Author Contributions
Conceptualization, V.R.; methodology, V.R.; software, V.R., T.K. and J.L. (Jihyun Lee); validation, V.R., S.J. and H.S.; formal analysis, V.R., S.J. and H.S.; investigation, V.R.; resources, Y.A., H.B., J.L. (Jihyun Lee), C.K., K.L., S.J. and H.S.; data curation, Y.A., H.B., V.R. and A.C.; writing—original draft preparation, V.R.; writing—review and editing, V.R.; visualization, V.R., T.K., A.C. and S.J.; supervision, S.J. and H.S.; project administration, V.R., J.L. (JongDae Lee), C.K., K.L., S.J. and H.S.; funding acquisition, S.J. and H.S. All authors have read and agreed to the published version of the manuscript.
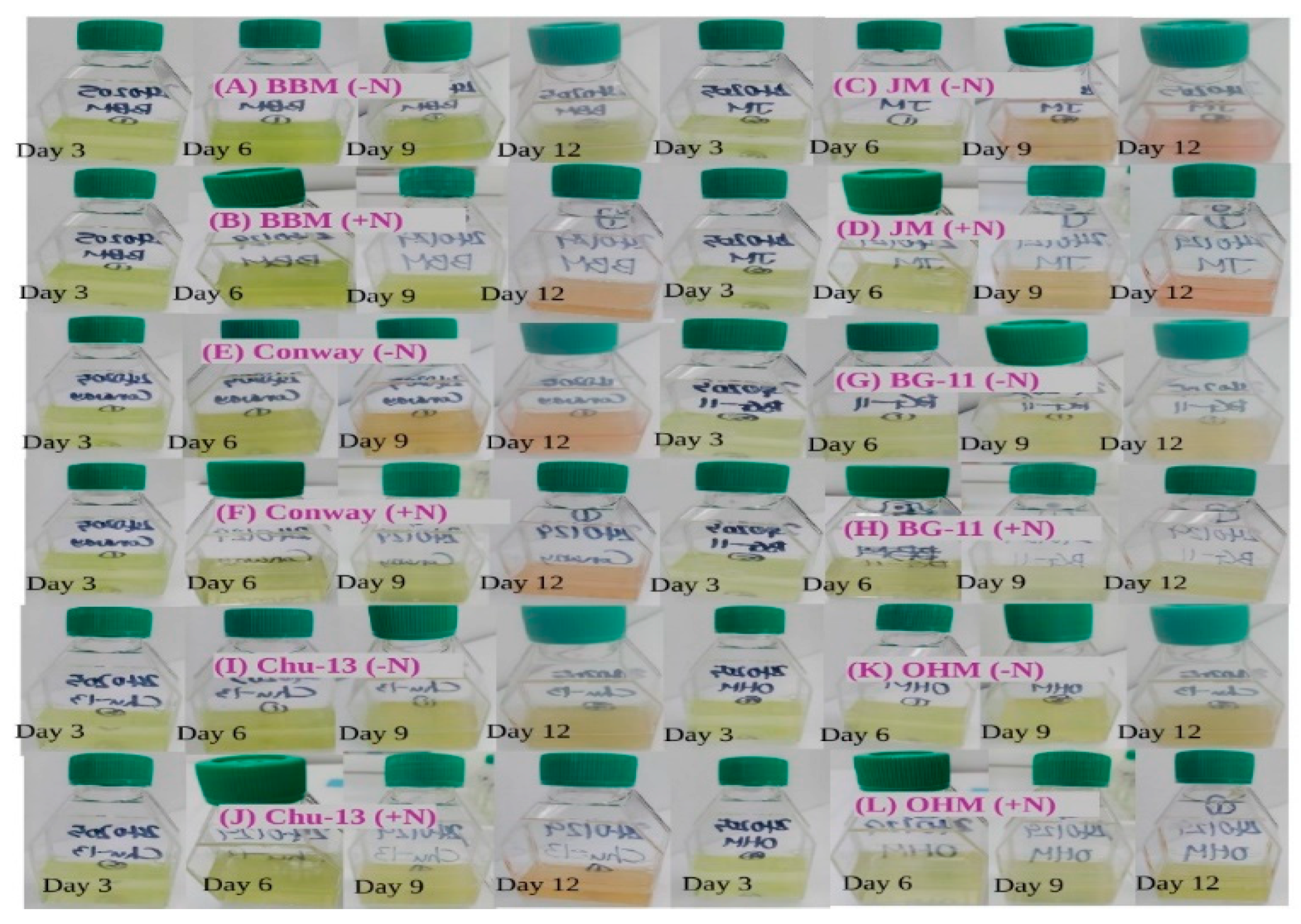
Figure 1.
Appearances of H. lacustris cultures grown in different media (Day 3, 6, 9, and 12). (A) H. lacustris cultures grown in BBM (−N). (B) H. lacustris cultures grown in BBM (+N). (C) H. lacustris cultures grown in JM (−N). (D) H. lacustris cultures grown in JM (+N). (E) H. lacustris cultures grown in Conway (−N). (F) H. lacustris cultures grown in Conway (+N). (G) H. lacustris cultures grown in BG-11 (−N). (H) H. lacustris cultures grown in BG-11 (+N). (I) H. lacustris cultures grown in CHU-13 (−N). (J) H. lacustris cultures grown in CHU-13 (+N). (K) H. lacustris cultures grown in OHM (−N). (L) H. lacustris cultures grown in OHM (+N).
Figure 1.
Appearances of H. lacustris cultures grown in different media (Day 3, 6, 9, and 12). (A) H. lacustris cultures grown in BBM (−N). (B) H. lacustris cultures grown in BBM (+N). (C) H. lacustris cultures grown in JM (−N). (D) H. lacustris cultures grown in JM (+N). (E) H. lacustris cultures grown in Conway (−N). (F) H. lacustris cultures grown in Conway (+N). (G) H. lacustris cultures grown in BG-11 (−N). (H) H. lacustris cultures grown in BG-11 (+N). (I) H. lacustris cultures grown in CHU-13 (−N). (J) H. lacustris cultures grown in CHU-13 (+N). (K) H. lacustris cultures grown in OHM (−N). (L) H. lacustris cultures grown in OHM (+N).
