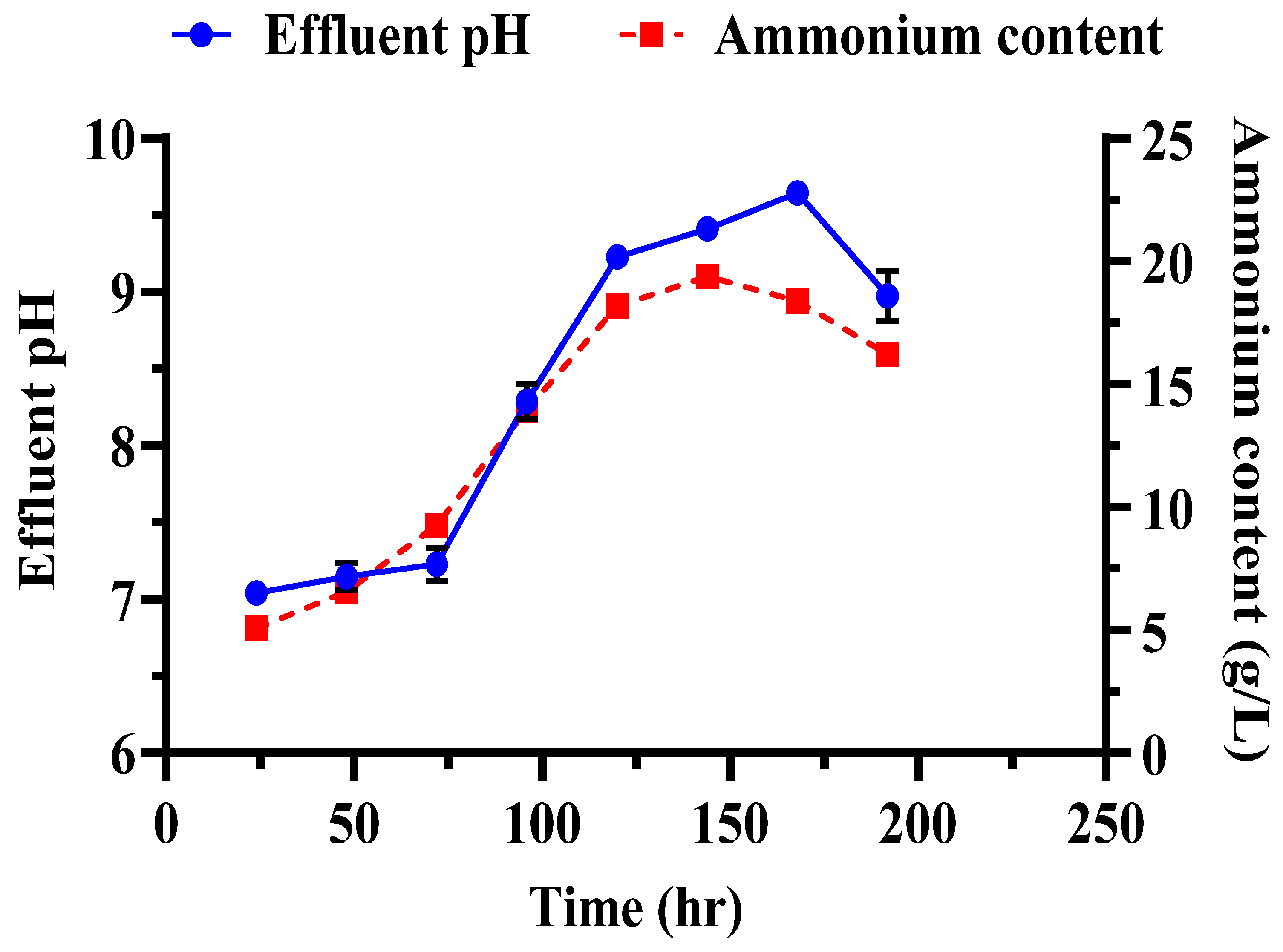
3.6. Strength, CaCO3 Contents, and Monitoring of Effluent
3.7. Durability of MICP and Future Direction
4. Broader Implications and Future Prospects
4.1. Practical Implication of the Study
4.2. Environmental Impact of MICP
4.3. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirilova, M.; Yotinov, I.; Todorova, Y.; Dinova, N.; Lincheva, S.; Schneider, I.; Topalova, Y. Microbiome Structure of Activated Sludge after Adaptation to Landfill Leachate Treatment in a Lab-Scale Sequencing Batch Reactor. Processes 2024, 12, 159. [Google Scholar] [CrossRef]
- Ibrahim Irka, C.; Prudent, P.; Théraulaz, F.; Farnet Da Silva, A.-M.; Asia, L.; Gori, D.; Vassalo, L.; Durand, A.; Demelas, C.; Höhener, P.; et al. Treatment of Sewage Sludge Compost Leachates on a Green Waste Biopile: A Case Study for an On-Site Application. Processes 2022, 10, 1196. [Google Scholar] [CrossRef]
- Annepogu, N.B.; Beese-Vasbender, P.F.; Himanshu, H.; Wolf, C.; Rehorek, A. Co-Treatment of Landfill Leachate and Liquid Fractions of Anaerobic Digestate in an Industrial-Scale Membrane Bioreactor System. Processes 2022, 10, 1140. [Google Scholar] [CrossRef]
- Hussein, M.; Yoneda, K.; Mohd-Zaki, Z.; Amir, A.; Othman, N. Heavy Metals in Leachate, Impacted Soils and Natural Soils of Different Landfills in Malaysia: An Alarming Threat. Chemosphere 2021, 267, 128874. [Google Scholar] [CrossRef]
- Yuan, Q.; Jia, H.; Poveda, M. Study on the Effect of Landfill Leachate on Nutrient Removal from Municipal Wastewater. J. Environ. Sci. 2016, 43, 153–158. [Google Scholar] [CrossRef]
- Wijekoon, P.; Koliyabandara, P.A.; Cooray, A.T.; Lam, S.S.; Athapattu, B.C.L.; Vithanage, M. Progress and Prospects in Mitigation of Landfill Leachate Pollution: Risk, Pollution Potential, Treatment and Challenges. J. Hazard. Mater. 2022, 421, 126627. [Google Scholar] [CrossRef]
- Bandala, E.R.; Liu, A.; Wijesiri, B.; Zeidman, A.B.; Goonetilleke, A. Emerging Materials and Technologies for Landfill Leachate Treatment: A Critical Review. Environ. Pollut. 2021, 291, 118133. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Ibrahim, A.M.; Al-Sulaiman, A.M.; Okasha, R.A. Landfill Leachate: Sources, Nature, Organic Composition, and Treatment: An Environmental Overview. Ain Shams Eng. J. 2024, 15, 102293. [Google Scholar] [CrossRef]
- Travar, I.; Uwayezu, J.N.; Kumpiene, J.; Yeung, L.W.Y. Challenges in the PFAS Remediation of Soil and Landfill Leachate: A Review. Adv. Environ. Eng. Res. 2021, 2, 006. [Google Scholar] [CrossRef]
- Fattahi, S.M.; Soroush, A.; Huang, N.; Zhang, J.; Jodari Abbasi, S.; Yu, Y. Durability of Biotechnologically Induced Crusts on Sand against Wind Erosion. J. Arid. Environ. 2021, 189, 104508. [Google Scholar] [CrossRef]
- Kang, B.; Zha, F.; Deng, W.; Wang, R.; Sun, X.; Lu, Z. Biocementation of Pyrite Tailings Using Microbially Induced Calcite Carbonate Precipitation. Molecules 2022, 27, 3608. [Google Scholar] [CrossRef] [PubMed]
