Effects of Different Farming Modes on Salmo trutta fario Growth and Intestinal Microbial Community
Abstract
1. Introduction
2. Materials and Methods
2.1. Aquaculture Farming Setup
2.2. Measurement of Growth Parameters and Aquaculture Water Environmental Factors
2.3. Collection and DNA Extraction of Intestinal Samples
2.4. Amplification and High-Throughput Sequencing of Intestinal Microbiota 16S rRNA
2.5. Statistical Analysis
3. Results
3.1. Varied Growth Performance Metrics among Salmo trutta fario Populations under Various Aquaculture Modes
3.2. Highly Variable Diversity in Salmo trutta fario Intestinal Microbiota Communities across Various Aquaculture Modes
3.3. Variations in Salmo trutta fario Intestinal Microbiota Community across Various Aquaculture Modes
3.4. Distribution and Functional Prediction of Core Microorganisms in Salmo trutta fario Intestinal Tract across Various Aquaculture Modes
3.5. Co-Occurrence Network Analysis of Salmo trutta fario Intestinal Microbiota Community across Diverse Aquaculture Modes
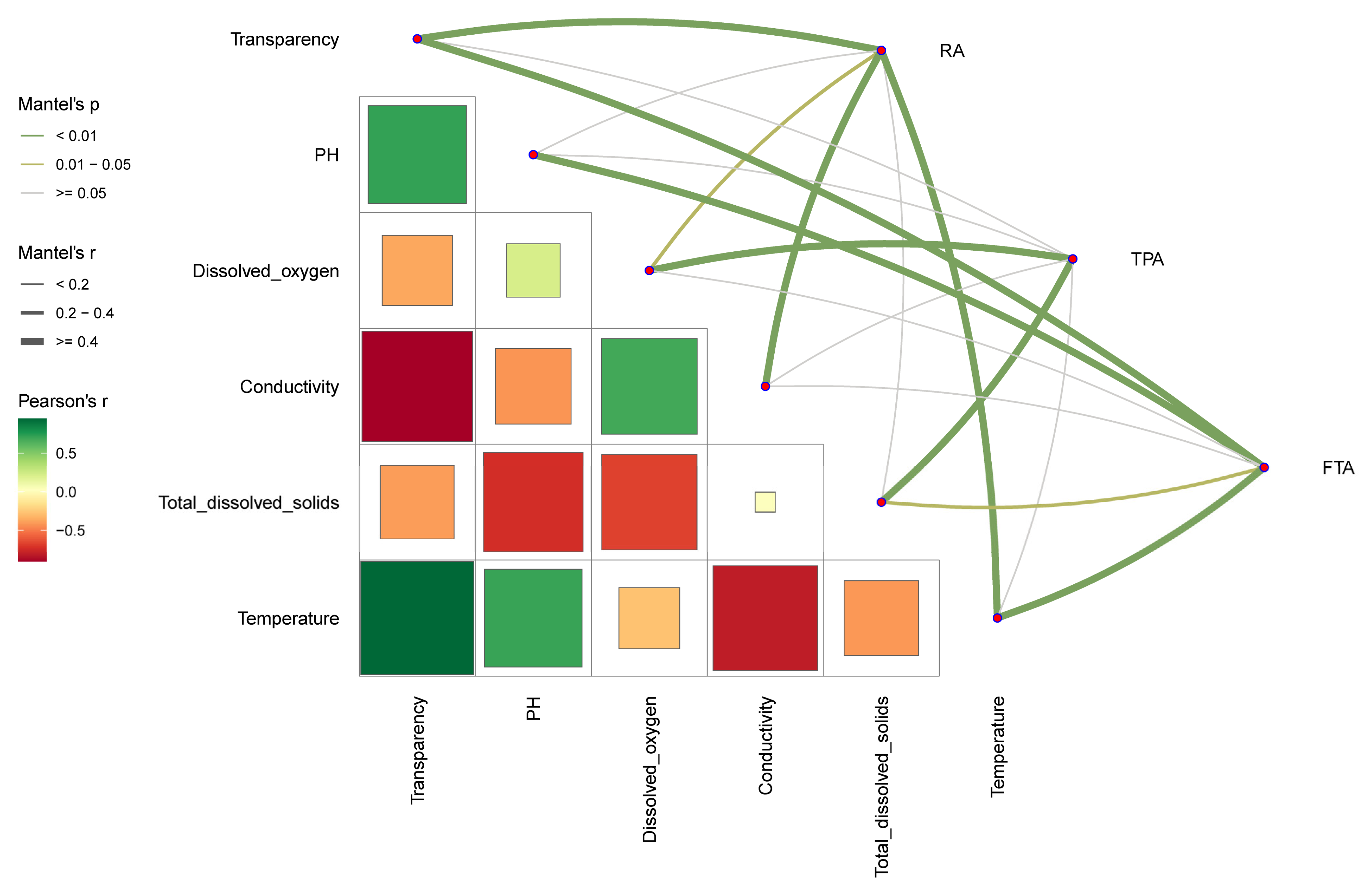
3.6. Different Aquaculture Modes Impact Salmo trutta fario Gut Microbiota Community Characteristics through Aquatic Environmental Factors
4. Discussion
4.1. Differences in Salmo trutta fario Gut Microbiota Characteristics and Distribution Patterns across Various Aquaculture Modes
4.2. Differences in Salmo trutta fario Growth Indicators and Aquaculture Water Environment across Various Aquaculture Modes
4.3. Water Environmental Factors: Key Influences on Salmo trutta fario Gut Microbiota across Various Aquaculture Modes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.L.; Wang, W.B. Fish of Xizang. Acta Zool. Sin. 1962, 14, 529–536. [Google Scholar]
