Virulence of Bacteria Causing Mastitis in Dairy Cows: A Literature Review
Abstract
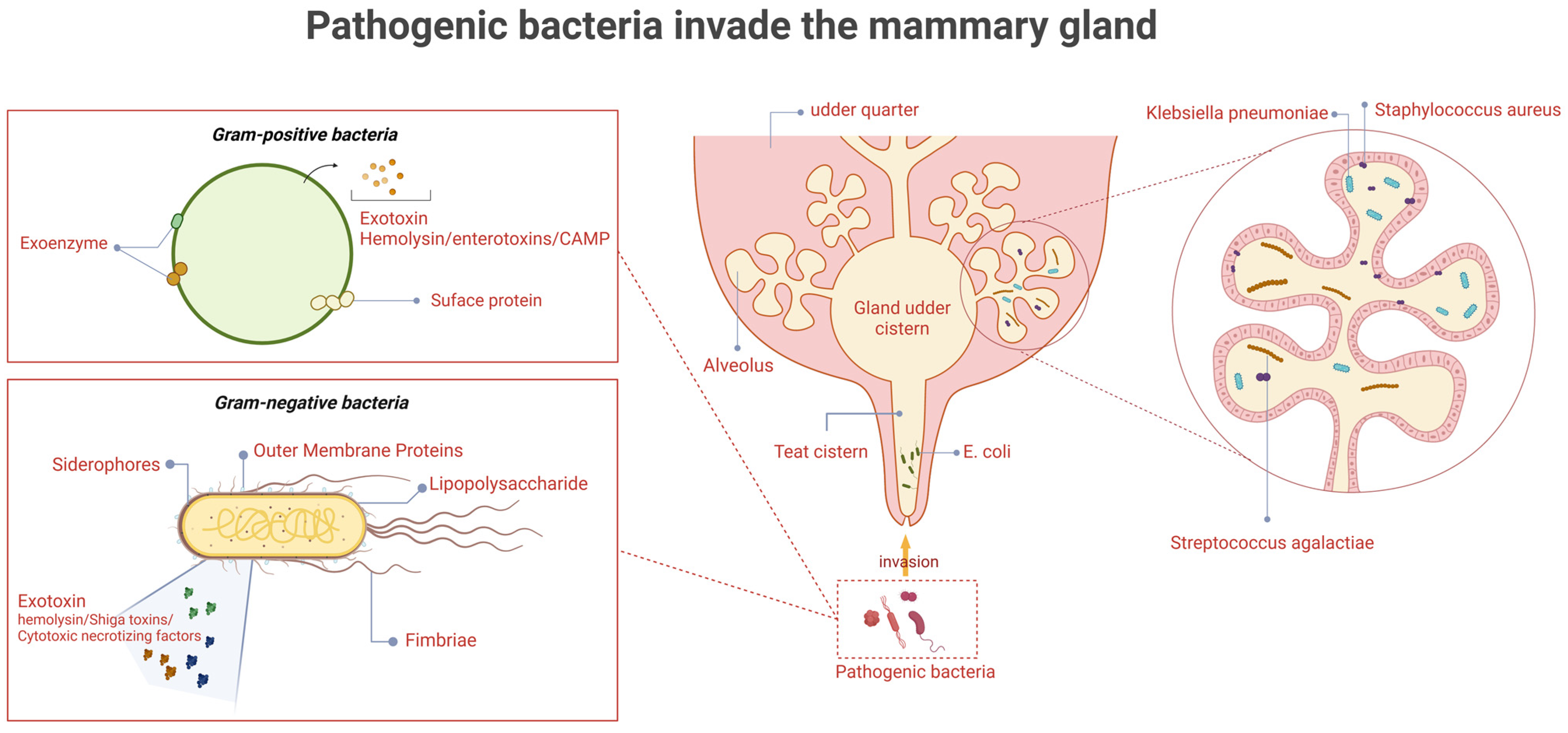
1. Introduction
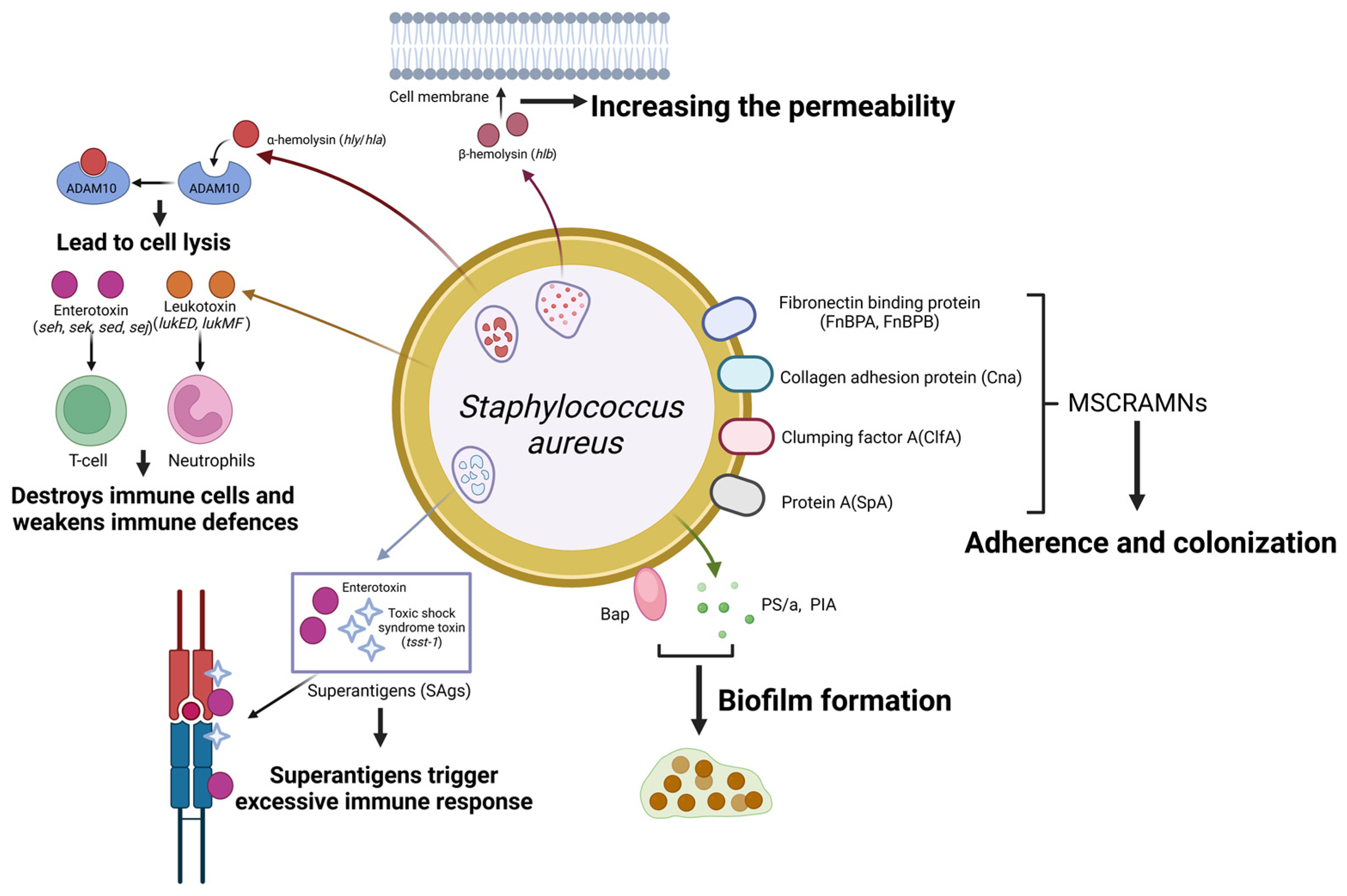
2. Staphylococcus aureus
2.1. Adherence
2.2. Exotoxins
2.3. Biofilms
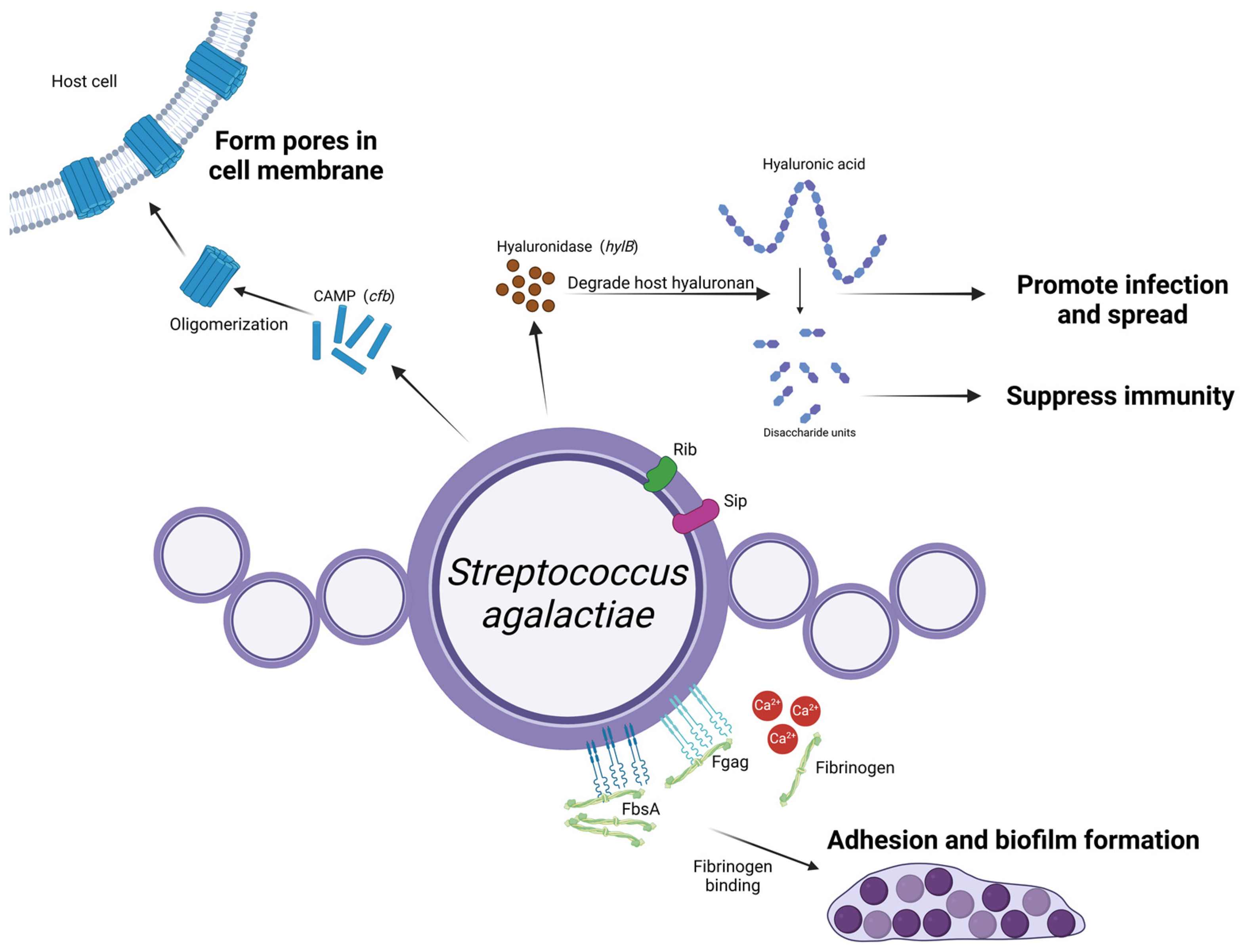
3. Streptococcus agalactiae
3.1. Adherence
3.2. Exotoxins
3.3. Others
4. Environmental Streptococci and Enterococci
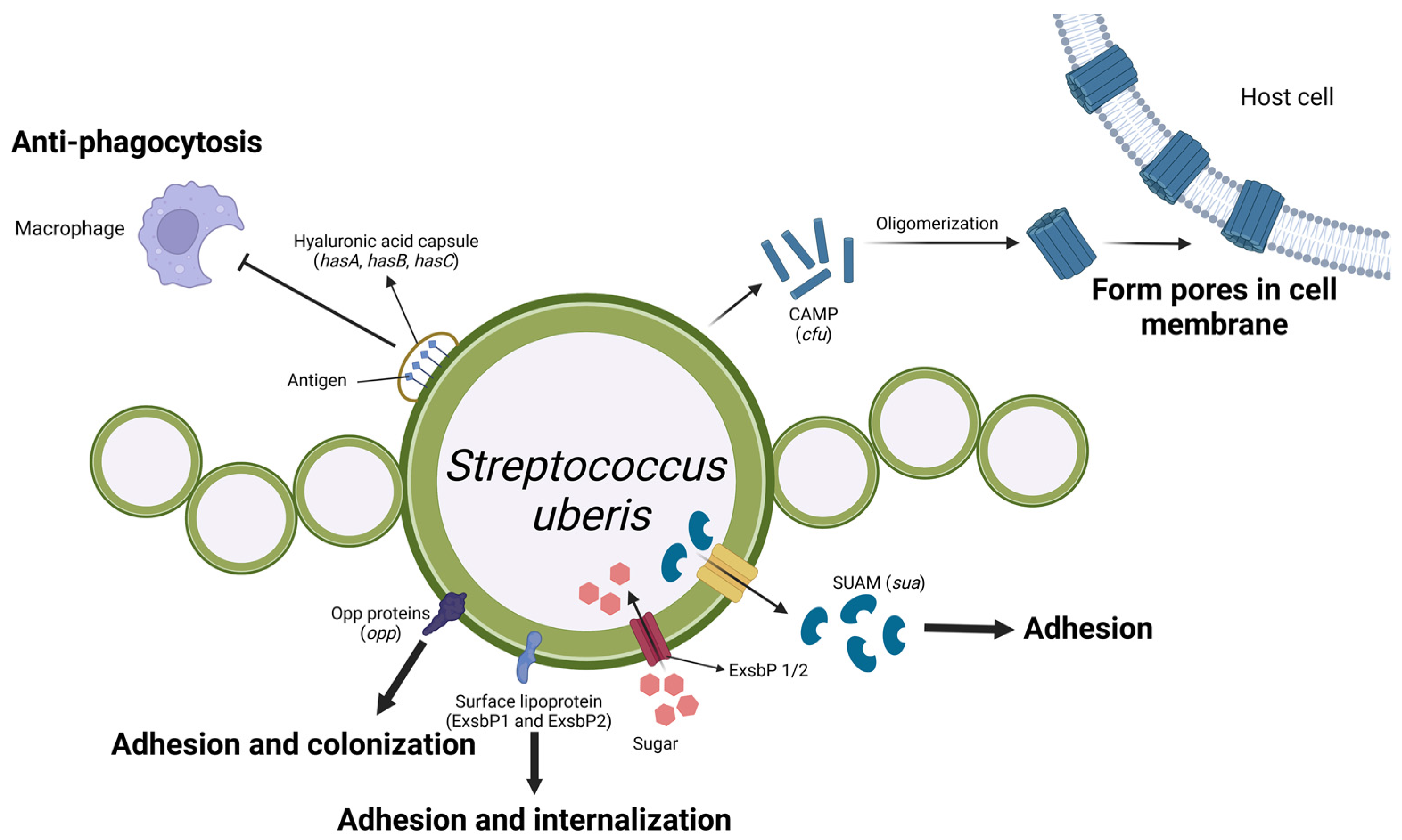
4.1. Streptococcus uberis
4.2. Streptococcus dysgalactiae
4.3. Enterococcus spp.
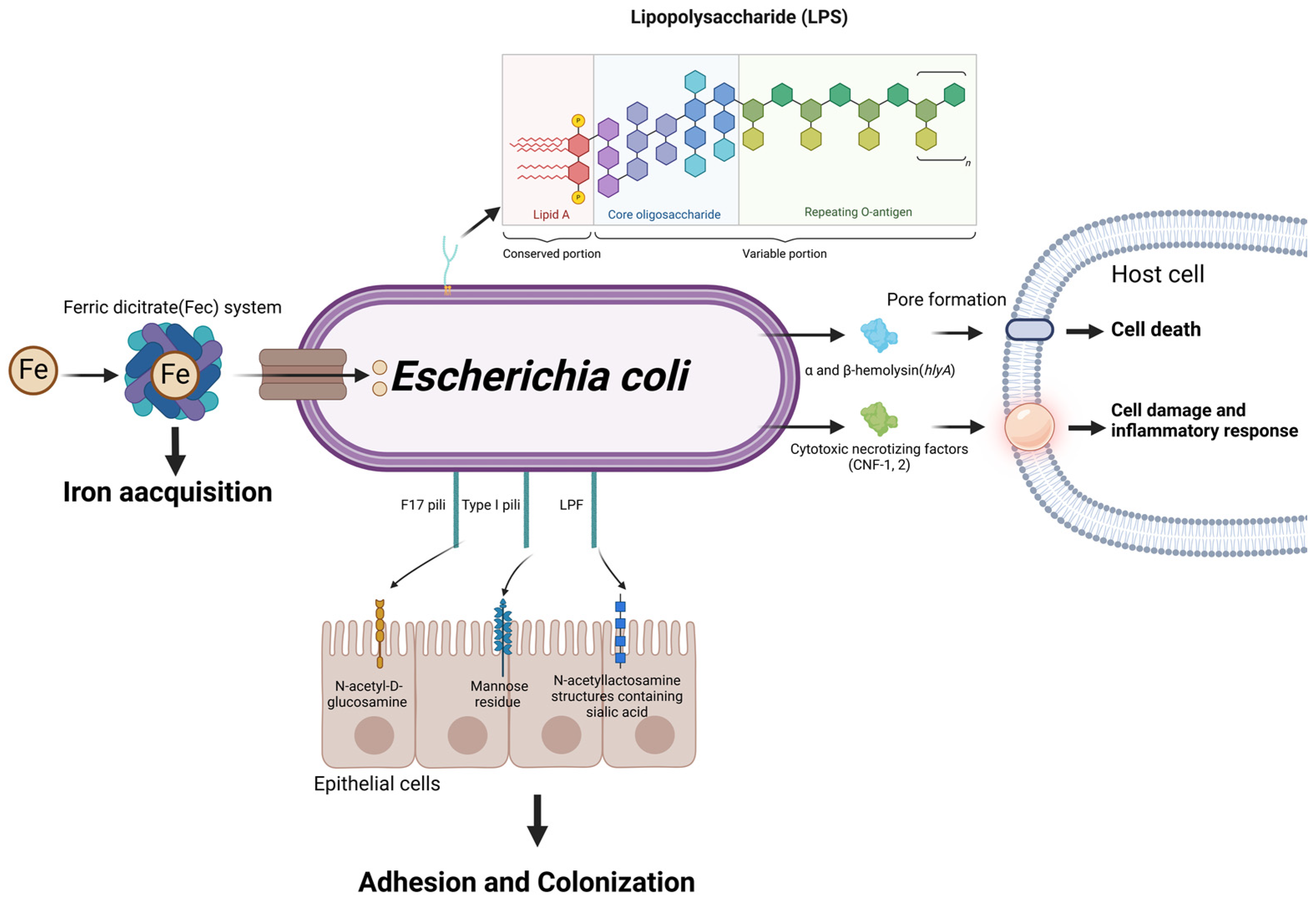
5. Escherichia coli
5.1. Adherence
5.2. Exotoxins
5.3. Endotoxin
5.4. Siderophore
6. Klebsiella pneumoniae
6.1. Capsular Polysaccharide
6.2. Adherence
6.3. Others
7. Prospects
7.1. Comparative Analysis of Virulence Factors in Mastitis-Causing Pathogens
7.2. Cross-Species Comparison of Virulence Factors in Mastitis Pathogens
7.3. Exploring and Validating Novel Virulence Factors in Mastitis Pathogens
7.4. Vaccine Development Based on Pathogen Virulence Factors
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Seegers, H.; Fourichon, C.; Beaudeau, F. Production Effects Related to Mastitis and Mastitis Economics in Dairy Cattle Herds. Vet. Res. 2003, 34, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Keane, O.M. Symposium Review: Intramammary Infections-Major Pathogens and Strain-Associated Complexity. J. Dairy Sci. 2019, 102, 4713–4726. [Google Scholar] [CrossRef]
- Ruegg, P.L. A 100-Year Review: Mastitis Detection, Management, and Prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef]
- Patel, K.; Godden, S.M.; Royster, E.; Crooker, B.A.; Timmerman, J.; Fox, L. Relationships among Bedding Materials, Bedding Bacteria Counts, Udder Hygiene, Milk Quality, and Udder Health in US Dairy Herds. J. Dairy Sci. 2019, 102, 10213–10234. [Google Scholar] [CrossRef] [PubMed]
- Condas, L.A.Z.; De Buck, J.; Nobrega, D.B.; Carson, D.A.; Naushad, S.; De Vliegher, S.; Zadoks, R.N.; Middleton, J.R.; Dufour, S.; Kastelic, J.P.; et al. Prevalence of Non-Aureus Staphylococci Species Causing Intramammary Infections in Canadian Dairy Herds. J. Dairy Sci. 2017, 100, 5592–5612. [Google Scholar] [CrossRef] [PubMed]
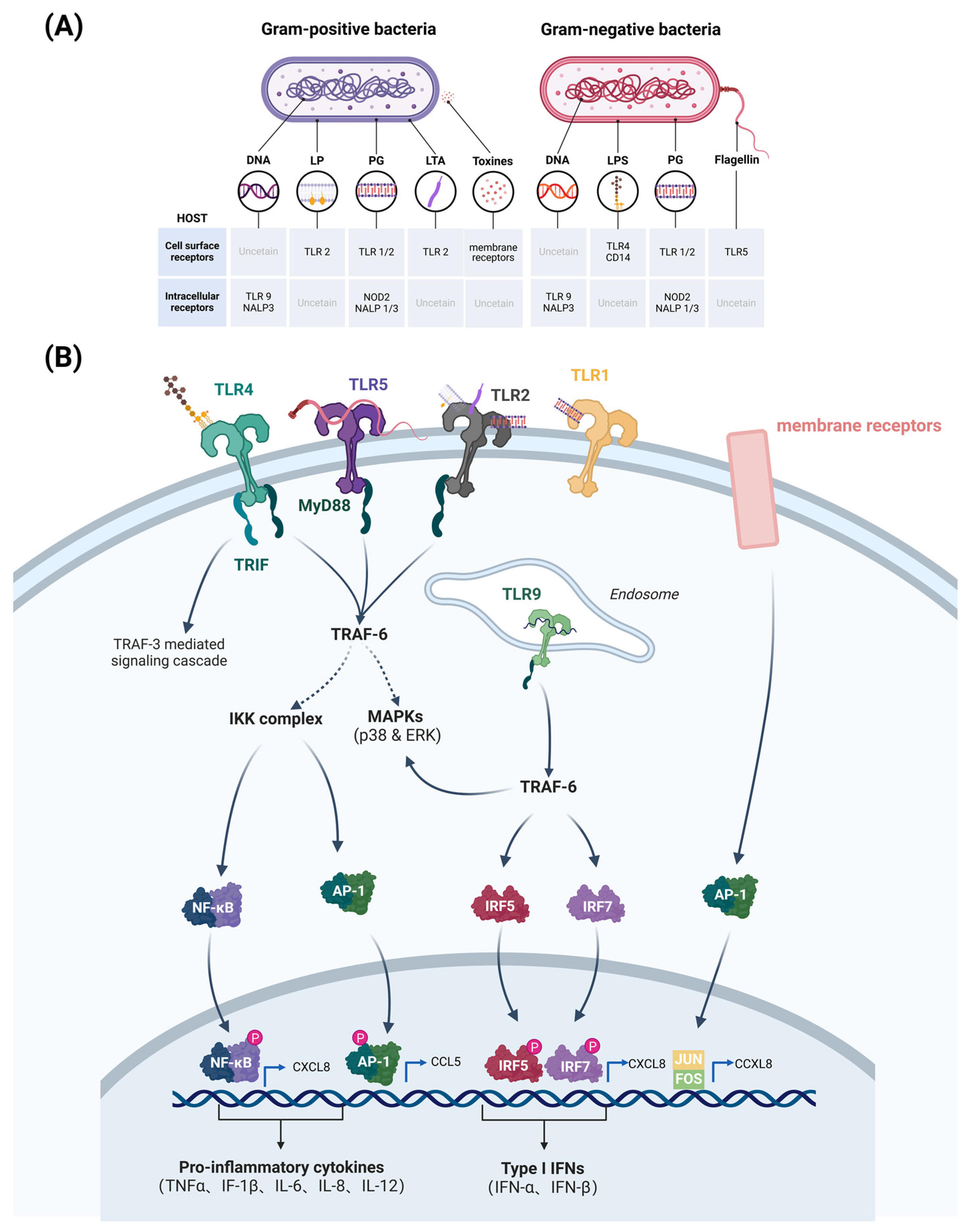
- Oviedo-Boyso, J.; Valdez-Alarcón, J.J.; Cajero-Juárez, M.; Ochoa-Zarzosa, A.; López-Meza, J.E.; Bravo-Patiño, A.; Baizabal-Aguirre, V.M. Innate Immune Response of Bovine Mammary Gland to Pathogenic Bacteria Responsible for Mastitis. J. Infect. 2007, 54, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, F.B.; Cunha, P.; Jensen, K.; Glass, E.J.; Foucras, G.; Robert-Granié, C.; Rupp, R.; Rainard, P. Differential Response of Bovine Mammary Epithelial Cells to Staphylococcus aureus or Escherichia coli Agonists of the Innate Immune System. Vet. Res. 2013, 44, 40. [Google Scholar] [CrossRef]
- Johnzon, C.-F.; Artursson, K.; Söderlund, R.; Guss, B.; Rönnberg, E.; Pejler, G. Mastitis Pathogens with High Virulence in a Mouse Model Produce a Distinct Cytokine Profile In Vivo. Front. Immunol. 2016, 7, 368. [Google Scholar] [CrossRef] [PubMed]
- Vrieling, M.; Koymans, K.J.; Heesterbeek, D.a.C.; Aerts, P.C.; Rutten, V.P.M.G.; de Haas, C.J.C.; van Kessel, K.P.M.; Koets, A.P.; Nijland, R.; van Strijp, J.a.G. Bovine Staphylococcus aureus Secretes the Leukocidin LukMF’ To Kill Migrating Neutrophils through CCR1. mBio 2015, 6, e00335. [Google Scholar] [CrossRef] [PubMed]
- Exel, C.E.; Halasa, T.; Koop, G.; Steeneveld, W.; Lam, T.J.G.M.; Benedictus, L.; Gussmann, M. A Stochastic Modelling Approach to Determine the Effect of Diverse Staphylococcus aureus Strains on the Economic and Epidemiological Outcomes of Mastitis Intervention Strategies in Dairy Cattle. Prev. Vet. Med. 2022, 199, 105566. [Google Scholar] [CrossRef]
- Rossi, B.F.; Bonsaglia, E.C.R.; Pantoja, J.C.F.; Santos, M.V.; Gonçalves, J.L.; Fernandes Júnior, A.; Rall, V.L.M. Short Communication: Association between the Accessory Gene Regulator (Agr) Group and the Severity of Bovine Mastitis Caused by Staphylococcus aureus. J. Dairy Sci. 2021, 104, 3564–3568. [Google Scholar] [CrossRef]
