DNA Methylation Levels of the ACE2 Promoter Are Not Associated with Post-COVID-19 Symptoms in Individuals Who Had Been Hospitalized Due to COVID-19
Abstract
1. Introduction
2. Methods
2.1. Participants
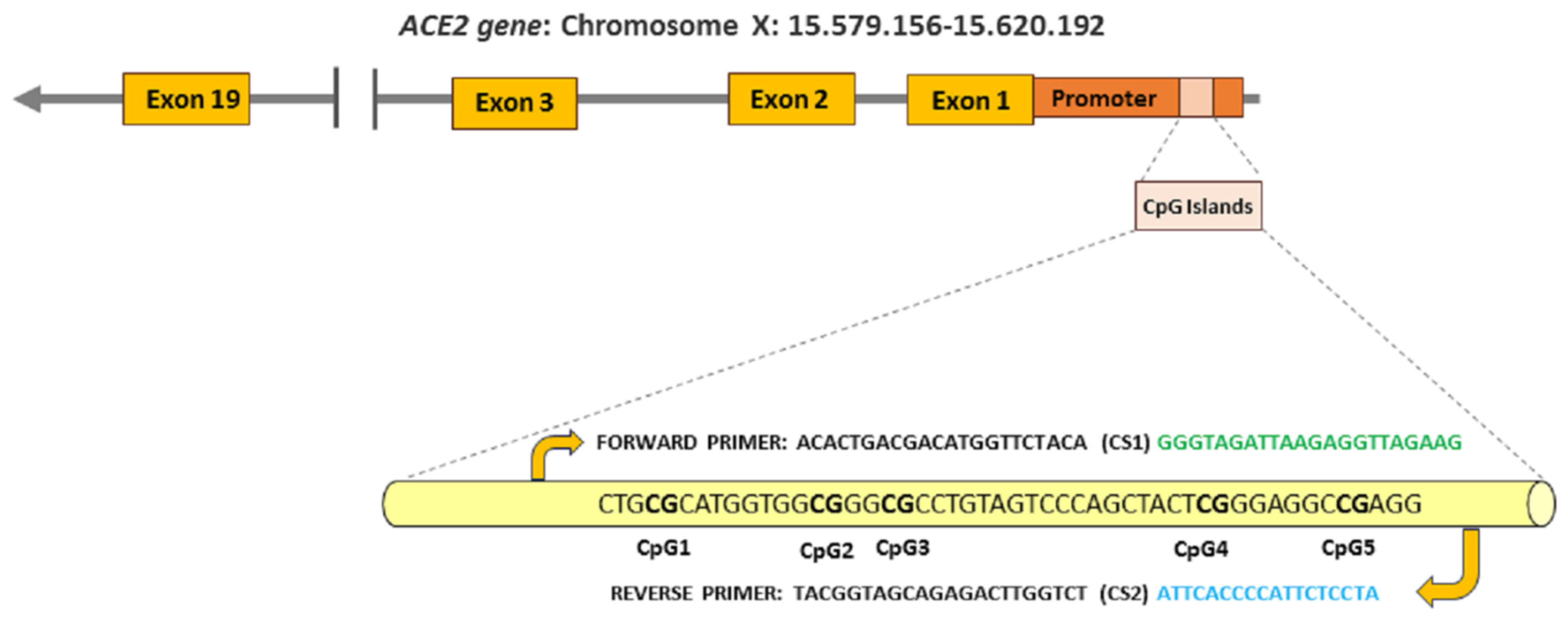
2.2. Genome DNA Collection
2.3. Differentially Methylation Profiling
2.4. Collection Data
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deans, C.; Maggert, K.A. What do you mean, “epigenetic”? Genetics 2015, 199, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Capp, J.P. Interplay between genetic, epigenetic, and gene expression variability: Considering complexity in evolvability. Evol. Appl. 2021, 14, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Netea, M.G. Trained innate immunity, epigenetics, and COVID-19. N. Engl. J. Med. 2020, 383, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Behura, A.; Naik, L.; Patel, S.; Das, M.; Kumar, A.; Mishra, A.; Nayak, D.K.; Manna, D.; Mishra, A.; Dhiman, R. Involvement of epigenetics in affecting host immunity during SARS-CoV-2 infection. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166634. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Vaishak, K.; Deka, D.; Radhakrishnan, A.K.; Paul, S.; Shanmugam, P.; Daniel, A.P.; Pathak, S.; Duttaroy, A.K.; Banerjee, A. Epigenetic perspectives associated with COVID-19 infection and related cytokine storm: An updated review. Infection 2023, 51, 1603–1618. [Google Scholar] [CrossRef] [PubMed]
- Balnis, J.; Madrid, A.; Hogan, K.J.; Drake, L.A.; Chieng, H.C.; Tiwari, A.; Vincent, C.E.; Chopra, A.; Vincent, P.A.; Robek, M.D.; et al. Blood DNA Methylation and COVID-19 outcomes. Clin. Epigenet. 2021, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.O.; Choudhari, R.; Nema, V.; Khan, A.A. ACE2 and TMPRSS2 polymorphisms in various diseases with special reference to its impact on COVID-19 disease. Microb. Pathog. 2021, 150, 104621. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, A.; Safaralizadeh, R.; Dastmalchi, N.; Teimourian, S. Epigenetic-related effects of COVID-19 on human cells. Infect. Disord. Drug Targets 2022, 22, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Najafipour, R.; Mohammadi, D.; Momeni, M.; Moghbelinejad, S. ACE-2 Expression and methylation pattern in bronchoalveolar lavage fluid and bloods of Iranian ARDS COVID-19 patients. Int. J. Mol. Cell Med. 2022, 11, 55–63. [Google Scholar] [PubMed]
