The Effect of Two Siderophore-Producing Bacillus Strains on the Growth Promotion of Perennial Ryegrass under Cadmium Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Cd2+ Tolerance
2.3. Indole Acetic Acid Production and Gibberellin, and Cytokinin Capacity
2.4. Phosphate Solubilization and Potassium Release Capacity
2.5. Determination of Siderophore-Producing Activity
2.5.1. Quantitative and Qualitative Measurements
2.5.2. Type Identification of Chelating Groups in Iron Carriers
- (1)
- FeCl3 experiment: We added 1−5 mL FeCl3 solution (2%) to 1 mL of supernatant. If it turned red or purple, there was ferrophilin, and if 1 mL of FeCl3 solution was added to 1 mL of supernatant and immediately turned red, it was of the hydroxamic acid type. If 1 mL of supernatant became red or purple after adding more than 1 mL of FeCl3 solution, it was catecholic.
- (2)
- Arnow’s experiment: A total of 1 mL of 0.5 mol/L HCl and 1 mL of 10% sodium molybdate-sodium nitrite solution were added to 1 mL of supernatant, and if there was a catechol structure in the solution, the nitrite decomposed to form a yellow ligand, and the solution turned yellow. We continued to add 1 mL of NaOH (1 mol/L) solution, which turned red (does not change color for at least 1 h) if it contained catechol ferrophile, and has a characteristic absorption peak at 510 nm on a UV spectrophotometer.
- (3)
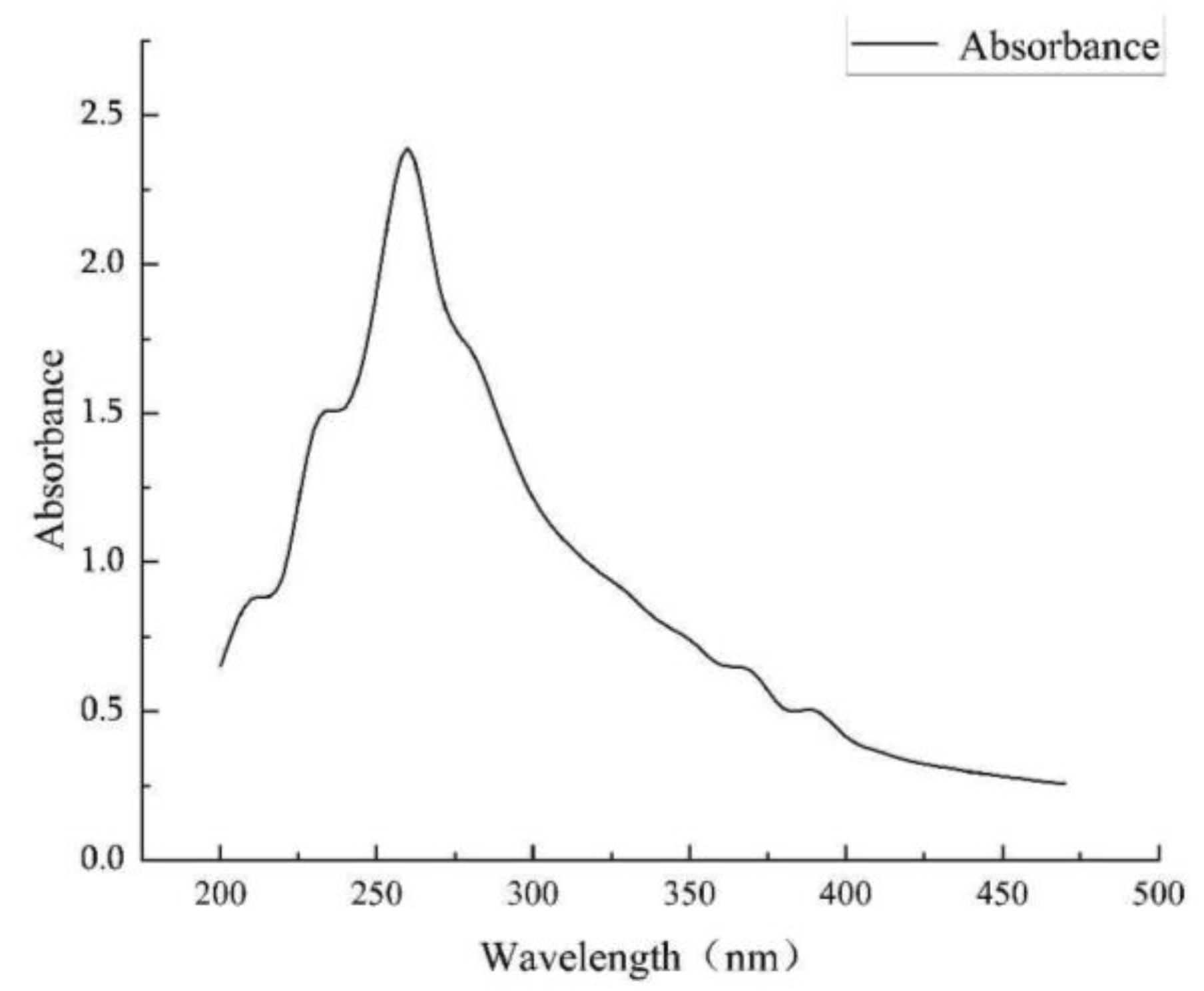
- Shenker’s experiment: A total of 1 mL of CuSO4 (750 μmol/L) solution and 2 mL of acetate buffer (pH = 4.0) were added to 1 mL of supernatant. The wavelengths in the range of 190−280 nm were scanned in a UV spectrophotometer to see if there were corresponding absorption peaks.