- Pereyra, E.A.L.; Picech, F.; Renna, M.S.; Baravalle, C.; Andreotti, C.S.; Russi, R.; Calvinho, L.F.; Diez, C.; Dallard, B.E. Detection of Staphylococcus aureus Adhesion and Biofilm-Producing Genes and Their Expression during Internalization in Bovine Mammary Epithelial Cells. Vet. Microbiol. 2016, 183, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Cheng, J.; Zhao, X. Clumping Factor A of Staphylococcus aureus Interacts with AnnexinA2 on Mammary Epithelial Cells. Sci. Rep. 2017, 7, 40608. [Google Scholar] [CrossRef] [PubMed]
- Artursson, K.; Söderlund, R.; Liu, L.; Monecke, S.; Schelin, J. Genotyping of Staphylococcus aureus in Bovine Mastitis and Correlation to Phenotypic Characteristics. Vet. Microbiol. 2016, 193, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Höök, M. Adhesion, Invasion and Evasion: The Many Functions of the Surface Proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Brouillette, E.; Talbot, B.G.; Malouin, F. The Fibronectin-Binding Proteins of Staphylococcus aureus May Promote Mammary Gland Colonization in a Lactating Mouse Model of Mastitis. Infect. Immun. 2003, 71, 2292–2295. [Google Scholar] [CrossRef] [PubMed]
- Castilho, I.G.; Dantas, S.T.A.; Langoni, H.; Araújo, J.P.; Fernandes, A.; Alvarenga, F.C.L.; Maia, L.; Cagnini, D.Q.; Rall, V.L.M. Host-Pathogen Interactions in Bovine Mammary Epithelial Cells and HeLa Cells by Staphylococcus aureus Isolated from Subclinical Bovine Mastitis. J. Dairy Sci. 2017, 100, 6414–6421. [Google Scholar] [CrossRef]
- Klein, R.C.; Fabres-Klein, M.H.; Brito, M.A.V.P.; Fietto, L.G.; Ribon, A.D.O.B. Staphylococcus aureus of Bovine Origin: Genetic Diversity, Prevalence and the Expression of Adhesin-Encoding Genes. Vet. Microbiol. 2012, 160, 183–188. [Google Scholar] [CrossRef] [PubMed]
- G Abril, A.; G Villa, T.; Barros-Velázquez, J.; Cañas, B.; Sánchez-Pérez, A.; Calo-Mata, P.; Carrera, M. Staphylococcus aureus Exotoxins and Their Detection in the Dairy Industry and Mastitis. Toxins 2020, 12, 537. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, J.; Barkema, H.W.; Ali, T.; Liu, G.; Deng, Y.; Naushad, S.; Kastelic, J.P.; Han, B. Virulence Gene Profiles: Alpha-Hemolysin and Clonal Diversity in Staphylococcus aureus Isolates from Bovine Clinical Mastitis in China. BMC Vet. Res. 2018, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Huseby, M.; Shi, K.; Brown, C.K.; Digre, J.; Mengistu, F.; Seo, K.S.; Bohach, G.A.; Schlievert, P.M.; Ohlendorf, D.H.; Earhart, C.A. Structure and Biological Activities of Beta Toxin from Staphylococcus aureus. J. Bacteriol. 2007, 189, 8719–8726. [Google Scholar] [CrossRef]
- Haveri, M.; Roslöf, A.; Rantala, L.; Pyörälä, S. Virulence Genes of Bovine Staphylococcus aureus from Persistent and Nonpersistent Intramammary Infections with Different Clinical Characteristics. J. Appl. Microbiol. 2007, 103, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, J.; Yang, D.; Tang, C.; Chen, J. Staphylococcal Enterotoxin M Induced Inflammation and Impairment of Bovine Mammary Epithelial Cells. J. Dairy Sci. 2020, 103, 8350–8359. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, W.; Ali, T.; Alkasir, R.; Yin, J.; Liu, G.; Han, B. Staphylococcal Enterotoxin H Induced Apoptosis of Bovine Mammary Epithelial Cells in Vitro. Toxins 2014, 6, 3552–3567. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Cui, J.; Cui, T.; Guo, H.; Ono, H.K.; Park, C.-H.; Okamura, M.; Nakane, A.; Hu, D.-L. Staphylococcal Enterotoxin C Is an Important Virulence Factor for Mastitis. Toxins 2019, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.D.; Proft, T. The Bacterial Superantigen and Superantigen-like Proteins. Immunol. Rev. 2008, 225, 226–243. [Google Scholar] [CrossRef]
- Wilson, G.J.; Tuffs, S.W.; Wee, B.A.; Seo, K.S.; Park, N.; Connelley, T.; Guinane, C.M.; Morrison, W.I.; Fitzgerald, J.R. Bovine Staphylococcus aureus Superantigens Stimulate the Entire T Cell Repertoire of Cattle. Infect. Immun. 2018, 86, e00505-18. [Google Scholar] [CrossRef] [PubMed]