- Ghezelbash, G.R.; Haddadi, M. Production of Nanocalcite Crystal by a Urease Producing Halophilic Strain of Staphylococcus Saprophyticus and Analysis of Its Properties by XRD and SEM. World J. Microbiol. Biotechnol. 2018, 34, 174. [Google Scholar] [CrossRef] [PubMed]
- Gowthaman, S.; Mohsenzadeh, A.; Nakashima, K.; Kawasaki, S. Removal of Ammonium By-Products from the Effluent of Bio-Cementation System through Struvite Precipitation. Mater. Today Proc. 2022, 61, 243–249. [Google Scholar] [CrossRef]
- Kang, C.-H.; Kwon, Y.-J.; So, J.-S. Bioremediation of Heavy Metals by Using Bacterial Mixtures. Ecol. Eng. 2016, 89, 64–69. [Google Scholar] [CrossRef]
- Zulaika, E.; Utomo, M.A.P.; Pangestu, A.S.; Alami, N.H.; Shovitri, M.; Prasetyo, E.N.; Setiawan, E.; Luqman, A.; Dwianita Kuswytasari, N.; Irawan, C. Novel Carbonatogenic Bacterial Strain Isolated from Limestone Quarry in East Java, Indonesia to Improve Concrete Performance. Biodiversitas 2021, 22, 3890–3898. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Khoshdelnezamiha, G.; Senian, N.; Ong, D.E.L.; Nissom, P.M. Experimental Optimisation of Various Cultural Conditions on Urease Activity for Isolated Sporosarcina Pasteurii Strains and Evaluation of Their Biocement Potentials. Ecol. Eng. 2017, 109, 65–75. [Google Scholar] [CrossRef]
- Sun, X.; Huang, J.; Sun, W.; Chen, B.; Shen, H.; Wang, Y.; Feng, J. Incubation Temperature Effect on Bacterial Self-Healing Capabilities of Cementitious Mortar Cracks: Deep Learning Based Crack Sealing Rates Evaluations. Constr. Build. Mater. 2024, 441, 137489. [Google Scholar] [CrossRef]
- Bonne, S.; Saleem, M.; Hanif, M.; Najjar, J.; Khan, S.; Zeeshan, M.; Tahir, T.; Ali, A.; Lu, C.; Chen, T. Synthesis, Urease Inhibition, Molecular Docking, and Optical Analysis of a Symmetrical Schiff Base and Its Selected Metal Complexes. Molecules 2024, 29, 4899. [Google Scholar] [CrossRef]
- Ramachandran, A.L.; Polat, P.; Mukherjee, A.; Dhami, N.K. Understanding and Creating Biocementing Beachrocks via Biostimulation of Indigenous Microbial Communities. Appl. Microbiol. Biotechnol. 2020, 104, 3655–3673. [Google Scholar] [CrossRef]
- Fang, C.; Achal, V. Biostimulation of Calcite Precipitation Process by Bacterial Community in Improving Cement Stabilized Rammed Earth as Sustainable Material. Appl. Microbiol. Biotechnol. 2019, 103, 7719–7727. [Google Scholar] [CrossRef]
- Gomez, M.G.; Anderson, C.M.; Graddy, C.M.R.; DeJong, J.T.; Nelson, D.C.; Ginn, T.R. Large-Scale Comparison of Bioaugmentation and Biostimulation Approaches for Biocementation of Sands. J. Geotech. Geoenviron. Eng. 2017, 143, 4016124–4016136. [Google Scholar] [CrossRef]
- Liu, S.; Sui, Y. Mitigation of Karst Soil Erosion by Optimizing a Biostimulation Strategy to Induce Mineralization. J. Test. Eval. 2022, 51, 918–944. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, N.-J.; Han, X. A Preliminary Study of Carbonate Sand Stabilization by Bio-Stimulation Based MICP Method. Jpn. Geotech. Soc. Spec. Publ. 2021, 9, 282–286. [Google Scholar] [CrossRef]
- Gorski, L. Selective Enrichment Media Bias the Types of Salmonella Enterica Strains Isolated from Mixed Strain Cultures and Complex Enrichment Broths. PLoS ONE 2012, 7, e34722. [Google Scholar] [CrossRef]
