Lacticaseibacillus rhamnosus Strains for Alleviation of Irritable Bowel Disease and Chronic Fatigue Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Fecal Sample Collection, Bacterial Isolation, and Identification
2.2. Genome Sequencing, Phylogenomic Tree Construction, and Annotations for Antibiotic Resistance, Carbohydrate Utilization, and Virulence Factors
2.3. Determination of Generation Time and Tests for Acidity and Alkalinity Tolerance
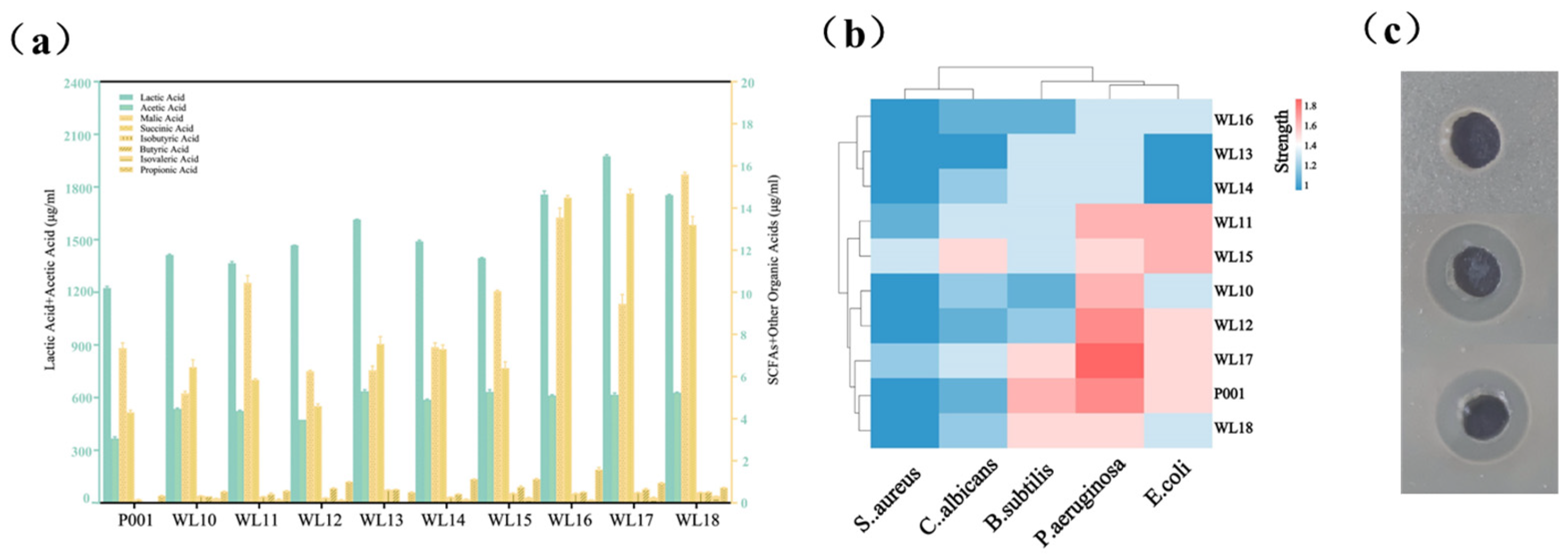
2.4. Carbohydrate Utilization, Production of Short-Chain Fatty Acids and Organic Acids, and Antibiotic Susceptibility Assays
2.5. Inhibition of the Growth of Causative Pathogenic Indicator Microbes
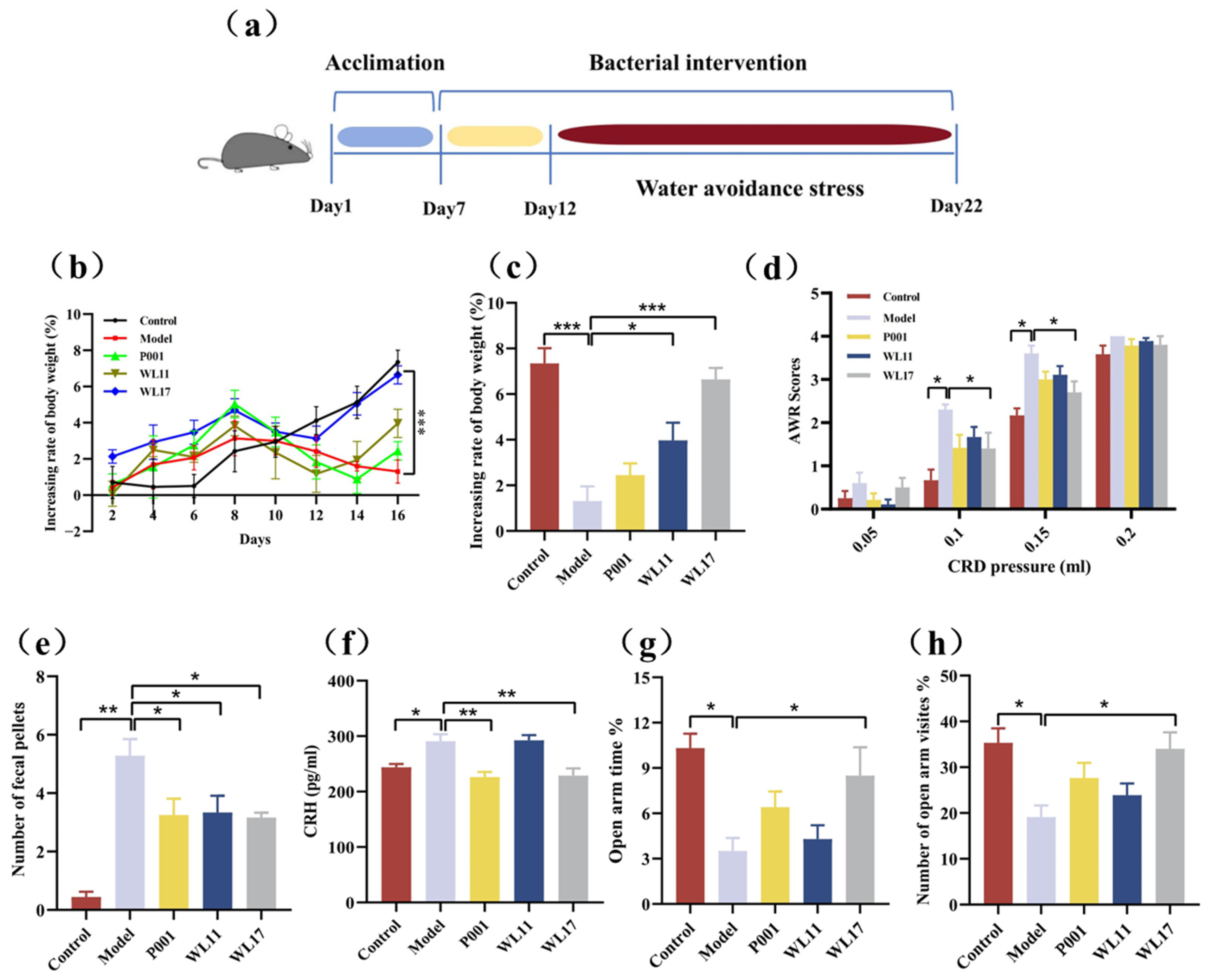
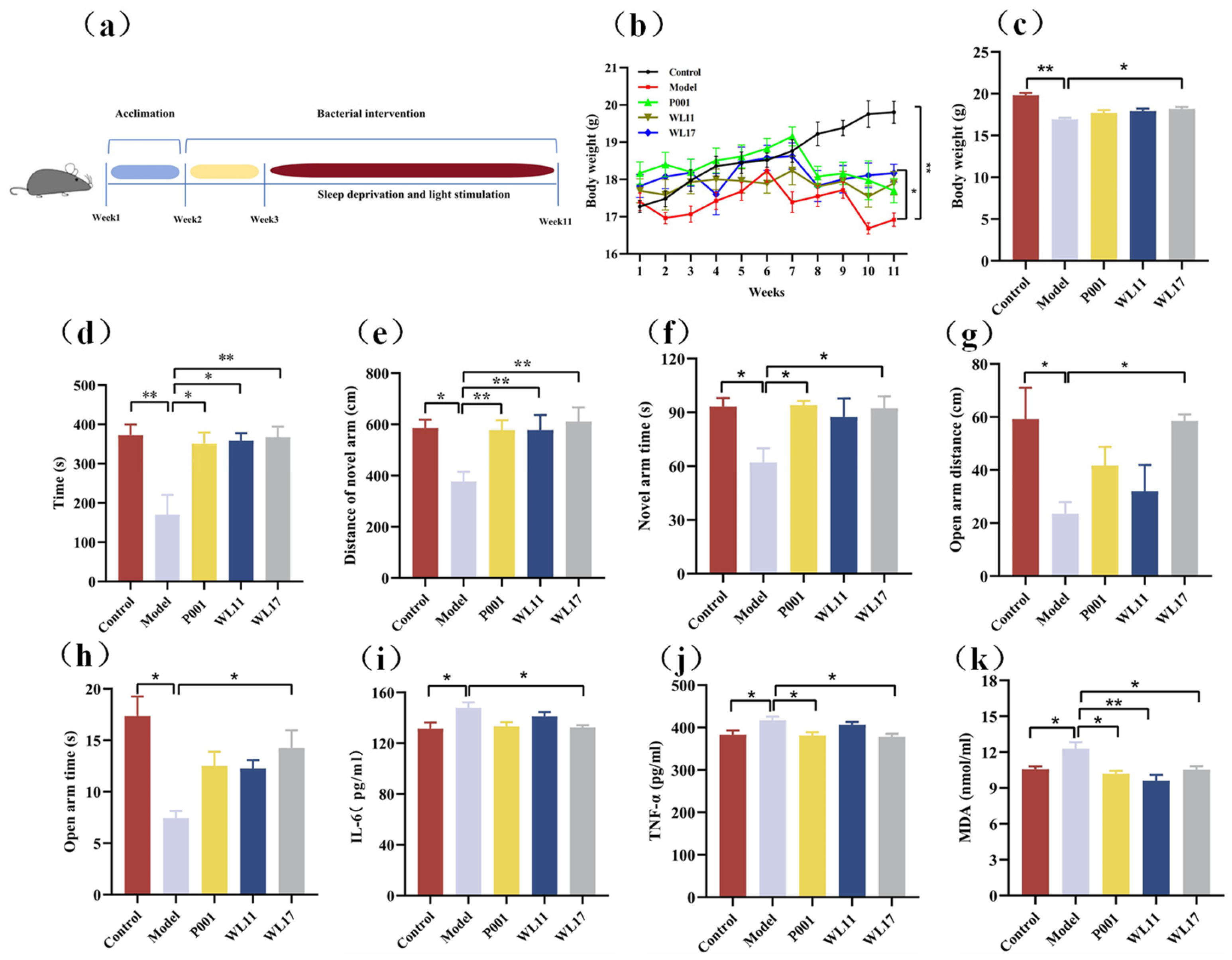
2.6. Experimental Design of CFS and IBS Model Animals
2.7. Visceral Sensitivity Assessment
2.8. Tests with Elevated Plus-Maze (EPM), Rotating Rod, and Y-Maze
2.9. Statistical Analysis
3. Results and Discussion
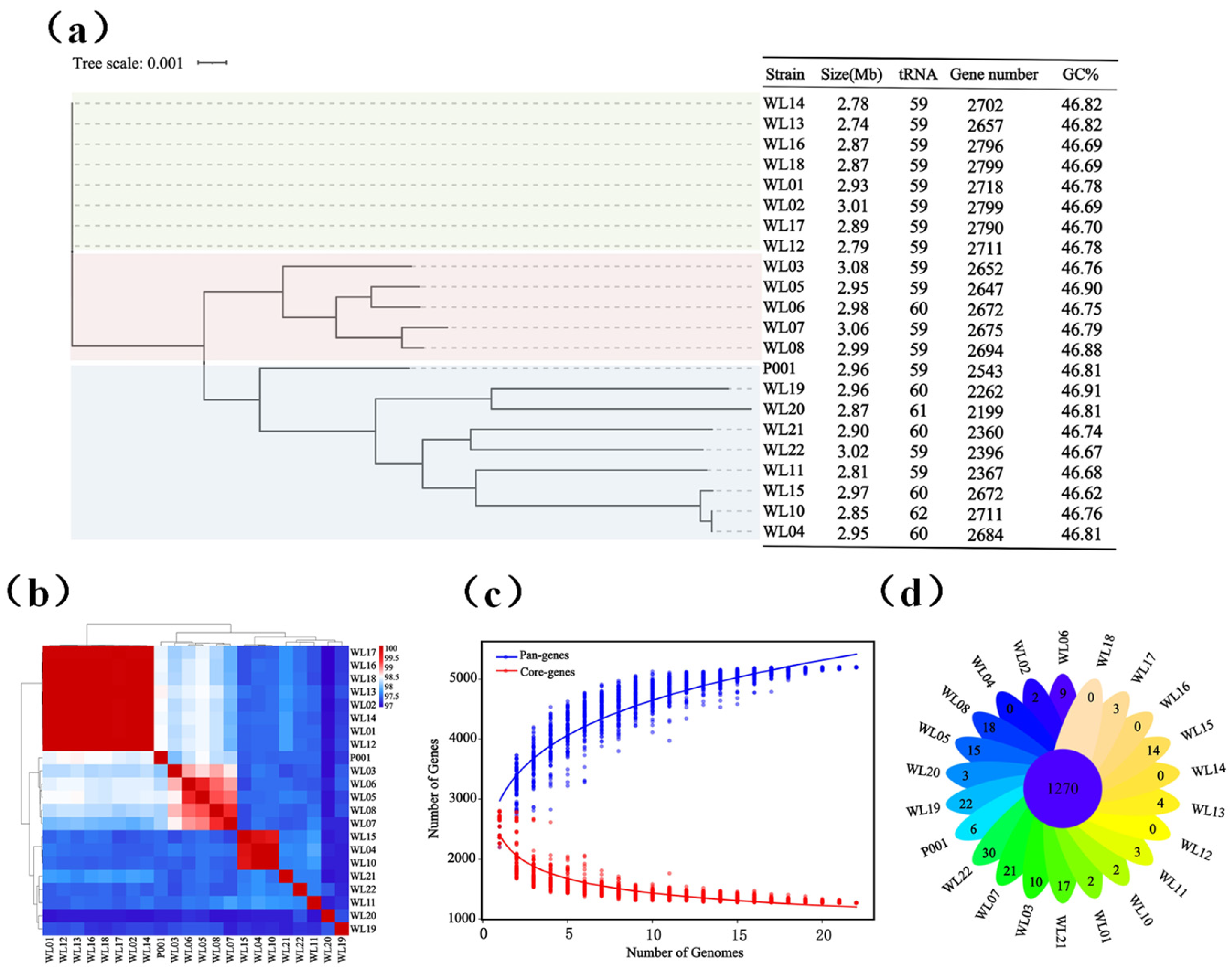
3.1. Ls. rhamnosus Strain Diversity and Their Genomic Features
3.2. Genome Annotation and Experimental Determination of Carbohydrate Utilization, Resistance to Antibiotics, and Prediction of Virulence Factors
3.3. Growth Rates, Tolerance to Low/High pH, Bile Acids and Production of Short-Chain Fatty Acids by Nine Selected Strains
3.4. Strains WL11 and WL17 Alleviate Symptoms in IBS Mouse Model
3.5. Strains WL11 and WL17 Alleviate the Anxiety-Like Behavior, Improve Motor Coordination and Memory Cognition, and Regulate the Oxidative Stress and Inflammatory Factors in CFS Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panelli, M.C. JTM advances in uncharted territories: Diseases and disorders of unknown etiology. J. Transl. Med. 2017, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D.; Dumitrascu, D.; Fukudo, S.; Gerson, C.; Ghoshal, U.C.; Gwee, K.A.; Hungin, A.P.S.; Kang, J.