Circulation of Respiratory Syncytial Virus (RSV) in Poland Between Seasons of 2009/2010 and 2022/2023 Based on SENTINEL System
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Material and Surveillance System
2.2. Retrospective Data
2.3. Samples Testing Methodology
2.4. Statistical Analysis
2.5. Limitations of the Study
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. 2021. Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/diseases/respiratory-syncytial-virus-(rsv) (accessed on 19 July 2021).
- Curns, A.T.; Rha, B.; Lively, J.Y.; Sahni, L.C.; Englund, J.A.; Weinberg, G.A.; Halasa, N.B.; Staat, M.A.; Selvarangan, R.; Michaels, M.; et al. Respiratory Syncytial Virus-Associated Hospitalizations Among Children < 5 Years Old: 2016 to 2020. Pediatrics 2024, 153, e2023062574. [Google Scholar] [CrossRef] [PubMed]
- Scheltema, N.M.; Gentile, A.; Lucion, F.; Nokes, D.J.; Munywoki, P.K.; Madhi, S.A.; Groome, M.J.; Cohen, C.; Moyes, J.; Thorburn, K.; et al. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): A retrospective case series. Lancet Glob. Health 2017, 5, e984–e991, Erratum in Lancet Glob. Health 2017, 5, e1190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carvajal, J.J.; Avellaneda, A.M.; Salazar-Ardiles, C.; Maya, J.E.; Kalergis, A.M.; Lay, M.K. Host Components Contributing to Respiratory Syncytial Virus Pathogenesis. Front. Immunol. 2019, 10, 2152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eiland, L.S. Respiratory syncytial virus: Diagnosis, treatment and prevention. J. Pediatr. Pharmacol. Ther. 2009, 14, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Walsh, E.E. Respiratory syncytial virus infection in adults. Clin. Microbiol. Rev. 2000, 13, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.H.; Ison, M.G. Respiratory syncytial virus infection in adults. BMJ 2019, 366, l5021. [Google Scholar] [CrossRef] [PubMed]
- Cegielska, K.; Pogonowska, M.; Kalicki, B. Analysis of respiratory syncytial virus infections in children up to 24 months old, hospitalized in the Department of Pediatrics, Pediatric Nephrology and Allergology of the Military Institute of Medicine between 2016 and 2017. Pediatr. Med. Rodz. 2018, 14, 69–77. [Google Scholar] [CrossRef]
- Falsey, A.R.; McElhaney, J.E.; Beran, J.; van Essen, G.A.; Duval, X.; Esen, M.; Galtier, F.; Gervais, P.; Hwang, S.-J.; Kremsner, P.; et al. Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza-like illness. J. Infect. Dis. 2014, 209, 1873–1881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thorburn, K. Pre-existing disease is associated with a significantly higher risk of death in severe respiratory syncytial virus infection. Arch. Dis Child. 2009, 94, 99–103. [Google Scholar] [CrossRef]
- Welliver, R.C. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J. Pediatr. 2003, 143 (Suppl. S5), S112–S117. [Google Scholar] [CrossRef] [PubMed]
- Popow-Kraupp, T.; Aberle, J.H. Diagnosis of respiratory syncytial virus infection. Open Microbiol. J. 2011, 5, 128–134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barenfanger, J.; Drake, C.; Leon, N.; Mueller, T.; Troutt, T. Clinical and financial benefits of rapid detection of respiratory viruses: An outcomes study. J. Clin. Microbiol. 2000, 38, 2824–2828. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, K.H.; Peiris, J.S.; Lim, W.; Nicholls, J.M.; Chiu, S.S. Comparison of nasopharyngeal flocked swabs and aspirates for rapid diagnosis of respiratory viruses in children. J. Clin. Virol. 2008, 42, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, P.; Denham, J.; Assous, J.; Hughes, C. RSV testing in bronchiolitis: Which nasal sampling method is best? Arch. Dis. Child. 2005, 90, 634–635. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, T.; Marttila, J.; Salmi, A.A.; Ruuskanen, O. Nasal swab versus nasopharyngeal aspirate for isolation of respiratory viruses. J. Clin. Microbiol. 2002, 40, 4337–4339. [Google Scholar] [CrossRef] [PubMed]