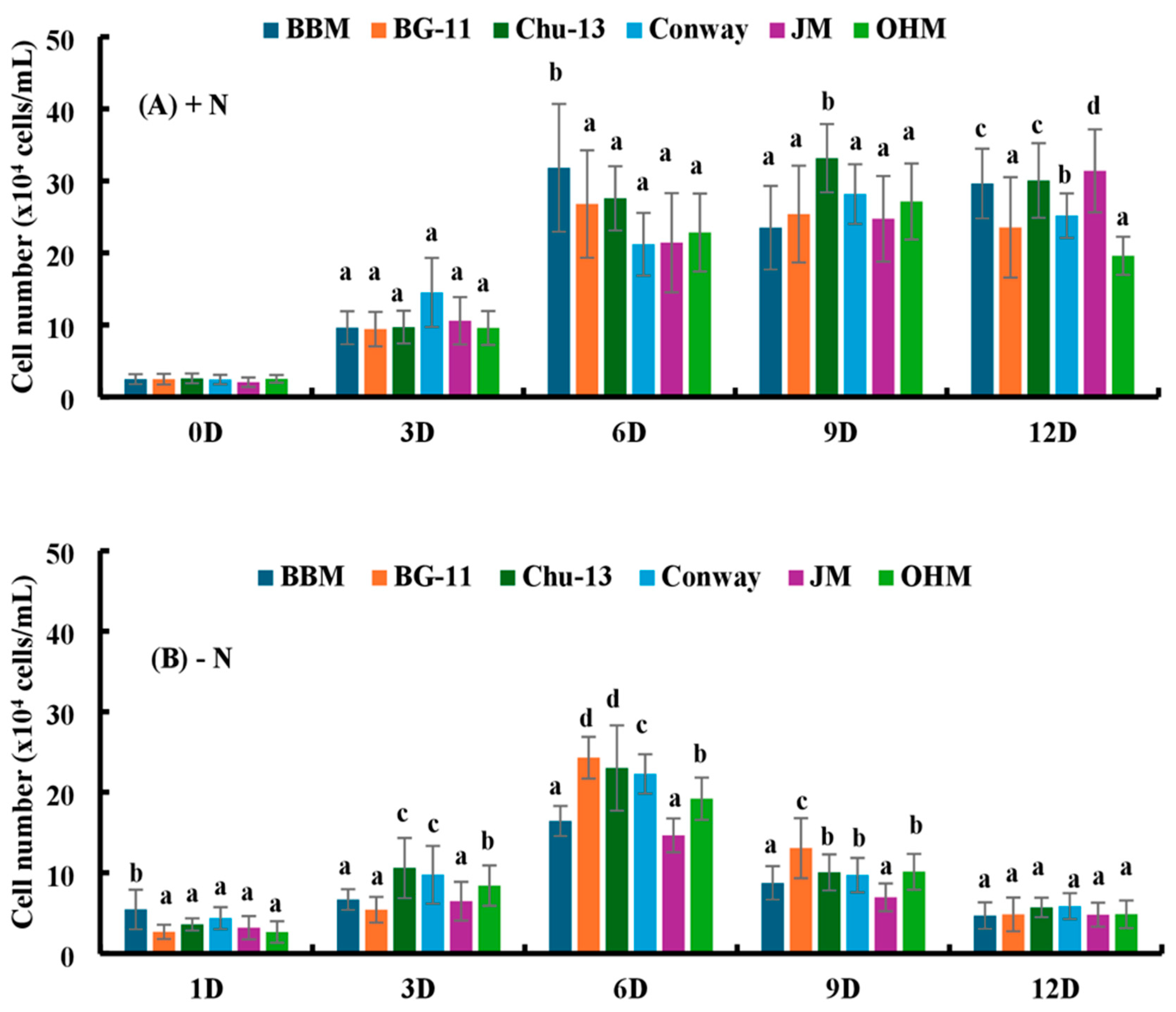
Figure 2.
(A) Comparison of the cell number of H. lacustris under the influence of different media with nitrogen source (0D—Day 0, 3D—Day 3, 6D—Day 6, 9D—Day 9, 12D—Day 12). (B) Comparison of the cell number of H. lacustris under the influence of different media without nitrogen source (1D—Day 1, 3D—Day 3, 6D—Day 6, 9D—Day 9, 12D—Day 12). Mean values that do not share the same letter are significantly different at p < 0.001.
Figure 2.
(A) Comparison of the cell number of H. lacustris under the influence of different media with nitrogen source (0D—Day 0, 3D—Day 3, 6D—Day 6, 9D—Day 9, 12D—Day 12). (B) Comparison of the cell number of H. lacustris under the influence of different media without nitrogen source (1D—Day 1, 3D—Day 3, 6D—Day 6, 9D—Day 9, 12D—Day 12). Mean values that do not share the same letter are significantly different at p < 0.001.