- Barrio, M.B.; Rainard, P.; Prévost, G. LukM/LukF’-PV Is the Most Active Staphylococcus aureus Leukotoxin on Bovine Neutrophils. Microbes Infect. 2006, 8, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Tochimaru, N.; Nakasuji, S.; Hata, E.; Kobayashi, H.; Eguchi, M.; Kaneko, J.; Kamio, Y.; Kaidoh, T.; Takeuchi, S. Leukotoxin Family Genes in Staphylococcus aureus Isolated from Domestic Animals and Prevalence of lukM-lukF-PV Genes by Bacteriophages in Bovine Isolates. Vet. Microbiol. 2005, 110, 97–103. [Google Scholar] [CrossRef]
- Rainard, P.; Corrales, J.-C.; Barrio, M.B.; Cochard, T.; Poutrel, B. Leucotoxic Activities of Staphylococcus aureus Strains Isolated from Cows, Ewes, and Goats with Mastitis: Importance of LukM/LukF’-PV Leukotoxin. Clin. Diagn. Lab. Immunol. 2003, 10, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, R.R.; Krömker, V.; Bjarnsholt, T.; Dahl-Pedersen, K.; Buhl, R.; Jørgensen, E. Biofilm Research in Bovine Mastitis. Front. Vet. Sci. 2021, 8, 656810. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, P.; Nair, M.K.M.; Annamalai, T.; Venkitanarayanan, K.S. Phenotypic and Genotypic Characterization of Bovine Mastitis Isolates of Staphylococcus aureus for Biofilm Formation. Vet. Microbiol. 2003, 92, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Cucarella, C.; Tormo, M.A.; Ubeda, C.; Trotonda, M.P.; Monzón, M.; Peris, C.; Amorena, B.; Lasa, I.; Penadés, J.R. Role of Biofilm-Associated Protein Bap in the Pathogenesis of Bovine Staphylococcus aureus. Infect. Immun. 2004, 72, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Taglialegna, A.; Navarro, S.; Ventura, S.; Garnett, J.A.; Matthews, S.; Penades, J.R.; Lasa, I.; Valle, J. Staphylococcal Bap Proteins Build Amyloid Scaffold Biofilm Matrices in Response to Environmental Signals. PLoS Pathog. 2016, 12, e1005711. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Latasa, C.; Gil, C.; Toledo-Arana, A.; Solano, C.; Penadés, J.R.; Lasa, I. Bap, a Biofilm Matrix Protein of Staphylococcus aureus Prevents Cellular Internalization through Binding to GP96 Host Receptor. PLoS Pathog. 2012, 8, e1002843. [Google Scholar] [CrossRef] [PubMed]
- Grunert, T.; Stessl, B.; Wolf, F.; Sordelli, D.O.; Buzzola, F.R.; Ehling-Schulz, M. Distinct Phenotypic Traits of Staphylococcus aureus Are Associated with Persistent, Contagious Bovine Intramammary Infections. Sci. Rep. 2018, 8, 15968. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Bexiga, R.; Nunes, S.F.; Vilela, C.L. Invasive Potential of Biofilm-Forming Staphylococci Bovine Subclinical Mastitis Isolates. J. Vet. Sci. 2011, 12, 95–97. [Google Scholar] [CrossRef]
- Bohl, L.P.; Isaac, P.; Breser, M.L.; Orellano, M.S.; Correa, S.G.; Tolosa de Talamoni, N.G.; Porporatto, C. Interaction between Bovine Mammary Epithelial Cells and Planktonic or Biofilm Staphylococcus aureus: The Bacterial Lifestyle Determines Its Internalization Ability and the Pathogen Recognition. Microb. Pathog. 2021, 152, 104604. [Google Scholar] [CrossRef] [PubMed]
- Buzzola, F.R.; Alvarez, L.P.; Tuchscherr, L.P.N.; Barbagelata, M.S.; Lattar, S.M.; Calvinho, L.; Sordelli, D.O. Differential Abilities of Capsulated and Noncapsulated Staphylococcus aureus Isolates from Diverse Agr Groups to Invade Mammary Epithelial Cells. Infect. Immun. 2007, 75, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Bardiau, M.; Detilleux, J.; Farnir, F.; Mainil, J.G.; Ote, I. Associations between Properties Linked with Persistence in a Collection of Staphylococcus aureus Isolates from Bovine Mastitis. Vet. Microbiol. 2014, 169, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Carra, E.; Russo, S.; Micheli, A.; Garbarino, C.; Ricchi, M.; Bergamini, F.; Bassi, P.; Prosperi, A.; Piva, S.; Cricca, M.; et al. Evidence of Common Isolates of Streptococcus agalactiae in Bovines and Humans in Emilia Romagna Region (Northern Italy). Front. Microbiol. 2021, 12, 673126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, H.; Fan, Y.; Chen, Z.; Li, M.; Mao, Y.; Karrow, N.A.; Loor, J.J.; Moore, S.; Yang, Z. Transcriptomics and iTRAQ-Proteomics Analyses of Bovine Mammary Tissue with Streptococcus agalactiae-Induced Mastitis. J. Agric. Food Chem. 2018, 66, 11188–11196. [Google Scholar] [CrossRef] [PubMed]
