Association between Exposure to Leptospira spp. and Abortion in Mares in Croatia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Population
2.2. Serology
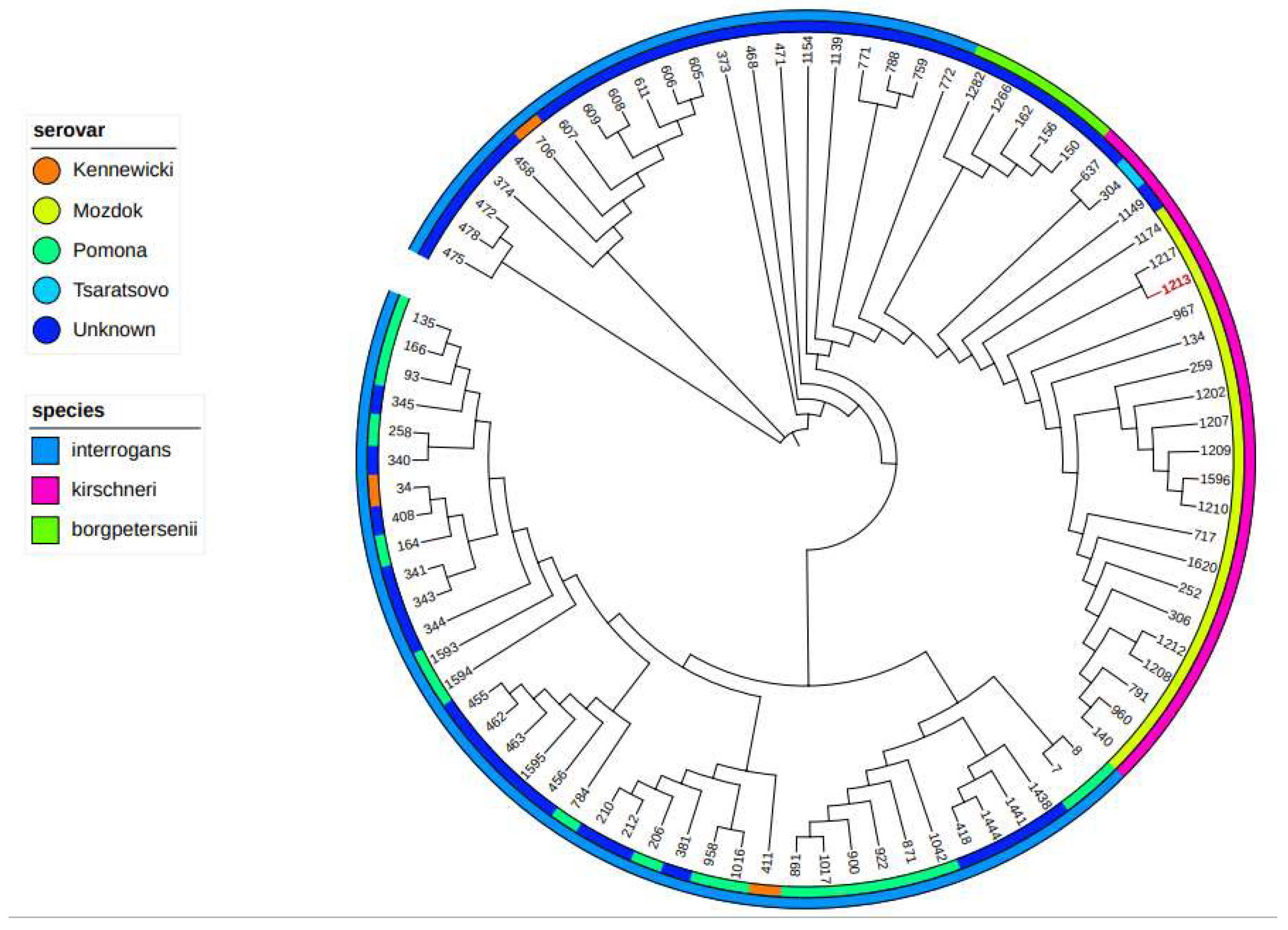
2.3. Isolation and Typing
2.4. Statistical Analyses
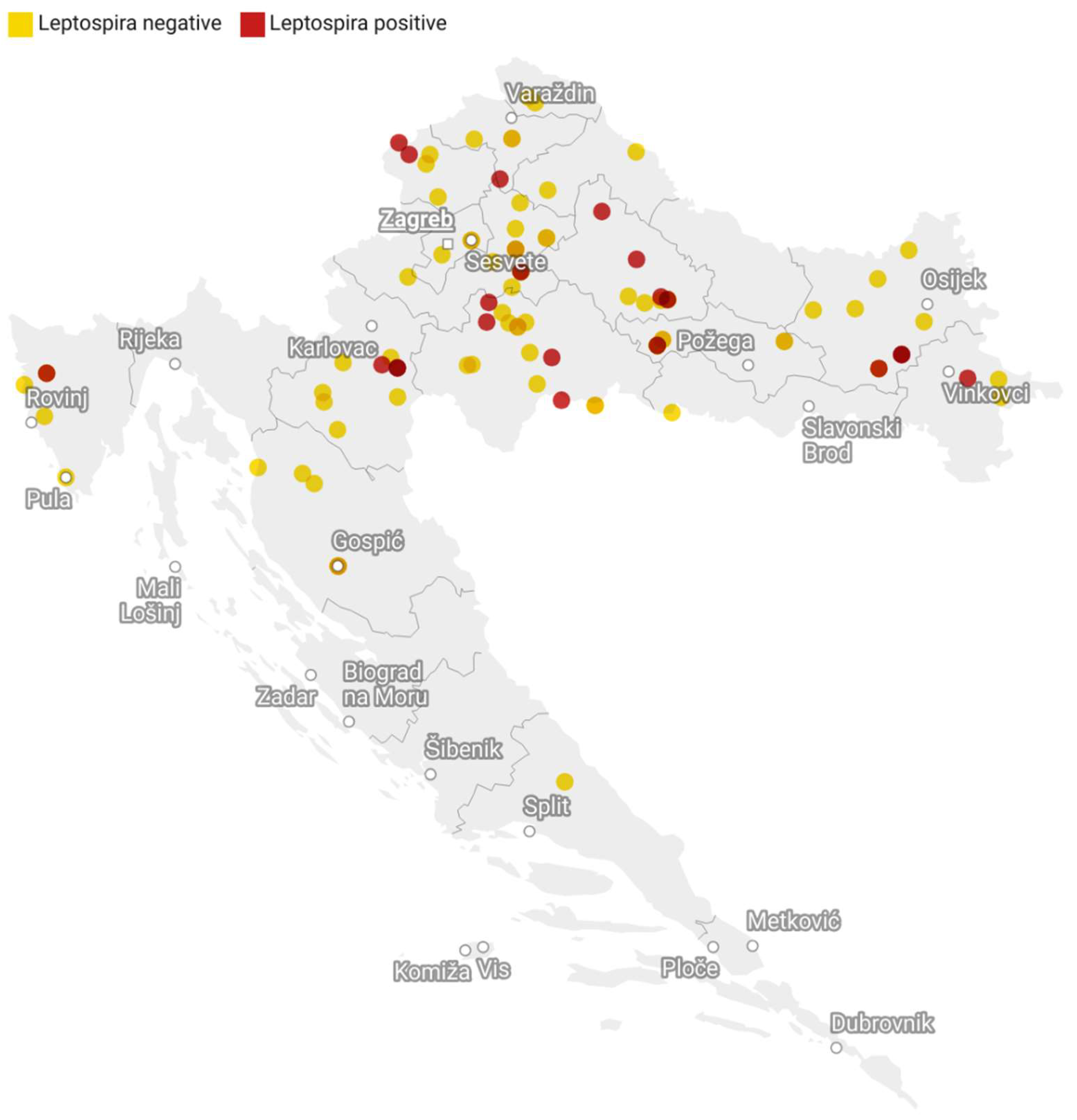
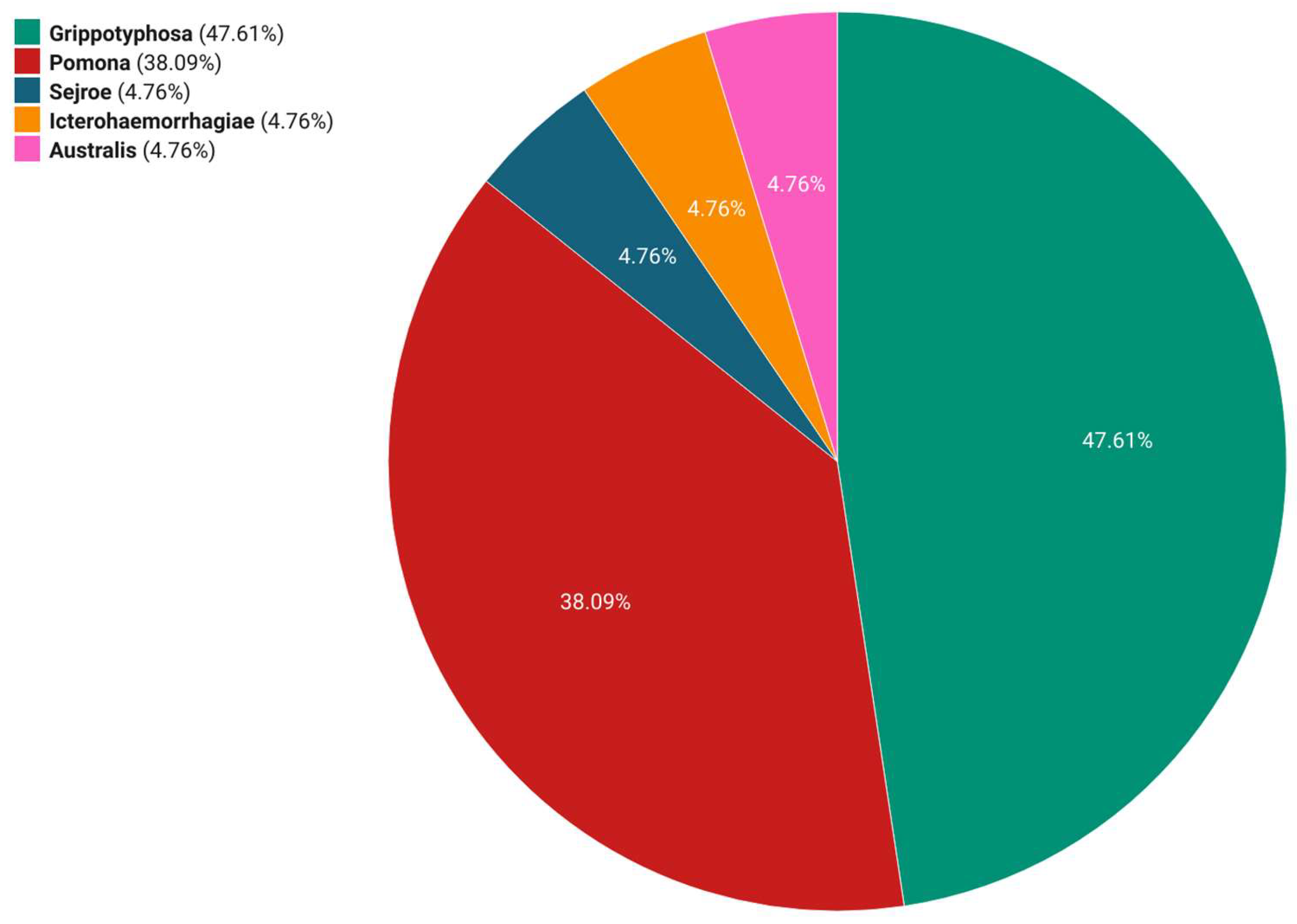
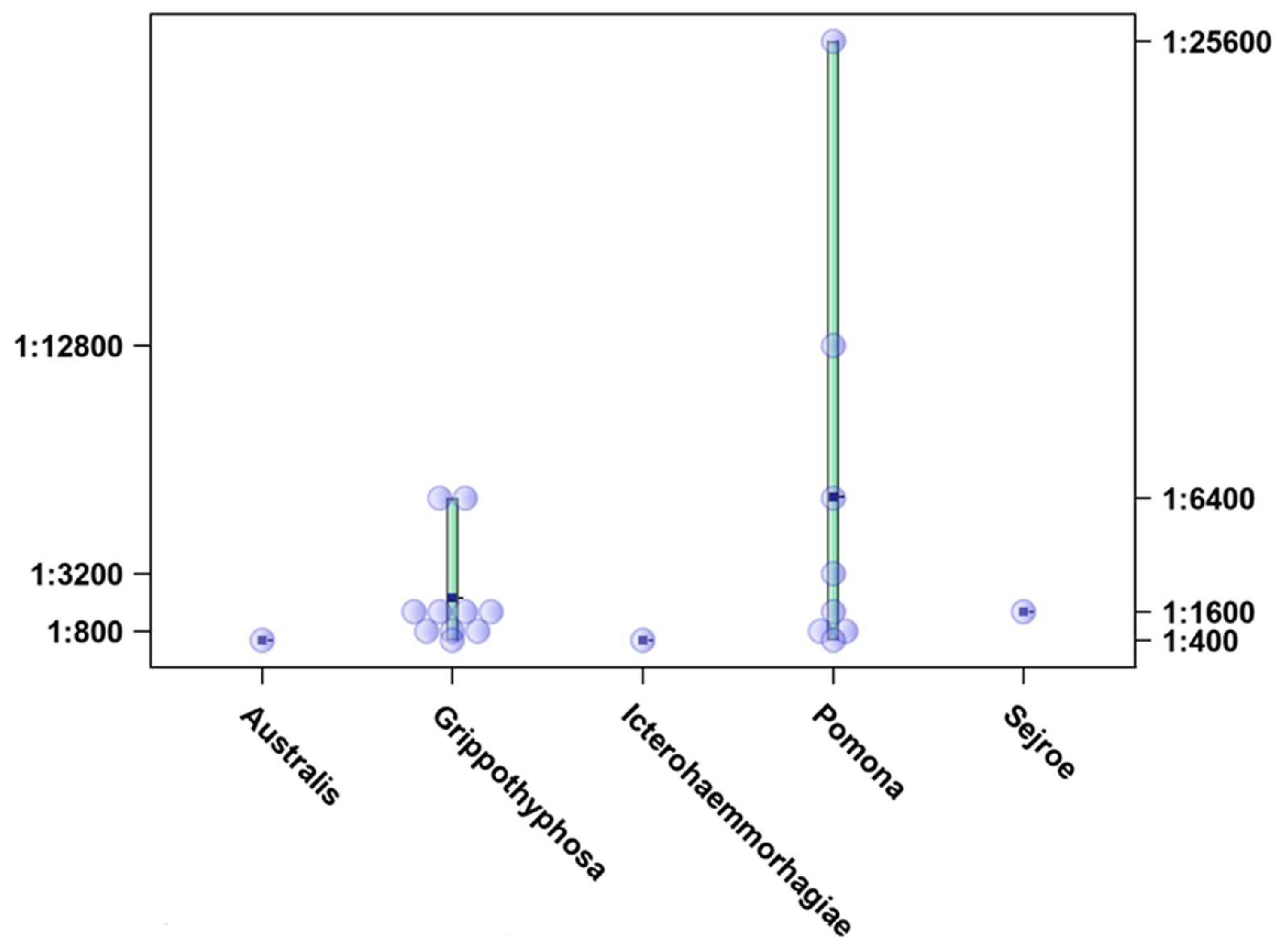
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hagedoorn, N.N.; Maze, M.J.; Carugati, M.; Cash-Goldwasser, S.; Allan, K.J.; Chen, K.; Cossic, B.; Demeter, E.; Gallagher, S.; German, R.; et