- Somerville, L.K.; Ratnamohan, V.M.; Dwyer, D.E.; Kok, J. Molecular diagnosis of respiratory viruses. Pathology 2015, 47, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Teirlinck, A.C.; Broberg, E.K.; Berg, A.S.; Campbell, H.; Reeves, R.M.; Carnahan, A.; Lina, B.; Pakarna, G.; Bøås, H.; Nohynek, H.; et al. Recommendations for respiratory syncytial virus surveillance at national level. Eur. Respir. J. 2021, 22, 2003766. [Google Scholar] [CrossRef] [PubMed]
- Obando-Pacheco, P.; Justicia-Grande, A.J.; Rivero-Calle, I.; Rodríguez-Tenreiro, C.; Sly, P.; Ramilo, O.; Mejías, A.; Baraldi, E.; Papadopoulos, N.G.; Nair, H.; et al. Respiratory Syncytial Virus Seasonality: A Global Overview. J. Infect. Dis. 2018, 217, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Bednarska, K.; Hallmann-Szelińska, E.; Kondratiuk, K.; Brydak, L.B. Nadzór nad grypą [Influenza surveillance]. Postepy. Hig. Med. Dosw. 2016, 70, 313–318. (In Polish) [Google Scholar] [CrossRef] [PubMed]
- Bednarska, K.; Hallmann-Szelińska, E.; Kondratiuk, K.; Rabczenko, D.; Brydak, L.B. Novelties in influenza surveillance in Poland. Probl. Hig. Epidemiol. 2016, 97, 101–105. (In Polish) [Google Scholar]
- WHO. 2024. Available online: https://www.who.int/teams/global-influenza-programme/surveillance-and-monitoring/influenza-surveillance-outputs (accessed on 14 August 2024).
- Trigueros Montes, J.B.; Montes, D.; Miele, A.; Baik-Han, W.; Gulati, G.; Lew, L.Q. The Impact of COVID-19 Pandemic on Respiratory Syncytial Virus Infection in Children. Pulm. Med. 2024, 2024, 2131098. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dalena, P.; Zago, A.; Troisi, A.; Trobia, G.L.; Lucarelli, A.; Bressan, S.; Fasoli, S.; Martelossi, S.; Lubrano, R.; Parrino, R.; et al. Lesson in understanding parents’ perspective: Perception of quality of care and COVID-19-related fears among users of paediatric health services over the COVID-19 pandemic in 11 facilities in Italy. BMJ Paediatr. Open 2024, 8, e002926. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cong, B.; Koç, U.; Bandeira, T.; Bassat, Q.; Bont, L.; Chakhunashvili, G.; Cohen, C.; Desnoyers, C.; Hammitt, L.L.; Heikkinen, T.; et al. Changes in the global hospitalisation burden of respiratory syncytial virus in young children during the COVID-19 pandemic: A systematic analysis. Lancet Infect. Dis. 2024, 24, 361–374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Masoom, S.I.; Aloisio, G.; Camp, E.A.; Dunn, J.J.; Meskill, S.D. Characterizing respiratory syncytial virus (RSV) infections before and during the COVID-19 pandemic. Am. J. Emerg Med. 2025, 87, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Meslé, M.M.I.; Sinnathamby, M.; Mook, P.; WHO European Region Respiratory Network Group; Pebody, R.; Lakhani, A.; Zambon, M.; Popovici, O.; Lazăr, M.; Ljubović, A.D.; et al. Seasonal and inter-seasonal RSV activity in the European Region during the COVID-19 pandemic from autumn 2020 to summer 2022. Influenza Other Respir Viruses 2023, 17, e13219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mazela, J.; Jackowska, T.; Czech, M.; Helwich, E.; Martyn, O.; Aleksiejuk, P.; Smaga, A.; Glazewska, J.; Wysocki, J. Epidemiology of Respiratory Syncytial Virus Hospitalizations in Poland: An Analysis from 2015 to 2023 Covering the Entire Polish Population of Children Aged under Five Years. Viruses 2024, 16, 704. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- CDC. 2024. Available online: https://www.cdc.gov/mmwr/volumes/72/wr/mm7214a1.htm (accessed on 14 August 2024).