2.6. Phylogenetic Analysis
2.7. Effect of the Strains on Ryegrass Growth under Cd2+ Stress
2.7.1. Determination of Cd2+ Effects on Ryegrass Seed Germination
2.7.2. The Effect of the Strain on Ryegrass Growth under Cd2+ Stress
2.7.3. The Effects of the Strain on the Physiological Indicators of Ryegrass under Cd2+ Stress
Measurement of the Chlorophyll Content
Measurement of the Proline Content
Measurement of the Protein Content
Measurement of the Superoxide Dismutase Content
2.8. Determination of Cadmium in Soil and Plants
2.9. Whole-Genome Sequencing
2.10. Statistical Analysis
3. Results
3.1. Cd2+ Tolerance of the Strains
3.2. Strains’ Ability to Produce CTK, GA, and IAA
3.3. Phosphorus Solubilization and Potassium Release Activity Assay
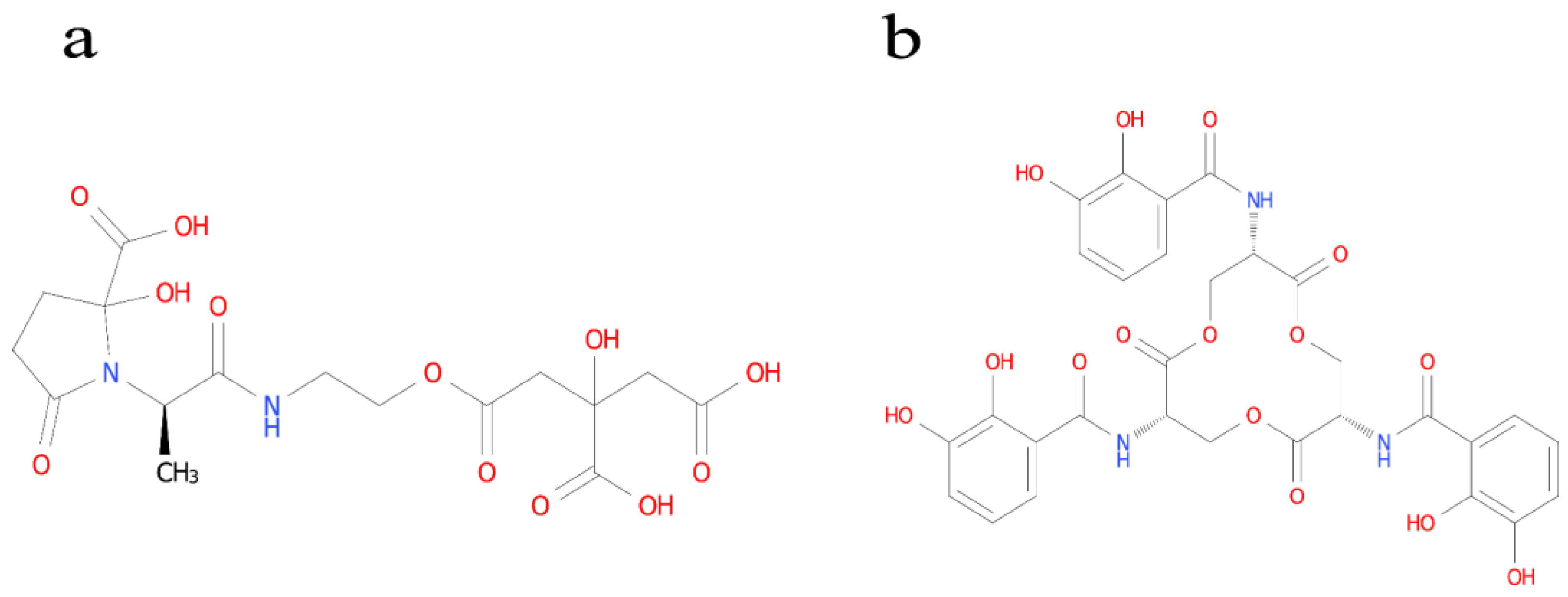
3.4. Siderophore-Producing Activities
3.5. Identification of the Type of Ferrophilin-Chelating Groups
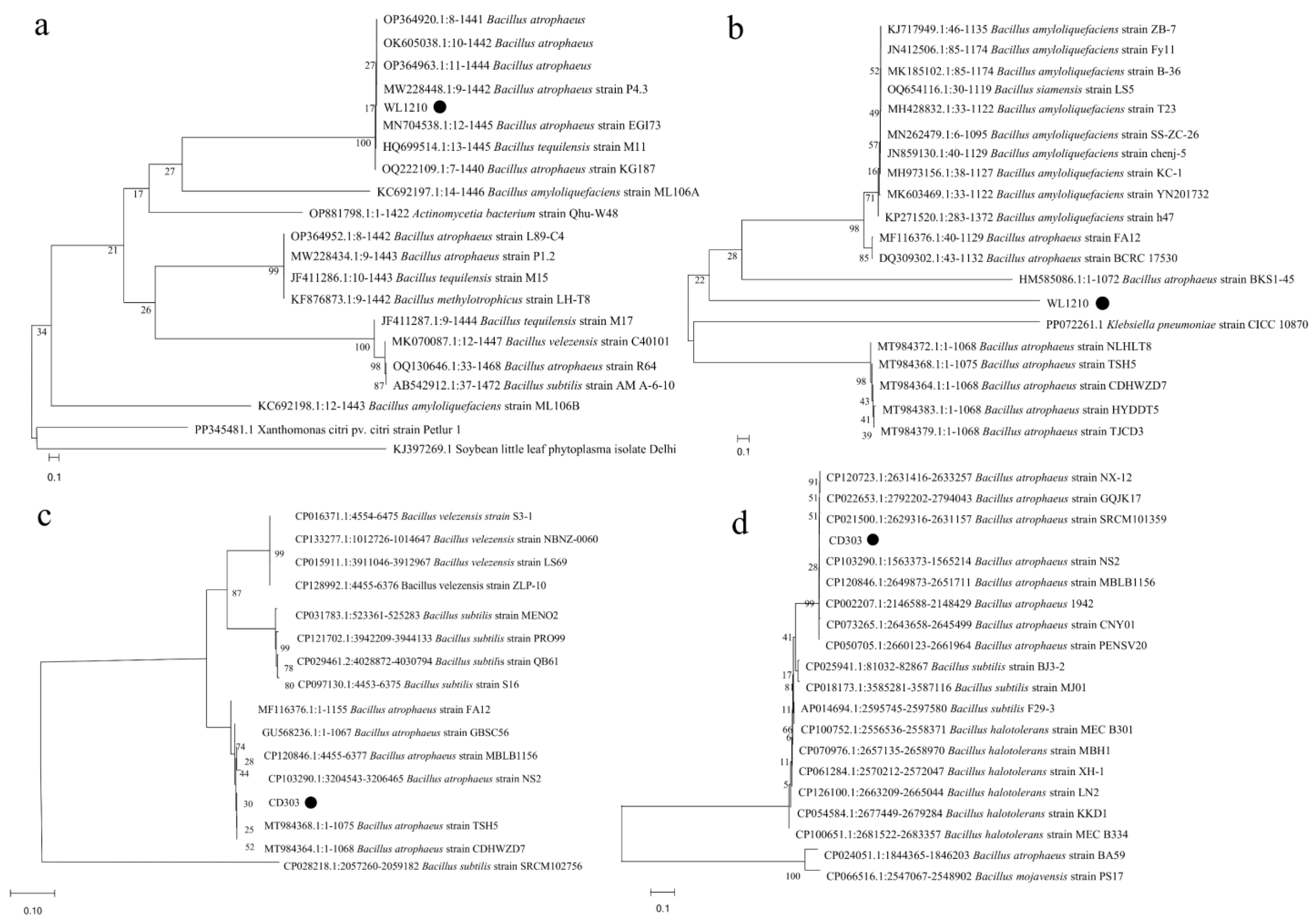
3.6. Phylogenetic Analysis
3.7. Effect of the Strains on Ryegrass Growth Promotion under Cd2+ Stress
3.7.1. Effects of Cd2+ Stress on Ryegrass Seed Germination
3.7.2. Effects of the Strains on Ryegrass Biomass under Cd2+ Stress
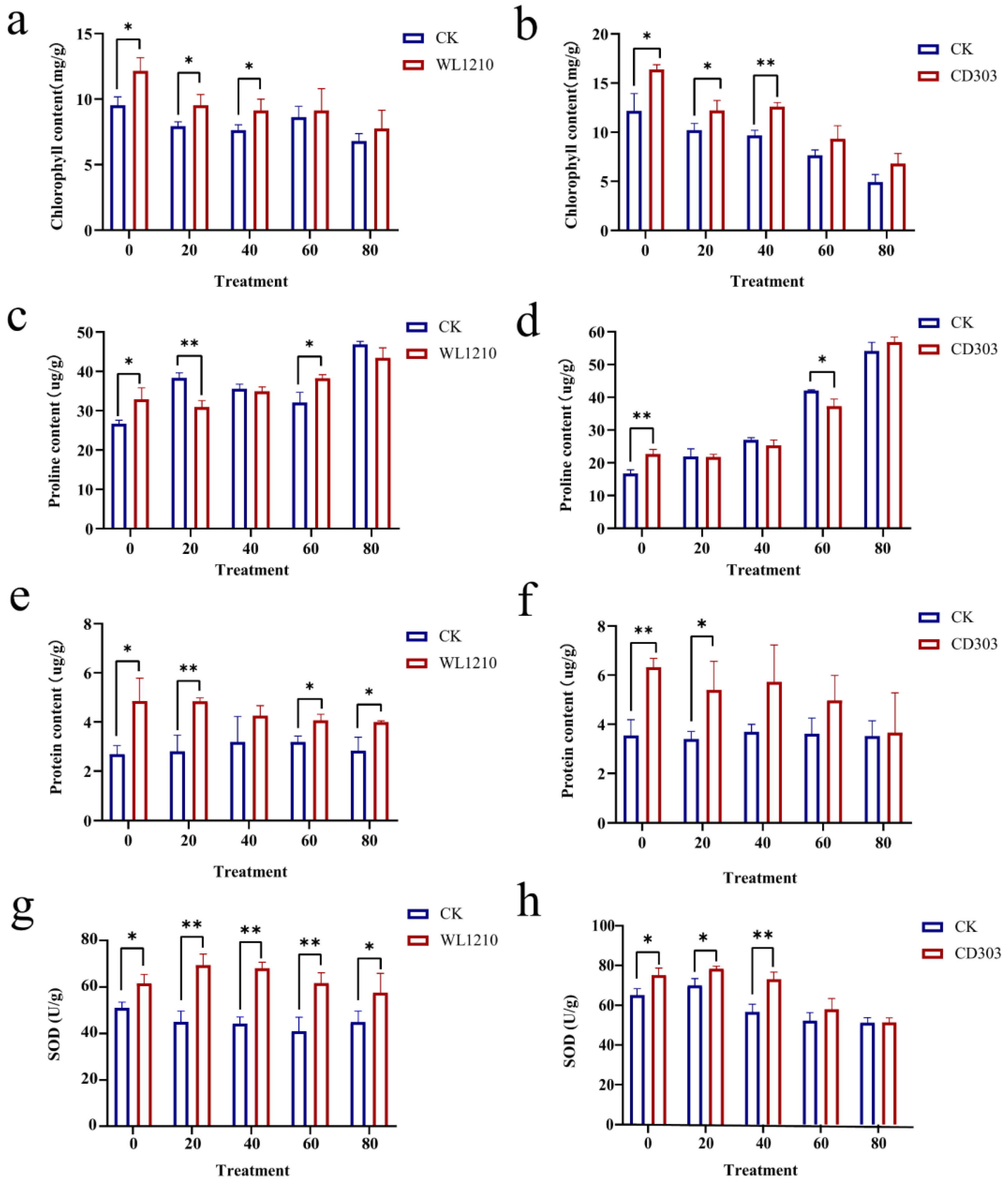
3.7.3. Effects of the Strains on the Chlorophyll Contents of Ryegrass under Cd2+ Stress
3.7.4. Effects of the Strains on the Proline Content of Ryegrass under Cd2+ Stress
3.7.5. Effect of Cd2+ and Strain Inoculation on Ryegrass Protein Concentrations
3.7.6. Effect of Cd2+ and Strain Inoculation on Ryegrass Superoxide Dismutase (SOD) Concentrations
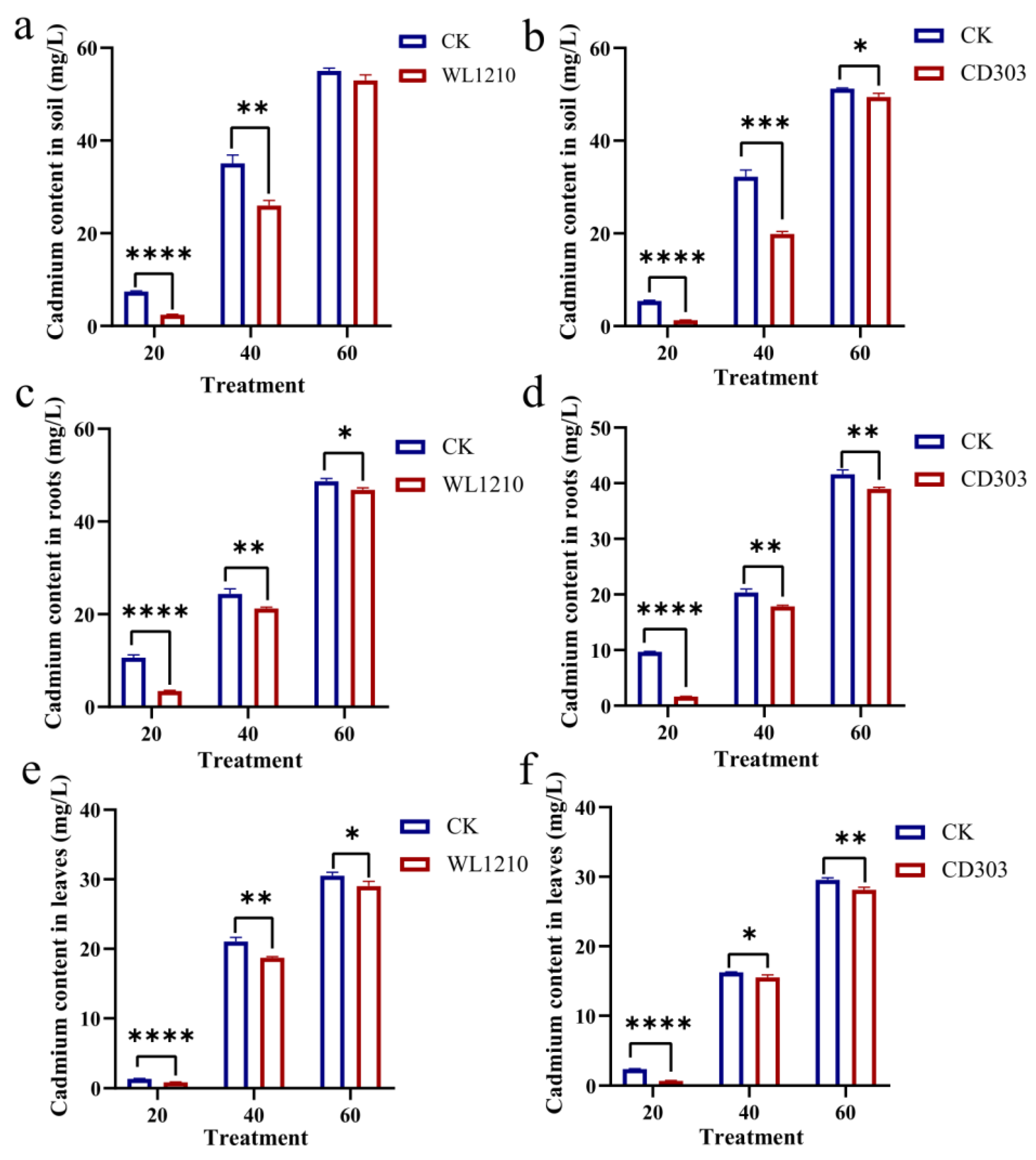
3.8. Determination of Cadmium in Soil and Plants
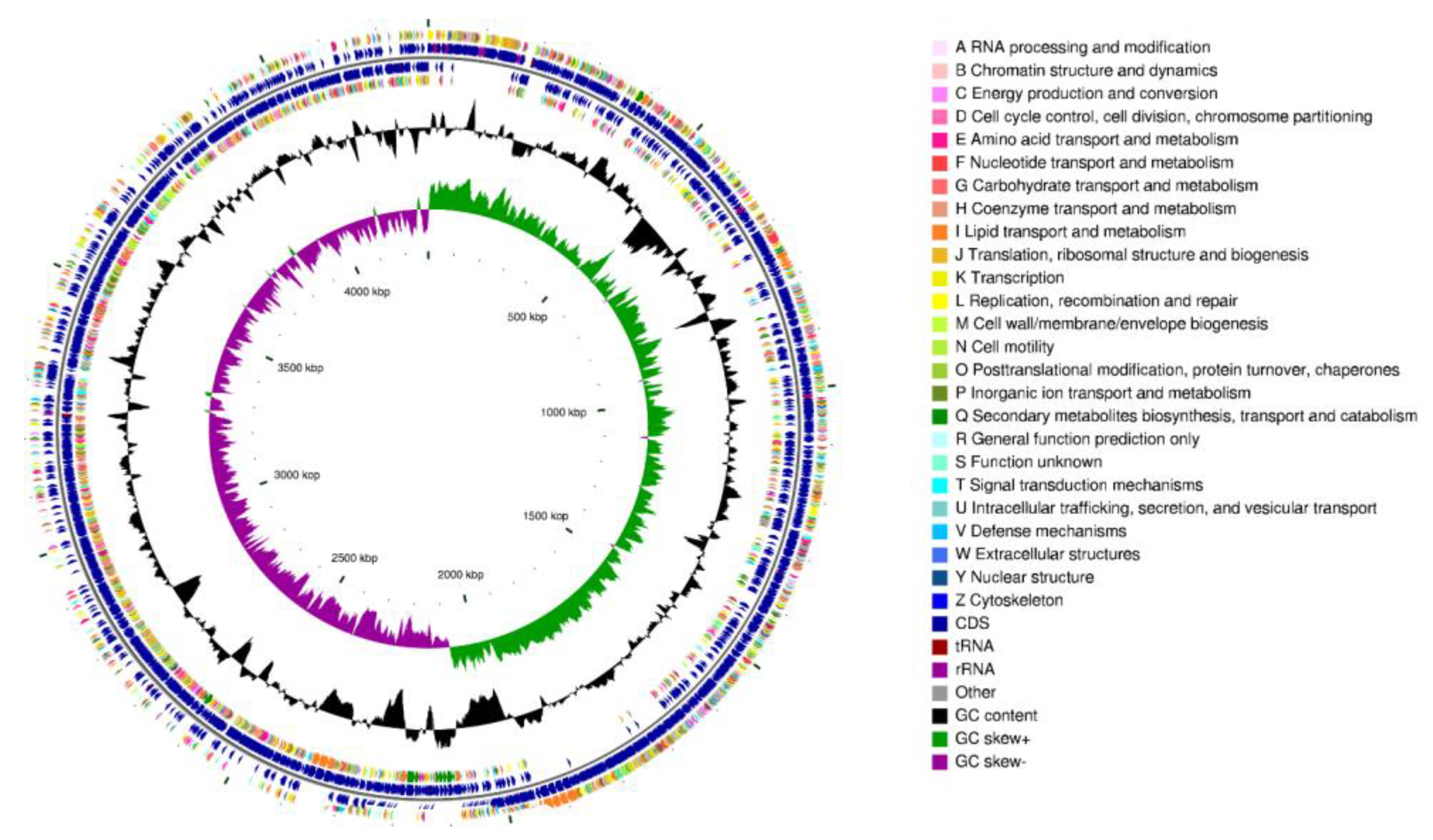
3.9. Genomic Characteristics of Strain CD303
3.9.1. Growth-Promoting Functional Genes
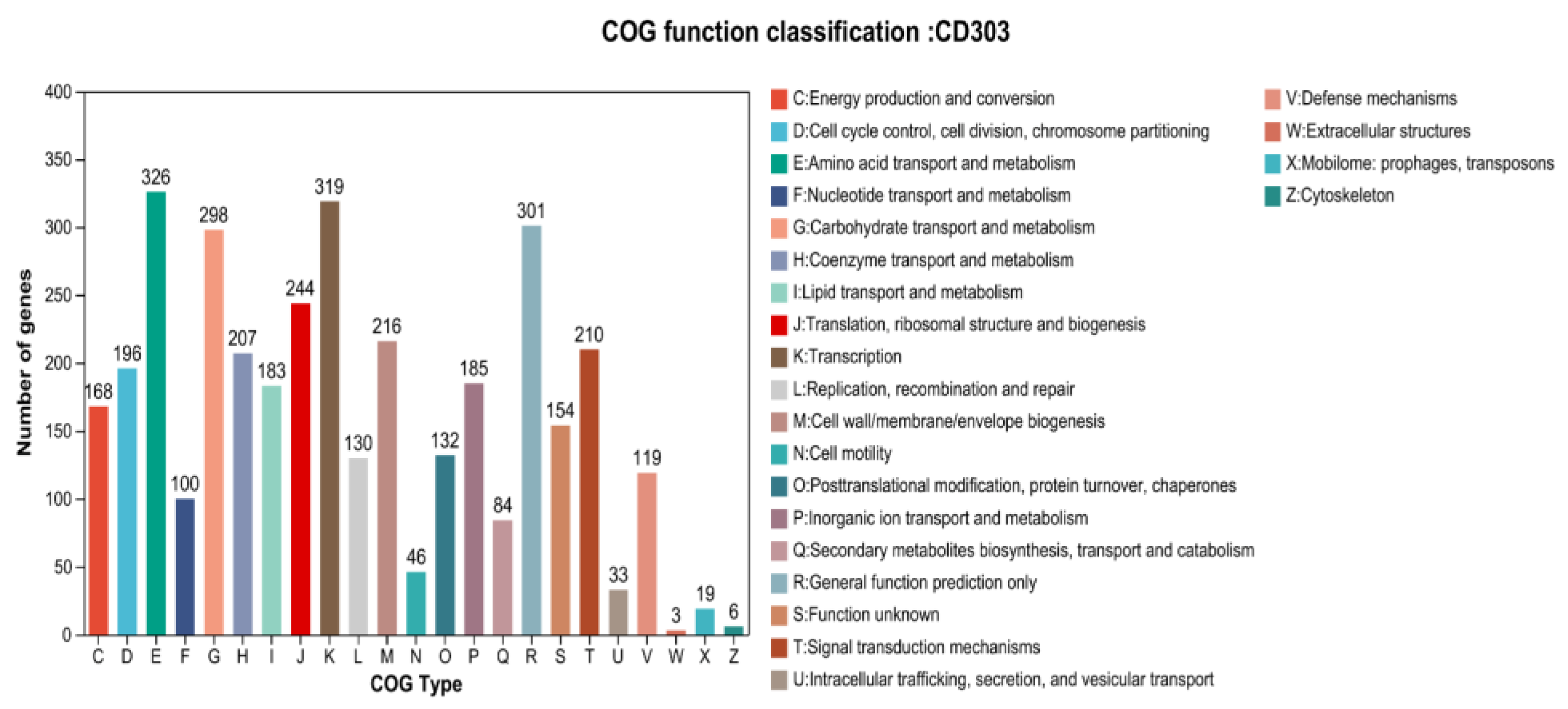
3.9.2. Functional Annotation of the CD303 Gene
3.9.3. Stress Tolerance-Related Functional Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zulfiqar, U.; Haider, F.U.; Maqsood, M.F.; Mohy-Ud-Din, W.; Shabaan, M.; Ahmad, M.; Kaleem, M.; Ishfaq, M.; Aslam, Z.; Shahzad, B. Recent Advances in Microbial-Assisted Remediation of Cadmium-Contaminated Soil. Plants 2023, 12, 3147. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, U.; Jiang, W.; Xiukang, W.; Hussain, S.; Ahmad, M.; Maqsood, M.F.; Ali, N.; Ishfaq, M.; Kaleem, M.; Haider, F.U.; et al. Cadmium Phytotoxicity, Tolerance, and Advanced Remediation Approaches in Agricultural Soils; A Comprehensive Review. Front. Plant Sci. 2022, 13, 773815. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.F.; Sehar, S.; Chen, G.; Chen, Z.-H.; Jilani, G.; Chaudhry, A.N.; Shamsi, I.H. Cadmium-zinc cross-talk delineates toxicity tolerance in rice via differential genes expression and physiological/ultrastructural adjustments. Ecotoxicol. Environ. Saf. 2020, 190, 110076. [Google Scholar] [CrossRef]
