The Impact of Bacterial Leaf Blight Disease (Pantoea agglomerans) on Grain Yield and Nutritional Quality of Oat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of a Bacterial Suspension
2.3. Seed Treatment and Seedling Cultivation
2.4. Bacterial Inoculation
2.5. Disease Investigation
2.6. Grain Yield and Nutritional Quality Analysis
2.7. Statistical Analysis
3. Results
3.1. Effects of LBD (P. agglomerans) on Average Disease Infection Rate (ADR) and Average Disease Index (ADI)
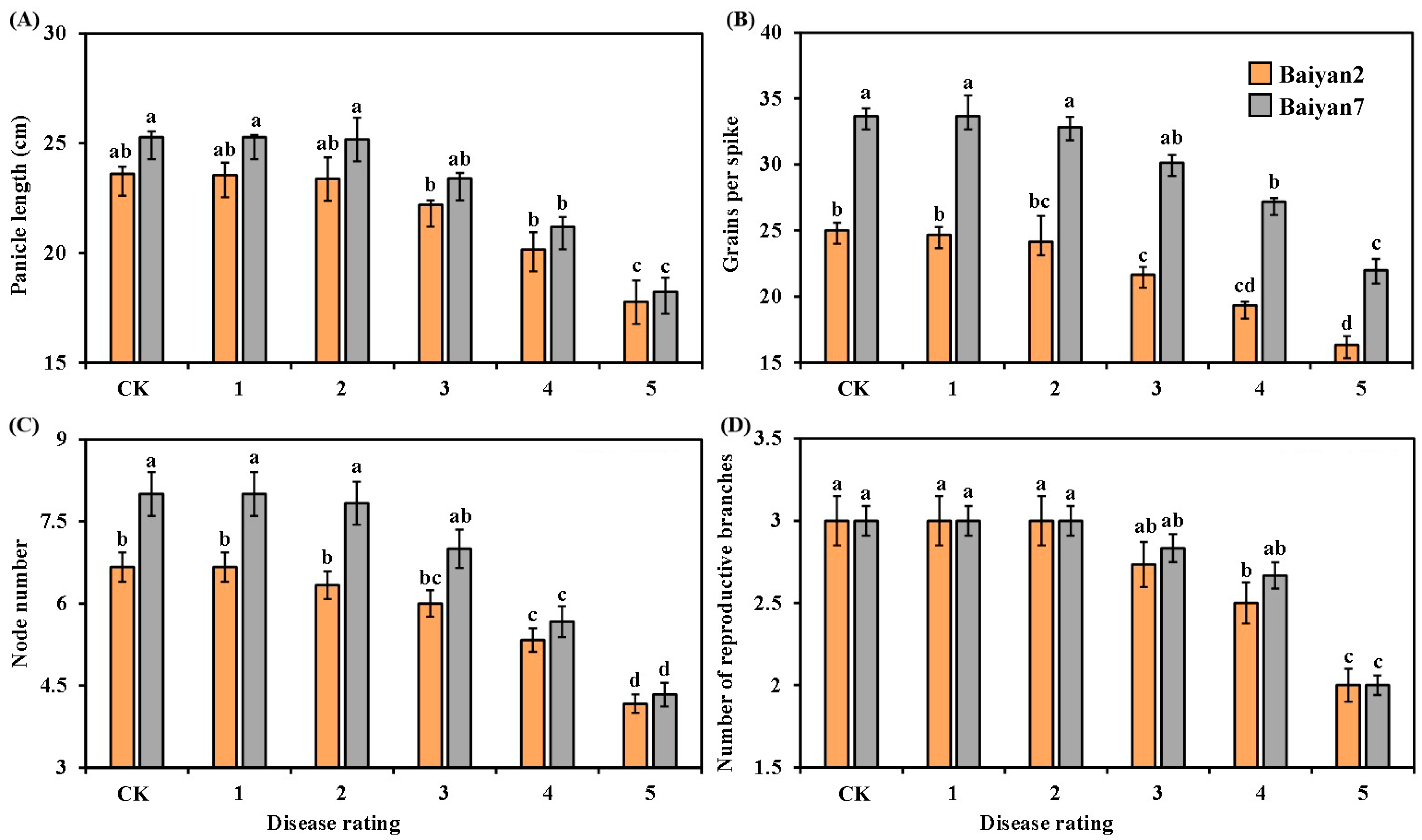
3.2. Effects of LBD (P. agglomerans) on Panicle Length, Grains Per Spike, Node Number and Reproductive Branch Number
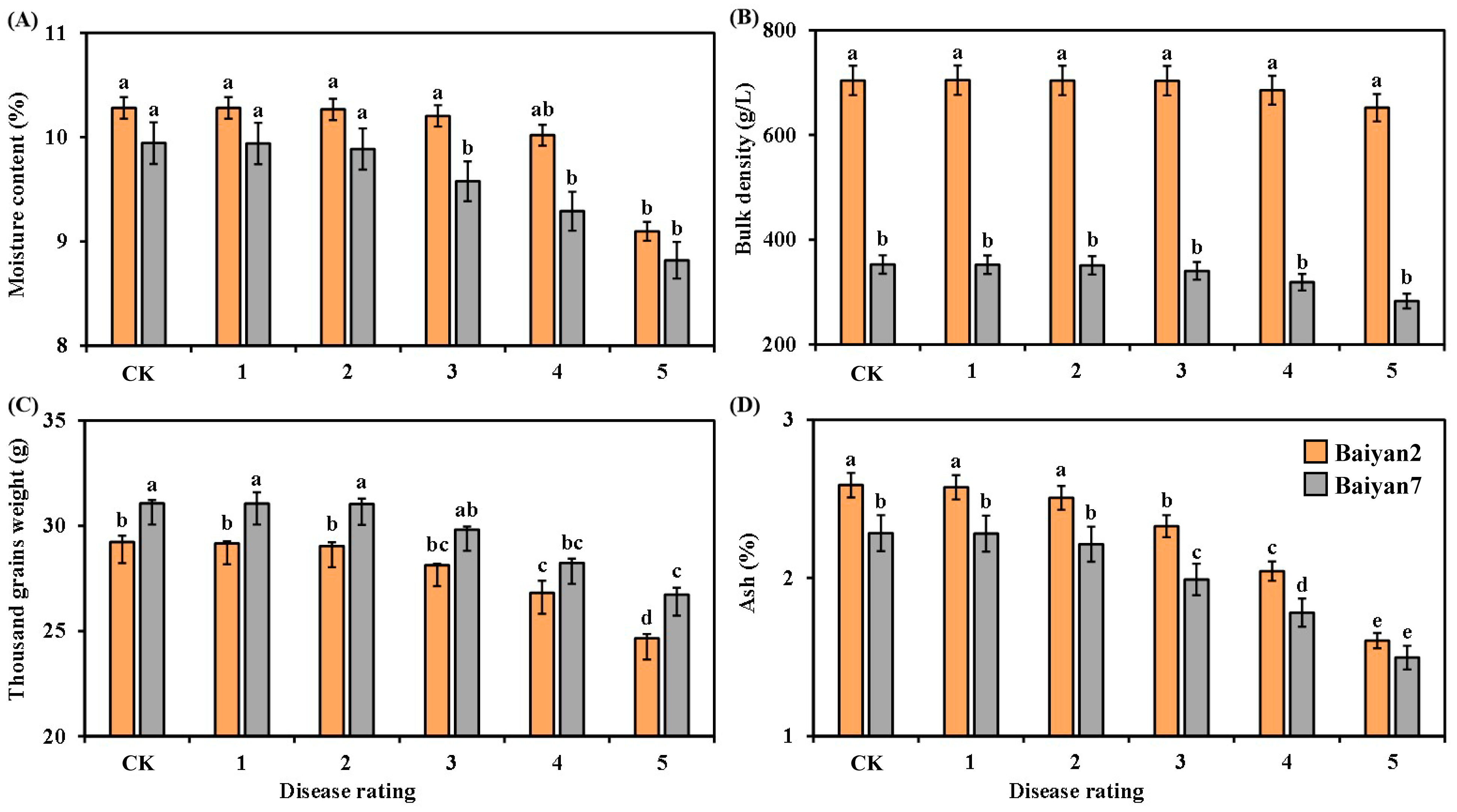
3.3. Effects of LBD (P. agglomerans) on Moisture Content, Bulk Density, Thousand Grain Weight and Ash
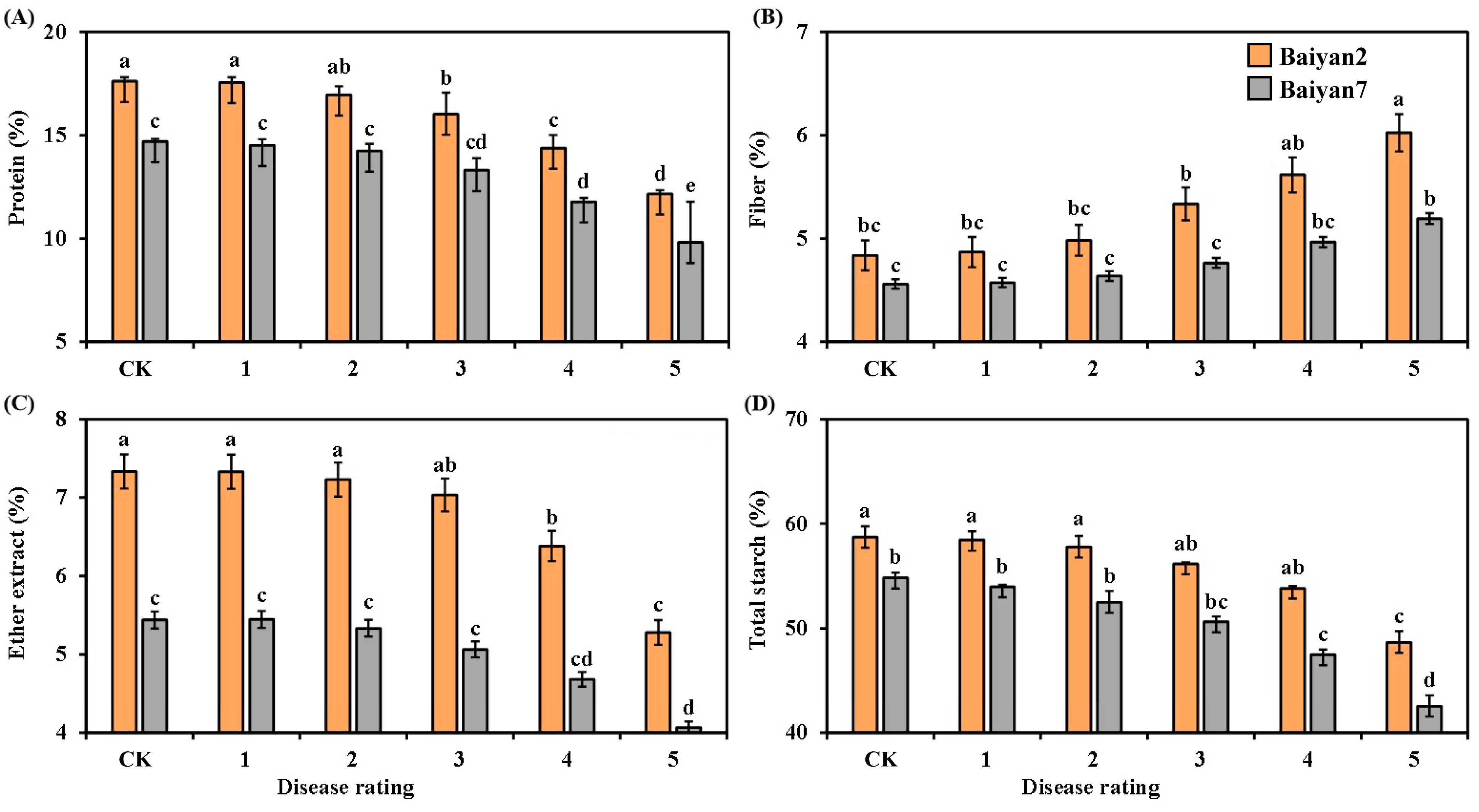
3.4. Effects of LBD (P. agglomerans) on Protein, Fiber, Ether Extract and Total Starch in Oat Seeds
3.5. Effects of LBD (P. agglomerans) on β-Glucan, Phytic Acid, Total Phosphorus and Ca in Oat Seeds
3.6. Correlations of LBD (P. agglomerans) and Oat Variety with Grain Yield and Nutritional Quality of Oat
4. Discussion
4.1. The Grain Yield of Oat Is Decreased by LBD (P. agglomerans)
4.2. The Nutritional Quality of Oat Seeds Is Reduced by LBD (P. agglomerans)
4.3. The Grain Yield and Nutritional Quality of A. sativa and A. nuda Are Different
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marshall, A.; Cowan, S.; Wards, S.; Griffiths, I.; White, E. Crops that feed the world 9. oats-a cereal crop for human and livestock feed with industrial applications. Food Secur. 2013, 5, 13–33. [Google Scholar] [CrossRef]
- Irfan, M.; Ansar, M.; Sher, A.; Wasaya, A.; Sattar, A. Improving forage yield and morphology of oat varieties through various row spacing and nitrogen application. J. Anim. Plant Sci. 2016, 26, 1718–1724. [Google Scholar]
- Maqbool, S.B.; Zhong, H.; Sticklen, M.B. Genetic engineering of oat (Avena sativa L.) via the biolistic bombardment of shoot apical meristems. Transgenic Crops World 2004, 5, 63–78. [Google Scholar]
- Mushtaq, A.; Gul-Zaffar, D.A.; Mehfuza, H. A review on oat (Avena sativa L.) as a dual-purpose crop. Acad. J. 2014, 9, 52–59. [Google Scholar]
- Ki-Seung, K.; Tinker, N.A.; Newell, M.A. Improvement of oat as a winter forage crop in the Southern United States. Crop Sci. 2014, 54, 1336–1346. [Google Scholar]
- Latif, N.; Mansoor, M.; Awan, A.A.; Khan, A.; Jamil, M. A review on multidimensional aspects of oat (Avena sativa) crop and its nutritional, medicinal and daily life importance. World Appl. Sci. J. 2016, 34, 1269–1275. [Google Scholar]
- Rispail, N.; Montilla-Bascón, G.; Sánchez-Martín, J.; Flores, F.; Howarth, C.; Langdon, T.; Rubiales, D.; Prats, E. Multi-environmental trials reveal genetic plasticity of oat agronomic traits associated with climate variable changes. Front. Plant Sci. 2018, 9, 1358. [Google Scholar] [CrossRef]