- Polish Ministry of Health Online Portal: E-Zdrowie. 2024. Available online: https://ezdrowie.gov.pl/15131 (accessed on 11 April 2024).
- Sadkowska-Todys, M.; Zieliński, A.; Czarkowski, M.P. Choroby zakaźne w Polsce w 2017 roku. [Infectious diseases in Poland in 2017] Przegląd Epidemiologiczny. Epidemiol. Rev. 2019, 73, 135-15. [Google Scholar] [CrossRef]
- WHO. 2024. Available online: https://www.who.int/teams/global-influenza-programme/global-respiratory-syncytial-virus-surveillance (accessed on 27 March 2024).
- ECDC. Operational Considerations for Respiratory Virus Surveillance in Europe; WHO Regional Office for Europe and Stockholm: Copenhagen, Denmark; European Centre for Disease Prevention and Control: Solna, Sweden, 2024; Available online: https://www.ecdc.europa.eu/en/publications-data/operational-considerations-respiratory-virus-surveillance-europe (accessed on 27 March 2024).
- WHO. Influenza Surveillance Outputs. 2024. Available online: https://worldhealthorg.shinyapps.io/flunetchart/ (accessed on 2 January 2025).
- Europe Medicines Agency. 2024. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/arexvy (accessed on 3 December 2024).
- Europe Medicines Agency. 2024. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/abrysvo (accessed on 3 December 2024).
- Europe Medicines Agency. 2024. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/synagis (accessed on 18 December 2024).
- Europe Medicines Agency. 2024. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/beyfortus (accessed on 18 December 2024).
- Centers for Disease Control and Prevention (CDC). Available online: https://www.cdc.gov/rsv/hcp/vaccine-clinical-guidance/infants-young-children.html (accessed on 18 December 2024).
- Assad, Z.; Romain, A.S.; Aupiais, C.; Shum, M.; Schrimpf, C.; Lorrot, M.; Corvol, H.; Prevost, B.; Ferrandiz, C.; Giolito, A.; et al. Nirsevimab and Hospitalization for RSV