- Cheng, L.; Cord-Ruwisch, R. Selective Enrichment and Production of Highly Urease Active Bacteria by Non-Sterile (Open) Chemostat Culture. J. Ind. Microbiol. Biotechnol. 2013, 40, 1095–1104. [Google Scholar] [CrossRef]
- Aoki, M.; Noma, T.; Yonemitsu, H.; Araki, N.; Yamaguchi, T.; Hayashi, K. A Low-Tech Bioreactor System for the Enrichment and Production of Ureolytic Microbes. Pol. J. Microbiol. 2018, 67, 59–65. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, C.S.; Jiang, N.J.; Pan, X.H.; Liu, B.; Wang, Y.J.; Shi, B. Microbial-induced Carbonate Precipitation (MICP) Technology: A Review on the Fundamentals and Engineering Applications. Environ. Earth Sci. 2023, 82, 229. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Muda, K.; Steven, R.; Mustapha, M.; Ibrahim, H.U.; Ouahbi, T. Insect Frass as a Substrate to Stimulate Native Ureolytic Bacteria for Microbial-Induced Carbonate Precipitation in Soil Biocementation. Biomass Convers. Biorefin. 2023, 14, 25849–25872. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Ong, D.E.L.; Li, P.Y.; Senian, N.; Hei, N.L.; Esnault-Filet, A.; Muda, K.; Nissom, P.M. Effects of Push-Pull Injection-Suction Spacing on Sand Biocementation Treatment. Geotech. Res. 2024, 11, 28–42. [Google Scholar] [CrossRef]
- Basri, H.F.; Omoregie, A.I.; Mokhter, M.A. Influence of Enriched Urease Producing Bacteria from Leachate and Restaurant Wastewater on Heavy Metal Removal. Malays. J. Fundam. Appl. Sci. 2023, 19, 956–969. [Google Scholar] [CrossRef]
- ASTM. Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System); ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar] [CrossRef]
- Hadi, S.; Abbas, H.; Almajed, A.; Binyahya, A.; Al-Salloum, Y. Biocementation by Sporosarcina Pasteurii ATCC6453 under Simulated Conditions in Sand Columns. J. Mater. Res. Technol. 2022, 18, 4375–4384. [Google Scholar] [CrossRef]
- Abdel-Aleem, H.; Dishisha, T.; Saafan, A.; AbouKhadra, A.A.; Gaber, Y. Biocementation of Soil by Calcite/Aragonite Precipitation Using Pseudomonas Azotoformans and Citrobacter Freundii Derived Enzymes. RSC Adv. 2019, 9, 17601–17611. [Google Scholar] [CrossRef] [PubMed]
- Giner-Sanz, J.J.; Leverick, G.M.; Pérez-Herranz, V.; Shao-Horn, Y. Salicylate Method for Ammonia Quantification in Nitrogen Electroreduction Experiments: The Correction of Iron III Interference. J. Electrochem. Soc. 2020, 167, 134519. [Google Scholar] [CrossRef]
- Gowthaman, S.; Nakashima, K.; Kawasaki, S. Durability Analysis of Bio-Cemented Slope Soil under the Exposure of Acid Rain. J. Soils Sediments 2021, 21, 2831–2844. [Google Scholar] [CrossRef]
- Lapierre, F.M.; Huber, R. Feeding Strategies for Sporosarcina Pasteurii Cultivation Unlock More Efficient Production of Ureolytic Biomass for MICP. Biotechnol. J. 2024, 19, 2300466. [Google Scholar] [CrossRef]
- Shirakawa, M.A.; Cincotto, M.A.; Atencio, D.; Gaylarde, C.C.; John, V.M. Effect of Culture Medium on Biocalcification by Pseudomonas Putida, Lysinibacillus Sphaericus and Bacillus Subtilis. Braz. J. Microbiol. 2011, 42, 499–507. [Google Scholar] [CrossRef]