- Nazareno, E.S.; Li, F.; Smith, M.; Park, R.F.; Kianian, S.F.; Figueroa, M. Puccinia coronata f. sp. avenae: A threat to global oat production. Mol. Plant Pathol. 2017, 19, 1047–1060. [Google Scholar] [CrossRef]
- Niekerk, B.D.; Pretorius, Z.A.; Boshoff, W.H.P. Pathogenic variability of Puccinia coronata f. sp. avenae and P. graminis f. sp. avenae on oat in South Africa. Plant Dis. 2001, 85, 1085–1090. [Google Scholar] [CrossRef]
- Tominaga, T.; Nishiyama, K. Bacterial stripe blight of oats caused by Pseudomonas striafaciens (elliott) starr et burkholder. Jpn. J. Grassl. Sci. 1968, 14, 51–55. [Google Scholar]
- Song, W.Y.; Kim, H.M.; Hwang, C.Y.; Schaad, N.W. Detection of Acidovorax avenae ssp. avenae in rice seeds using Biocccr. J. Phytopathol. 2004, 152, 667–676. [Google Scholar] [CrossRef]
- Menzies, J.G.; Turkington, T.K.; Knox, R.E. Testing for resistance to smut diseases of barley, oats and wheat in Western Canada. Can. J. Plant Pathol. 2009, 31, 265–279. [Google Scholar] [CrossRef]
- Jackson, E.W.; Obert, D.E.; Avant, J.B.; Harrison, S.A.; Bonman, J.M. Quantitative trait loci in the Ogle/Tam O-301 oat mapping population controlling resistance to Puccinia coronata in the field. Phytopathology 2010, 100, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Soovli, P.; Kangor, T.; Tamm, I. The incidence of fungal diseases in oat leaves and yields as affected by fertilizer and chemical inputs in Estonia. Agron. Res. 2010, 8, 475–480. [Google Scholar]
- Aggarwal, R.; Purwar, S.; Kharbikar, L.; Gupta, S. Induction of a wheat β-1,3-glucanase gene during the defense response to Bipolaris sorokiniana. Acta Phytopathol. Entomol. Hung. 2011, 46, 39–47. [Google Scholar] [CrossRef]
- Xue, A.G.; Chen, Y.; Marchand, G.; Wei, G.; Mcelroy, A. Timing of inoculation and Fusarium species affect the severity of fusarium head blight on oat. Can. J. Plant Sci. 2015, 95, 517–524. [Google Scholar] [CrossRef]
- Foresman, B. Molecular Markers Associated with Barley Yellow Dwarf Virus Tolerance in Spring Oat and Their Utilization in Predictive Breeding. Ph.D. Thesis, University of Illinois Urbana-Champaign, Champaign-Urbana, IL, USA, 2016. [Google Scholar]
- Bhardwaj, N.R.; Banyal, D.K.; Roy, A.K. Prediction model for assessing powdery mildew disease in common oat (Avena sativa L.). Crop Prot. 2021, 146, 105677. [Google Scholar] [CrossRef]
- Wang, J.; Chen, T.; Xue, L.; Wei, X.; White, J.F.; Qin, Z.; Li, C. A new bacterial leaf blight disease of oat (Avena sativa) caused by Pantoea agglomerans in China. Plant Pathol. 2022, 71, 470–478. [Google Scholar] [CrossRef]