-Y.; Minhu, C.; Schmulson, M.; et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: A Rome Foundation working team literature review. Gut 2017, 66, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Penfold, S.; Denis, E.S.; Mazhar, M.N. The association between borderline personality disorder, fibromyalgia and chronic fatigue syndrome: Systematic review. BJPsych Open 2018, 2, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Cortes Rivera, M.; Mastronardi, C.; Silva-Aldana, C.; Arcos-Burgos, M.; Lidbury, B. Myalgic encephalomyelitis/chronic fatigue syndrome: A comprehensive review. Diagnostics 2019, 9, 91. [Google Scholar] [CrossRef]
- Lim, E.-J.; Ahn, Y.-C.; Jang, E.-S.; Lee, S.-W.; Lee, S.-H.; Son, C.-G. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J. Transl. Med. 2020, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Brurberg, K.G.; Fønhus, M.S.; Larun, L.; Flottorp, S.; Malterud, K. Case definitions for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME): A systematic review. BMJ Open 2014, 4, e003973. [Google Scholar] [CrossRef]
- Bested, A.C.; Marshall, L.M. Review of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: An evidence-based approach to diagnosis and management by clinicians. Rev. Environ. Health 2015, 30, 223–249. [Google Scholar] [CrossRef]
- Johnston, S.; Staines, D.; Marshall-Gradisnik, S. Epidemiological characteristics of chronic fatigue syndrome/myalgic encephalomyelitis in Australian patients. Clin. Epidemiol. 2016, 8, 97–107. [Google Scholar] [CrossRef]
- Corbitt, M.; Campagnolo, N.; Staines, D.; Marshall-Gradisnik, S. A systematic review of probiotic interventions for gastrointestinal symptoms and irritable bowel syndrome in chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). Probiotics Antimicrob. Proteins 2018, 10, 466–477. [Google Scholar] [CrossRef]
- Frändemark, Å.; Törnblom, H.; Jakobsson, S.; Simrén, M. Work productivity and activity impairment in irritable bowel syndrome (IBS): A multifaceted problem. Am. J. Gastroenterol. 2018, 113, 1540–1549. [Google Scholar] [CrossRef]