- Yan, Z.-X.; Li, Y.; Peng, S.-Y.; Wei, L.; Zhang, B.; Deng, X.-Y.; Zhong, M.; Cheng, X. Cadmium Biosorption and Mechanism Investigation Using Two Cadmium-Tolerant Microorganisms Isolated from Rhizosphere Soil of Rice. J. Hazard. Mater. 2024, 470, 134134. [Google Scholar] [CrossRef]
- Chaparro, S.; Rojas, H.A.; Caicedo, G.; Romanelli, G.; Pineda, A.; Luque, R.; Martínez, J.J. Whey as an Alternative Nutrient Medium for Growth of Sporosarcina Pasteurii and Its Effect on CaCO3 Polymorphism and Fly Ash Bioconsolidation. Materials 2021, 14, 2470. [Google Scholar] [CrossRef]
- Kang, C.-H.; Shin, Y.J.; Anbu, P.; Nam, I.-H.; So, J.-S. Biosequestration of Copper by Bacteria Isolated from an Abandoned Mine by Using Microbially Induced Calcite Precipitation. J. General. Appl. Microbiol. 2016, 62, 206–212. [Google Scholar] [CrossRef]
- Marín, S.; Cabestrero, O.; Demergasso, C.; Olivares, S.; Zetola, V.; Vera, M. An Indigenous Bacterium with Enhanced Performance of Microbially-Induced Ca-Carbonate Biomineralization under Extreme Alkaline Conditions for Concrete and Soil-Improvement Industries. Acta Biomater. 2021, 120, 304–317. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, N.; Zhao, S.; Wang, J. Exploring the Diversity of Active Ureolytic Bacteria in the Rumen by Comparison of CDNA and GDNA. Animals 2020, 10, 2162. [Google Scholar] [CrossRef] [PubMed]
- Oshiki, M.; Araki, M.; Hirakata, Y.; Hatamoto, M.; Yamaguchi, T.; Araki, N. Ureolytic Prokaryotes in Soil: Community Abundance and Diversity. Microbes Environ. 2018, 33, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, X.; Qiao, L.; Zhang, S.; Su, K.; Qiu, Z.; Li, X.; Zhao, Q.; Yu, C. Study on the Spatial Distribution of Ureolytic Microorganisms in Farmland Soil around Tailings with Different Heavy Metal Pollution. Sci. Total Environ. 2021, 775, 144946. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chu, J.; Cao, B.; Liu, H.; Cheng, L. Biocementation of Soil Using Non-Sterile Enriched Urease-Producing Bacteria from Activated Sludge. J. Clean. Prod. 2020, 262, 121315. [Google Scholar] [CrossRef]
- Rahmaninezhad, S.A.; Houshmand, M.; Sadighi, A.; Ahmari, K.; Kamireddi, D.; Street, R.M.; Farnam, Y.; Schauer, C.L.; Najafi, A.R.; Sales, C.M. Overcoming the Inhibitory Effects of Urea to Improve the Kinetics of Microbial-Induced Calcium Carbonate Precipitation (MICCP) by Lysinibacillus Sphaericus Strain MB284. J. Biosci. Bioeng. 2024, 138, 63–72. [Google Scholar] [CrossRef]
- Chung, H.; Kim, S.H.; Nam, K. Inhibition of Urea Hydrolysis by Free Cu Concentration of Soil Solution in Microbially Induced Calcium Carbonate Precipitation. Sci. Total Environ. 2020, 740, 140194. [Google Scholar] [CrossRef]
- Zhou, Y.; Elchalakani, M.; Cheng, L.; Shahin, M.A. Impact of Calcium Content and PH Value on MICP Crack Healing of Geopolymer Concrete. Cem. Concr. Compos. 2024, 146, 105410. [Google Scholar] [CrossRef]