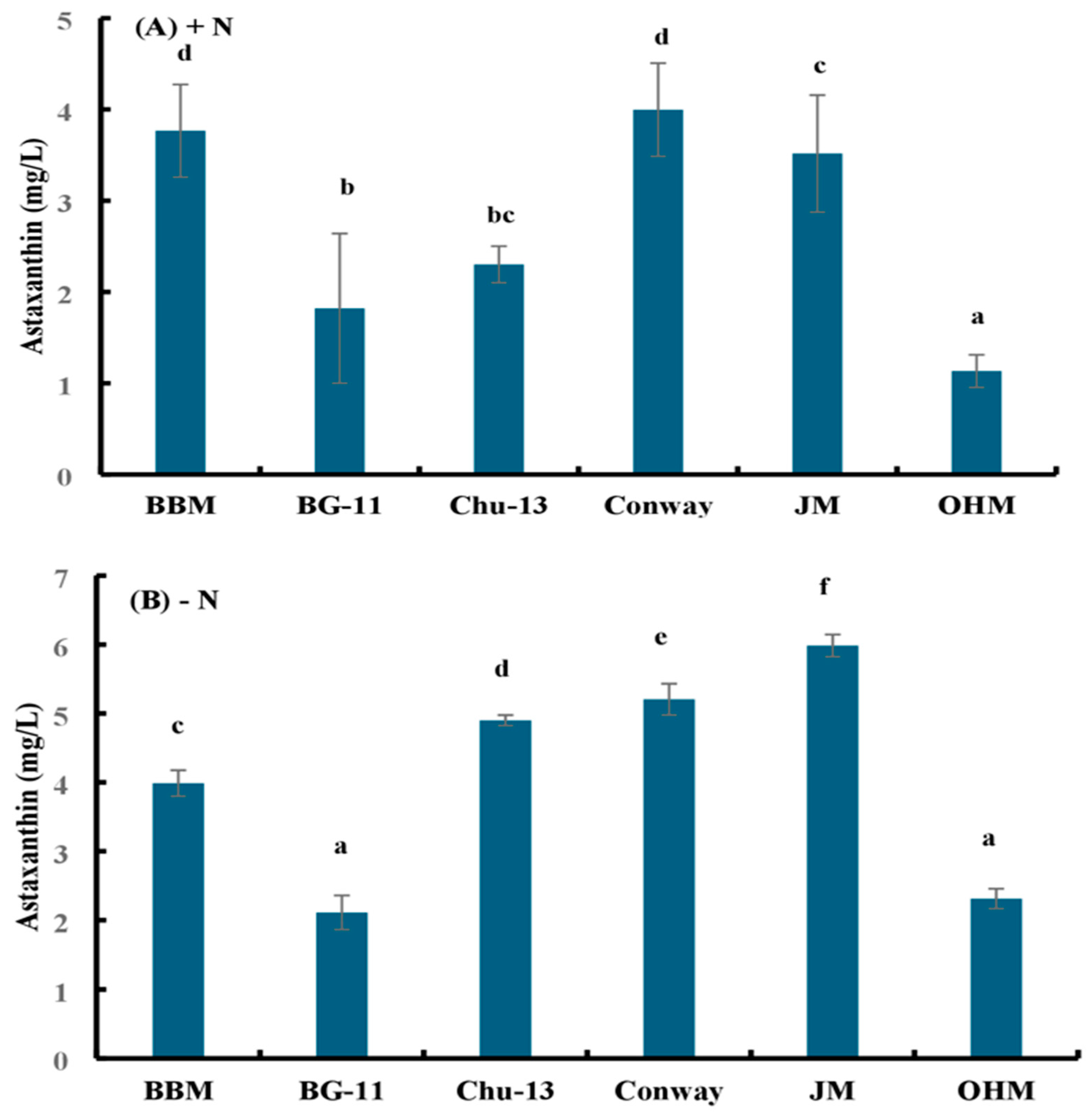
Figure 3.
Astaxanthin accumulation by H. lacustris grown in different media in 14 days (A) with a nitrogen source and (B) without a nitrogen source. Mean values that do not share the same letter are significantly different at p < 0.001.
Figure 3.
Astaxanthin accumulation by H. lacustris grown in different media in 14 days (A) with a nitrogen source and (B) without a nitrogen source. Mean values that do not share the same letter are significantly different at p < 0.001.
Table 1.
Chemical composition of six different media used in this study.
Table 1.
Chemical composition of six different media used in this study.
| Chemical | Walne/Conway | OHM | BG-11 | Mod. CHU 13 | BBM | JM |
|---|
| KNO3 | 100 g/L | 410 mg/L | - | 0.2 g/L | - | 0.05 mg/L |
| NaNO3 | - | - | 1.5 g/L | - | 1.5 g/L | 0.08 mg/L |
| Ca(NO3)2·4H2O | - | - | - | - | - | 0.02 mg/L |
| Ferric ammonium citrate | - | - | 0.012 g/L | - | 6 mg/L | - |
| Na2EDTA | - | - | 0.001 g/L | - | 1 mg/L | 2.25 µg/L |
| K2HPO4 | - | - | 0.04 g/L | 0.04 g/L | 40 mg/L | 0.0124 mg/L |
| Na3PO4 | 20 g/L | - | - | - | - | - |
| Na2HPO4 | - | 30 mg/L | - | - | - | 0.036 mg/L |
| EDTAFeNa | - | - | - | - | - | 2.25 µg/L |
| Na2CO3 | - | - | 0.02 g/L | - | - | - |
| MgSO4·7H2O | - | 246.5 mg/L | 36.1 mg/L | 0.1 g/L | 75 mg/L | - |
| CaCl2·2H2O | - | 110.9 mg/L | 27.2 mg/L | 0.054 g/L | 36 mg/L | - |
| Citric acid | - | - | - | 100.1 mg/L | 6 mg/L | - |
| Sodium citrate | - | - | 8.82 mg/L | - | - | - |
| NaHCO3 | - | - | 0.036 g/L | - | - | 15.9 µg/L |
| FeC6H5O7·5H2O | - | 2.62 mg/L | - | 0.01 g/L | - | - |
| Na2H2EDTA.2H2O | 45 g/L | - | - | - | - | - |
| FeCl3·6H2O | 1.3 g/L | - | - | - | - | - |
| ZnCl2 | 4.2 g/L | - | - | - | - | - |
| MnCl2·4H2O | 0.36 g/L | 0.989 mg/L | 1.81 mg/L | 1.8 mg/L | 1.81 mg/L | 0.139 µg/L |
| CoCl2·6H2O | 4 g/L | 0.011 mg/L | - | 0.08 mg/L | - | - |
| CuSO4·5H2O | 4 g/L | 0.012 mg/L | 0.079 mg/L | 0.08 mg/L | 0.08 mg/L | - |
| Cr2O3 | - | 0.076 mg/L | - | - | - | - |
| Na2MoO4·2H2O | - | 0.12 mg/L | 0.39 mg/L | 0.05 mg/L | 0.39 mg/L | - |
| (NH4)6Mo7O24·4H2O | 1.8 g/L | - | - | - | - | 0.001 mg/L |
| H3BO3 | 33.4 g/L | - | 2.86 mg/L | 2.85 mg/L | 2.86 mg/L | 2.48 µg/L |
| Thiamine—HCl | 200 mg/L | - | - | - | - | 0.04 µg/L |
| Cyanocobalamin | 10 mg/L | 15 µg/mL | - | - | - | 0.04 µg/L |
| Biotin | | 25 µg/mL | -- | - | - | 0.04 µg/L |
| Thiamine | - | 17.5 µg/mL | - | - | - | - |
| ZnSO4·7H2O | - | - | 0.222 mg/L | 0.02 mg/L | 0.222 mg/L | - |
| Co(NO3)2·6H2O | - | - | 0.049 mg/L | - | - | - |
Table 2.