- Rosenau, A.; Martins, K.; Amor, S.; Gannier, F.; Lanotte, P.; van der Mee-Marquet, N.; Mereghetti, L.; Quentin, R. Evaluation of the Ability of Streptococcus agalactiae Strains Isolated from Genital and Neonatal Specimens to Bind to Human Fibrinogen and Correlation with Characteristics of the fbsA and fbsB Genes. Infect. Immun. 2007, 75, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, K. A Novel Family of Fibrinogen-Binding Proteins in Streptococcus agalactiae. Vet. Microbiol. 2003, 96, 103–113. [Google Scholar] [CrossRef]
- Devi, A.S.; Ponnuraj, K. Cloning, Expression, Purification and Ligand Binding Studies of Novel Fibrinogen-Binding Protein FbsB of Streptococcus agalactiae. Protein Expr. Purif. 2010, 74, 148–155. [Google Scholar] [CrossRef]
- Lauer, P.; Rinaudo, C.D.; Soriani, M.; Margarit, I.; Maione, D.; Rosini, R.; Taddei, A.R.; Mora, M.; Rappuoli, R.; Grandi, G.; et al. Genome Analysis Reveals Pili in Group B Streptococcus. Science 2005, 309, 105. [Google Scholar] [CrossRef]
- Rosini, R.; Margarit, I. Biofilm Formation by Streptococcus agalactiae: Influence of Environmental Conditions and Implicated Virulence Factors. Front. Cell. Infect. Microbiol. 2015, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.; Bottini, E.; Cadona, J.; Cacciato, C.; Monteavaro, C.; Bustamante, A.; Sanso, A.M. Multidrug Resistance and Molecular Characterization of Streptococcus agalactiae Isolates from Dairy Cattle with Mastitis. Front. Cell. Infect. Microbiol. 2021, 11, 647324. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hu, C.; Gong, R.; Chen, Y.; Ren, N.; Xiao, G.; Xie, Q.; Zhang, M.; Liu, Q.; Guo, A.; et al. Evaluation of a Novel Chimeric B Cell Epitope-Based Vaccine against Mastitis Induced by Either Streptococcus agalactiae or Staphylococcus aureus in Mice. Clin. Vaccine Immunol. 2011, 18, 893–900. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jin, T.; Brefo-Mensah, E.; Fan, W.; Zeng, W.; Li, Y.; Zhang, Y.; Palmer, M. Crystal Structure of the Streptococcus agalactiae CAMP Factor Provides Insights into Its Membrane-Permeabilizing Activity. J. Biol. Chem. 2018, 293, 11867–11877. [Google Scholar] [CrossRef]
- Liu, G.; Yin, J.; Barkema, H.W.; Chen, L.; Shahid, M.; Szenci, O.; De Buck, J.; Kastelic, J.P.; Han, B. Development of a Single-Dose Recombinant CAMP Factor Entrapping Poly(Lactide-Co-Glycolide) Microspheres-Based Vaccine against Streptococcus agalactiae. Vaccine 2017, 35, 1246–1253. [Google Scholar] [CrossRef]
- Fontaine, M.C.; Perez-Casal, J.; Song, X.-M.; Shelford, J.; Willson, P.J.; Potter, A.A. Immunisation of Dairy Cattle with Recombinant Streptococcus uberis GapC or a Chimeric CAMP Antigen Confers Protection against Heterologous Bacterial Challenge. Vaccine 2002, 20, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, C.; Xu, Y.; Liu, G.; Lu, C.; Liu, Y. Two Novel Functions of Hyaluronidase from Streptococcus agalactiae Are Enhanced Intracellular Survival and Inhibition of Proinflammatory Cytokine Expression. Infect. Immun. 2014, 82, 2615–2625. [Google Scholar] [CrossRef]
- Kolar, S.L.; Kyme, P.; Tseng, C.W.; Soliman, A.; Kaplan, A.; Liang, J.; Nizet, V.; Jiang, D.; Murali, R.; Arditi, M.; et al. Group B Streptococcus Evades Host Immunity by Degrading Hyaluronan. Cell Host Microbe 2015, 18, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Razik, K.A.E.-H.; Arafa, A.A.; Fouad, E.A.; Younes, A.M.; Almuzaini, A.M.; Abdou, A.M. Isolation, Identification and Virulence Determinants of Streptococcus agalactiae from Bovine Subclinical Mastitis in Egypt. J. Infect. Dev. Ctries. 2021, 15, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Abureema, S.; Deighton, M.; Mantri, N. A Novel Subtraction Diversity Array Distinguishes between Clinical and Non-Clinical Streptococcus uberis and Identifies Potential Virulence Determinants. Vet. Microbiol. 2019, 237, 108385. [Google Scholar] [CrossRef]
- Tomita, T.; Meehan, B.; Wongkattiya, N.; Malmo, J.; Pullinger, G.; Leigh, J.; Deighton, M. Identification of Streptococcus uberis Multilocus Sequence Types Highly Associated with Mastitis. Appl. Environ. Microbiol. 2008, 74, 114–124. [Google Scholar] [CrossRef]
- Tassi, R.; McNeilly, T.N.; Fitzpatrick, J.L.; Fontaine, M.C.; Reddick, D.; Ramage, C.; Lutton, M.; Schukken, Y.H.; Zadoks, R.N. Strain-Specific Pathogenicity of Putative Host-Adapted and Nonadapted Strains of Streptococcus uberis in Dairy Cattle. J. Dairy Sci. 2013, 96, 5129–5145. [Google Scholar] [CrossRef]