- Guan, X.; Liu, Y.; Zhang, X.; Hou, X.; Yang, H. Enhancement Mechanism of Microbial Mineralization Modified Coal Gangue Aggregate Properties. Constr. Build. Mater. 2024, 440, 137477. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Soga, K.; DeJong, J.T.; Kabla, A.J. Microscale Investigations of Temperature-Dependent Microbially Induced Carbonate Precipitation (MICP) in the Temperature Range 4–50 °C. Acta Geotech. 2022, 18, 2239–2261. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Wang, S.; Evans, T.M.; Stuedlein, A.W.; Chu, J.; Zhao, C.; Wu, H.; Liu, H. Homogeneity and Mechanical Behaviors of Sands Improved by a Temperature-Controlled One-Phase MICP Method. Acta Geotech. 2021, 16, 1417–1427. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Tong, T.; Wang, C. Study of the Effect of Temperature on Microbially Induced Carbonate Precipitation. Acta Geotech. 2019, 14, 627–638. [Google Scholar] [CrossRef]
- Kim, G.; Kim, J.; Youn, H. Effect of Temperature, PH, and Reaction Duration on Microbially Induced Calcite Precipitation. Appl. Sci. 2018, 8, 1277. [Google Scholar] [CrossRef]
- Al Disi, Z.A.; Bontognali, T.R.R.; Jaoua, S.; Attia, E.; Al-Kuwari, H.A.S.; Zouari, N. Influence of Temperature, Salinity and Mg2+:Ca2+ Ratio on Microbially-Mediated Formation of Mg-Rich Carbonates by Virgibacillus Strains Isolated from a Sabkha Environment. Sci. Rep. 2019, 9, 19633. [Google Scholar] [CrossRef]
- Kim, H.; Son, H.M.; Park, S.; Seo, J.; Lee, H.K. Effects of Temperature and Salinity on Concrete-Surface Treatment by Bacteria in Marine Environment. ACI Mater. J. 2020, 117, 57–65. [Google Scholar] [CrossRef]
- Sepúlveda, S.; Duarte-Nass, C.; Rivas, M.; Azócar, L.; Ramírez, A.; Toledo-Alarcón, J.; Gutiérrez, L.; Jeison, D.; Torres-Aravena, Á. Testing the Capacity of Staphylococcus Equorum for Calcium and Copper Removal through MICP Process. Minerals 2021, 11, 905. [Google Scholar] [CrossRef]
- Yang, J.; Pan, X.; Zhao, C.; Mou, S.; Achal, V.; Al-Misned, F.A.; Mortuza, M.G.; Gadd, G.M. Bioimmobilization of Heavy Metals in Acidic Copper Mine Tailings Soil. Geomicrobiol. J. 2016, 33, 261–266. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, W.-C.; Xue, Z.-F.; Rahman, M.M.; Xie, Y.-X.; Hu, W. Immobilizing Lead and Copper in Aqueous Solution Using Microbial- and Enzyme-Induced Carbonate Precipitation. Front. Bioeng. Biotechnol. 2023, 11, 1146858. [Google Scholar] [CrossRef]
- Rajasekar, A.; Zhao, C.; Wu, S.; Murava, R.T.; Wilkinson, S. Synergistic Biocementation: Harnessing Comamonas and Bacillus Ureolytic Bacteria for Enhanced Sand Stabilization. World J. Microbiol. Biotechnol. 2024, 40, 229. [Google Scholar] [CrossRef]
- Cardoso, R.; Borges, I.; Vieira, J.; Duarte, S.O.D.; Monteiro, G.A. Interactions between Clay Minerals, Bacteria Growth and Urease Activity on Biocementation of Soils. Appl. Clay Sci. 2023, 240, 106972. [Google Scholar] [CrossRef]
- Zheng, L.; Lin, H.; Dong, Y.; Li, B.; Lu, Y. A Promising Approach for Simultaneous Removal of Ammonia and Multiple Heavy Metals from Landfill Leachate by Carbonate Precipitating Bacterium. J. Hazard. Mater. 2023, 456, 131662. [Google Scholar] [CrossRef]