- Maccrimmon, H.R.; Marshall, T.L.; Gots, B.L. World Distribution of Brown Trout, Salmo trutta: Further Observations. J. Fish. Res. Board Can. 1970, 27, 811–818. [Google Scholar] [CrossRef]
- Peng, S.S. Fish and Their Resources in Tibet; China Agriculture Press: Beijing, China, 1995; pp. 32–33. [Google Scholar]
- Elliott, J.M. Wild brown trout Salmo trutta: An important national and international resource. Freshw. Biol. 1989, 21, 1–5. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, C.L.; Li, J.; Re, D.; Duoji, O.Z. Development status and countermeasures of salmon industry in Yadong, Xizang Province. China Fish. Econ. 2022, 40, 20–26. [Google Scholar]
- Karatas, T.; Cakir, M. Assessment of deltamethrin-induced DNA damage, neurotoxic and neuroimmune effects in the brain tissue of brown trout (Salmo trutta fario). Vet. Med. 2024, 69, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Drechsel, V.; Krais, S.; Peschke, K.; Ziegler, M.; Köhler, H.-R.; Triebskorn, R. Glyphosate-and aminomethylphosphonic acid (AMPA)-induced mortality and residues in juvenile brown trout (Salmo trutta f. fario) exposed at different temperatures. Environ. Sci. Eur. 2024, 36, 30. [Google Scholar] [CrossRef]
- Santos, M.E.S.; Grabicová, K.; Steinbach, C.; Schmidt-Posthaus, H.; Šálková, E.; Kolářová, J.; Staňová, A.V.; Grabic, R.; Randák, T. Environmental concentration of methamphetamine induces pathological changes in brown trout (Salmo trutta fario). Chemosphere 2020, 254, 126882. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.Y.; Gao, B.W.; Xu, Z.; Yang, H.; Li, X.Q.; Leng, X.J. Study on the suitable types and levels of starch in the feed of juvenile salmon in Yadong. Chin. J. Hydrobiol. 2022, 46, 69–78. [Google Scholar]