- Bombardier, C.H.; Buchwald, D. Chronic fatigue, chronic fatigue syndrome, and fibromy disability and health-care use. Med. Care 1996, 34, 924–930. [Google Scholar] [CrossRef]
- Martoni, C.J.; Srivastava, S.; Leyer, G.J. Lactobacillus acidophilus DDS-1 and Bifidobacterium lactis UABla-12 improve abdominal pain severity and symptomology in irritable bowel syndrome: Randomized controlled trial. Nutrients 2020, 12, 363. [Google Scholar] [CrossRef]
- So, D.; Quigley, E.M.M.; Whelan, K. Probiotics in irritable bowel syndrome and inflammatory bowel disease: Review of mechanisms and effectiveness. Curr. Opin. Gastroenterol. 2023, 39, 103–109. [Google Scholar] [CrossRef]
- Tziatzios, G.; Gkolfakis, P.; Leite, G.; Mathur, R.; Damoraki, G.; Giamarellos-Bourboulis, E.J.; Triantafyllou, K. Probiotics in functional dyspepsia. Microorganisms 2023, 11, 351. [Google Scholar] [CrossRef]
- Cao, Y.-N.; Feng, L.-J.; Liu, Y.-Y.; Jiang, K.; Zhang, M.-J.; Gu, Y.-X.; Wang, B.-M.; Gao, J.; Wang, Z.-L.; Wang, Y.-M. Effect of Lactobacillus rhamnosus GG supernatant on serotonin transporter expression in rats with post-infectious irritable bowel syndrome. World J. Gastroenterol. 2018, 24, 315–444. [Google Scholar] [CrossRef]
- Bonfrate, L.; Di Palo, D.M.; Celano, G.; Albert, A.; Vitellio, P.; De Angelis, M.; Gobbetti, M.; Portincasa, P. Effects of Bifidobacterium longum BB536 and Lactobacillus rhamnosus HN001 in IBS patients. Eur. J. Clin. Investig. 2020, 50, e13201. [Google Scholar] [CrossRef]
- Montoya, C.A.; Young, W.; Ryan, L.; Dunstan, K.; Peters, J.; Dewhurst, H.; Dekker, J.; Haggarty, N.; Dilger, R.N.; Roy, N.C. The probiotic Lacticaseibacillus rhamnosus HN001 influences the architecture and gene expression of small intestine tissue in a piglet model. Brit. J. Nutr. 2023, 131, 1289–1297. [Google Scholar] [CrossRef]