- Rajasekar, A.; Wilkinson, S.; Sekar, R.; Bridge, J.; Medina-Roldán, E.; Moy, C.K.S. Biomineralisation Performance of Bacteria Isolated from a Landfill in China. Can. J. Microbiol. 2018, 64, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Meier, A.; Kastner, A.; Harries, D.; Wierzbicka-Wieczorek, M.; Majzlan, J.; Büchel, G.; Kothe, E. Calcium Carbonates: Induced Biomineralization with Controlled Macromorphology. Biogeosciences 2017, 14, 4867–4878. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, L.; Dong, Y.; Gao, Z. Comparative Study on the Effect of SRB and Sporosarcina Pasteurii on the MICP Cementation and Solidification of Lead–Zinc Tailings. Chem. Eng. J. 2024, 495, 153446. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, Y.; Wang, Y.; Zheng, L.; Zhang, Y.; Li, L.; Sun, B.; Li, S.; Zhu, Y. Study on MICP Dust Suppression Technology in Open Pit Coal Mine: Preparation and Mechanism of Microbial Dust Suppression Material. J. Environ. Manag. 2023, 343, 118181. [Google Scholar] [CrossRef]
- Wen, K.; Li, Y.; Amini, F.; Li, L. Impact of Bacteria and Urease Concentration on Precipitation Kinetics and Crystal Morphology of Calcium Carbonate. Acta Geotech. 2020, 15, 17–27. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, W.; Zhang, Q.; Hu, X. Investigation on Mineralization Performance and Spore Germination Conditions of Calcium Carbonate Mineralizing Bacteria. Mater. Res. Express 2022, 9, 65403. [Google Scholar] [CrossRef]
- Zhan, Q.; Qian, C. Microbial-Induced Remediation of Zn2+ Pollution Based on the Capture and Utilization of Carbon Dioxide. Electron. J. Biotechnol. 2016, 19, 29–32. [Google Scholar] [CrossRef]
- Yu, X.; Zhan, Q.; Qian, C.; Ma, J.; Liang, Y. The Optimal Formulation of Bio-Carbonate and Bio-Magnesium Phosphate Cement to Reduce Ammonia Emission. J. Clean. Prod. 2019, 240, 118156. [Google Scholar] [CrossRef]
- Rajasekar, A.; Moy, C.K.S.; Wilkinson, S.; Sekar, R. Microbially Induced Calcite Precipitation Performance of Multiple Landfill Indigenous Bacteria Compared to a Commercially Available Bacteria in Porous Media. PLoS ONE 2021, 16, e0254676. [Google Scholar] [CrossRef]
- Amini Kiasari, M.; Pakbaz, M.S.; Ghezelbash, G.R. Comparison of Effects of Different Nutrients on Stimulating Indigenous Soil Bacteria for Biocementation. J. Mater. Civil. Eng. 2019, 31, 04019067–04019079. [Google Scholar] [CrossRef]
- Lee, M.; Kolbus, C.M.; Yepez, A.D.; Gomez, M.G. Investigating Ammonium By-Product Removal Following Stimulated Ureolytic Microbially-Induced Calcite Precipitation. In Geo-Congress 2019, Proceedings of the Geotechnical Special Publication; Eighth International Conference on Case Histories in Geotechnical Engineering Philadelphia, PA, USA, 24–27 March 2019; Meehan, C.L., Kumar, S., Pando, M.A., Coe, J.T., Eds.; American Society of Civil Engineers: Reston, VA, USA, 2019; pp. 260–272. [Google Scholar] [CrossRef]
- Van Den Heede, P.; De Belie, N. Environmental Impact and Life Cycle Assessment (LCA) of Traditional and “green” Concretes: Literature Review and Theoretical Calculations. Cem. Concr. Compos. 2012, 34, 431–442. [Google Scholar] [CrossRef]