- Labianca, L.; Montanaro, A.; Turturro, F.; Calderaro, C.; Rretti, F.A. Osteomyelitis caused by Pantoea agglomerans in a closed fracture in a child. Orthopedics 2013, 36, 252–256. [Google Scholar] [CrossRef]
- José, A.G.B.; Cazorla, F.M.; Juan, A.T.; Vicente, A.D. Pantoea agglomerans as a new etiological agent of a bacterial necrotic disease of mango trees. Phytopathology 2018, 109, 17–26. [Google Scholar]
- Tiwari, S.; Beriha, S.S. Pantoea species causing early onset neonatal sepsis: A case report. J. Med. Case Rep. 2015, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, E.M.; Ceyssens, P.J.; Dunon, V.; Ackermann, H.W.; Van, V.J.; Maes, M. Bacteriophages LIMElight and LIMEzero of Pantoea agglomerans, belonging to the “phiKMV-Like viruses”. Appl. Environ. Microbiol. 2011, 77, 3443–3450. [Google Scholar] [CrossRef] [PubMed]
- Medrano, E.G.; Bell, A.A. Role of Pantoea agglomerans in opportunistic bacterial seed and boll rot of cotton (Gossypium hirsutum) grown in the field. J. Appl. Microbiol. 2010, 102, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, R.; Safaie, N.; Shams-Bakhsh, M. Association of Pantoea ananatis and Pantoea agglomerans with leaf spot disease on ornamental plants of Araceae family. Eur. J. Plant Pathol. 2018, 150, 167–178. [Google Scholar] [CrossRef]
- Yao, B.; He, P.F.; Huang, M.; Wu, Y.X.; Li, X.Y.; He, Y.Q. A study on the environmental adaptation of Pantoea agglomerans C3 strain, a causal agent of maize top rot disease. J. Yunnan Agric. Univ. (Nat. Sci.) 2019, 34, 210–215. [Google Scholar]
- Edens, D.G.; Gitaitis, R.D.; Sanders, F.H.; Nischwitz, C. First report of Pantoea agglomerans causing a leaf blight and bulb rot of onions in Georgia. Plant Dis. 2006, 90, 1551. [Google Scholar] [CrossRef]
- Lee, H.B.; Hong, J.P.; Kim, S.B. First report of leaf blight caused by Pantoea agglomerans on rice in Korea. Plant Dis. 2010, 94, 1372. [Google Scholar] [CrossRef]
- Wang, H.; Wang, R.J.; Tian, F.M.; Zhao, S.L.; He, J.J.; Li, Z.H.; Wang, Y.F.; Zhuo, P.Q.; Wang, M.X. Identification and pathogenicity pssay of pathogen of plight disease on walnut trees in Longnan. Fujian J. Agric. Sci. 2016, 31, 1086–1090. [Google Scholar]
- She, X.; Yu, L.; Lan, G.; Tang, Y.; He, Z. Pantoea agglomerans causing blight disease on pepino melon (Solanum muricatum) in China. Crop Prot. 2021, 139, 105385. [Google Scholar] [CrossRef]
- Morales, V.; Silva, R.; Ochoa, M.; Valadez, M.; Farfán, G. First report of Pantoea agglomerans causing leaf blight and vascular wilt in maize and sorghum in Mexico. Plant Dis. 2007, 91, 1365. [Google Scholar] [CrossRef]
- Wang, P.; Souma, K.; Kobayashi, Y.; Iwabuchi, K.; Sato, C.; Masuko, T. Influences of northern leaf blight on corn silage fermentation quality, nutritive value and feed intake by sheep. Anim. Sci. J. 2010, 81, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Suman, A.; Shukla, L.; Marag, P.S.; Verma, P.; Prasad, J.S. Potential use of plant colonizing Pantoea as generic plant growth promoting bacteria for cereal crops. J. Environ. Biol. 2020, 41, 987–994. [Google Scholar] [CrossRef]