- Freedman, S.B.; Williamson-Urquhart, S.; Farion, K.J.; Gouin, S.; Willan, A.R.; Poonai, N.; Hurley, K.; Sherman, P.M.; Finkelstein, Y.; Lee, B.E.; et al. Multicenter trial of a combination probiotic for children with gastroenteritis. N. Engl. J. Med. 2018, 379, 2015–2026. [Google Scholar] [CrossRef]
- Barnett, A.; Roy, N.; Cookson, A.; McNabb, W. Metabolism of caprine milk carbohydrates by probiotic bacteria and Caco-2:HT29–MTX epithelial co-cultures and their impact on intestinal barrier integrity. Nutrients 2018, 10, 949. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Maillard, F.; Meynier, M.; Mondot, S.; Pepke, F.; Galbert, C.; Torres Maravilla, E.; Kropp, C.; Sokol, H.; Carvalho, F.A.; Jacouton, E.; et al. From in vitro to in vivo: A rational flowchart for the selection and characterization of candidate probiotic strains in intestinal disorders. Microorganisms 2023, 11, 906. [Google Scholar] [CrossRef]
- You, L.; Jin, H.; Kwok, L.Y.; Lv, R.; Zhao, Z.; Bilige, M.; Sun, Z.; Liu, W.; Zhang, H. Intraspecific microdiversity and ecological drivers of lactic acid bacteria in naturally fermented milk ecosystem. Sci. Bull. 2023, 68, 2405–2417. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhou, N.; Du, M.-X.; Sun, Y.-T.; Wang, K.; Wang, Y.-J.; Li, D.-H.; Yu, H.-Y.; Song, Y.; Bai, B.-B.; et al. The mouse gut microbial biobank expands the coverage of cultured bacteria. Nat. Commun. 2020, 11, 79. [Google Scholar] [CrossRef]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.G.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Lee, I.; Ouk Kim, Y.; Park, S.-C.; Chun, J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 2016, 66, 1100–1103. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree: Computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.-L.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2019, 48, D517–D525. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef]