- Wang, W.L.; Mou, Z.B.; Zhou, J.J.; Wang, Q.L.; Chen, M.Q.; Zhang, C. Effects of β-glucan on survival and growth of juvenile Brown Trout Salmo trutta. J. Fish. Sci. 2019, 40, 273–278. [Google Scholar]
- Sun, S.J.; Zhou, J.S.; Wang, W.L.; Chen, M.Q.; Zhang, C.; Li, M. Isolation and identification of the pathogenic bacteria of bacterial gill disease of salmon. Fish. Sci. 2019, 42, 288–295. [Google Scholar]
- Wang, C.G.; Hu, G.; Sun, P.; Gu, W.; Wang, B.; Xu, Q. Effects of Dietary Protein and Fat Levels on Growth Performance, Digestive enzyme activities and Serum Indexes of Salmonid parents. Chin. J. Anim. Nutr. 2017, 29, 571–582. [Google Scholar]
- Qi, C.Q. Effects of salinity on growth status and survival rate of Salmo trutta fario. Heilongjiang Fish. 2022, 41, 40–42. [Google Scholar]
- Wang, J.L.; Wang, W.L.; Zhang, B.B.; Tan, D.M. Comparison of the anesthetic effect of MS-222 and eugenol on Salmo trutta fario. J. Gansu Agric. Univ. 2021, 56, 26–32+40. [Google Scholar]
- Zhang, C.; Zhang, B.B.; Mou, Z.B.; Zhou, J.S.; Zeng, B.H.; Liu, H.P.; Wang, W.L. The effect of salinity on the growth and survival of Salmo trutta fario fry. Southwest Agric. J. 2019, 32, 1659–1663. [Google Scholar]
- Wang, W.L.; Zhang, B.B.; Zhou, J.S.; Zeng, B.H.; Wang, Q.L.; Wang, J.L.; Pan, Y.Z. Effects of different water temperatures on the growth and survival of young Salmo trutta fario. Aquat. Sci. Technol. Inf. 2019, 46, 24–27. [Google Scholar]
- Wang, W.L.; Zhang, B.B.; Tan, D.M. Effects of different cultivation modes on growth performance, serum biochemical indexes and muscle nutrient composition of young Salmo trutta fario. J. Hydrobiol. 2023, 47, 488–494. [Google Scholar]