- Ge, J.; Liu, L.-L.; Cui, Z.-G.; Talukder, M.; Lv, M.-W.; Li, J.-L. Comparative study on protective effect of different selenium sources against cadmium-induced nephrotoxicity via regulating the transcriptions of selenoproteome. Ecotoxicol. Environ. Saf. 2021, 215, 112135. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Moosa, A.; Ali, H.M.; Hancock, J.T.; Yong, J.W.H. Synergistic interplay between melatonin and hydrogen sulfide enhances cadmium-induced oxidative stress resistance in stock (Matthiola incana L.). Plant Signal. Behav. 2024, 19, 2331357. [Google Scholar] [CrossRef] [PubMed]
- Naqqash, T.; Aziz, A.; Baber, M.; Shahid, M.; Sajid, M.; Emanuele, R.; Gaafar, A.-R.Z.; Hodhod, M.S.; Haider, G. Metal-tolerant morganella morganii isolates can potentially mediate nickel stress tolerance in Arabidopsis by upregulating antioxidative enzyme activities. Plant Signal. Behav. 2024, 19, 2318513. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xing, Y.; Huang, B.; Chen, X.; Ji, L.; Fu, X.; Li, T.; Wang, J.; Chen, G.; Zhang, Q. Rhizospheric mechanisms of Bacillus subtilis bioaugmentation-assisted phytostabilization of cadmium-contaminated soil. Sci. Total Environ. 2022, 825, 154136. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Li, B.Z.; Wang, T.; Wang, L. Study of Two Microbes Combined to Remediate Field Soil Cadmium Pollution. J. Soil Water Conserv. 2020, 34, 364–369. [Google Scholar]
- Gao, J.-J.; Peng, R.-H.; Zhu, B.; Tian, Y.-S.; Xu, J.; Wang, B.; Fu, X.-Y.; Han, H.-J.; Wang, L.-J.; Zhang, F.-J.; et al. Enhanced phytoremediation of TNT and cobalt co-contaminated soil by AfSSB transformed plant. Ecotoxicol. Environ. Saf. 2021, 220, 112407. [Google Scholar] [CrossRef]
- Cruz, Y.; Villar, S.; Gutiérrez, K.; Montoya-Ruiz, C.; Gallego, J.L.; Delgado, M.d.P.; Saldarriaga, J.F. Gene expression and morphological responses of Lolium perenne L. exposed to cadmium (Cd2+) and mercury (Hg2+). Sci. Rep. 2021, 11, 11257. [Google Scholar] [CrossRef]
- Zheng, Y.; Cui, M.; Ni, L.; Qin, Y.; Li, J.; Pan, Y.; Zhang, X. Heterologous Expression of Human Metallothionein Gene HsMT1L Can Enhance the Tolerance of Tobacco (Nicotiana nudicaulis Watson) to Zinc and Cadmium. Genes 2022, 13, 2413. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Desoky, E.-S.M.; El-Tarabily, K.A.; AbuQamar, S.F.; Saad, A.M. Exploiting the role of plant growth promoting rhizobacteria in reducing heavy metal toxicity of pepper (Capsicum annuum L.). Environ. Sci. Pollut. Res. 2024, 31, 27465–27484. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huang, Z.; Zhong, Z.; Bian, F.; Zhang, X. Integrated Genomics and Transcriptomics Provide Insights into Salt Stress Response in Bacillus subtilis ACP81 from Moso Bamboo Shoot (Phyllostachys praecox) Processing Waste. Microorganisms 2024, 12, 285. [Google Scholar] [CrossRef]
- Wang, D.S.; Wang, L.L.; Li, Q.; Zhou, T.; Zhou, X.; Dao, Q. Enhancing effect of siderophore-producting bacteria on remediation of cadmium-contaminated soil by Solanum nigrum L. J. Environ. Eng. 2018, 12, 2311–2319. [Google Scholar]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The Significance of Bacillus spp. in Disease Suppression and Growth Promotion of Field and Vegetable Crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Z.; Jiang, X.L.; Cai, Q.Z.; Zhou, L.Y.; Yang, Q. Screening of plant growth-promoting rhizobacteria and its effect on seed germination of Polygonum multiflorum. Zhongguo Zhong Yao Za Zhi 2021, 46, 5247–5252. [Google Scholar] [PubMed]