- Azad, H.R.; Holmes, G.J.; Cooksey, D.A. A new leaf blotch disease of sudangrass caused by Pantoea ananas and Pantoea stewartii. Plant Dis. 2000, 84, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, Z.; Liu, J.; Cui, H.; Long, J.; Xue, L.; Li, C. Identification, Pathogenicity, and Fungicide Sensitivity of Colletotrichum Species Associated with Anthracnose on Italian Ryegrass in Southwestern China. Plant Dis. 2024, 108, 3540–3549. [Google Scholar] [CrossRef]
- Wang, J.; Chen, T.; Wei, X.; Kamran, M.; White, J.; Li, C. Photosynthetic responses of oat to leaf blight disease caused by Pantoea agglomerans. J. Plant Pathol. 2022, 104, 721–733. [Google Scholar] [CrossRef]
- Ashfaq, M.; Rizwan, M.; Rashid, A.; Akhter, M.; Khan, F.; Ali, M.; Chattha, M.B.; Sujjad, M.M.; Mubashar, U. Disease response of exotic rice genotypes against bacterial leaf blight and its effect on various morphological traits. Pak. J. Phytopathol. 2016, 28, 269–274. [Google Scholar]
- Toledo, M.Z.; Castro, G.S.A.; Crusciol, C.A.C.; Soratto, R.P.; Cavariani, C.; Ishizuka, M.S. Silicon leaf application and physiological quality of white oat and wheat seeds. Semin. Ciênc. Agrár. 2012, 33, 1693–1701. [Google Scholar] [CrossRef]
- Asghari, M.J.; Karimi, E.; Mousavi, S.B. Tillage effects on wheat yield and soil water content and bulk density in dryland wheat fallow rotation, in Maragheh. J. Sci. Technol. Agric. Nat. Resour. 2012, 16, 119–128. [Google Scholar]
- Tian, L.H.; Bell, L.W.; Shen, Y.Y.; Whish, J.P.M. Dual-purpose use of winter wheat in western china: Cutting time and nitrogen application effects on phenology, forage production, and grain yield. Crop Pasture Sci. 2012, 63, 520–528. [Google Scholar] [CrossRef]
- Zakirullah, M.; Ali, N.; Jan, T.; Aka Khil, H.; Ikramullah, M. Effect of different nitrogen levels and cutting stages on crude protein, crude fiber, dry matter and green fodder yield of oat (Avena sativa L.). Pure Appl. Biol. 2017, 6, 448–453. [Google Scholar] [CrossRef]
- Chen, L.; Guo, G.; Yu, C.; Zhang, J.; Shimojo, M.; Shao, T. The effects of replacement of whole-plant corn with oat and common vetch on the fermentation quality, chemical composition and aerobic stability of total mixed ration silage in Tibet. Anim. Sci. J. 2015, 86, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Al-Attar, H. Effect of drying method on rheological, thermal, and structural properties of chestnut flour doughs. Food Hydrocoll. 2015, 51, 76–87. [Google Scholar] [CrossRef]