- Wang, Q.L.; Wang, J.L.; Liu, F.; Zeng, B.H.; Wang, W.L.; Zhou, J.S.; Pan, Y.Z. Effects of different cultivation densities on the growth performance of Asian salmon sub adults. Aquaculture 2020, 41, 8–10. [Google Scholar]
- Chen, M.L.; Li, X.; Yuan, D.D.; Sun, S.J.; Yu, G.Q.; Hu, G.; Luan, P.X.; Wang, W.L.; Zhaxi, L.M.; Zhou, J.S. Genetic background analysis of Asian salmon breeding population based on molecular marker co ancestor. Freshw. Fish. 2023, 53, 82–90. [Google Scholar]
- Liu, J.H.; Liu, C.L.; Duojiou, Z.; Re, D.; Labaluo, B.; Liu, B.L.; Li, J.; Ma, Q.; Wei, P.L.; Liang, M.Q.; et al. Comparative analysis of nutritional composition of different colored Asian salmon egg. Prog. Fish. Sci. 2023, 44, 133–141. [Google Scholar]
- Chen, M.Q.; Zaxi, L.; Sun, S.J.; Zhang, C.; Zhou, J.S.; Wang, Q.L.; Wang, W.L. Identification and pathogenicity and drug sensitivity analysis of 2 strains of Pseudomonas aeruginosa from Asian salmon. Chin. J. Aquat. Sci. 2022, 29, 914–927. [Google Scholar]
- Delghandi, M.R.; Menanteau-Ledouble, S.; Waldner, K.; El-Matbouli, M. Renibacterium salmoninarum and Mycobacterium spp.: Two bacterial pathogens present at low levels in wild brown trout (Salmo trutta fario) populations in Austrian rivers. BMC Vet. Res. 2020, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Rawls, J.F. Intestinal microbiota composition in fishes is influenced by host ecology and environment. Mol. Ecol. 2012, 21, 3100–3102. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.H.; Olafsen, J.A. Bacterial Colonization of Cod (Gadus morhua L.) and Halibut (Hippoglossus hippoglossus) Eggs in Marine Aquaculture. Appl. Environ. Microbiol. 1989, 55, 1435. [Google Scholar] [CrossRef]
- Nayak, S.K. Role of gastrointestinal microbiota in fish. Aquac. Res. 2010, 41, 1553–1573. [Google Scholar] [CrossRef]