Cell number of H. lacustris grown in JM (+N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of benzoic acid. Data are presented as the mean ± standard deviation.
Table 2.
Cell number of H. lacustris grown in JM (+N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of benzoic acid. Data are presented as the mean ± standard deviation.
| H. lacustris Culture Grown with the Supplementation of Benzoic Acid | Day 1 Cell Number (×104 Cells/mL) | Day 3 Cell Number (×104 Cells/mL) | Day 6 Cell Number (×104 Cells/mL) | Day 9 Cell Number (×104 Cells/mL) | Day 12 Cell Number (×104 Cells/mL) | Day 14 Astaxanthin Content (mg/L) |
|---|
| Control | 0.20 ± 0.000 | 3.20 ± 0.566 | 12.93 ± 1.320 | 11.47 ± 1.676 | 22.40 ± 5.571 | 11.64 ± 0.077 |
| Benzoic acid (0.325 g/L) | 0.20 ± 0.000 | 0.07 ± 0.094 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 |
| Benzoic acid (0.65 g/L) | 0.20 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 |
| Benzoic acid (1.3 g/L) | 0.13 ± 0.094 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 |
| Benzoic acid (2.6 g/L) | 0.27 ± 0.249 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 |
Table 3.
Cell number of H. lacustris grown in JM (+N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of cellulose. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
Table 3.
Cell number of H. lacustris grown in JM (+N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of cellulose. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
| H. lacustris Culture Grown with the Supplementation of Cellulose | Day 1 Cell Number (×104 Cells/mL) | Day 3 Cell Number (×104 Cells/mL) | Day 6 Cell Number (×104 Cells/mL) | Day 9 Cell Number (×104 Cells/mL) | Day 12 Cell Number (×104 Cells/mL) | Day 14 Astaxanthin Content (mg/L) |
|---|
| Control | 0.20 ± 0.000 | 3.20 ± 0.566 | 12.93 ± 1.320 b | 11.47 ± 1.676 | 22.40 ± 5.571 ab | 11.64 ± 0.077 a |
| Cellulose (0.325 g/L) | 0.27 ± 0.094 | 3.93 ± 0.660 | 10.93 ± 1.268 ab | 14.47 ± 3.151 | 29.40 ± 0.432 b | 17.76 ± 0.067 d |
| Cellulose (0.65 g/L) | 0.40 ± 0.283 | 2.93 ± 0.411 | 9.93 ± 1.76 ab | 13.27 ± 2.131 | 24.33 ± 0.772 ab | 12.17 ± 0.017 b |
| Cellulose (1.3 g/L) | 0.27 ± 0.094 | 9.93 ± 1.761 | 8.33 ± 0.525 a | 12.40 ± 0.589 | 20.73 ± 0.340 a | 11.79 ± 0.048 ab |
| Cellulose (2.6 g/L) | 0.13 ± 0.094 | 2.47 ± 0.411 | 9.00 ± 0.993 ab | 13.20 ± 2.592 | 20.13 ± 0.838 a | 12.60 ± 0.294 c |
Table 4.
Cell number of H. lacustris grown in JM (+N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of glutamine. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
Table 4.
Cell number of H. lacustris grown in JM (+N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of glutamine. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
| H. lacustris Culture Grown with the Supplementation of Glutamine | Day 1 Cell Number (×104 Cells/mL) | Day 3 Cell Number (×104 Cells/mL) | Day 6 Cell Number (×104 Cells/mL) | Day 9 Cell Number (×104 Cells/mL) | Day 12 Cell Number (×104 Cells/mL) | Day 14 Astaxanthin Content (mg/L) |
|---|
| Control | 0.20 ± 0.000 | 3.20 ± 0.566 b | 12.93 ± 1.320 c | 11.47 ± 1.676 c | 22.40 ± 5.571 c | 11.64 ± 0.077 e |
| Glutamine (0.325 g/L) | 0.47 ± 0.094 | 3.07 ± 0.822 b | 6.87 ± 1.389 b | 3.13 ± 0.736 a | 1.73 ± 0.411 b | 8.80 ± 0.106 d |
| Glutamine (0.65 g/L) | 0.60 ± 0.432 | 1.27 ± 0.189 a | 2.60 ± 0.589 a | 2.20 ± 0.909 a | 0.40 ± 0.163 a | 5.92 ± 0.006 c |
| Glutamine (1.3 g/L) | 0.33 ± 0.094 | 0.33 ± 0.094 a | 0.07 ± 0.094 | 0.07 ± 0.094 | 0.07 ± 0.094 | 1.31 ± 0.029 b |
| Glutamine (2.6 g/L) | 0.33 ± 0.094 | 0.73 ± 0.411 a | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.01 ± 0.003 a |
Table 5.
Cell number of H. lacustris grown in JM (+N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of alanine. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
Table 5.
Cell number of H. lacustris grown in JM (+N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of alanine. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
| H. lacustris Culture Grown with the Supplementation of Alanine | Day 1 Cell Number (×104 Cells/mL) | Day 3 Cell Number (×104 Cells/mL) | Day 6 Cell Number (×104 Cells/mL) | Day 9 Cell Number (×104 Cells/mL) | Day 12 Cell Number (×104 Cells/mL) | Day 14 Astaxanthin Content (mg/L) |
|---|
| Control | 0.20 ± 0.000 | 3.20 ± 0.566 | 12.93 ± 1.320 d | 11.47 ± 1.676 c | 22.40 ± 5.571 c | 11.64 ± 0.077 c |
| Alanine (0.325 g/L) | 0.33 ± 0.094 | 3.40 ± 0.864 | 10.27 ± 0.249 c | 5.27 ± 0.660 b | 4.60 ± 0.163 b | 7.03 ± 0.048 b |
| Alanine (0.65 g/L) | 0.47 ± 0.094 | 0.07 ± 0.094 | 3.00 ± 0.432 b | 3.00 ± 0.748 a | 0.73 ± 0.249 a | 7.05 ± 0.006 b |
| Alanine (1.3 g/L) | 0.13 ± 0.094 | 0.07 ± 0.094 | 0.07 ± 0.094 a | 0.00 ± 0.000 | 0.07 ± 0.094 | 1.34 ± 0.076 a |
| Alanine (2.6 g/L) | 0.40 ± 0.163 | 0.07 ± 0.094 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.23 ± 0.017 a |
Table 6.