- Leandro, D.C.O.; Maurício, O.; Meneghetti, V.L.; Mazzutti, S.; Gutkoski, L.C. Effect of drying temperature on quality of β-glucan in white oat grains. Cienc. Tecnol. Aliment. 2012, 32, 775–783. [Google Scholar]
- Li, G.R. Study on Effect Factors of Grain Phytic Acid Content and Physiological Mechanism in Oat. Ph.D. Thesis, China Agricultural University, Beijing, China, 2007. [Google Scholar]
- Rajarajeswari, N.; Muralidharan, K. Assessments of farm yield and district production loss from bacterial leaf blight epidemics in rice. Crop Prot. 2006, 25, 244–252. [Google Scholar] [CrossRef]
- Noh, T.H.; Lee, D.K.; Park, J.C.; Shim, H.K.; Kim, J.D. Effects of bacterial leaf blight occurrence on rice yield and grain quality in different rice growth stage. Res. Plant Dis. 2007, 13, 20–23. [Google Scholar] [CrossRef]
- Boersma, J.G.; Hou, A.; Gillard, C.L.; Mcrae, K.B.; Conner, R.L. Impact of common bacterial blight on the yield, seed weight and seed discoloration of different market classes of dry beans (Phaseolus vulgaris L.). Can. J. Plant Sci. 2015, 95, 703–710. [Google Scholar] [CrossRef]
- Salgado, J.D.; Madden, L.V.; Paul, P.A. Quantifying the effects of fusarium head blight on grain yield and test weight in soft red winter wheat. Phytopathology 2015, 105, 295–306. [Google Scholar] [CrossRef]
- Arazi, T.; Slutsky, S.G.; Shiboleth, Y.M.; Wang, Y.; Rubinstein, M.; Barak, S.; Yang, J.; Gal-On, A. Engineering zucchini yellow mosaic potyvirus as a non-pathogenic vector for expression of heterologous proteins in cucurbits. J. Biotechnol. 2001, 87, 67–82. [Google Scholar] [CrossRef]
- Drew, H.; Eric, J.D.; Susan, M.; Eugene, R.; Laurie, R.; Garriet, S.; Ernesto, W.; Bette, W. Coral disease, environmental drivers, and the balance between coral and microbial associates. Oceanography 2007, 20, 58–81. [Google Scholar]
- Mistry, P.M.; Patel, V.J.; Desai, N.M.; Chaudhari, M.H. Relative heterosis and heterobeltiosis for grain yield and yield attributing traits in rice (Oryza sativa L.). Trends Biosci. 2015, 8, 74–81. [Google Scholar]
- Ning, N.; Yuan, X.; Dong, S.; Wen, Y.; Gao, Z.; Guo, M.; Guo, P. Grain yield and quality of foxtail millet (Setaria italica L.) in response to tribenuron-methyl. PLoS ONE 2015, 10, e0142557. [Google Scholar] [CrossRef] [PubMed]
- Herawati, R.; Masdar, M.; Alnopri. Genetic analysis of grain yield of F4 populations for developing new type of upland rice. SABRAO J. Breed. Genet. 2019, 51, 68–79. [Google Scholar]
- Zarbafi, S.S.; Rabiei, B.; Ebadi, A.A.; Ham, J.H. Statistical analysis of phenotypic traits of rice (Oryza sativa L.) related to grain yield under neck blast disease. J. Plant Dis. Prot. 2019, 126, 293–306. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Aminuzzaman, F.M.; Islam, M.R.; Zaman, R. Effect of different levels of seed infection by Bipolaris sorokiniana on leaf blight severity, grain formation, yield and subsequent seed infection of wheat. Int. J. Agric. Enviornment Biotechnol. 2010, 3, 219–224. [Google Scholar]