- Chen, X.X.; Wu, Z.X.; Zhou, W.H. Research progress on the role and influencing factors of fish digestive tract microbiota. J. Huazhong Agric. Univ. 2005, 101–106. [Google Scholar]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of infammatory bowel diseasely. Clin. J. Gastroenterol. 2018, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Spanggaard, B.; Huber, I.; Nielsen, J.; Nielsen, T.; Appel, K.; Gram, L. The microflora of rainbow trout intestine: A comparison of traditional and molecular identification. Aquaculture 2000, 182, 1–15. [Google Scholar] [CrossRef]
- Navarrete, P.; Mardones, P.; Opazo, R.; Espejo, R.; Romero, J. Oxytetracycline Treatment Reduces Bacterial Diversity of Intestinal Microbiota of Atlantic Salmon. J. Aquat. Anim. Health 2008, 20, 77–183. [Google Scholar] [CrossRef] [PubMed]
- Givens, C.E.; Ransom, B.; Bano, N.; Hollibaugh, J.T. Comparson of the gut microbiomes of 12 bony fish and 3 shark species. Mar. Ecol. Prog. 2015, 518, 209–223. [Google Scholar] [CrossRef]
- Xu, S.G.; Zhang, L.; Yang, H.L.; Xu, X.L. Research progress on gut microbiota of salmon fish. China Fish. 2019, 1, 104–106. [Google Scholar]
- Ding, X.; Liu, J.; Liu, W.; Dai, S.; Ke, Z.; Guo, J.; Lai, Y.; Tan, Y. Phytoplankton communities miniaturization driven by extreme weather in subtropical estuary under climate changes. Water Res. 2023, 245, 120588. [Google Scholar] [CrossRef]
- Ding, X.; Liu, J.; Zhang, H.; Ke, Z.; Li, J.; Liu, W.; Li, K.; Zhao, C.; Tan, Y. Phytoplankton community patterns in the Northeastern South China Sea: Implications of intensified kuroshio intrusion during the 2015/16 El Niño. J. Geophys. Res. Ocean. 2022, 127, e2021JC017998. [Google Scholar] [CrossRef]