- Jiao, H.; Wang, R.; Qin, W.; Yang, J. Screening of rhizosphere nitrogen fixing, phosphorus and potassium solubilizing bacteria of Malus sieversii (Ldb.) Roem. and the effect on apple growth. J. Plant Physiol. 2024, 292, 154142. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.H.; Abdellatif, M.M.; Mostafa, H.M.; Elsehemy, I.A.; Kobisi, A.E.A. Biodegradation and antimicrobial capability-induced heavy metal resistance of the marine-derived actinomycetes Nocardia harenae JJB5 and Amycolatopsis marina JJB11. World J. Microbiol. Biotechnol. 2024, 40, 202. [Google Scholar] [CrossRef] [PubMed]
- Sarvepalli, M.; Velidandi, A.; Korrapati, N. Optimization of Siderophore Production in Three Marine Bacterial Isolates along with Their Heavy-Metal Chelation and Seed Germination Potential Determination. Microorganisms 2023, 11, 2873. [Google Scholar] [CrossRef]
- Ghorbel, S.; Aldilami, M.; Zouari-Mechichi, H.; Mechichi, T.; AlSherif, E.A. Isolation and characterization of a plant growth-promoting rhizobacterium strain MD36 that promotes barley seedlings and growth under heavy metals stress. 3 Biotech 2023, 13, 145. [Google Scholar] [CrossRef]
- Ballén, V.; Gabasa, Y.; Ratia, C.; Sánchez, M.; Soto, S. Correlation Between Antimicrobial Resistance, Virulence Determinants and Biofilm Formation Ability Among Extraintestinal Pathogenic Escherichia coli Strains Isolated in Catalonia, Spain. Front. Microbiol. 2022, 12, 803862. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xie, Y.L.; Wu, X.H.; Yang, X.; Wang, T.; Wu, L.L. Avena sativa growth-promoting activity of Bacillus atrophaeus CKL1 under salt stress and the functional genes. Microbiol. Bull. 2022, 49, 3150–3164. [Google Scholar]
- Jin, Y.L.; Yao, T.; Lan, X.J.; Li, C.N.; Ma, Y.C. Screening of Antagonistic Bacteria of Oat Root Rot and Studies of Antibacterial Properties. J. Grassl. Sci. 2022, 30, 1095–1101. [Google Scholar]
- Zhang, Z.; Ma, Q.L.; Zhang, D.K.; Wei, L.Y.; Chen, F.; Qi, F.J. Effects of Exogenous Hormone on Seed Germination of Agriophyllum squarrosum. Chin. Agron. Bull. 2023, 39, 40–44. [Google Scholar]
- Amri, M.; Rjeibi, M.R.; Gatrouni, M.; Mateus, D.M.R.; Asses, N.; Pinho, H.J.O.; Abbes, C. Isolation, Identification, and Characterization of Phosphate-Solubilizing Bacteria from Tunisian Soils. Microorganisms 2023, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.A.; Clément, E.; Arlt, J.; Douarche, C.; Dawson, A.; Schwarz-Linek, J.; Creppy, A.K.; Škultéty, V.; Morozov, A.N.; Auradou, H.; et al. A combined rheometry and imaging study of viscosity reduction in bacterial suspensions. Proc. Natl. Acad. Sci. USA 2020, 117, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, W.; Li, Y.; Yu, F.; Penttinen, P. Isolation, characterization, and evaluation of a high-siderophore-yielding bacterium from heavy metal-contaminated soil. Environ. Sci. Pollut. Res. 2022, 29, 3888–3899. [Google Scholar] [CrossRef] [PubMed]
- Chieb, M.; Gachomo, E.W. The role of plant growth promoting rhizobacteria in plant drought stress responses. BMC Plant Biol. 2023, 23, 407. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef]