- Lalitha, D.G.; Pranitha, K.; Vinay, S.; Lalitha, S.M. Making an Indian traditional rice variety mahsuri, bacterial blight resistant using marker-assisted selection. J. Crop Sci. Biotechnol. 2013, 16, 111–121. [Google Scholar]
- Spanic, V.; Dvojkovic, K.; Babic, J.; Drezner, G.; Zdunic, Z. Fusarium head blight infestation in relation to winter wheat end-use quality—A three-year study. Agronomy 2021, 11, 1648. [Google Scholar] [CrossRef]
- Camaille, M.; Fabre, N.; Clément, C.; Ait Barka, E. Advances in Wheat Physiology in Response to Drought and the Role of Plant Growth Promoting Rhizobacteria to Trigger Drought Tolerance. Microorganisms 2021, 9, 687. [Google Scholar] [CrossRef]
- Timper, P.; Wilson, J.P.; Johnson, A.W.; Hanna, W.W. Evaluation of pearl millet grain hybrids for resistance to Meloidogyne spp. and leaf blight caused by Pyricularia grisea. Plant Dis. 2002, 86, 909–914. [Google Scholar] [CrossRef]
- Chang, S.W.; Hwang, B.K. Relationship of host genotype to Bipolaris leaf blight severities and yield components of adlay. Plant Dis. 2002, 86, 774–779. [Google Scholar] [CrossRef]
- Spyridon, M.; Conley, S.P.; Gaska, J.M. Agronomic management and fungicide effects on oat yield and quality. Crop Sci. 2014, 55, 1290. [Google Scholar]
- Bai, X.; Zhang, M.L.; Zhang, Y.; Zhang, J.; Liu, R. Effects of steaming, microwaving, and hot-air drying on the physicochemical properties and storage stability of oat bran. J. Food Qual. 2021, 240, 4058645. [Google Scholar] [CrossRef]
- Sawan, Z.M. Direct and residual effects of plant nutrition’s and plant growth retardants, on cotton seed. Agric. Sci. 2013, 4, 66–88. [Google Scholar]
- Derek, S.; Gordon, M.D. Oat agriculture, cultivation and breeding targets: Implications for human nutrition and health. Br. J. Nutr. 2014, 112, S50–S57. [Google Scholar]
- Long, J.; Holland, J.B.; Munkvold, G.P.; Jannink, J.L. Responses to selection for partial resistance to crown rust in oat. Crop Sci. 2006, 46, 1260–1265. [Google Scholar] [CrossRef]
- Potter, L.R. Interaction between barley yellow dwarf virus and rust in wheat, barley and oats, and the effects on grain yield and quality. Ann. Appl. Biol. 2010, 100, 321–329. [Google Scholar] [CrossRef]
- Boyacioglu, D.; Hettiarachchy, N.S. Changes in some biochemical components of wheat grain that was infected with Fusarium graminearum. J. Cereal Sci. 1995, 21, 57–62. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Kumar, R.; Sharma, S.; Sagar, V.; Kumar, A.; Singh, B.; Aggarwal, R. Impact of Fusarium infection on potato quality, starch digestibility, in vitro glycemic response, and resistant starch content. J. Fungi 2023, 9, 466. [Google Scholar] [CrossRef]