- Yang, B.B.; Shao, Q.J. Research progress on gut microbiota in fish. China Feed 2013, 23, 1–4+8. [Google Scholar]
- Kim, D.H.; Brunt, J.; Austin, B. Microbial diversity of intestinal contents and mucus in rainbow trout (Oncorhynchus mykiss). J. Appl. Microbiol. 2007, 102, 1654–1664. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.L.; Li, W.J.; Nie, G.X. Research progress on factors affecting intestinal flora of fish. J. Water Prod. 2019, 43, 143–155. [Google Scholar]
- Roeselers, G.; Mittge, E.K.; Stephens, W.Z.; Parichy, D.M.; Cavanaugh, C.M.; Guillemin, K.; Rawls, J.F. Evidence for a core gut microbiota in the zebrafish. ISME J. 2011, 5, 1595–1608. [Google Scholar] [CrossRef]
- Li, T.; Long, M.; Gatesoupe, F.J.; Zhang, Q.; Li, A.; Gong, X. Comparative analysis of the intestinal bacterial communities in different species of carp by pyrosequencing. Microb. Ecol. 2015, 69, 25–36. [Google Scholar] [CrossRef]
- Shi, S.; Nuccio, E.E.; Shi, Z.J.; He, Z.; Zhou, J.; Firestone, M.K. The interconnected rhizosphere: High network complexity dominates rhizo sphere assemblages. Ecol. Lett. 2016, 19, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Matthews, A.; Pierce, S.; Hipperson, H.; Raymond, B. Rhizobacterial community assembly patterns vary between crop species. Front. Microbiol. 2019, 10, 441189. [Google Scholar] [CrossRef]
- Bergman, K.; Henriksson, P.J.; Hornborg, S.; Troell, M.; Borthwick, L.; Jonell, M.; Philis, G.; Ziegler, F. Recirculating aquaculture is possible without major energy tradeoff: Life cycle assessment of warmwater fish farming in Sweden. Environ. Sci. Technol. 2020, 54, 16062–16070. [Google Scholar] [CrossRef]