Bronchiolitis. N. Engl. J. Med. 2024, 391, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Brault, A.; Pontais, I.; Enouf, V.; Debeuret, C.; Bloch, E.; Paireau, J.; Rameix-Welti, M.A.; White, M.; Baudemont, G.; Lina, B.; et al. Effect of nirsevimab on hospitalisations for respiratory syncytial virus bronchiolitis in France, 2023–2024: A modelling study. Lancet Child Adolesc. Health 2024, 8, 721–729. [Google Scholar] [CrossRef] [PubMed]
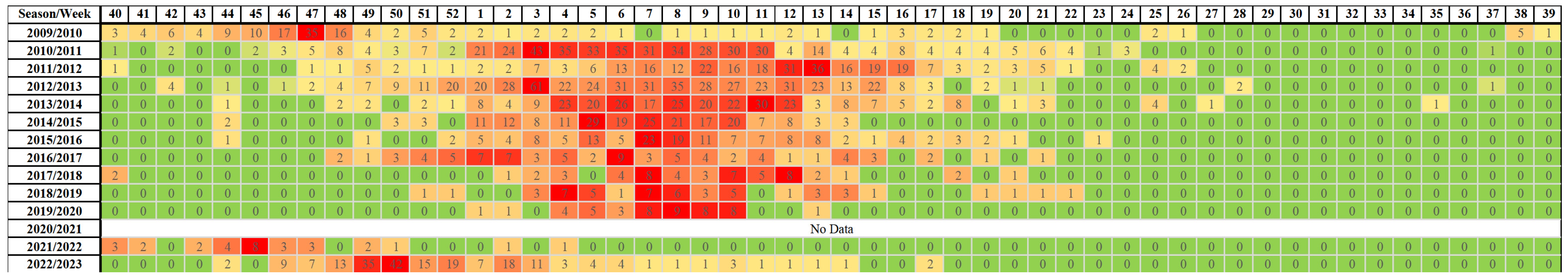
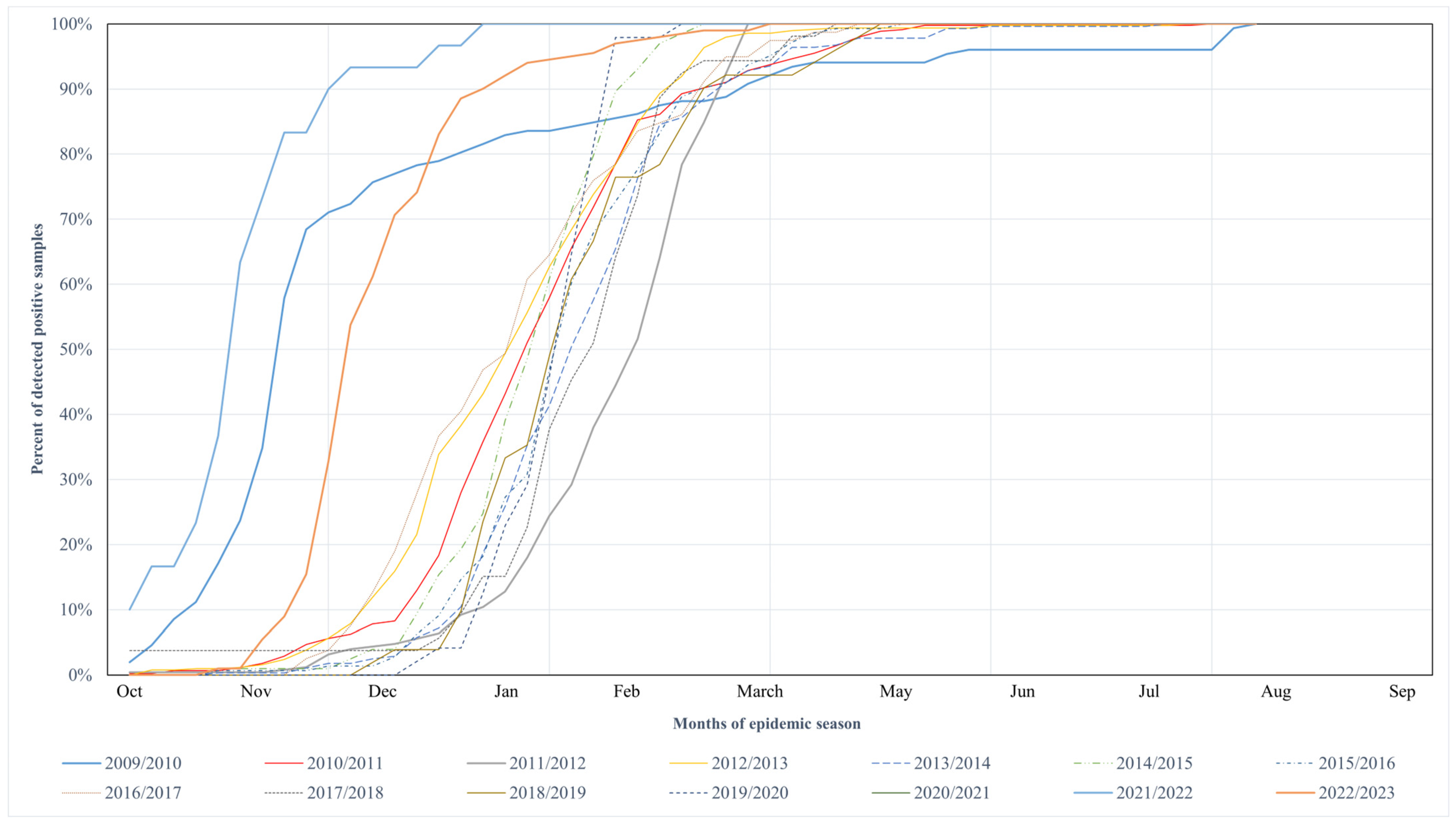
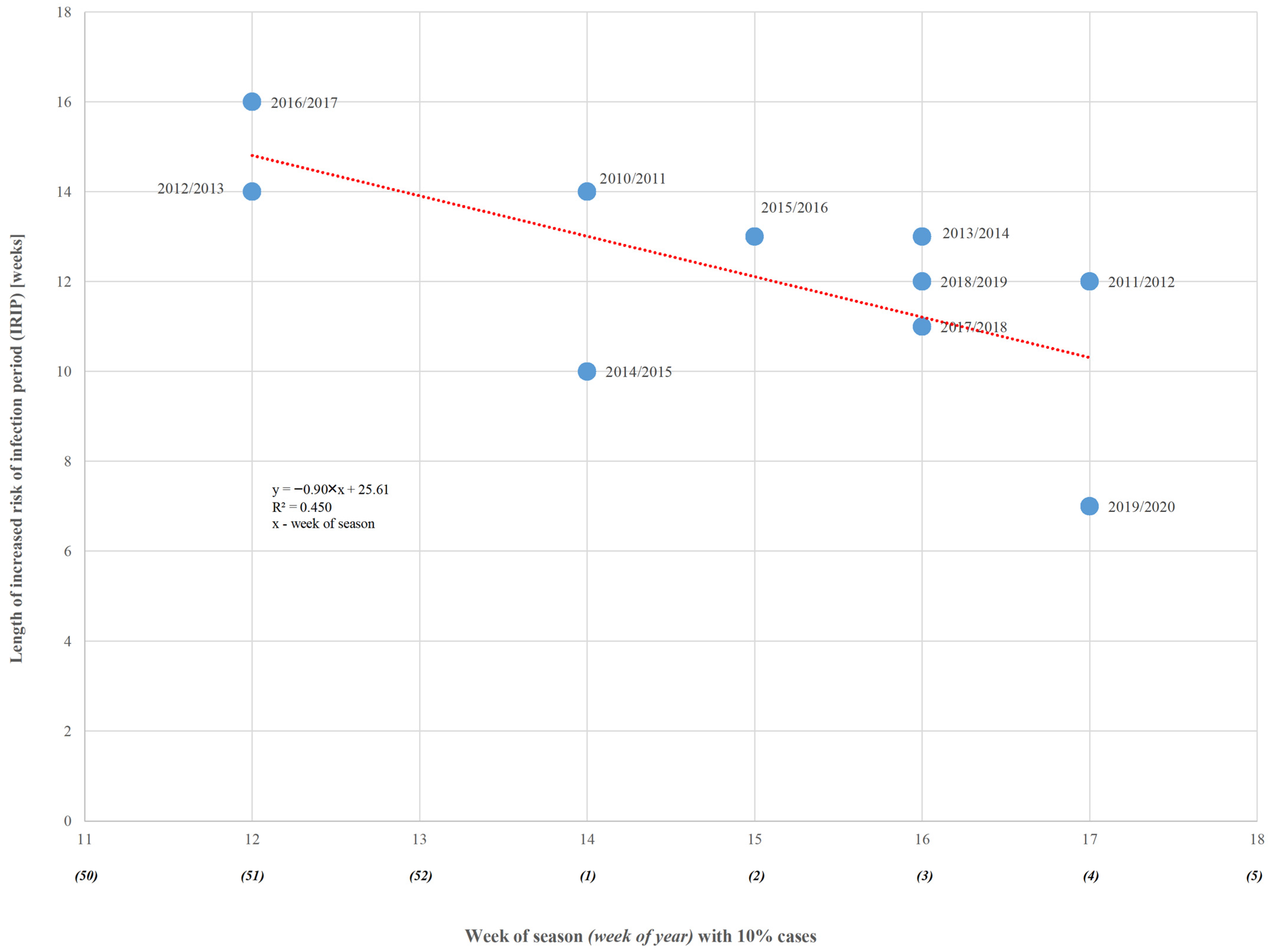
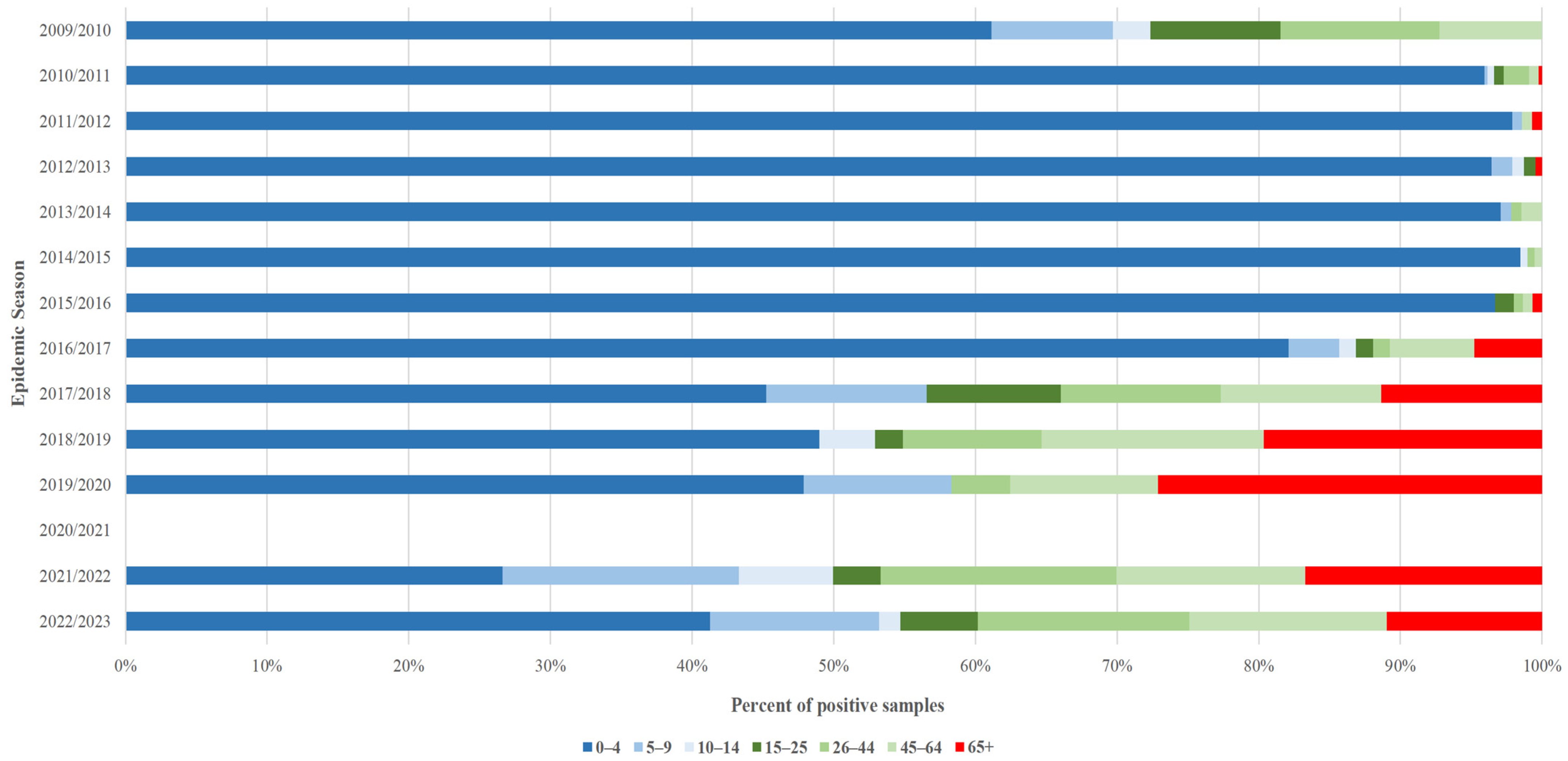
| Season (Season Group I/II) | RSV Samples | Number of the Week | Length of Increased Risk of Infection (in Weeks) | |||
|---|---|---|---|---|---|---|
| Tested | Positive | 10% of Cases | 50% of Cases | 90% of Cases | ||
| 2009/2010 (I) | 479 | 152 | 43 | 47 | 16 | 26 |
| 2010/2011 (II) | 1692 | 447 | 1 | 6 | 14 | 14 |