- Chen, L.; Yang, J.; Yu, J.; Yao, Z.; Sun, L.; Shen, Y.; Jin, Q. VFDB: A reference database for bacterial virulence factors. Nucleic Acids Res. 2004, 33, D325–D328. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, J.; Kellingray, L.; Zhang, H.; Narbad, A.; Zhai, Q.; Chen, W. In vitro and in vivo evaluation of Lactobacillus strains and comparative genomic analysis of Lactobacillus plantarum CGMCC12436 reveal candidates of colonise-related genes. Food Res. Int. 2019, 119, 813–821. [Google Scholar] [CrossRef]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 2019, 26, 222–235. [Google Scholar] [CrossRef]
- Halder, D.; Mandal, M.; Chatterjee, S.; Pal, N.; Mandal, S. Indigenous probiotic Lactobacillus isolates presenting antibiotic like activity against human pathogenic bacteria. Biomedicines 2017, 5, 31. [Google Scholar] [CrossRef]
- Hai, D.; Lu, Z.; Huang, X.; Lv, F.; Bie, X. In vitro screening of chicken-derived Lactobacillus strains that effectively inhibit Salmonella colonization and adhesion. Foods 2021, 10, 569. [Google Scholar] [CrossRef]
- Rao, Z.-L.; Wang, N.; Zheng, J.-L.; Wang, Y.; Yao, J.-Q.; Kuang, S.-S.; Tang, X.-J. Establishment of rat model of chronic fatigue syndrome. Lab. Anim. Sci. 2014, 31, 24–27. [Google Scholar]
- Zheng, Y.; Zhang, L.; Bonfili, L.; de Vivo, L.; Eleuteri, A.M.; Bellesi, M. Probiotics supplementation attenuates inflammation and oxidative stress induced by chronic sleep restriction. Nutrients 2023, 15, 1518. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.; Wang, Y.; Liu, C.; Zhang, L.; Zhu, S.; Liu, S.; Duan, L. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J. Gastroenterol. Hepatol. 2018, 34, 1368–1376. [Google Scholar] [CrossRef]
- Rodriguez-R, L.M.; Jain, C.; Conrad, R.E.; Aluru, S.; Konstantinidis, K.T. Reply to: “Re-evaluating the evidence for a universal genetic boundary among microbial species”. Nat. Commun. 2021, 12, 4060. [Google Scholar] [CrossRef]
- Jin, H.; Ma, T.; Chen, L.; Kwok, L.Y.; Quan, K.; Li, Y.; Zhang, Z.; Chen, T.; Zhang, J.; Sun, Z.; et al. The iLABdb: A web-based integrated lactic acid bacteria database. Sci. Bull. 2023, 68, 2527–2530. [Google Scholar] [CrossRef]