- Lang, P.; Lefébure, T.; Wang, W.; Zadoks, R.N.; Schukken, Y.; Stanhope, M.J. Gene Content Differences across Strains of Streptococcus uberis Identified Using Oligonucleotide Microarray Comparative Genomic Hybridization. Infect. Genet. Evol. 2009, 9, 179–188. [Google Scholar] [CrossRef]
- Abureema, S.; Smooker, P.; Malmo, J.; Deighton, M. Molecular Epidemiology of Recurrent Clinical Mastitis Due to Streptococcus uberis: Evidence of Both an Environmental Source and Recurring Infection with the Same Strain. J. Dairy Sci. 2014, 97, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.H.; Haider, W.; Hill, A.W.; Cook, R.S. Pathologic Findings of Experimentally Induced Streptococcus uberis Infection in the Mammary Gland of Cows. Am. J. Vet. Res. 1994, 55, 1723–1728. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.L.; Ward, P.N.; Rapier, C.D.; Leigh, J.A.; Bowler, L.D. Identification of a Differentially Expressed Oligopeptide Binding Protein (OppA2) in Streptococcus uberis by Representational Difference Analysis of cDNA. J. Bacteriol. 2003, 185, 5210–5219. [Google Scholar] [CrossRef]
- Hossain, M.; Egan, S.A.; Coffey, T.; Ward, P.N.; Wilson, R.; Leigh, J.A.; Emes, R.D. Virulence Related Sequences; Insights Provided by Comparative Genomics of Streptococcus uberis of Differing Virulence. BMC Genom. 2015, 16, 334. [Google Scholar] [CrossRef] [PubMed]
- Superti, F. Lactoferrin from Bovine Milk: A Protective Companion for Life. Nutrients 2020, 12, 2562. [Google Scholar] [CrossRef] [PubMed]
- Collado, R.; Prenafeta, A.; González-González, L.; Pérez-Pons, J.A.; Sitjà, M. Probing Vaccine Antigens against Bovine Mastitis Caused by Streptococcus uberis. Vaccine 2016, 34, 3848–3854. [Google Scholar] [CrossRef]
- Kerro Dego, O.; Almeida, R.A.; Saxton, A.M.; Abdi, R.D.; Ensermu, D.B.; Oliver, S.P. Bovine Intramammary Infection Associated Immunogenic Surface Proteins of Streptococcus uberis. Microb. Pathog. 2018, 115, 304–311. [Google Scholar] [CrossRef]
- Collado, R.; Montbrau, C.; Sitjà, M.; Prenafeta, A. Study of the Efficacy of a Streptococcus uberis Mastitis Vaccine against an Experimental Intramammary Infection with a Heterologous Strain in Dairy Cows. J. Dairy Sci. 2018, 101, 10290–10302. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.N.; Field, T.R.; Ditcham, W.G.; Maguin, E.; Leigh, J.A. Identification and Disruption of Two Discrete Loci Encoding Hyaluronic Acid Capsule Biosynthesis Genes hasA, hasB, and hasC in Streptococcus uberis. Infect. Immun. 2001, 69, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Field, T.R.; Ward, P.N.; Pedersen, L.H.; Leigh, J.A. The Hyaluronic Acid Capsule of Streptococcus uberis Is Not Required for the Development of Infection and Clinical Mastitis. Infect. Immun. 2003, 71, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Srithanasuwan, A.; Pangprasit, N.; Suriyasathaporn, W. Comparison of Virulence Patterns Between Streptococcus uberis Causing Transient and Persistent Intramammary Infection. Front. Vet. Sci. 2022, 9, 806674. [Google Scholar] [CrossRef]
- Kabelitz, T.; Aubry, E.; van Vorst, K.; Amon, T.; Fulde, M. The Role of Streptococcus Spp. in Bovine Mastitis. Microorganisms 2021, 9, 1497. [Google Scholar] [CrossRef]
- Shen, J.; Wu, X.; Yang, Y.; Lv, Y.; Li, X.; Ding, X.; Wang, S.; Yan, Z.; Yan, Y.; Yang, F.; et al. Antimicrobial Resistance and Virulence Factor of Streptococcus Dysgalactiae Isolated from Clinical Bovine Mastitis Cases in Northwest China. Infect. Drug Resist. 2021, 14, 3519–3530. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, Y.; Gao, J.; Zhou, M.; Yang, J.; He, F.; Kastelic, J.P.; Deng, Z.; Han, B. Comparative Genomic Analysis of Streptococcus Dysgalactiae Subspecies Dysgalactiae Isolated From Bovine Mastitis in China. Front. Microbiol. 2021, 12, 751863. [Google Scholar] [CrossRef]
- Kaczorek, E.; Małaczewska, J.; Wójcik, R.; Siwicki, A.K. Biofilm Production and Other Virulence Factors in Streptococcus Spp. Isolated from Clinical Cases of Bovine Mastitis in Poland. BMC Vet. Res. 2017, 13, 398. [Google Scholar] [CrossRef] [PubMed]