| 2011/2012 (II) | 1613 | 277 | 4 | 11 | 15 | 12 |
| 2012/2013 (II) | 3022 | 496 | 51 | 5 | 12 | 14 |
| 2013/2014 (II) | 1843 | 278 | 3 | 8 | 15 | 13 |
| 2014/2015 (II) | 1237 | 202 | 1 | 6 | 10 | 10 |
| 2015/2016 (II) | 1748 | 143 | 2 | 8 | 14 | 13 |
| 2016/2017 (II) | 656 | 79 | 51 | 5 | 14 | 16 |
| 2017/2018 (II) | 963 | 53 | 3 | 9 | 13 | 11 |
| 2018/2019 (II) | 1013 | 51 | 3 | 7 | 14 | 12 |
| 2019/2020 (II) | 605 | 48 | 4 | 8 | 10 | 7 |
| 2020/2021 (I) | No cases reported | |||||
| 2021/2022 (I) | 295 | 30 | 40 | 45 | 49 | 10 |
| 2022/2023 (I) | 2835 | 201 | 48 | 50 | 4 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymański, K.; Poznańska, A.; Kondratiuk, K.; Hallmann, E.; Łuniewska, K.; Masny, A.; Brydak, L.B. Circulation of Respiratory Syncytial Virus (RSV) in Poland Between Seasons of 2009/2010 and 2022/2023 Based on SENTINEL System. Microorganisms 2025, 13, 140. https://doi.org/10.3390/microorganisms13010140
Szymański K, Poznańska A, Kondratiuk K, Hallmann E, Łuniewska K, Masny A, Brydak LB. Circulation of Respiratory Syncytial Virus (RSV) in Poland Between Seasons of 2009/2010 and 2022/2023 Based on SENTINEL System. Microorganisms. 2025; 13(1):140. https://doi.org/10.3390/microorganisms13010140
Chicago/Turabian StyleSzymański, Karol, Anna Poznańska, Katarzyna Kondratiuk, Ewelina Hallmann, Katarzyna Łuniewska, Aleksander Masny, and Lidia B. Brydak. 2025. "Circulation of Respiratory Syncytial Virus (RSV) in Poland Between Seasons of 2009/2010 and 2022/2023 Based on SENTINEL System" Microorganisms 13, no. 1: 140. https://doi.org/10.3390/microorganisms13010140
APA StyleSzymański, K., Poznańska, A., Kondratiuk, K., Hallmann, E., Łuniewska, K., Masny, A., & Brydak, L. B. (2025). Circulation of Respiratory Syncytial Virus (RSV) in Poland Between Seasons of 2009/2010 and 2022/2023 Based on SENTINEL System. Microorganisms, 13(1), 140. https://doi.org/10.3390/microorganisms13010140