- Damodharan, K.; Palaniyandi, S.A.; Yang, S.H.; Suh, J.-W. Functional probiotic characterization and in vivo cholesterol-lowering activity of Lactobacillus helveticus isolated from fermented cow milk. J. Microbiol. Biotechnol. 2016, 26, 1675–1686. [Google Scholar] [CrossRef]
- Huang, D.; Yang, B.; Chen, Y.; Stanton, C.; Ross, R.P.; Zhao, J.; Zhang, H.; Chen, W. Comparative genomic analyses of Lactobacillus rhamnosus isolated from Chinese subjects. Food Biosci. 2020, 36, 100659. [Google Scholar] [CrossRef]
- Zhou, J.S.; Shu, Q.; Rutherfurd, K.J.; Prasad, J.; Birtles, M.J.; Gopal, P.K.; Gill, H.S. Safety assessment of potential probiotic lactic acid bacterial strains Lactobacillus rhamnosus HN001, Lb. acidophilus HN017, and Bifidobacterium lactis HN019 in BALB/c mice. Int. J. Food Microbiol. 2000, 56, 87–96. [Google Scholar] [CrossRef]
- Gude, S.; Pinçe, E.; Taute, K.M.; Seinen, A.-B.; Shimizu, T.S.; Tans, S.J. Bacterial coexistence driven by motility and spatial competition. Nature 2020, 578, 588–592. [Google Scholar] [CrossRef]
- Han, S.; Du, M.; Liu, C.; Niu, P.; Feng, J.; Zhang, Z.; Liu, S. Colonization of germ-free pig guts by Enterococcus xiangfangensis and Lactobacillus reuteri strains from different origins. Acta Microbiol. Sin. 2022, 62, 3981–3996. [Google Scholar]
- Succi, M.; Tremonte, P.; Reale, A.; Sorrentino, E.; Grazia, L.; Pacifico, S.; Coppola, R. Bile salt and acid tolerance of Lactobacillus rhamnosus strains isolated from Parmigiano Reggiano cheese. FEMS Microbiol. Lett. 2005, 244, 129–137. [Google Scholar] [CrossRef]
- Yan, F.; Li, N.; Yue, Y.; Wang, C.; Zhao, L.; Evivie, S.E.; Li, B.; Huo, G. Screening for potential novel probiotics with dipeptidyl peptidase IV-inhibiting activity for type 2 diabetes attenuation in vitro and in vivo. Front. Microbiol. 2020, 10, 2855. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Yu, X.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Physiological characteristics of Lactobacillus casei strains and their alleviation effects against inflammatory bowel disease. J. Microbiol. Biotechnol. 2021, 31, 92–103. [Google Scholar] [CrossRef]