- Rato, M.G.; Bexiga, R.; Nunes, S.F.; Vilela, C.L.; Santos-Sanches, I. Human Group A Streptococci Virulence Genes in Bovine Group C Streptococci. Emerg. Infect. Dis. 2010, 16, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Alves-Barroco, C.; Caço, J.; Roma-Rodrigues, C.; Fernandes, A.R.; Bexiga, R.; Oliveira, M.; Chambel, L.; Tenreiro, R.; Mato, R.; Santos-Sanches, I. New Insights on Streptococcus Dysgalactiae Subsp. Dysgalactiae Isolates. Front. Microbiol. 2021, 12, 686413. [Google Scholar] [CrossRef]
- Gao, J.; Barkema, H.W.; Zhang, L.; Liu, G.; Deng, Z.; Cai, L.; Shan, R.; Zhang, S.; Zou, J.; Kastelic, J.P.; et al. Incidence of Clinical Mastitis and Distribution of Pathogens on Large Chinese Dairy Farms. J. Dairy Sci. 2017, 100, 4797–4806. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Shpigel, N.Y.; Elazar, S.; Rosenshine, I. Mammary Pathogenic Escherichia coli. Curr. Opin. Microbiol. 2008, 11, 60–65. [Google Scholar] [CrossRef]
- Kempf, F.; Slugocki, C.; Blum, S.E.; Leitner, G.; Germon, P. Genomic Comparative Study of Bovine Mastitis Escherichia coli. PLoS ONE 2016, 11, e0147954. [Google Scholar] [CrossRef] [PubMed]
- Bihannic, M.; Ghanbarpour, R.; Auvray, F.; Cavalié, L.; Châtre, P.; Boury, M.; Brugère, H.; Madec, J.-Y.; Oswald, E. Identification and Detection of Three New F17 Fimbrial Variants in Escherichia coli Strains Isolated from Cattle. Vet. Res. 2014, 45, 76. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Liu, W.; Liu, Y.; Ali, T.; Chen, W.; Yin, J.; Han, B. Phylogenetic Group, Virulence Factors and Antimicrobial Resistance of Escherichia coli Associated with Bovine Mastitis. Res. Microbiol. 2014, 165, 273–277. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Yin, J.; Deng, Y.; Ali, T.; Zhang, J.; Cheng, J.; Ur Rahman, S.; Gao, J.; Han, B. Cloning, Expression, and Immunogenicity of Fimbrial-F17A Subunit Vaccine against Escherichia coli Isolated from Bovine Mastitis. BioMed Res. Int. 2017, 2017, 3248483. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yang, Y.; Wu, M.; Ma, F.; Xu, Y.; Deng, B.; Zhang, J.; Zhu, G.; Lu, Y. Role of Long Polar Fimbriae Type 1 and 2 in Pathogenesis of Mammary Pathogenic Escherichia coli. J. Dairy Sci. 2021, 104, 8243–8255. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.T.; Orsi, H.; Joaquim, S.F.; Guimarães, F.F.; Lopes, B.C.; Dalanezi, F.M.; Leite, D.S.; Langoni, H.; Pantoja, J.C.F.; Rall, V.L.M.; et al. Short Communication: Investigation of Extra-Intestinal Pathogenic Escherichia coli Virulence Genes, Bacterial Motility, and Multidrug Resistance Pattern of Strains Isolated from Dairy Cows with Different Severity Scores of Clinical Mastitis. J. Dairy Sci. 2020, 103, 3606–3614. [Google Scholar] [CrossRef] [PubMed]
- Dogan, B.; Klaessig, S.; Rishniw, M.; Almeida, R.A.; Oliver, S.P.; Simpson, K.; Schukken, Y.H. Adherent and Invasive Escherichia coli Are Associated with Persistent Bovine Mastitis. Vet. Microbiol. 2006, 116, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Nüesch-Inderbinen, M.; Käppeli, N.; Morach, M.; Eicher, C.; Corti, S.; Stephan, R. Molecular Types, Virulence Profiles and Antimicrobial Resistance of Escherichia coli Causing Bovine Mastitis. Vet. Rec. Open 2019, 6, e000369. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, E.; Mardani, K.; Amiri, A. Molecular Detection and Antimicrobial Resistance Patterns of Shiga Toxigenic Escherichia coli Isolated from Bovine Subclinical Mastitis Milk Samples in Kurdistan, Iran. Arch. Razi Inst. 2020, 75, 169–177. [Google Scholar] [CrossRef]
- Sun, M.; Gao, X.; Zhao, K.; Ma, J.; Yao, H.; Pan, Z. Insight Into the Virulence Related Secretion Systems, Fimbriae, and Toxins in O2:K1 Escherichia coli Isolated From Bovine Mastitis. Front. Vet. Sci. 2021, 8, 622725. [Google Scholar] [CrossRef]