Cell number of H. lacustris grown in JM (+N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of leucine. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
Table 6.
Cell number of H. lacustris grown in JM (+N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of leucine. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
| H. lacustris Culture Grown with the Supplementation of Leucine | Day 1 Cell Number (×104 Cells/mL) | Day 3 Cell Number (×104 Cells/mL) | Day 6 Cell Number (×104 Cells/mL) | Day 9 Cell Number (×104 Cells/mL) | Day 12 Cell Number (×104 Cells/mL) | Day 14 Astaxanthin Content (mg/L) |
|---|
| Control | 0.20 ± 0.000 | 3.20 ± 0.566 ab | 12.93 ± 1.320 a | 11.47 ± 1.676 a | 22.40 ± 5.571 | 11.64 ± 0.077 e |
| Leucine (0.325 g/L) | 0.27 ± 0.094 | 3.47 ± 0.525 b | 23.20 ± 2.953 b | 23.27 ± 0.806 c | 21.40 ± 2.673 | 11.48 ± 0.073 d |
| Leucine (0.65 g/L) | 0.33 ± 0.094 | 2.93 ± 0.680 ab | 15.93 ± 1.746 a | 15.80 ± 1.766 b | 19.47 ± 1.636 | 7.33 ± 0.017 c |
| Leucine (1.3 g/L) | 0.20 ± 0.163 | 1.60 ± 0.163 a | 10.27 ± 1.310 a | 19.73 ± 0.411 c | 23.13 ± 3.465 | 5.33 ± 0.011 b |
| Leucine (2.6 g/L) | 0.27 ± 0.094 | 2.27 ± 0.618 ab | 12.33 ± 1.087 a | 15.07 ± 1.676 ab | 20.60 ± 0.748 | 5.18 ± 0.006 a |
Table 7.
Cell number of H. lacustris grown in JM (+N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of lysine. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
Table 7.
Cell number of H. lacustris grown in JM (+N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of lysine. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
| H. lacustris Culture Grown with the Supplementation of Lysine | Day 1 Cell Number (×104 Cells/mL) | Day 3 Cell Number (×104 Cells/mL) | Day 6 Cell Number (×104 Cells/mL) | Day 9 Cell Number (×104 Cells/mL) | Day 12 Cell Number (×104 Cells/mL) | Day 14 Astaxanthin Content (mg/L) |
|---|
| Control | 0.20 ± 0.000 | 3.20 ± 0.566 b | 12.93 ± 1.320 b | 11.47 ± 1.676 bc | 22.40 ± 5.571 bc | 11.64 ± 0.077 d |
| Lysine (0.325 g/L) | 0.27 ± 0.094 | 4.67 ± 1.087 b | 12.73 ± 0.411 b | 14.60 ± 0.589 c | 20.73 ± 2.205 bc | 0.04 ± 0.000 a |
| Lysine (0.65 g/L) | 0.80 ± 0.327 | 4.13 ± 0.806 b | 12.87 ± 1.668 b | 26.47 ± 3.356 d | 38.67 ± 11.927 c | 10.15 ± 0.006 c |
| Lysine (1.3 g/L) | 0.27 ± 0.094 | 1.07 ± 0.525 a | 1.93 ± 0.618 a | 7.00 ± 1.395 b | 15.27 ± 0.660 ab | 13.62 ± 0.061 e |
| Lysine (2.6 g/L) | 0.20 ± 0.000 | 0.07 ± 0.094 a | 0.13 ± 0.094 a | 0.53 ± 0.340 a | 1.00 ± 0.327 a | 7.65 ± 0.013 b |
Table 8.
Cell number of H. lacustris grown in JM (+N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of malic acid.
Table 8.
Cell number of H. lacustris grown in JM (+N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of malic acid.
| H. lacustris Culture Grown with the Supplementation of Malic Acid | Day 1 Cell Number (×104 Cells/mL) | Day 3 Cell Number (×104 Cells/mL) | Day 6 Cell Number (×104 Cells/mL) | Day 9 Cell Number (×104 Cells/mL) | Day 12 Cell Number (×104 Cells/mL) | Day 14 Astaxanthin Content (mg/L) |
|---|
| Control | 0.20 ± 0.000 | 3.20 ± 0.566 | 12.93 ± 1.320 | 11.47 ± 1.676 | 22.40 ± 5.571 | 11.64 ± 0.077 |
| Malic acid (0.325 g/L) | 0.47 ± 0.094 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.13 ± 0.006 |
| Malic acid (0.65 g/L) | 0.67 ± 0.094 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.23 ± 0.048 |
| Malic acid (1.3 g/L) | 0.13 ± 0.094 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.03 ± 0.006 |
| Malic acid (2.6 g/L) | 0.20 ± 0.163 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 |
Table 9.
Cell number of H. lacustris grown in JM (+N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of maltose. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
Table 9.
Cell number of H. lacustris grown in JM (+N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of maltose. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
| H. lacustris Culture Grown with the Supplementation of Maltose | Day 1 Cell Number (×104 Cells/mL) | Day 3 Cell Number (×104 Cells/mL) | Day 6 Cell Number (×104 Cells/mL) | Day 9 Cell Number (×104 Cells/mL) | Day 12 Cell Number (×104 Cells/mL) | Day 14 Astaxanthin Content (mg/L) |
|---|
| Control | 0.20 ± 0.000 | 3.20 ± 0.566 | 12.93 ± 1.320 a | 11.47 ± 1.676 | 22.40 ± 5.571 b | 11.64 ± 0.077 |
| Maltose (0.325 g/L) | 0.13 ± 0.094 | 2.00 ± 0.163 | 22.67 ± 2.391 b | 16.87 ± 2.584 | 22.13 ± 4.620 b | 0.00 ± 0.000 |
| Maltose (0.65 g/L) | 0.40 ± 0.163 | 3.40 ± 0.712 | 16.80 ± 1.337 a | 13.40 ± 0.589 | 14.67 ± 1.236 ab | 0.00 ± 0.000 |
| Maltose (1.3 g/L) | 0.20 ± 0.163 | 3.33 ± 0.340 | 15.00 ± 2.007 a | 13.13 ± 0.680 | 9.60 ± 1.020 a | 0.00 ± 0.000 |
| Maltose (2.6 g/L) | 0.27 ± 0.094 | 3.60 ± 0.849 | 16.20 ± 2.068 a | 12.73 ± 2.553 | 11.87 ± 2.007 ab | 0.00 ± 0.000 |
Table 10.