- Atta, M.I.; Zehra, S.S.; Dai, D.-Q.; Ali, H.; Naveed, K.; Ali, I.; Sarwar, M.; Ali, B.; Iqbal, R.; Bawazeer, S.; et al. Amassing of heavy metals in soils, vegetables and crop plants irrigated with wastewater: Health risk assessment of heavy metals in Dera Ghazi Khan, Punjab, Pakistan. Front. Plant Sci. 2023, 13, 1080635. [Google Scholar] [CrossRef]
- Sharma, J.K.; Kumar, N.; Singh, N.P.; Santal, A.R. Phytoremediation technologies and their mechanism for removal of heavy metal from contaminated soil: An approach for a sustainable environment. Front. Plant Sci. 2023, 14, 1076876. [Google Scholar] [CrossRef]
- Hou, D.; O’Connor, D.; Igalavithana, A.D.; Alessi, D.S.; Luo, J.; Tsang, D.C.W.; Sparks, D.L.; Yamauchi, Y.; Rinklebe, J.; Ok, Y.S. Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat. Rev. Earth Environ. 2020, 1, 366–381. [Google Scholar] [CrossRef]
- Sodhi, K.K.; Singh, C.K.; Kumar, M.; Singh, D.K. Whole-genome sequencing of Alcaligenes sp. strain MMA: Insight into the antibiotic and heavy metal resistant genes. Front. Pharmacol. 2023, 14, 1144561. [Google Scholar] [CrossRef]
- Hou, D. Sustainable Remediation of Contaminated Soil and Groundwater: Materials, Processes, and Assessment; Butterworth-Heinemann: Oxford, UK, 2019. [Google Scholar]
- Taghavi, S.; Garafola, C.; Monchy, S.; Newman, L.; Hoffman, A.; Weyens, N.; Barac, T.; Vangronsveld, J.; van der Lelie, D. Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar Trees. Appl. Environ. Microbiol. 2009, 75, 748–757. [Google Scholar] [CrossRef]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef]
- Gavrilescu, M. Enhancing phytoremediation of soils polluted with heavy metals. Curr. Opin. Biotechnol. 2022, 74, 21–31. [Google Scholar] [CrossRef]
- Carlos, M.-H.J.; Stefani, P.-V.Y.; Janette, A.-M.; Melani, M.-S.S.; Gabriela, P.-O. Assessing the effects of heavy metals in ACC deaminase and IAA production on plant growth-promoting bacteria. Microbiol. Res. 2016, 188–189, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.H.; Kuhl-Nagel, T.; Sommermann, L.; Moradtalab, N.; Chowdhury, S.P.; Schloter, M.; Windisch, S.; Schellenberg, I.; Maccario, L.; Sorensen, S.J.; et al. Long-term conservation tillage with reduced nitrogen fertilization intensity can improve winter wheat health via positive plant-microorganism feedback in the rhizosphere. FEMS Microbiol. Ecol. 2024, 100, fiae003. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-M.; Zheng, H.-X.; Zhang, X.-S.; Sui, N. Cytokinins as central regulators during plant growth and stress response. Plant Cell Rep. 2021, 40, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Tassi, E.; Pouget, J.; Petruzzelli, G.; Barbafieri, M. The effects of exogenous plant growth regulators in the phytoextraction of heavy metals. Chemosphere 2008, 71, 66–73. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, S.; Xue, T.; Yuan, Y. Physiology and transcriptome of Eucommia ulmoides seeds at different germination stages. Plant Signal. Behav. 2024, 19, 2329487. [Google Scholar] [CrossRef]