- Garbacz, K. Anticancer activity of lactic acid bacteria. Semin. Cancer Biol. 2022, 86, 356–366. [Google Scholar] [CrossRef]
- Alessandri, G.; van Sinderen, D.; Ventura, M. The genus Bifidobacterium: From genomics to functionality of an important component of the mammalian gut microbiota. Comput. Struct. Biotechnol. J. 2021, 19, 1472–1487. [Google Scholar] [CrossRef]
- Brestoff, J.R.; Artis, D. Commensal bacteria at the interface of host metabolism and the immune system. Nat. Immunol. 2013, 14, 676–684. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain–gut axis alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Ni, X.; Jiang, M.; Du, M.; Zhang, S.; Jiang, H.; Liu, C.; Liu, S. Lacticaseibacillus rhamnosus Strains for Alleviation of Irritable Bowel Disease and Chronic Fatigue Syndrome. Microorganisms 2024, 12, 1081. https://doi.org/10.3390/microorganisms12061081
Zhang L, Ni X, Jiang M, Du M, Zhang S, Jiang H, Liu C, Liu S. Lacticaseibacillus rhamnosus Strains for Alleviation of Irritable Bowel Disease and Chronic Fatigue Syndrome. Microorganisms. 2024; 12(6):1081. https://doi.org/10.3390/microorganisms12061081
Chicago/Turabian StyleZhang, Liang, Xue Ni, Minzhi Jiang, Mengxuan Du, Shuwen Zhang, He Jiang, Chang Liu, and Shuangjiang Liu. 2024. "Lacticaseibacillus rhamnosus Strains for Alleviation of Irritable Bowel Disease and Chronic Fatigue Syndrome" Microorganisms 12, no. 6: 1081. https://doi.org/10.3390/microorganisms12061081
APA StyleZhang, L., Ni, X., Jiang, M., Du, M., Zhang, S., Jiang, H., Liu, C., & Liu, S. (2024). Lacticaseibacillus rhamnosus Strains for Alleviation of Irritable Bowel Disease and Chronic Fatigue Syndrome. Microorganisms, 12(6), 1081. https://doi.org/10.3390/microorganisms12061081