Cell number of H. lacustris grown in JM (+N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of sodium glutamate. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
Table 10.
Cell number of H. lacustris grown in JM (+N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of sodium glutamate. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
| H. lacustris Culture Grown with the Supplementation of Sodium Glutamate | Day 1 Cell Number (×104 Cells/mL) | Day 3 Cell Number (×104 Cells/mL) | Day 6 Cell Number (×104 Cells/mL) | Day 9 Cell Number (×104 Cells/mL) | Day 12 Cell Number (×104 Cells/mL) | Day 14 Astaxanthin Content (mg/L) |
|---|
| Control | 0.20 ± 0.000 | 3.20 ± 0.566 a | 12.93 ± 1.320 c | 11.47 ± 1.676 c | 22.40 ± 5.571 b | 11.64 ± 0.077 d |
| Sodium Glutamate (0.325 g/L) | 0.40 ± 0.000 | 1.93 ± 0.340 c | 15.13 ± 0.998 c | 12.73 ± 0.411 c | 25.00 ± 2.592 b | 20.48 ± 0.017 e |
| Sodium Glutamate (0.65 g/L) | 0.33 ± 0.094 | 1.40 ± 0.163 bc | 6.87 ± 0.957 b | 5.53 ± 0.573 b | 5.27 ± 0.754 a | 8.30 ± 0.048 c |
| Sodium Glutamate (1.3 g/L) | 0.40 ± 0.163 | 0.80 ± 0.000 ab | 3.07 ± 0.943 a | 3.33 ± 0.411 b | 0.53 ± 0.094 a | 5.62 ± 0.011 b |
| Sodium Glutamate (2.6 g/L) | 0.53 ± 0.249 | 0.07 ± 0.094 a | 0.53 ± 0.189 a | 0.20 ± 0.000 a | 0.13 ± 0.094 a | 0.55 ± 0.019 a |
Table 11.
Cell number of H. lacustris grown in JM (−N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of benzoic acid. Data are presented as the mean ± standard deviation.
Table 11.
Cell number of H. lacustris grown in JM (−N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of benzoic acid. Data are presented as the mean ± standard deviation.
| H. lacustris Culture Grown with the Supplementation of Benzoic Acid | Day 1 Cell Number (×104 Cells/mL) | Day 3 Cell Number (×104 Cells/mL) | Day 6 Cell Number (×104 Cells/mL) | Day 9 Cell Number (×104 Cells/mL) | Day 12 Cell Number (×104 Cells/mL) | Day 14 Astaxanthin Content (mg/L) |
|---|
| Control | 0.20 ± 0.163 | 0.20 ± 0.163 | 0.53 ± 0.189 | 0.87 ± 0.094 | 0.80 ± 0.163 | 0.91 ± 0.035 |
| Benzoic acid (0.325 g/L) | 0.13 ± 0.094 | 0.13 ± 0.094 | 0.20 ± 0.163 | 0.07 ± 0.094 | 0.00 ± 0.000 | 0.00 ± 0.000 |
| Benzoic acid (0.65 g/L) | 0.20 ± 0.163 | 0.07 ± 0.094 | 0.13 ± 0.094 | 0.13 ± 0.094 | 0.00 ± 0.000 | 0.00 ± 0.000 |
| Benzoic acid (1.3 g/L) | 0.20 ± 0.163 | 0.27 ± 0.249 | 0.13 ± 0.189 | 0.13 ± 0.189 | 0.00 ± 0.000 | 0.00 ± 0.000 |
| Benzoic acid (2.6 g/L) | 0.20 ± 0.000 | 0.13 ± 0.094 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 | 0.00 ± 0.000 |
Table 12.
Cell number of H. lacustris grown in JM (−N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of cellulose. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
Table 12.
Cell number of H. lacustris grown in JM (−N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of cellulose. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
| H. lacustris Culture Grown with the Supplementation of Cellulose | Day 1 Cell Number (×104 Cells/mL) | Day 3 Cell Number (×104 Cells/mL) | Day 6 Cell Number (×104 Cells/mL) | Day 9 Cell Number (×104 Cells/mL) | Day 12 Cell Number (×104 Cells/mL) | Day 14 Astaxanthin Content (mg/L) |
|---|
| Control | 0.20 ± 0.163 | 0.20 ± 0.163 | 0.53 ± 0.189 a | 0.87 ± 0.094 | 0.80 ± 0.163 | 0.91 ± 0.035 a |
| Cellulose (0.325 g/L) | 0.27 ± 0.094 | 0.47 ± 0.249 | 0.67 ± 0.094 a | 1.07 ± 0.094 | 1.47 ± 0.822 | 0.90 ± 0.006 a |
| Cellulose (0.65 g/L) | 0.20 ± 0.000 | 0.40 ± 0.163 | 0.73 ± 0.094 a | 0.93 ± 0.189 | 1.00 ± 0.589 | 1.04 ± 0.028 b |
| Cellulose (1.3 g/L) | 0.07 ± 0.094 | 0.47 ± 0.249 | 1.38 ± 0.189 b | 1.00 ± 0.163 | 1.13 ± 0.377 | 1.40 ± 0.023 c |
| Cellulose (2.6 g/L) | 0.07 ± 0.094 | 0.53 ± 0.340 | 1.47 ± 0.189 b | 1.87 ± 0.660 | 2.13 ± 0.525 | 2.02 ± 0.017 d |
Table 13.
Cell number of H. lacustris grown in JM (−N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of glutamine. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
Table 13.