- Wahab, N.A.; Roshan, M.J.; Rashid, A.S.A.; Hezmi, M.A.; Jusoh, S.N.; Norsyahariati, N.D.N.; Tamassoki, S. Strength and Durability of Cement-Treated Lateritic Soil. Sustainability 2021, 13, 6430. [Google Scholar] [CrossRef]
- Sohail, M.G.; Disi, Z.A.; Zouari, N.; Nuaimi, N.A.; Kahraman, R.; Gencturk, B.; Rodrigues, D.F.; Yildirim, Y. Bio Self-Healing Concrete Using MICP by an Indigenous Bacillus Cereus Strain Isolated from Qatari Soil. Constr. Build. Mater. 2022, 328, 126943. [Google Scholar] [CrossRef]
- Zhang, J.; Su, P.; Wen, K.; Li, Y.; Li, L. Environmental Impact and Mechanical Improvement of Microbially Induced Calcium Carbonate Precipitation-Treated Coal Fly Ash-Soil Mixture. Environ. Geotech. 2021, 11, 29–39. [Google Scholar] [CrossRef]
- Iezzi, B.; Brady, R.; Sardag, S.; Eu, B.; Skerlos, S. Growing Bricks: Assessing Biocement for Lower Embodied Carbon Structures. Procedia CIRP 2019, 80, 470–475. [Google Scholar] [CrossRef]
- Hu, X.; Yu, C.; Shi, J.; He, B.; Wang, X.; Ma, Z. Biomineralization Mechanism and Remediation of Cu, Pb and Zn by Indigenous Ureolytic Bacteria B. Intermedia TSBOI. J. Clean. Prod. 2024, 436, 140508. [Google Scholar] [CrossRef]
- Kasra, A.I.A.; Erkurt, H.A. Biomineralization of Different Trace Metals by Using Ureolytic Bacteria Isolated from Soil. J. Adv. Res. Dyn. Control Syst. 2020, 12, 370–377. [Google Scholar] [CrossRef]
- Sugata, M.; Arviana, N.; Tampubolon, L.; Widjajakusuma, J.; Victor, H.; Jan, T.T. Identification and Characterization of Calcite Producing Bacteria Isolated from Soils in West Java, Indonesia. Biodiversitas 2022, 23, 3921–3927. [Google Scholar] [CrossRef]
- Lors, C.; Gassie, C.; Guyoneaud, R.; Damidot, D. Impact of a Biorepair Treatment on the Diversity of Calcifying Bacterial Communities at the Surface of Cracked Concrete Walls. Appl. Microbiol. Biotechnol. 2023, 107, 187–200. [Google Scholar] [CrossRef]
- Naeimi, M.; Haddad, A. Environmental Impacts of Chemical and Microbial Grouting. Environ. Sci. Pollut. Res. 2020, 27, 2264–2272. [Google Scholar] [CrossRef]
- Babakhani, S.; Fahmi, A.; Katebi, H.; Ouria, A.; Majnouni-Toutakhane, A.; Ganbarov, K.; Kafil, H.S. Non-Sterile Corn Steep Liquor a Novel, Cost Effective and Powerful Culture Media for Sporosarcina Pasteurii Cultivation for Sand Improvement. J. Appl. Microbiol. 2021, 130, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Kahani, M.; Kalantary, F.; Soudi, M.R.; Pakdel, L.; Aghaalizadeh, S. Optimization of Cost Effective Culture Medium for Sporosarcina Pasteurii as Biocementing Agent Using Response Surface Methodology: Up Cycling Dairy Waste and Seawater. J. Clean. Prod. 2020, 253, 120022. [Google Scholar] [CrossRef]
- Gowthaman, S.; Koizumi, H.; Nakashima, K.; Kawasaki, S. Field Experimentation of Bio-Cementation Using Low-Cost Cementation Media for Preservation of Slope Surface. Case Stud. Constr. Mater. 2023, 18, e02086. [Google Scholar] [CrossRef]