- Crozier, L.G.; Zabel, R.W.; Hockersmith, E.E.; Achord, S. Interacting effects of density and temperature on body size in multiple populations of Chinook salmon. J. Anim. Ecol. 2010, 79, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.-Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun. 2019, 10, 5029. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, B.; Jonsson, N. A review of the likely effects of climate change on anadromous Atlantic salmon Salmo salar and brown trout Salmo trutta, with particular reference to water temperature and flow. J. Fish Biol. 2009, 75, 2381–2447. [Google Scholar] [CrossRef] [PubMed]
- Beemelmanns, A.; Zanuzzo, F.S.; Xue, X.; Sandrelli, R.M.; Rise, M.L.; Gamperl, A.K. The transcriptomic responses of Atlantic salmon (Salmo salar) to high temperature stress alone, and in combination with moderate hypoxia. BMC Genom. 2021, 22, 261. [Google Scholar] [CrossRef] [PubMed]
- Stephens, W.Z.; Burns, A.R.; Stagaman, K.; Wong, S.; Rawls, J.F.; Guillemin, K.; Bohannan, B.J.M. The composition of the zebrafish intestinal microbial community varies across development. ISME J. 2016, 10, 644–654. [Google Scholar] [CrossRef]
- Sullam, K.E.; Essinger, S.D.; Lozupone, C.A.; O’connor, M.P.; Rosen, G.L.; Knight, R.; Kilham, S.S.; Russell, J.A. Environmental and ecological factors that shape the gut bacterial communities of fish: A meta-analysis. Mol. Ecol. 2012, 21, 3363–3378. [Google Scholar] [CrossRef]
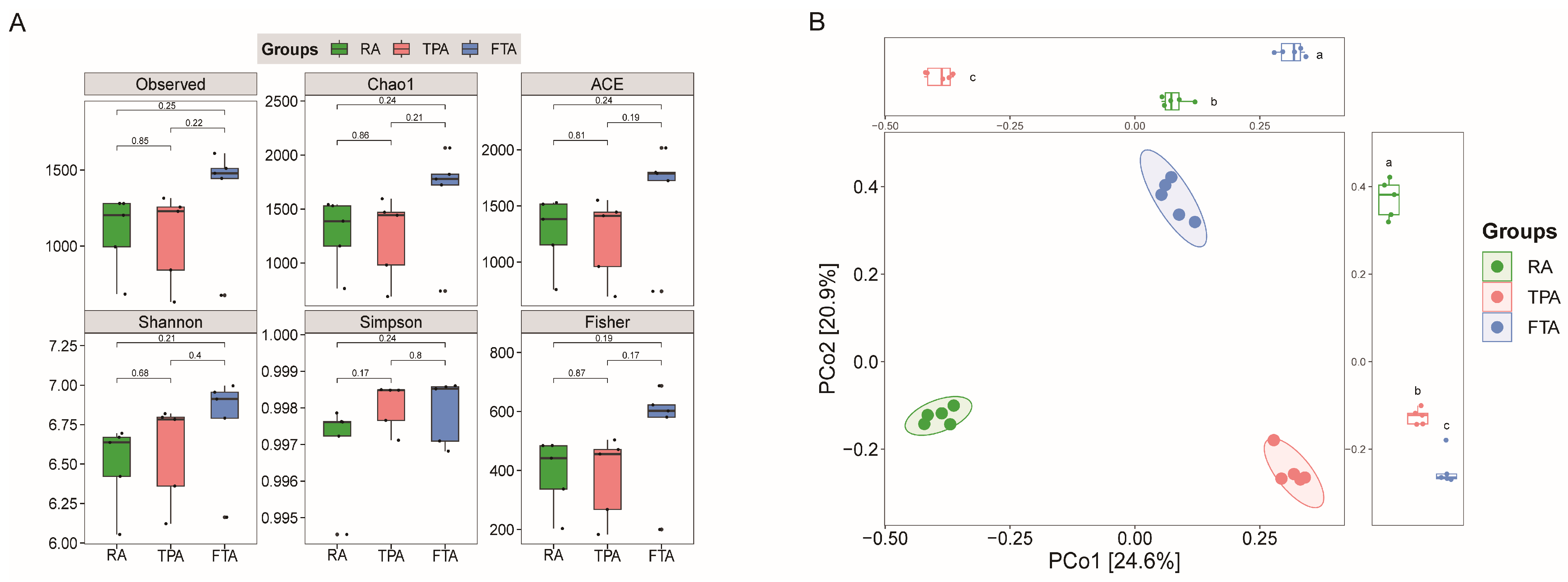
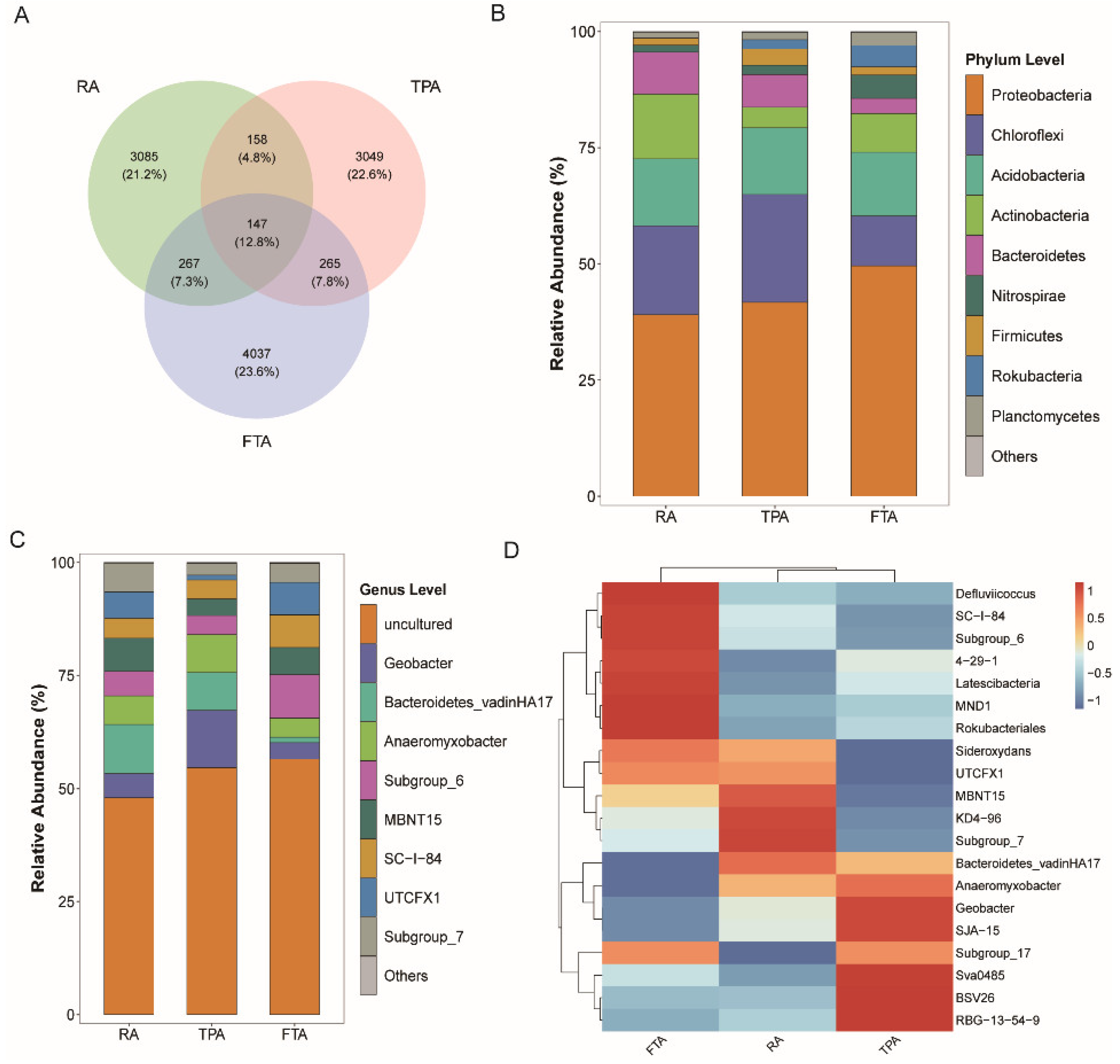
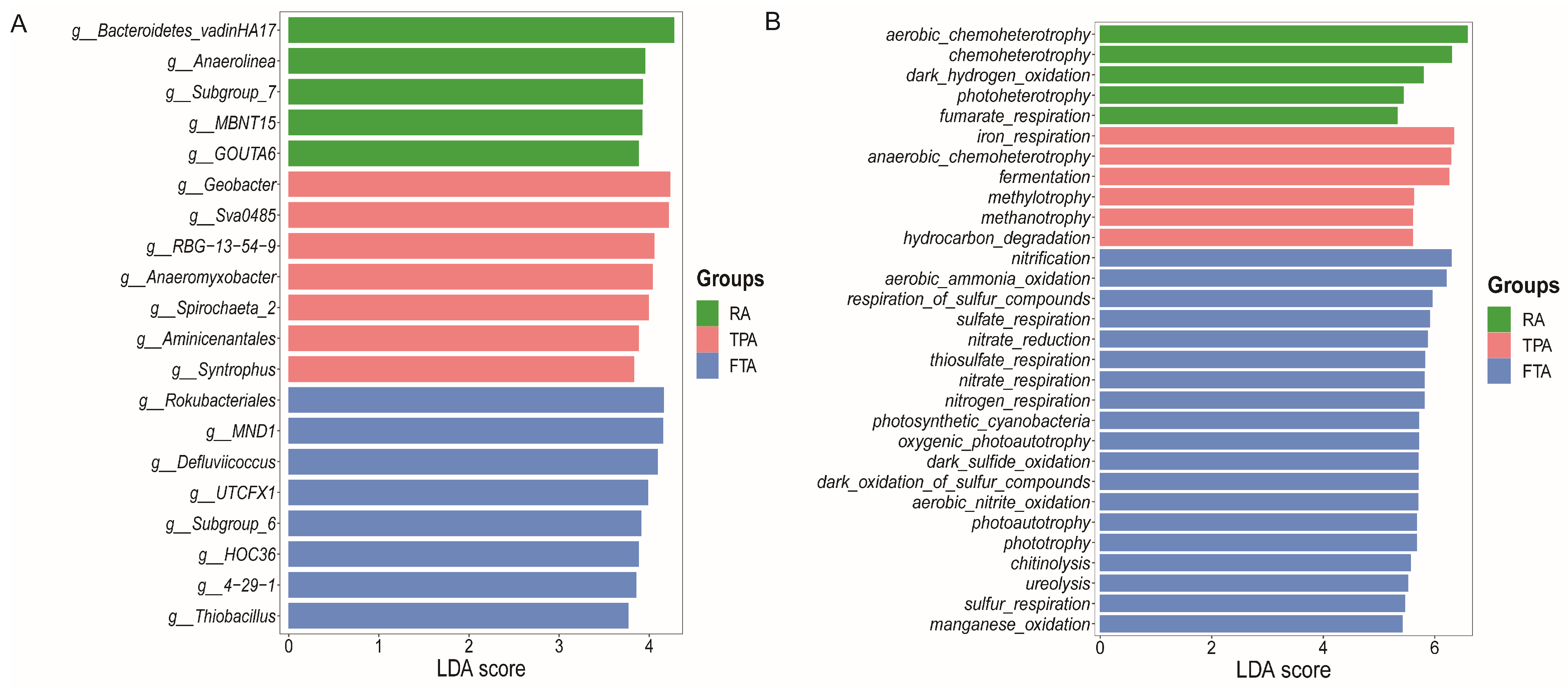
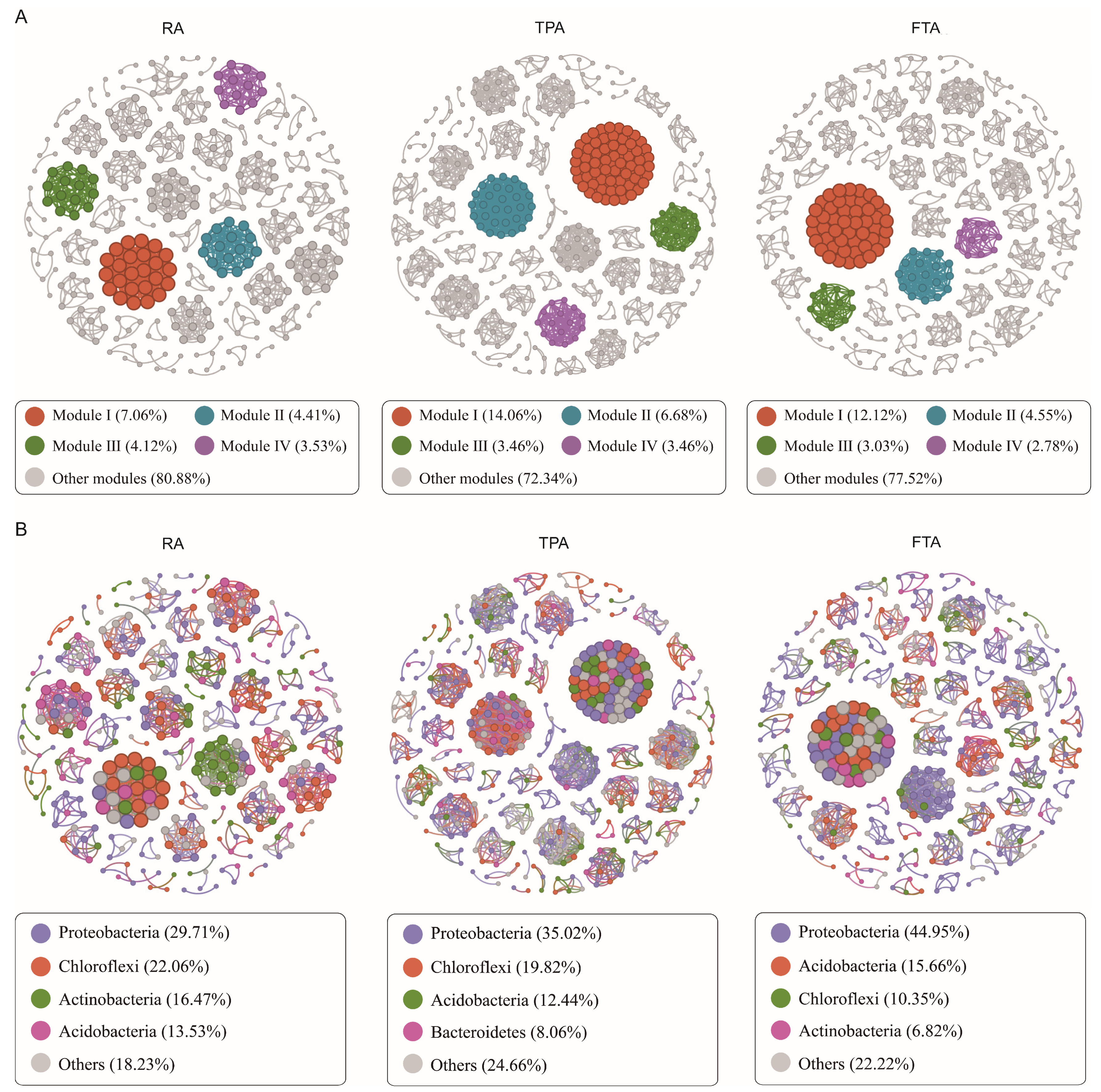
| Aquaculture Modes | Density (kg/m3) | Initial Body Weight (g) | Final Body Weight (g) | GR% | WGR% |
|---|---|---|---|---|---|
| TPA | 7.83 ± 0.06 a | 100.35 ± 2.89 a | 363.00 ± 4.56 b | 85.46 ± 1.23 b | 262.34 ± 7.45 b |