- Lira, W.M.; Macedo, C.; Marin, J.M. The Incidence of Shiga Toxin-Producing Escherichia coli in Cattle with Mastitis in Brazil. J. Appl. Microbiol. 2004, 97, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Bihannic, M.; Haenni, M.; Oswald, E.; Madec, J.-Y. Divergent Evolution of the repFII Replicon of IncF Plasmids Carrying Cytotoxic Necrotizing Factor Cnf2, Cytolethal Distending Toxin cdtIII, and f17Ae Fimbrial Variant Genes in Type 2 Necrotoxigenic Escherichia coli Isolates from Calves. Appl. Environ. Microbiol. 2016, 82, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, C.; Wei, Z.; He, X.; Kou, J.; Zhou, E.; Yang, Z.; Fu, Y. Morin Suppresses Inflammatory Cytokine Expression by Downregulation of Nuclear Factor-κB and Mitogen-Activated Protein Kinase (MAPK) Signaling Pathways in Lipopolysaccharide-Stimulated Primary Bovine Mammary Epithelial Cells. J. Dairy Sci. 2016, 99, 3016–3022. [Google Scholar] [CrossRef] [PubMed]
- Needham, B.D.; Trent, M.S. Fortifying the Barrier: The Impact of Lipid A Remodelling on Bacterial Pathogenesis. Nat. Rev. Microbiol. 2013, 11, 467–481. [Google Scholar] [CrossRef]
- Duda, K.A.; Lindner, B.; Brade, H.; Leimbach, A.; Brzuszkiewicz, E.; Dobrindt, U.; Holst, O. The Lipopolysaccharide of the Mastitis Isolate Escherichia coli Strain 1303 Comprises a Novel O-Antigen and the Rare K-12 Core Type. Microbiology (Reading) 2011, 157, 1750–1760. [Google Scholar] [CrossRef]
- Wenz, J.R.; Barrington, G.M.; Garry, F.B.; Ellis, R.P.; Magnuson, R.J. Escherichia coli Isolates’ Serotypes, Genotypes, and Virulence Genes and Clinical Coliform Mastitis Severity. J. Dairy Sci. 2006, 89, 3408–3412. [Google Scholar] [CrossRef] [PubMed]
- Blum, S.E.; Heller, E.D.; Sela, S.; Elad, D.; Edery, N.; Leitner, G. Genomic and Phenomic Study of Mammary Pathogenic Escherichia coli. PLoS ONE 2015, 10, e0136387. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, R.J.; Harris, S.; Smith, D.G.E. Genomic Content Typifying a Prevalent Clade of Bovine Mastitis-Associated Escherichia coli. Sci. Rep. 2016, 6, 30115. [Google Scholar] [CrossRef]
- Aslam, N.; Khan, S.-U.-H.; Usman, T.; Ali, T. Phylogenetic Genotyping, Virulence Genes and Antimicrobial Susceptibility of Escherichia coli Isolates from Cases of Bovine Mastitis. J. Dairy. Res. 2021, 88, 78–79. [Google Scholar] [CrossRef]
- Olson, M.A.; Siebach, T.W.; Griffitts, J.S.; Wilson, E.; Erickson, D.L. Genome-Wide Identification of Fitness Factors in Mastitis-Associated Escherichia coli. Appl. Environ. Microbiol. 2018, 84, e02190-17. [Google Scholar] [CrossRef]
- Blum, S.E.; Goldstone, R.J.; Connolly, J.P.R.; Répérant-Ferter, M.; Germon, P.; Inglis, N.F.; Krifucks, O.; Mathur, S.; Manson, E.; Mclean, K.; et al. Postgenomics Characterization of an Essential Genetic Determinant of Mammary Pathogenic Escherichia coli. mBio 2018, 9, e00423-18. [Google Scholar] [CrossRef] [PubMed]
- Suojala, L.; Pohjanvirta, T.; Simojoki, H.; Myllyniemi, A.-L.; Pitkälä, A.; Pelkonen, S.; Pyörälä, S. Phylogeny, Virulence Factors and Antimicrobial Susceptibility of Escherichia coli Isolated in Clinical Bovine Mastitis. Vet. Microbiol. 2011, 147, 383–388. [Google Scholar] [CrossRef]
- Schukken, Y.; Chuff, M.; Moroni, P.; Gurjar, A.; Santisteban, C.; Welcome, F.; Zadoks, R. The “Other” Gram-Negative Bacteria in Mastitis: Klebsiella, Serratia, and More. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 239–256. [Google Scholar] [CrossRef]
- Ko, K.S. The Contribution of Capsule Polysaccharide Genes to Virulence of Klebsiella Pneumoniae. Virulence 2017, 8, 485–486. [Google Scholar] [CrossRef]
- Gao, J.; Li, S.; Zhang, J.; Zhou, Y.; Xu, S.; Barkema, H.W.; Nobrega, D.B.; Zhu, C.; Han, B. Prevalence of Potential Virulence Genes in Klebsiella Spp. Isolated from Cows with Clinical Mastitis on Large Chinese Dairy Farms. Foodborne Pathog. Dis. 2019, 16, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhou, M.; Nobrega, D.B.; Cao, Z.; Yang, J.; Zhu, C.; Han, B.; Gao, J. Virulence Profiles of Klebsiella Pneumoniae Isolated from 2 Large Dairy Farms in China. J. Dairy Sci. 2021, 104, 9027–9036. [Google Scholar] [CrossRef] [PubMed]
- Kanevsky-Mullarky, I.; Nedrow, A.J.; Garst, S.; Wark, W.; Dickenson, M.; Petersson-Wolfe, C.S.; Zadoks, R.N. Short Communication: Comparison of Virulence Factors in Klebsiella Pneumoniae Strains Associated with Multiple or Single Cases of Mastitis. J. Dairy Sci. 2014, 97, 2213–2218. [Google Scholar] [CrossRef] [PubMed]
- Struve, C.; Bojer, M.; Krogfelt, K.A. Identification of a Conserved Chromosomal Region Encoding Klebsiella Pneumoniae Type 1 and Type 3 Fimbriae and Assessment of the Role of Fimbriae in Pathogenicity. Infect. Immun. 2009, 77, 5016–5024. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella Pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef]