- Nagel, R.; Bieber, J.E.; Schmidt-Dannert, M.G.; Nett, R.S.; Peters, R.J. A Third Class: Functional Gibberellin Biosynthetic Operon in Beta-Proteobacteria. Front. Microbiol. 2018, 9, 2916. [Google Scholar] [CrossRef]
- Joo, G.-J.; Kang, S.-M.; Hamayun, M.; Kim, S.-K.; Na, C.-I.; Shin, D.-H.; Lee, I.-J. Burkholderia sp. KCTC 11096BP as a newly isolated gibberellin producing bacterium. J. Microbiol. 2009, 47, 167–171. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Nguyen, Q.T.; Dang, D.H.; Emery, R.J.N. Phytohormones enhance heavy metal responses in Euglena gracilis: Evidence from uptake of Ni, Pb and Cd and linkages to hormonomic and metabolomic dynamics. Environ. Pollut. 2023, 320, 121094. [Google Scholar] [CrossRef]
- Ben Massoud, M.; Sakouhi, L.; Karmous, I.; Zhu, Y.; El Ferjani, E.; Sheehan, D.; Chaoui, A. Protective role of exogenous phytohormones on redox status in pea seedlings under copper stress. J. Plant Physiol. 2018, 221, 51–61. [Google Scholar] [CrossRef]
- Liang, Y.; Xiao, X.; Guo, Z.; Peng, C.; Zeng, P.; Wang, X. Co-application of indole-3-acetic acid/gibberellin and oxalic acid for phytoextraction of cadmium and lead with Sedum alfredii Hance from contaminated soil. Chemosphere 2021, 285, 131420. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yang, J.; Zhang, Y.; Yang, S.; Zhang, Y.; Chen, Y.; Shao, Y.; Gao, D.; Yuan, Z.; Zhang, Y. Mulches assist degraded soil recovery via stimulating biogeochemical cycling: Metagenomic analysis. Appl. Microbiol. Biotechnol. 2023, 108, 20. [Google Scholar] [CrossRef]
- Chandwani, S.; Amaresan, N. Siderophore-producing bacteria mitigate cobalt stress in black gram (Vigna mungo L.), and the mitigation strategies are associated with iron concentration. Environ. Sci. Pollut. Res. 2023, 30, 123556–123569. [Google Scholar] [CrossRef]
- Santos, K.F.D.N.; Moure, V.R.; Hauer, V.; Santos, A.R.; Donatti, L.; Galvão, C.W.; Pedrosa, F.O.; Souza, E.M.; Wassem, R.; Steffens, M.B. Wheat colonization by an Azospirillum brasilense ammonium-excreting strain reveals upregulation of nitrogenase and superior plant growth promotion. Plant Soil 2017, 415, 245–255. [Google Scholar] [CrossRef]
- Çakmakçı, R.; Erat, M.; Erdoğan, Ü.; Dönmez, M.F. The influence of plant growth–promoting rhizobacteria on growth and enzyme activities in wheat and spinach plants. J. Plant Nutr. Soil Sci. 2007, 170, 288–295. [Google Scholar] [CrossRef]
- Dhiman, V.K.; Rana, N.; Dhiman, V.K.; Pandey, H.; Verma, P.; Singh, D. Effect of rhizobial isolates and nitrogen fertilizers on nursery performance, nodulation behavior and nitrogenase activity of Dalbergia sissoo Roxb. seedlings. Plant Stress 2022, 4, 100080. [Google Scholar] [CrossRef]
- Jalloh, A.A.; Khamis, F.M.; Yusuf, A.A.; Subramanian, S.; Mutyambai, D.M. Long-term push–pull cropping system shifts soil and maize-root microbiome diversity paving way to resilient farming system. BMC Microbiol. 2024, 24, 92. [Google Scholar] [CrossRef]