- Daniel, G.; Paola, A.R.; Nancy, G.; Fernando, S.O.; Beatriz, A.; Zulema, R.; Julieth, A.; Claudia, C.; Adriana, R. Epigenetic mechanisms and host factors impact ACE2 gene expression: Implications in COVID-19 susceptibility. Infect. Genet. Evol. 2022, 104, 105357. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C. Long COVID: Current definition. Infection 2022, 50, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V.; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Hayes, L.D.; Ingram, J.; Sculthorpe, N.F. More Than 100 Persistent Symptoms of SARS-CoV-2 (Long COVID): A scoping review. Front. Med. 2021, 8, 750378. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Long COVID Collaborators; Wulf Hanson, S.; Abbafati, C.; Aerts, J.G.; Al-Aly, Z.; Ashbaugh, C.; Ballouz, T.; Blyuss, O.; Bobkova, P.; Bonsel, G.; et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA 2022, 328, 1604–1615. [Google Scholar] [PubMed]
- Fernández-de-las-Peñas, C.; Notarte, K.I.; Macasaet, R.; Velasco, J.V.; Catahay, J.A.; Therese Ver, A.; Chung, W.; Valera-Calero, J.A.; Navarro-Santana, M. Persistence of post-COVID symptoms in the general population two years after SARS-CoV-2 infection: A systematic review and meta-analysis. J. Infect. 2024, 88, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Raveendran, A.V.; Giordano, R.; Arendt-Nielsen, L. Long COVID or post-COVID-19 condition: Past, present and future research directions. Microorganisms 2023, 11, 2959. [Google Scholar] [CrossRef] [PubMed]
- Balnis, J.; Madrid, A.; Hogan, K.J.; Drake, L.A.; Adhikari, A.; Vancavage, R.; Singer, H.A.; Alisch, R.S.; Jaitovich, A. Whole-Genome methylation sequencing reveals that COVID-19-induced epigenetic dysregulation remains 1 year after hospital discharge. Am. J. Respir. Cell Mol. Biol. 2023, 68, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Nikesjö, F.; Sayyab, S.; Karlsson, L.; Apostolou, E.; Rosén, A.; Hedman, K.; Lerm, M. Defining post-acute COVID-19 syndrome (PACS) by an epigenetic biosignature in peripheral blood mononuclear cells. Clin. Epigenet. 2022, 14, 172. [Google Scholar] [CrossRef] [PubMed]
- Mikeska, T.; Felsberg, J.; Hewitt, C.A.; Dobrovic, A. Analysing DNA methylation using bisulphite pyrosequencing. Methods Mol. Biol. 2011, 791, 33–53. [Google Scholar]
- Fan, R.; Mao, S.Q.; Gu, T.L.; Zhong, F.D.; Gong, M.L.; Hao, L.M.; Yin, F.Y.; Dong, C.Z.; Zhang, L.N. Preliminary analysis of the association between methylation of the ACE2 promoter and essential hypertension. Mol. Med. Rep. 2017, 15, 3905–3911. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Notarte, K.I.; Peligro, P.J.; Velasco, J.V.; Ocampo, M.J.; Henry, B.M.; Arendt-Nielsen, L.; Torres-Macho, J.; Plaza-Manzano, G. Long-COVID symptoms in individuals infected with different SARS-CoV-2 variants of concern: A systematic review of the literature. Viruses 2022, 14, 2629. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Ma, Y.; Deng, J.; Liu, M.; Liu, J. Comparison of long COVID-19 caused by different SARS-CoV-2 strains: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2022, 19, 16010. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Iwagami, M.; Yasuhara, J.; Takagi, H.; Kuno, T. Protective effect of COVID-19 vaccination against long COVID syndrome: A systematic review and meta-analysis. Vaccine 2023, 41, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Lv, Z.H.; Sun, C.R.; Wen, Y.Y.; Tao, T.Y.; Qian, D.; Tao, F.P.; Yu, J.H. Post-acute COVID-19 symptom risk in hospitalized and non-hospitalized COVID-19 survivors: A systematic review and meta-analysis. Front. Public Health 2023, 11, 1112383. [Google Scholar] [CrossRef] [PubMed]
- Møller Johansen, L.; Gerra, M.C.; Arendt-Nielsen, L. Time course of DNA methylation in pain conditions: From experimental models to humans. Eur. J. Pain 2021, 25, 296–312. [Google Scholar] [CrossRef] [PubMed]
- Tajerian, M.; Alvarado, S.; Millecamps, M.; Vachon, P.; Crosby, C.; Bushnell, M.C.; Szyf, M.; Stone, L.S. Peripheral nerve injury is associated with chronic, reversible changes in global DNA methylation in the mouse prefrontal cortex. PLoS ONE 2013, 8, e55259. [Google Scholar] [CrossRef] [PubMed]
| Post-COVID-19 Fatigue (n = 174) | No Post-COVID-19 Fatigue (n = 105) | p Value | |
|---|---|---|---|
| Age, mean (SD), years | 57.0 (12.5) | 55.7 (13.1) | 0.423 |
| Gender, male/female (%) * | 74 (42.5%)/100 (57.5%) | 69 (65.7%)/36 (34.3%) | 0.007 * |
| Weight, mean (SD), kg | 81.5 (18.0) | 80.5 (15.0) | 0.675 |
| Height, mean (SD), cm | 166.5 (11.5) | 169.0 (9.2) | 0.679 |
| Number of medical conditions | 1.3 (1.0) | 1.1 (1.0) | 0.523 |
| Pre-existing medical conditions, n (%) | |||
| Hypertension | 58 (33.3%) | 37 (35.25%) | 0.791 |
| Diabetes | 21 (12.1%) | 8 (7.6%) | 0.264 |
| Cardiovascular Diseases | 12 (6.9%) | 8 (7.6%) | 0.829 |
| Asthma | 20 (11.5%) | 11 (10.5%) | 0.805 |
| Obesity | 60 (34.5%) | 25 (23.8%) | 0.118 |
| Chronic Obstructive Pulmonary Disease | 3 (1.7%) | 2 (1.9%) | 0.913 |
| Number of COVID-19-onset symptoms, mean (SD) | 3.25 (1.0) | 3.1 (1.0) | 0.218 |
| Days at hospital, mean (SD) | 7.0 (5.8) | 8.6 (10.0) | 0.136 |
| Intensive Care Unit (ICU) admission | |||
| Yes/No, n (%) | 8 (4.5%)/166 (95.5%) | 2 (2%)/103 (98%) | 0.326 |
| CpG1 methylation (%) | 93.3 (4.0) | 93.7 (3.2) | 0.335 |
| CpG2 methylation (%) | 40.4 (7.4) | 39.4 (7.3) | 0.259 |