- Michaela, H.; Andrea, H.; Alžbeta, Ž.; Peter, K.; Daniela, D.; Ľubomíra, D. Effect of fertilization on β-d-glucan content in oat grain (Avena sativa L.). Agriculture 2013, 59, 111. [Google Scholar]
- Li, X.R.; Wang, S.X.; Yao, Y.; Yun, T.T.; Liu, S.; Ren, G.X.; Qi, W.T. A comparative analysis of nutrition components and functional active ingredients in Avena nuda and Avena sativa. Sci. Technol. Cereals Oils Foods 2015, 5, 50–54. [Google Scholar]
- Liu, H.; Mu, P.; Zhao, G.Q.; Zhou, X.R. The impact of herbicides concentrations on photosynthetic characters and production of covered and naked oat. Agric. Res. Arid. Areas 2017, 35, 124–133. [Google Scholar]
- Ma, X.Q.; Zhao, G.Q.; Gong, J.J. Effect of sowing date and nitrogen fertilizer on seed yield and its components of oats in alpine area. Pratacultural Sci. 2010, 27, 88–92. [Google Scholar]
- Ma, B.L.; Biswas, D.K.; Zhou, Q.P.; Ren, C.Z. Comparisons among cultivars of wheat, hulled and hulless oats: Effects of N fertilization on growth and yield. Can. J. Plant Sci. 2012, 92, 1213–1222. [Google Scholar] [CrossRef]
- Li, G.R.; Zhao, B.P.; Hu, Y.G.; Cheng, F.M.; Zeng, Z.H.; Zhao, N.C. Effect of irrigation regimes on phytic acid, protein, and mineral element contents in two oat cultivars. Acta Agron. Sin. 2007, 26, 866–870. [Google Scholar]
- Zhang, M. Study on quality and chromatographic fingerprint characteristics of oat in Inner Mongolia. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2014. [Google Scholar]
- Wang, J.; Chen, T.; Wei, X.; Kamran, M.; White, J.; Zhao, G.; Li, C. Evaluation of different antimicrobial agents for laboratory and field against pantoea agglomerans, the causative agent of bacterial leaf blight disease on oat (Avena sativa). Plant Pathol. 2023, 72, 1585–1594. [Google Scholar] [CrossRef]
- Rempelos, L.; Barański, M.; Sufar, E.K.; Gilroy, J.; Shotton, P.; Leifert, H.; Średnicka-Tober, D.; Hasanaliyeva, G.; Rosa, E.A.S.; Hajslova, J.; et al. Effect of Climatic Conditions, and Agronomic Practices Used in Organic and Conventional Crop Production on Yield and Nutritional Composition Parameters in Potato, Cabbage, Lettuce and Onion; Results from the Long-Term NFSC-Trials. Agronomy 2023, 13, 1225. [Google Scholar] [CrossRef]



| Disease Rating | Rating Criteria |
|---|---|
| grade 0 | Asymptomatic |
| grade 1 | Lesion length 2–3 cm |
| grade 2 | Lesion length less than 1/4 of leaf length |
| grade 3 | Lesion length more than 1/4 of leaf length, but less than 1/2 |
| grade 4 | Lesion length more than 1/2 of leaf length, but less than 3/4 |
| grade 5 | Lesion length less than 3/4 of leaf length |
| Source | df | Panicle Length | Grains per Spike | Node Number | Number of Reproductive Branches | ||||