- De Zutter, N.; Ameye, M.; Debode, J.; De Tender, C.; Ommeslag, S.; Verwaeren, J.; Vermeir, P.; Audenaert, K.; De Gelder, L. Shifts in the rhizobiome during consecutive in planta enrichment for phosphate-solubilizing bacteria differentially affect maize P status. Microb. Biotechnol. 2021, 14, 1594–1612. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Oliveira, R.S.; Freitas, H.; Zhang, C. Biochemical and Molecular Mechanisms of Plant-Microbe-Metal Interactions: Relevance for Phytoremediation. Front. Plant Sci. 2016, 7, 918. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. 2016, 23, 3984–3999. [Google Scholar] [CrossRef] [PubMed]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, C.; Bei, S.; Wang, G.; Geisen, S.; Bedoussac, L.; Christie, P.; Zhang, J. High bacterial diversity and siderophore-producing bacteria collectively suppress Fusarium oxysporum in maize/faba bean intercropping. Front. Microbiol. 2022, 13, 972587. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhang, M.; Liu, K.; Xue, Y.; Li, X.; Dong, C. Phylogeny of the HO family in cyprinus carpio and the response of the HO-1 gene to adding Bacillus coagulans in feed under Cd2+ stress. Fish Physiol. Biochem. 2022, 48, 117–131. [Google Scholar] [CrossRef]
- Raza, A.; Habib, M.; Kakavand, S.N.; Zahid, Z.; Zahra, N.; Sharif, R.; Hasanuzzaman, M. Phytoremediation of Cadmium: Physiological, Biochemical, and Molecular Mechanisms. Biology 2020, 9, 177. [Google Scholar] [CrossRef]
- French, K.S.; Chukwuma, E.; Linshitz, I.; Namba, K.; Duckworth, O.W.; Cubeta, M.A.; Baars, O. Inactivation of siderophore iron-chelating moieties by the fungal wheat root symbiont Pyrenophora biseptata. Environ. Microbiol. Rep. 2024, 16, e13234. [Google Scholar] [CrossRef]
- Khan, A.; Singh, P.; Srivastava, A. Synthesis, nature and utility of universal iron chelator—Siderophore: A review. Microbiol. Res. 2018, 212–213, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, P.; Keutgen, A.J.; Keutgen, N.; Sierka, E.; Baczewska-Dąbrowska, A.H.; Mojski, J.; Pawluśkiewicz, B.; Sieczko, L.; Kalaji, H.M. Photosynthetic efficiency of perennial ryegrass (Lolium perenne L.) seedlings in response to Ni and Cd stress. Sci. Rep. 2023, 13, 5357. [Google Scholar] [CrossRef] [PubMed]





| Strain | Siderophore Activity (%) | A/Ar |
|---|---|---|
| WL1210 | 36.16 | ++ |
| CD303 | 49.88 | +++ |
| Treatment Concentration (mg/L) | Germination Rate per Day after Bed Placement (%) | |||
|---|---|---|---|---|
| 7 d | 8 d | 9 d | 10 d | |
| 0 | 78.0 ± 4.00 | 81.3 ± 5.77 | 85.3 ± 9.45 | 91.3 ± 6.11 |
| 20 | 76.0 ± 5.29 | 78.6 ± 8.08 | 84.0 ± 12.00 | 88.0 ± 12.41 |
| 40 | 78.0 ± 3.46 | 83.3 ± 6.42 | 84.0 ± 9.16 | 88.7 ± 9.45 |
| 60 | 77.3 ± 6.11 | 81.3 ± 5.77 | 83.0 ± 8.71 | 83.3 ± 9.45 |
| 80 | 18.0 ± 4.00 | 18.6 ± 3.06 | 29.3 ± 11.37 | 34.7 ± 11.79 |
| Concentration (mg/L) | GV (%) | GR (%) | GI | VI |
|---|---|---|---|---|
| 0 | 78.0 a | 91.3 a | 5.0 ± 0.44 a | 15.9 ± 3.04 a |
| 20 | 76.0 a | 88.0 a | 4.9 ± 0.44 ab | 13.5 ± 1.09 ab |
| 40 | 78.0 a | 88.7 a | 5.0 ± 0.50 a | 10.9 ± 1.06 bc |
| 60 | 77.3 a | 83.3 a | 4.9 ± 0.69 a | 8.7 ± 1.14 c |
| 80 | 18.0 b | 34.7 b | 1.5 ± 0.27 b | 1.4 ± 0.59 d |
| Gene Name | Location | Length | Function |
|---|---|---|---|
| glpQ | 236,068–236,355 | 288 bp | Glycerophosphodiester phosphodiesterase |
| hxlA | 380,639–381,271 | 633 bp | 3-hexulose-6-phosphate synthase |