| CpG3 methylation (%) | 43.6 (9.0) | 42.8 (8.1) | 0.437 |
| CpG4 methylation (%) | 45.5 (8.0) | 45.6 (7.8) | 0.937 |
| CpG5 methylation (%) | 0.6 (0.3) | 0.6 (0.4) | 0.804 |
| Post-COVID-19 Dyspnea at Rest (n = 36) | No Post-COVID-19 Dyspnea at Rest (n = 243) | p Value | |
|---|---|---|---|
| Age, mean (SD), years | 55.0 (15.5) | 57.7 (12.4) | 0.421 |
| Gender, male/female (%) | 11 (30.5%)/25 (69.5%) | 132 (64.3%)/111 (45.7%) | 0.07 |
| Weight, mean (SD), kg | 81.0 (20.0) | 81.9 (16.5) | 0.933 |
| Height, mean (SD), cm | 165.5 (8.7) | 168.0 (9.5) | 0.501 |
| Number of medical conditions | 1.3 (1.0) | 1.3 (1.0) | 0.724 |
| Pre-existing medical conditions, n (%) | |||
| Hypertension | 13 (36.1%) | 82 (33.7%) | 0.820 |
| Diabetes | 7 (19.4%) | 22 (9.0%) | 0.081 |
| Cardiovascular Diseases | 1 (2.8%) | 19 (7.8%) | 0.291 |
| Asthma | 4 (11.1%) | 27 (11.1%) | 0.636 |
| Obesity | 14 (38.9%) | 71 (29.2%) | 0.327 |
| Chronic Obstructive Pulmonary Disease | 1 (2.8%) | 4 (1.7%) | 0.636 |
| Number of COVID-19-onset symptoms, mean (SD) | 3.4 (1.0) | 3.2 (1.0) | 0.125 |
| Days at hospital, mean (SD) | 8.7 (5.8) | 7.9 (9.0) | 0.617 |
| Intensive Care Unit (ICU) admission | |||
| Yes/No, n (%) | 1 (2.8%)/35 (97.2%) | 9 (3.7%)/107 (96.3%) | 0.623 |
| CpG1 methylation (%) | 93.0 (4.5) | 93.5 (3.6) | 0.350 |
| CpG2 methylation (%) | 37.9 (8.3) | 40.3 (7.2) | 0.061 |
| CpG3 methylation (%) | 40.7 (10.0) | 43.7 (8.4) | 0.054 |
| CpG4 methylation (%) | 44.3 (9.7) | 45.7 (7.6) | 0.322 |
| CpG5 methylation (%) | 0.6 (0.25) | 0.6 (0.4) | 0.623 |
| Post-COVID-19 Dyspnea on Exertion (n = 188) | No Post-COVID-19 Dyspnea Exertion (n = 91) | p Value | |
|---|---|---|---|
| Age, mean (SD), years | 56.5 (13.2) | 56.5 (12.0) | 0.997 |
| Gender, male/female (%) * | 82 (43.6%)/106 (56.4%) | 61 (67.0%)/30 (33.0%) | 0.008 * |
| Weight, mean (SD), kg | 81.0 (17.5) | 81.1 (15.0) | 0.967 |
| Height, mean (SD), cm | 166.5 (9.5) | 169.0 (9.6) | 0.282 |
| Number of medical conditions | 1.3 (1.0) | 1.1 (1.0) | 0.205 |
| Pre-existing medical conditions, n (%) | |||
| Hypertension | 62 (33.0%) | 33 (36.25%) | 0.659 |
| Diabetes | 20 (10.6%) | 9 (9.9%) | 0.855 |
| Cardiovascular Diseases | 14 (7.5%) | 6 (6.6%) | 0.802 |
| Asthma | 24 (12.8%) | 7 (7.7%) | 0.233 |
| Obesity | 64 (34.0%) | 21 (23.1%) | 0.120 |
| Chronic Obstructive Pulmonary Disease | 3 (1.6%) | 2 (2.2%) | 0.727 |
| Number of COVID-19-onset symptoms, mean (SD) | 3.2 (1.0) | 3.25 (1.0) | 0.637 |
| Days at hospital, mean (SD) | 8.2 (9.6) | 7.6 (6.2) | 0.608 |
| Intensive Care Unit (ICU) admission | |||