|---|---|---|---|---|---|---|---|---|---|
| F-Value | p | F-Value | p | F-Value | p | F-Value | p | ||
| V | 1 | 196.983 | <0.001 | 172.456 | <0.001 | 26.691 | <0.001 | 0.131 | 0.002 |
| D | 5 | 56.751 | <0.001 | 37.327 | <0.001 | 30.422 | <0.001 | 17.767 | <0.001 |
| V×D | 5 | 7.786 | <0.001 | 1.052 | 0.436 | 1.082 | 0.415 | 9.575 | <0.001 |
| Source | df | Moisture Content | Bulk Density | Thousand Grain Weight | Ash | ||||
|---|---|---|---|---|---|---|---|---|---|
| F-Value | p | F-Value | p | F-Value | p | F-Value | p | ||
| V | 1 | 38.867 | <0.001 | 191.286 | <0.001 | 87.710 | <0.001 | 5.725 | 0.022 |
| D | 5 | 4.099 | 0.006 | 0.017 | 1.000 | 2.295 | 0.007 | 31.320 | <0.001 |
| V×D | 5 | 7.447 | <0.001 | 21.297 | <0.001 | 2.653 | 0.022 | 2.459 | 0.032 |
| Source | df | Protein | Fiber | Ether Extract | Total Starch | ||||
|---|---|---|---|---|---|---|---|---|---|
| F-Value | p | F-Value | p | F-Value | p | F-Value | p | ||
| V | 1 | 24.479 | <0.001 | 17.331 | <0.001 | 67.439 | <0.001 | 35.134 | <0.001 |
| D | 5 | 198.774 | <0.001 | 57.843 | <0.001 | 2.769 | 0.036 | 206.821 | <0.001 |
| V × D | 5 | 9.097 | <0.001 | 6.760 | <0.001 | 24.699 | <0.001 | 30.135 | <0.001 |
| Source | df | β-Glucan | Phytic Acid | Total Phosphorus | Ca | ||||
|---|---|---|---|---|---|---|---|---|---|
| F-Value | p | F-Value | p | F-Value | p | F-Value | p | ||
| V | 1 | 365.654 | <0.001 | 6.169 | 0.018 | 2.687 | 0.110 | 288.485 | <0.001 |
| D | 5 | 21.780 | <0.001 | 30.869 | <0.001 | 67.975 | <0.001 | 18.755 | <0.001 |
| V×D | 5 | 1.748 | <0.001 | 1.748 | 0.122 | 1.081 | 0.415 | 0.623 | 0.792 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Wang, J.; Xue, L.; Kamran, M.; Wang, Y.; Wei, X.; Zhao, G.; Li, C. The Impact of Bacterial Leaf Blight Disease (Pantoea agglomerans) on Grain Yield and Nutritional Quality of Oat. Microorganisms 2025, 13, 141. https://doi.org/10.3390/microorganisms13010141
Zhang R, Wang J, Xue L, Kamran M, Wang Y, Wei X, Zhao G, Li C. The Impact of Bacterial Leaf Blight Disease (Pantoea agglomerans) on Grain Yield and Nutritional Quality of Oat. Microorganisms. 2025; 13(1):141. https://doi.org/10.3390/microorganisms13010141
Chicago/Turabian StyleZhang, Ruochen, Jianjun Wang, Longhai Xue, Malik Kamran, Yue Wang, Xuekai Wei, Guiqin Zhao, and Chunjie Li. 2025. "The Impact of Bacterial Leaf Blight Disease (Pantoea agglomerans) on Grain Yield and Nutritional Quality of Oat" Microorganisms 13, no. 1: 141. https://doi.org/10.3390/microorganisms13010141
APA StyleZhang, R., Wang, J., Xue, L., Kamran, M., Wang, Y., Wei, X., Zhao, G., & Li, C. (2025). The Impact of Bacterial Leaf Blight Disease (Pantoea agglomerans) on Grain Yield and Nutritional Quality of Oat. Microorganisms, 13(1), 141. https://doi.org/10.3390/microorganisms13010141