- Rosen, D.A.; Hilliard, J.K.; Tiemann, K.M.; Todd, E.M.; Morley, S.C.; Hunstad, D.A. Klebsiella Pneumoniae FimK Promotes Virulence in Murine Pneumonia. J. Infect. Dis. 2016, 213, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Kline, K.A.; Fälker, S.; Dahlberg, S.; Normark, S.; Henriques-Normark, B. Bacterial Adhesins in Host-Microbe Interactions. Cell Host Microbe 2009, 5, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Jagnow, J.; Clegg, S. Klebsiella Pneumoniae MrkD-Mediated Biofilm Formation on Extracellular Matrix- and Collagen-Coated Surfaces. Microbiology (Reading) 2003, 149, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Schroll, C.; Barken, K.B.; Krogfelt, K.A.; Struve, C. Role of Type 1 and Type 3 Fimbriae in Klebsiella Pneumoniae Biofilm Formation. BMC Microbiol. 2010, 10, 179. [Google Scholar] [CrossRef]
- Guerra, M.E.S.; Destro, G.; Vieira, B.; Lima, A.S.; Ferraz, L.F.C.; Hakansson, A.P.; Darrieux, M.; Converso, T.R. Klebsiella Pneumoniae Biofilms and Their Role in Disease Pathogenesis. Front. Cell. Infect. Microbiol. 2022, 12, 877995. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Cao, Z.; Cheng, S.; Zhang, B.; Li, X.; Kastelic, J.P.; Xu, C.; Han, B.; Gao, J. Immunoprotective Efficacy of 3 Klebsiella Pneumoniae Type I Fimbriae Proteins in a Murine Model. Vet. Microbiol. 2024, 297, 110197. [Google Scholar] [CrossRef] [PubMed]
- Tsuka, T.; Kumashiro, S.; Kihara, T.; Iida, T. Correlation between Polymerase Chain Reaction Identification of Iron Acquisition Genes and an Iron-Deficient Incubation Test for Klebsiella Pneumoniae Isolates from Bovine Mastitis. Microorganisms 2022, 10, 1138. [Google Scholar] [CrossRef] [PubMed]
- Gorden, P.J.; Kleinhenz, M.D.; Ydstie, J.A.; Brick, T.A.; Slinden, L.M.; Peterson, M.P.; Straub, D.E.; Burkhardt, D.T. Efficacy of Vaccination with a Klebsiella Pneumoniae Siderophore Receptor Protein Vaccine for Reduction of Klebsiella Mastitis in Lactating Cattle. J. Dairy Sci. 2018, 101, 10398–10408. [Google Scholar] [CrossRef] [PubMed]
- Holt, K.E.; Wertheim, H.; Zadoks, R.N.; Baker, S.; Whitehouse, C.A.; Dance, D.; Jenney, A.; Connor, T.R.; Hsu, L.Y.; Severin, J.; et al. Genomic Analysis of Diversity, Population Structure, Virulence, and Antimicrobial Resistance in Klebsiella Pneumoniae, an Urgent Threat to Public Health. Proc. Natl. Acad. Sci. USA 2015, 112, E3574–E3581. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Higgins, C.H.; Rehman, I.; Galvao, K.N.; Brito, I.L.; Bicalho, M.L.; Song, J.; Wang, H.; Bicalho, R.C. Genomic Diversity, Virulence, and Antimicrobial Resistance of Klebsiella Pneumoniae Strains from Cows and Humans. Appl. Environ. Microbiol. 2019, 85, e02654-18. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Gorden, P.J.; Xia, X.; Zheng, Y.; Li, G. Whole-Genome Analysis of Klebsiella Pneumoniae from Bovine Mastitis Milk in the U.S. Environ. Microbiol. 2022, 24, 1183–1199. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, X.; Barkema, H.W.; Nobrega, D.B.; Xu, C.; Han, B.; Zhang, C.; Yang, J.; Li, X.; Gao, J. Virulence of Bacteria Causing Mastitis in Dairy Cows: A Literature Review. Microorganisms 2025, 13, 167. https://doi.org/10.3390/microorganisms13010167
Tong X, Barkema HW, Nobrega DB, Xu C, Han B, Zhang C, Yang J, Li X, Gao J. Virulence of Bacteria Causing Mastitis in Dairy Cows: A Literature Review. Microorganisms. 2025; 13(1):167. https://doi.org/10.3390/microorganisms13010167
Chicago/Turabian StyleTong, Xiaofang, Herman W. Barkema, Diego B. Nobrega, Chuang Xu, Bo Han, Chenyibo Zhang, Jingyue Yang, Xiaoping Li, and Jian Gao. 2025. "Virulence of Bacteria Causing Mastitis in Dairy Cows: A Literature Review" Microorganisms 13, no. 1: 167. https://doi.org/10.3390/microorganisms13010167
APA StyleTong, X., Barkema, H. W., Nobrega, D. B., Xu, C., Han, B., Zhang, C., Yang, J., Li, X., & Gao, J. (2025). Virulence of Bacteria Causing Mastitis in Dairy Cows: A Literature Review. Microorganisms, 13(1), 167. https://doi.org/10.3390/microorganisms13010167