| Yes/No, n (%) | 7 (3.7%)/181 (96.3%) | 3 (3.3%)/88 (96.7%) | 0.538 |
| CpG1 methylation (%) | 93.2 (4.2) | 94.1 (2.5) | 0.052 |
| CpG2 methylation (%) | 40.2 (7.6) | 39.7 (6.9) | 0.578 |
| CpG3 methylation (%) | 43.4 (8.9) | 43.0 (8.5) | 0.681 |
| CpG4 methylation (%) | 45.7 (8.1) | 45.4 (7.5) | 0.742 |
| CpG5 methylation (%) | 0.6 (0.35) | 0.6 (0.35) | 0.517 |
| Post-COVID-19 Memory Loss (n = 87) | No Post-COVID-19 Memory Loss (n = 192) | p Value | |
|---|---|---|---|
| Age, mean (SD), years | 57.9 (12.3) | 55.8 (13.0) | 0.204 |
| Gender, male/female (%) | 8 (44.7%)/49 (56.3%) | 107 (54.7%)/87 (45.3%) | 0.222 |
| Weight, mean (SD), kg | 81.2 (16.9) | 81.0 (16.8) | 0.867 |
| Height, mean (SD), cm | 166.7 (9.5) | 168.0 (9.5) | 0.469 |
| Number of medical conditions | 1.45 (1.0) | 1.2 (1.0) | 0.07 |
| Pre-existing medical conditions, n (%) | |||
| Hypertension | 35 (40.2%) | 60 (31.25%) | 0.233 |
| Diabetes * | 14 (16.1%) | 15 (7.8%) | 0.046 * |
| Cardiovascular Diseases | 6 (6.9%) | 14 (7.3%) | 0.909 |
| Asthma * | 16 (18.4%) | 15 (7.8%) | 0.014 * |
| Obesity | 24 (27.6%) | 61 (31.8%) | 0.557 |
| Chronic Obstructive Pulmonary Disease | 0 (0.0%) | 5 (2.6%) | 0.132 |
| Number of COVID-19-onset symptoms, mean (SD) * | 3.4 (0.8) | 3.1 (1.1) | 0.04 * |
| Days at hospital, mean (SD) | 9.1 (12.3) | 7.5 (6.4) | 0.159 |
| Intensive Care Unit (ICU) admission | |||
| Yes/No, n (%) | 4 (4.6%)/83 (95.4%) | 6 (3.1%)/186 (96.9%) | 0.09 |
| CpG1 methylation (%) | 93.1 (4.9) | 93.6 (3.1) | 0.263 |
| CpG2 methylation (%) | 41.1 (7.1) | 39.5 (7.4) | 0.096 |
| CpG3 methylation (%) | 44.6 (8.3) | 42.7 (8.7) | 0.087 |
| CpG4 methylation (%) | 46.5 (7.8) | 45.1 (7.9) | 0.177 |
| CpG5 methylation (%) | 0.6 (0.25) | 0.6 (0.4) | 0.086 |
| Post-COVID-19 Brain Fog (n = 41) | No Post-COVID-19 Brain Fog (n = 238) | p Value | |
|---|---|---|---|
| Age, mean (SD), years | 55.1 (12.8) | 56.7 (12.8) | 0.459 |
| Gender, male/female (%) | 17 (41.5%)/24 (58.5%) | 126 (52.9%)/112 (47.1%) | 0.331 |
| Weight, mean (SD), kg | 81.6 (19.0) | 81.0 (16.3) | 0.805 |
| Height, mean (SD), cm | 166.5 (9.9) | 167.5 (9.5) | 0.580 |
| Number of medical conditions | 1.35 (1.0) | 1.3 (1.0) | 0.331 |
| Pre-existing medical conditions, n (%) | |||
| Hypertension | 11 (26.83%) | 84 (35.3%) | 0.390 |
| Diabetes | 6 (14.6%) | 23 (9.7%) | 0.361 |
| Cardiovascular Diseases | 1 (2.5%) | 19 (8.0%) | 0.221 |
| Asthma | 7 (17.1%) | 24 (10.1%) | 0.215 |
| Obesity | 14 (34.1%) | 71 (29.8%) | 0.643 |
| Chronic Obstructive Pulmonary Disease | 0 (0.0%) | 5 (2.1%) | 0.353 |