Cell number of H. lacustris grown in JM (−N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of glutamine. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
| H. lacustris Culture Grown with the Supplementation of Glutamine | Day 1 Cell Number (×104 Cells/mL) | Day 3 Cell Number (×104 Cells/mL) | Day 6 Cell Number (×104 Cells/mL) | Day 9 Cell Number (×104 Cells/mL) | Day 12 Cell Number (×104 Cells/mL) | Day 14 Astaxanthin Content (mg/L) |
|---|
| Control | 0.20 ± 0.163 | 0.20 ± 0.163 a | 0.53 ± 0.189 ab | 0.87 ± 0.094 c | 0.80 ± 0.163 b | 0.91 ± 0.035 a |
| Glutamine (0.325 g/L) | 0.20 ± 0.000 | 1.20 ± 0.163 b | 1.40 ± 0.283 c | 0.53 ± 0.189 b | 0.13 ± 0.189 a | 8.60 ± 0.046 e |
| Glutamine (0.65 g/L) | 0.13 ± 0.094 | 0.13 ± 0.094 a | 0.87 ± 0.094 bc | 0.13 ± 0.094 a | 0.00 ± 0.000 | 7.61 ± 0.011 d |
| Glutamine (1.3 g/L) | 0.07 ± 0.094 | 0.20 ± 0.163 a | 0.67 ± 0.094 ab | 0.07 ± 0.094 a | 0.00 ± 0.000 | 4.86 ± 0.006 c |
| Glutamine (2.6 g/L) | 0.13 ± 0.094 | 0.33 ± 0.094 a | 0.20 ± 0.283 a | 0.00 ± 0.000 | 0.00 ± 0.000 | 1.63 ± 0.025 b |
Table 14.
Cell number of H. lacustris grown in JM (−N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of alanine. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
Table 14.
Cell number of H. lacustris grown in JM (−N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of alanine. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
| H. lacustris Culture Grown with the Supplementation of Alanine | Day 1 Cell Number (×104 Cells/mL) | Day 3 Cell Number (×104 Cells/mL) | Day 6 Cell Number (×104 Cells/mL) | Day 9 Cell Number (×104 Cells/mL) | Day 12 Cell Number (×104 Cells/mL) | Day 14 Astaxanthin Content (mg/L) |
|---|
| Control | 0.20 ± 0.163 | 0.20 ± 0.163 a | 0.53 ± 0.189 | 0.87 ± 0.094 | 0.80 ± 0.163 | 0.91 ± 0.035 a |
| Alanine (0.325 g/L) | 0.20 ± 0.000 | 1.20 ± 0.163 b | 1.60 ± 0.589 | 1.20 ± 0.566 | 1.07 ± 0.471 | 5.76 ± 0.011 c |
| Alanine (0.65 g/L) | 0.27 ± 0.094 | 0.27 ± 0.094 a | 1.67 ± 0.499 | 1.00 ± 0.163 | 0.87 ± 0.094 | 6.06 ± 0.092 d |
| Alanine (1.3 g/L) | 0.07 ± 0.094 | 0.40 ± 0.163 a | 0.60 ± 0.163 | 0.87 ± 0.094 | 1.93 ± 0.525 | 6.13 ± 0.011 d |
| Alanine (2.6 g/L) | 0.20 ± 0.000 | 0.53 ± 0.499 ab | 0.53 ± 0.189 | 0.87 ± 0.094 | 1.47 ± 0.377 | 1.80 ± 0.013 b |
Table 15.
Cell number of H. lacustris grown in JM (−N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of leucine. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
Table 15.
Cell number of H. lacustris grown in JM (−N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of leucine. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
| H. lacustris Culture Grown with the Supplementation of Leucine | Day 1 Cell Number (×104 Cells/mL) | Day 3 Cell Number (×104 Cells/mL) | Day 6 Cell Number (×104 Cells/mL) | Day 9 Cell Number (×104 Cells/mL) | Day 12 Cell Number (×104 Cells/mL) | Day 14 Astaxanthin Content (mg/L) |
|---|
| Control | 0.20 ± 0.163 | 0.20 ± 0.163 a | 0.53 ± 0.189 a | 0.87 ± 0.094 a | 0.80 ± 0.163 a | 0.91 ± 0.035 a |
| Leucine (0.325 g/L) | 0.07 ± 0.094 | 0.93 ± 0.189 b | 1.73 ± 0.411 b | 3.13 ± 0.660 ab | 10.80 ± 0.589 c | 8.30 ± 0.090 d |
| Leucine (0.65 g/L) | 0.20 ± 0.000 | 0.67 ± 0.094 ab | 2.47 ± 0.618 bc | 10.13 ± 0.618 bc | 20.93 ± 2.614 e | 9.10 ± 0.029 e |
| Leucine (1.3 g/L) | 0.13 ± 0.094 | 0.47 ± 0.094 ab | 3.27 ± 0.249 c | 5.93 ± 1.112 b | 16.40 ± 0.993 d | 5.82 ± 0.032 c |
| Leucine (2.6 g/L) | 0.07 ± 0.094 | 0.53 ± 0.189 ab | 2.73 ± 0.499 bc | 4.93 ± 1.543 b | 7.07 ± 0.660 b | 2.17 ± 0.011 b |
Table 16.
Cell number of H. lacustris grown in JM (−N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of lysine. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
Table 16.
Cell number of H. lacustris grown in JM (−N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of lysine. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
| H. lacustris Culture Grown with the Supplementation of Lysine | Day 1 Cell Number (×104 Cells/mL) | Day 3 Cell Number (×104 Cells/mL) | Day 6 Cell Number (×104 Cells/mL) | Day 9 Cell Number (×104 Cells/mL) | Day 12 Cell Number (×104 Cells/mL) | Day 14 Astaxanthin Content (mg/L) |
|---|
| Control | 0.20 ± 0.163 | 0.20 ± 0.163 | 0.53 ± 0.189 a | 0.87 ± 0.094 ab | 0.80 ± 0.163 a | 0.91 ± 0.035 a |
| Lysine (0.325 g/L) | 0.20 ± 0.000 | 0.40 ± 0.163 | 1.47 ± 0.249 b | 7.53 ± 0.499 c | 12.93 ± 1.236 c | 1.99 ± 0.061 c |
| Lysine (0.65 g/L) | 0.07 ± 0.094 | 0.20 ± 0.163 | 0.93 ± 0.411 ab | 1.67 ± 0.499 b | 3.73 ± 0.499 b | 2.12 ± 0.006 d |
| Lysine (1.3 g/L) | 0.07 ± 0.094 | 0.20 ± 0.163 | 0.27 ± 0.377 a | 0.07 ± 0.094 a | 0.07 ± 0.094 | 1.85 ± 0.000 b |
| Lysine (2.6 g/L) | 0.13 ± 0.094 | 0.07 ± 0.094 | 0.13 ± 0.189 a | 0.00 ± 0.000 | 0.00 ± 0.000 | 7.36 ± 0.023 e |
Table 17.