- Maleki-Kakelar, M.; Azarhoosh, M.J.; Golmohammadi Senji, S.; Aghaeinejad-Meybodi, A. Urease Production Using Corn Steep Liquor as a Low-Cost Nutrient Source by Sporosarcina Pasteurii: Biocementation and Process Optimization via Artificial Intelligence Approaches. Environ. Sci. Pollut. Res. 2022, 29, 13767–13781. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Nakashima, K.; Takano, C.; Kawasaki, S. Kitchen Waste Bone-Driven Enzyme-Induced Calcium Phosphate Precipitation under Microgravity for Space Biocementation. Biogeotechnics 2024, 100156. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Muda, K.; Bin Bakri, M.K.; Rahman, R.; Yusof, F.A.M.; Ojuri, O.O. Calcium Carbonate Bioprecipitation Mediated by Ureolytic Bacteria Grown in Pelletized Organic Manure Medium. Biomass Convers. Biorefin. 2024, 14, 13005–13026. [Google Scholar] [CrossRef]
- Chen, L.; Song, Y.; Fang, H.; Feng, Q.; Lai, C.; Song, X. Systematic Optimization of a Novel, Cost-Effective Fermentation Medium of Sporosarcina Pasteurii for Microbially Induced Calcite Precipitation (MICP). Constr. Build. Mater. 2022, 348, 128632. [Google Scholar] [CrossRef]
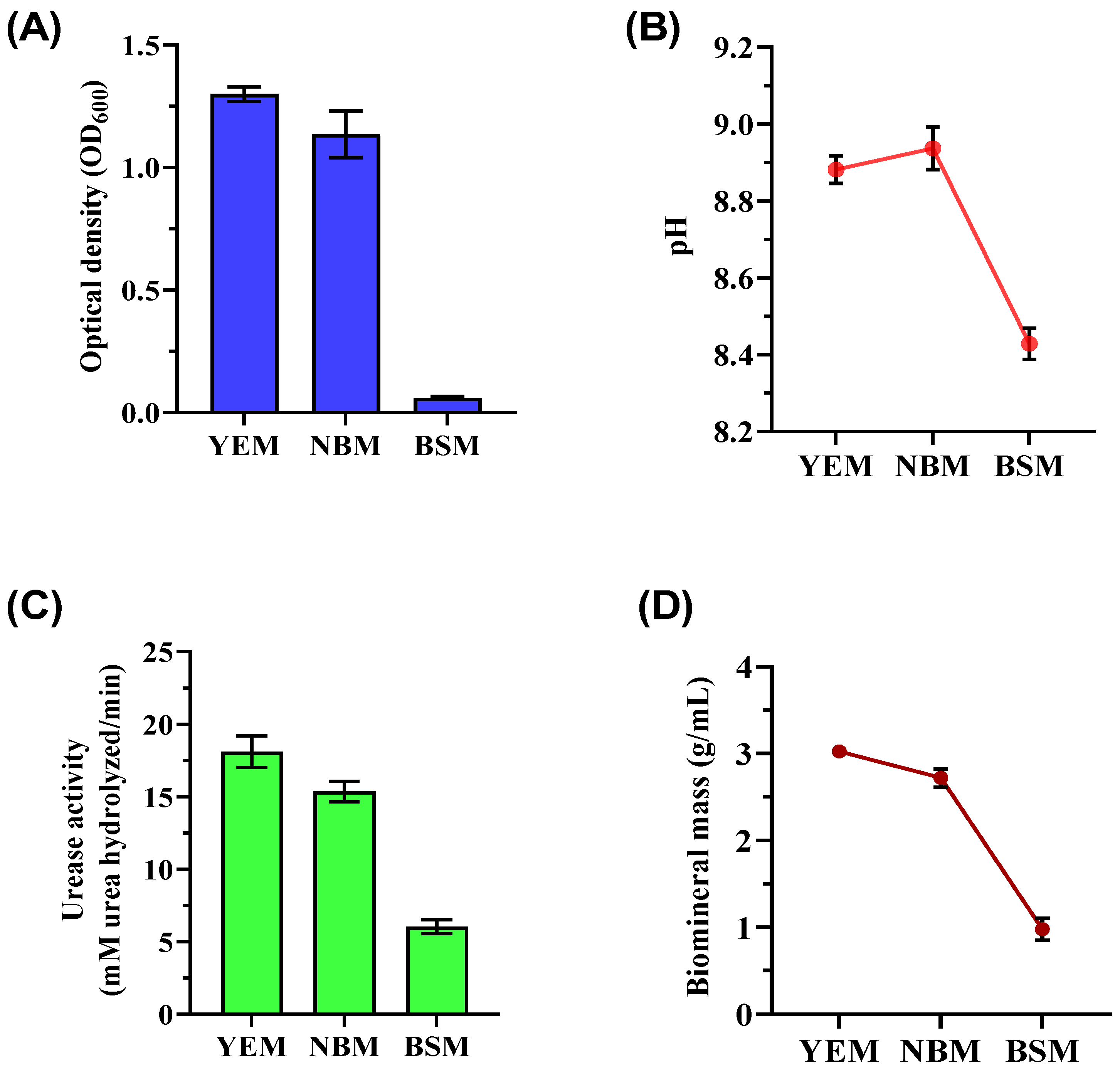



| Component | YEM | NBM | BSM |
|---|---|---|---|
| Yeast extract | 20 g/L | 0 | 0 |
| Nutrient broth | 0 | 13 g/L | 0 |
| Brown sugar | 0 | 0 | 10 g/L |
| Urea | 40 g/L | 40 g/L | 40 g/L |
| Nickel chloride | 0.02 g/L | 0.02 g/L | 0.02 g/L |
| Ammonium chloride | 5 g/L | 5 g/L | 5 g/L |
| Sodium chloride | 5 g/L | 0 | 5 g/L |
| Initial pH | 6.26 | 6.92 | 5.4 |
| Element | Atomic Number | Mass Normalized % | Atom % |
|---|---|---|---|
| O | 8 | 49.87 | 58.76 |
| Ca | 20 | 16.35 | 7.69 |
| Si | 14 | 10.54 | 7.08 |
| C | 6 | 10.22 | 16.0467 |
| N | 7 | 3.78 | 5.08 |
| Al | 13 | 3.00 | 2.10 |
| Cl | 17 | 3.10 | 1.39 |
| K | 19 | 2.42 | 1.17 |
| Fe | 26 | 0.75 | 0.27 |
| Na | 11 | 0.59 | 0.48 |
| Samples | Cycle 1 (% Loss) | Cycle 2 (% Loss) | Cycle (% Loss) |
|---|---|---|---|
| Wet–Dry | 1.24 ± 0.72 | 2.15 ± 0.92 | 2.83 ± 0.48 |
| Freeze–Thaw | 2.10 ± 0.84 | 3.47 ± 0.49 | 3.82 ± 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omoregie, A.I.; Kan, F.-K.; Basri, H.F.; Silini, M.O.E.; Rajasekar, A. Enhanced MICP for Soil Improvement and Heavy Metal Remediation: Insights from Landfill Leachate-Derived Ureolytic Bacterial Consortium. Microorganisms 2025, 13, 174. https://doi.org/10.3390/microorganisms13010174
Omoregie AI, Kan F-K, Basri HF, Silini MOE, Rajasekar A. Enhanced MICP for Soil Improvement and Heavy Metal Remediation: Insights from Landfill Leachate-Derived Ureolytic Bacterial Consortium. Microorganisms. 2025; 13(1):174. https://doi.org/10.3390/microorganisms13010174
Chicago/Turabian StyleOmoregie, Armstrong Ighodalo, Fock-Kui Kan, Hazlami Fikri Basri, Muhammad Oliver Ensor Silini, and Adharsh Rajasekar. 2025. "Enhanced MICP for Soil Improvement and Heavy Metal Remediation: Insights from Landfill Leachate-Derived Ureolytic Bacterial Consortium" Microorganisms 13, no. 1: 174. https://doi.org/10.3390/microorganisms13010174
APA StyleOmoregie, A. I., Kan, F.-K., Basri, H. F., Silini, M. O. E., & Rajasekar, A. (2025). Enhanced MICP for Soil Improvement and Heavy Metal Remediation: Insights from Landfill Leachate-Derived Ureolytic Bacterial Consortium. Microorganisms, 13(1), 174. https://doi.org/10.3390/microorganisms13010174