| Number of COVID-19-onset symptoms, mean (SD) | 3.4 (0.9) | 3.15 (1.0) | 0.142 |
| Days at hospital, mean (SD) | 7.5 (7.0) | 8.1 (9.0) | 0.673 |
| Intensive Care Unit (ICU) admission | |||
| Yes/No, n (%) | 2 (4.9%)/39 (95.1%) | 8 (3.3%)/230 (96.7%) | 0.657 |
| CpG1 methylation (%) | 93.7 (4.4) | 93.4 (3.7) | 0.748 |
| CpG2 methylation (%) | 41.4 (6.6) | 39.8 (7.5) | 0.197 |
| CpG3 methylation (%) | 44.6 (8.1) | 43.1 (8.8) | 0.306 |
| CpG4 methylation (%) | 46.7 (7.5) | 45.4 (8.0) | 0.319 |
| CpG5 methylation (%) | 0.6 (0.4) | 0.65 (0.35) | 0.612 |
| Post-COVID-19 Concentration Loss (n = 42) | No Post-COVID-19 Concentration Loss (n = 237) | p Value | |
|---|---|---|---|
| Age, mean (SD), years | 54.5 (12.4) | 57.0 (12.9) | 0.311 |
| Gender, male/female (%) * | 16 (28.1%)/26 (61.9%) | 127 (54.6%)/110 (46.4%) | 0.007 * |
| Weight, mean (SD), kg | 80.7 (16.4) | 81.0 (16.9) | 0.898 |
| Height, mean (SD), cm | 165.5 (9.75) | 167.0 (9.5) | 0.202 |
| Number of medical conditions | 1.3 (1.0) | 1.3 (1.0) | 0.989 |
| Pre-existing medical conditions, n (%) | |||
| Hypertension | 14 (33.3%) | 81 (34.2%) | 0.931 |
| Diabetes | 2 (4.8%) | 27 (11.4%) | 0.219 |
| Cardiovascular Diseases | 2 (4.8%) | 18 (7.6%) | 0.525 |
| Asthma | 4 (9.5%) | 27 (11.4%) | 0.737 |
| Obesity | 19 (45.2%) | 66 (27.8%) | 0.06 |
| Chronic Obstructive Pulmonary Disease | 0 (0.0%) | 5 (2.1%) | 0.346 |
| Number of COVID-19-onset symptoms, mean (SD) | 3.3 (1.1) | 3.2 (1.0) | 0.554 |
| Days at hospital, mean (SD) | 8.5 (7.7) | 7.9 (8.8) | 0.708 |
| Intensive Care Unit (ICU) admission | |||
| Yes/No, n (%) | 2 (4.7%)/40 (95.3%) | 8 (3.4%)/229 (94.6%) | 0.657 |
| CpG1 methylation (%) | 93.1 (4.5) | 93.5 (3.6) | 0.497 |
| CpG2 methylation (%) | 38.9 (7.6) | 40.1 (7.3) | 0.292 |
| CpG3 methylation (%) | 42.2 (8.6) | 43.5 (8.7) | 0.368 |
| CpG4 methylation (%) | 43.5 (7.6) | 45.9 (8.0) | 0.066 |
| CpG5 methylation (%) | 0.6 (0.3) | 0.6 (0.4) | 0.506 |
| Post-COVID-19 Gastrointestinal Symptoms (n = 25) | No Post-COVID-19 Gastrointestinal Symptoms (n = 254) | p Value | |
|---|---|---|---|
| Age, mean (SD), years | 55.0 (14.1) | 56.6 (12.7) | 0.536 |
| Gender, male/female (%) | 11 (44.0%)/14 (56.0%) | 132 (52.0%)/122 (48.0%) | 0.586 |
| Weight, mean (SD), kg | 81.2 (21.2) | 81.0 (16.4) | 0.947 |
| Height, mean (SD), cm | 168.5 (12.7) | 167.5 (9.2) | 0.475 |
| Number of medical conditions | 1.2 (1.0) | 1.3 (1.0) | 0.523 |
| Pre-existing medical conditions, n (%) | |||
| Hypertension | 10 (40.0%) | 85 (33.5%) | 0.593 |
| Diabetes | 1 (4.0%) | 28 (11.0%) | 0.299 |
| Cardiovascular Diseases | 1 (4.0%) | 19 (7.5%) | 0.535 |