| RA | 7.81 ± 0.02 a | 103.04 ± 2.85 a | 315.29 ± 3.26 c | 97.08 ± 1.91 a | 206.13 ± 5.59 c |
| FTA | 7.85 ± 0.03 a | 102.67 ± 3.08 a | 423.72 ± 3.34 a | 99.17 ± 0.72 a | 314.10 ± 9.46 a |
| Aquaculture Modes | Transparency (cm) | pH | Dissolved Oxygen (mg/L) | Conductivity (μS/cm) | Total Dissolved Solids (mg/L) | Temperature (°C) |
|---|---|---|---|---|---|---|
| TPA | 0.32 ± 0.02 c | 7.60 ± 0.12 b | 6.67 ± 0.19 b | 306.80 ± 4.87 b | 211.67 ± 6.69 a | 9.30 ± 0.15 c |
| RA | 0.15 ± 0.01 b | 7.47 ± 0.09 b | 7.77 ± 0.09 a | 362.20 ± 5.63 a | 195.33 ± 3.28 a | 7.07 ± 0.15 b |
| FTA | 0.48 ± 0.02 a | 8.20 ± 0.06 a | 7.40 ± 0.06 a | 296.33 ± 3.71 b | 174.67 ± 3.48 b | 11.57 ± 0.41 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.-Z.; Wang, Z.-T.; Wang, W.-L.; Lei, K.-K.; Zhou, J.-S. Effects of Different Farming Modes on Salmo trutta fario Growth and Intestinal Microbial Community. Microorganisms 2024, 12, 1082. https://doi.org/10.3390/microorganisms12061082
Wang Z-Z, Wang Z-T, Wang W-L, Lei K-K, Zhou J-S. Effects of Different Farming Modes on Salmo trutta fario Growth and Intestinal Microbial Community. Microorganisms. 2024; 12(6):1082. https://doi.org/10.3390/microorganisms12061082
Chicago/Turabian StyleWang, Zhuang-Zhuang, Zhi-Tong Wang, Wan-Liang Wang, Kuan-Kuan Lei, and Jian-She Zhou. 2024. "Effects of Different Farming Modes on Salmo trutta fario Growth and Intestinal Microbial Community" Microorganisms 12, no. 6: 1082. https://doi.org/10.3390/microorganisms12061082
APA StyleWang, Z.-Z., Wang, Z.-T., Wang, W.-L., Lei, K.-K., & Zhou, J.-S. (2024). Effects of Different Farming Modes on Salmo trutta fario Growth and Intestinal Microbial Community. Microorganisms, 12(6), 1082. https://doi.org/10.3390/microorganisms12061082