Cell number of H. lacustris grown in JM (−N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of malic acid. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
Table 17.
Cell number of H. lacustris grown in JM (−N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of malic acid. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
| H. lacustris Culture Grown with the Supplementation of Malic Acid | Day 1 Cell Number (×104 Cells/mL) | Day 3 Cell Number (×104 Cells/mL) | Day 6 Cell Number (×104 Cells/mL) | Day 9 Cell Number (×104 Cells/mL) | Day 12 Cell Number (×104 Cells/mL) | Day 14 Astaxanthin Content (mg/L) |
|---|
| Control | 0.20 ± 0.163 | 0.20 ± 0.163 | 0.53 ± 0.189 | 0.87 ± 0.094 | 0.80 ± 0.163 | 0.91 ± 0.035 c |
| Malic acid (0.325 g/L) | 0.07 ± 0.094 | 0.07 ± 0.094 | 1.27 ± 0.189 | 0.40 ± 0.327 | 0.00 ± 0.000 | 0.07 ± 0.000 a |
| Malic acid (0.65 g/L) | 0.20 ± 0.000 | 0.00 ± 0.000 | 1.00 ± 0.432 | 0.13 ± 0.094 | 0.00 ± 0.000 | 0.00 ± 0.000 |
| Malic acid (1.3 g/L) | 0.13 ± 0.094 | 0.00 ± 0.000 | 0.67 ± 0.340 | 0.07 ± 0.094 | 0.00 ± 0.000 | 0.00 ± 0.000 |
| Malic acid (2.6 g/L) | 0.13 ± 0.094 | 0.00 ± 0.000 | 0.93 ± 0.411 | 0.07 ± 0.094 | 0.00 ± 0.000 | 0.89 ± 0.055 b |
Table 18.
Cell number of H. lacustris grown in JM (−N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of maltose. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
Table 18.
Cell number of H. lacustris grown in JM (−N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of maltose. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
| H. lacustris Culture Grown with the Supplementation of Maltose | Day 1 Cell Number (×104 Cells/mL) | Day 3 Cell Number (×104 Cells/mL) | Day 6 Cell Number (×104 Cells/mL) | Day 9 Cell Number (×104 Cells/mL) | Day 12 Cell Number (×104 Cells/mL) | Day 14 Astaxanthin Content (mg/L) |
|---|
| Control | 0.20 ± 0.163 | 0.20 ± 0.163 a | 0.53 ± 0.189 | 0.87 ± 0.094 ab | 0.80 ± 0.163 | 0.91 ± 0.035 a |
| Maltose (0.325 g/L) | 0.13 ± 0.094 | 0.47 ± 0.094 ab | 0.87 ± 0.249 | 0.47 ± 0.189 a | 0.73 ± 0.094 | 0.89 ± 0.055 a |
| Maltose (0.65 g/L) | 0.20 ± 0.000 | 0.67 ± 0.189 ab | 1.00 ± 0.327 | 0.60 ± 0.283 a | 0.73 ± 0.094 | 0.73 ± 0.000 b |
| Maltose (1.3 g/L) | 0.13 ± 0.094 | 1.20 ± 0.566 b | 1.27 ± 0.499 | 1.33 ± 0.249 b | 1.27 ± 1.087 | 0.70 ± 0.006 ab |
| Maltose (2.6 g/L) | 0.20 ± 0.000 | 0.80 ± 0.189 ab | 0.87 ± 0.189 | 1.13 ± 0.094 ab | 0.60 ± 0.094 | 0.62 ± 0.046 a |
Table 19.
Cell number of H. lacustris grown in JM (−N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of sodium glutamate. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
Table 19.
Cell number of H. lacustris grown in JM (−N) media (Day 1, Day 3, Day 6, Day 9, Day 12) and astaxanthin contents of H. lacustris on Day 14 grown with the supplementation of sodium glutamate. Data are presented as the mean ± standard deviation. Lowercase letters indicate significant differences (p < 0.001).
| H. lacustris Culture Grown with the Supplementation of Sodium Glutamate | Day 1 Cell Number (×104 Cells/mL) | Day 3 Cell Number (×104 Cells/mL) | Day 6 Cell Number (×104 Cells/mL) | Day 9 Cell Number (×104 Cells/mL) | Day 12 Cell Number (×104 Cells/mL) | Day 14 Astaxanthin Content (mg/L) |
|---|
| Control | 0.20 ± 0.163 | 0.20 ± 0.163 a | 0.53 ± 0.189 a | 0.87 ± 0.094 a | 0.80 ± 0.163 a | 0.91 ± 0.035 a |
| Sodium Glutamate (0.325 g/L) | 0.20 ± 0.000 | 1.27 ± 0.340 b | 10.27 ± 0.618 c | 11.87 ± 1.087 c | 11.67 ± 0.525 b | 6.03 ± 0.017 c |
| Sodium Glutamate (0.65 g/L) | 0.13 ± 0.094 | 1.27 ± 0.249 b | 2.87 ± 0.499 c | 1.93 ± 0.411 b | 0.73 ± 0.189 a | 8.01 ± 0.092 d |
| Sodium Glutamate (1.3 g/L) | 0.13 ± 0.094 | 0.33 ± 0.094 a | 0.33 ± 0.094 a | 0.20 ± 0.000 a | 0.07 ± 0.094 a | 8.45 ± 0.017 e |
| Sodium Glutamate (2.6 g/L) | 0.07 ± 0.094 | 0.33 ± 0.340 a | 0.27 ± 0.094 a | 0.27 ± 0.094 a | 0.13 ± 0.189 a | 2.00 ± 0.017 b |