| Asthma | 3 (12.0%) | 28 (11.0%) | 0.889 |
| Obesity | 50 (20.0%) | 80 (31.5%) | 0.320 |
| Chronic Obstructive Pulmonary Disease | 1 (4.0%) | 4 (1.6%) | 0.387 |
| Number of COVID-19-onset symptoms, mean (SD) | 3.35 (0.8) | 3.2 (1.0) | 0.408 |
| Days at hospital, mean (SD) | 5.7 (2.5) | 8.2 (9.0) | 0.168 |
| Intensive Care Unit (ICU) admission | |||
| Yes/No, n (%) | 0 (0.0%)/25 (100%) | 10 (3.9%)/244 (96.1%) | 0.412 |
| CpG1 methylation (%) | 91.3 (6.0) | 93.7 (3.4) | 0.002 |
| CpG2 methylation (%) | 40.5 (8.0) | 40.0 (7.3) | 0.711 |
| CpG3 methylation (%) | 42.2 (9.2) | 43.4 (8.6) | 0.527 |
| CpG4 methylation (%) | 44.3 (9.5) | 45.7 (7.7) | 0.411 |
| CpG5 methylation (%) | 0.55 (0.45) | 0.6 (0.35) | 0.295 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-de-las-Peñas, C.; Díaz-Gil, G.; Gil-Crujera, A.; Gómez-Sánchez, S.M.; Ambite-Quesada, S.; Torres-Macho, J.; Ryan-Murua, P.; Franco-Moreno, A.; Pellicer-Valero, O.J.; Arendt-Nielsen, L.; et al. DNA Methylation Levels of the ACE2 Promoter Are Not Associated with Post-COVID-19 Symptoms in Individuals Who Had Been Hospitalized Due to COVID-19. Microorganisms 2024, 12, 1304. https://doi.org/10.3390/microorganisms12071304
Fernández-de-las-Peñas C, Díaz-Gil G, Gil-Crujera A, Gómez-Sánchez SM, Ambite-Quesada S, Torres-Macho J, Ryan-Murua P, Franco-Moreno A, Pellicer-Valero OJ, Arendt-Nielsen L, et al. DNA Methylation Levels of the ACE2 Promoter Are Not Associated with Post-COVID-19 Symptoms in Individuals Who Had Been Hospitalized Due to COVID-19. Microorganisms. 2024; 12(7):1304. https://doi.org/10.3390/microorganisms12071304
Chicago/Turabian StyleFernández-de-las-Peñas, César, Gema Díaz-Gil, Antonio Gil-Crujera, Stella M. Gómez-Sánchez, Silvia Ambite-Quesada, Juan Torres-Macho, Pablo Ryan-Murua, Anabel Franco-Moreno, Oscar J. Pellicer-Valero, Lars Arendt-Nielsen, and et al. 2024. "DNA Methylation Levels of the ACE2 Promoter Are Not Associated with Post-COVID-19 Symptoms in Individuals Who Had Been Hospitalized Due to COVID-19" Microorganisms 12, no. 7: 1304. https://doi.org/10.3390/microorganisms12071304
APA StyleFernández-de-las-Peñas, C., Díaz-Gil, G., Gil-Crujera, A., Gómez-Sánchez, S. M., Ambite-Quesada, S., Torres-Macho, J., Ryan-Murua, P., Franco-Moreno, A., Pellicer-Valero, O. J., Arendt-Nielsen, L., & Giordano, R. (2024). DNA Methylation Levels of the ACE2 Promoter Are Not Associated with Post-COVID-19 Symptoms in Individuals Who Had Been Hospitalized Due to COVID-19. Microorganisms, 12(7), 1304. https://doi.org/10.3390/microorganisms12071304