| hxlB | 380,075–380,632 | 558 bp | Carbohydrate derivative metabolic process, isomerase activity, and carbohydrate derivative binding |
| miaA | 1,957,705–1,958,649 | 945 bp | Isopentenyl adenine gene, considered the main cytokinin |
| miaB | 1,861,144–1,862,673 | 1530 bp | Isopentenyl adenine gene, considered the main cytokinin |
| ndhF | 197,749–199,266 | 1518 bp | NADH dehydrogenase subunit 5, chloroplast functional genes |
| YBCL | 204,425–205,597 | 1173 bp | MFS transporter, encodes the Rubisco enzymes in plants, and is capable of converting carbon dioxide into organic matter |
| frmA | 372,000–373,136 | 1137 bp | Threonine dehydrogenase or related Zn-dependent dehydrogenase |
| phnB | 408,798–409,163 | 366 bp | Zn-dependent glyoxalase, PhnB family |
| fnr | 3,382,739–3,383,740 | 1002 bp | Ferredoxin and NADP reductase 2 |
| iscA | 3,385,318–3,385,680 | 363 bp | Iron-sulfur cluster assembly protein |
| ggt | 2,146,580–2,148,346 | 1767 bp | Plant stress tolerance enhancer |
| mgtC | 791,225–791,917 | 693 bp | Magnesium uptake protein YhiD/SapB, involved in acid resistance |
| proB | 2,158,357–2,159,481 | 1125 bp | Involved in proline or betaine synthesis and alleviates cell apoptosis |
| proC | 2,159,505–2,160,305 | 801 bp | Involved in proline or betaine synthesis and alleviates cell apoptosis |
| glnA | 1,969,335–1,970,669 | 1335 bp | Involved in the regulation of nitrogen fixation |
| glnR | 1,968,870–1,969,277 | 408 bp | Involved in the regulation of nitrogen fixation |
| licA | 382,536–393,302 | 10,767 bp | Surfactin non-ribosomal peptide synthetase SrfAA |
| licB | 393,324–404,093 | 10,770 bp | Surfactin non-ribosomal peptide synthetase SrfAB |
| licC | 404,112–407,942 | 3831 bp | Non-ribosomal peptide synthetase |
| srfATE | 407,970–408,704 | 735 bp | Surfactin biosynthesis thioesterase SrfAD |
| acpT | 411,550–412,224 | 675 bp | 4′-phosphopantetheinyl transferase superfamily protein |
| tcyC | 413,260–414,003 | 744 bp | Amino acid ABC transporter ATP-binding protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Xie, Y.; Li, J.; Han, M.; Yang, X.; Chang, F. The Effect of Two Siderophore-Producing Bacillus Strains on the Growth Promotion of Perennial Ryegrass under Cadmium Stress. Microorganisms 2024, 12, 1083. https://doi.org/10.3390/microorganisms12061083
Wu L, Xie Y, Li J, Han M, Yang X, Chang F. The Effect of Two Siderophore-Producing Bacillus Strains on the Growth Promotion of Perennial Ryegrass under Cadmium Stress. Microorganisms. 2024; 12(6):1083. https://doi.org/10.3390/microorganisms12061083
Chicago/Turabian StyleWu, Lingling, Yongli Xie, Junxi Li, Mingrong Han, Xue Yang, and Feifei Chang. 2024. "The Effect of Two Siderophore-Producing Bacillus Strains on the Growth Promotion of Perennial Ryegrass under Cadmium Stress" Microorganisms 12, no. 6: 1083. https://doi.org/10.3390/microorganisms12061083
APA StyleWu, L., Xie, Y., Li, J., Han, M., Yang, X., & Chang, F. (2024). The Effect of Two Siderophore-Producing Bacillus Strains on the Growth Promotion of Perennial Ryegrass under Cadmium Stress. Microorganisms, 12(6), 1083. https://doi.org/10.3390/microorganisms12061083