al. Global distribution of Leptospira serovar isolations and detections from animal host species: A systematic review and online database. Trop. Med. Int. Health 2024, 29, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.; de la Peña Moctezuma, A. Leptospira and leptospirosis. Vet. Microbiol. 2010, 140, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Ellis, W.A. Animal Leptospirosis. Curr. Top. Microbiol. Immunol. 2015, 387, 99–137. [Google Scholar] [CrossRef]
- Divers, T.J.; Chang, Y.F.; Irby, N.L.; Smith, J.L.; Carter, C.N. Leptospirosis: An important infectious disease in North American horses. Equine Vet. J. 2019, 51, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Stevenson, B.; Adler, B. Leptospirosis in horses. Vet. Microbiol. 2013, 167, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Broux, B.; Torfs, S.; Wegge, B.; Deprez, P.; van Loon, G. Acute Respiratory Failure Caused by Leptospira spp. in 5 Foals. J. Vet. Intern. Med. 2012, 26, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Hamond, C.; Pestana, C.P.; Rocha-de-Souza, C.M.; Cunha LE, R.; Brandão, F.Z.; Medeiros, M.A.; Lilenbaum, W. Presence of leptospires on genital tract of mares with reproductive problems. Vet. Microbiol. 2015, 179, 264–269. [Google Scholar] [CrossRef]
- Picardeau, M. Diagnosis and epidemiology of leptospirosis. Med. Mal. Infect. 2013, 43, 1–9. [Google Scholar] [CrossRef]
- Caimi, K.; Ruybal, P. Leptospira spp., a genus in the stage of diversity and genomic data expansion. Infect. Genet. Evol. 2020, 81, 104241. [Google Scholar] [CrossRef]
- Erol, E.; Jackson, C.B.; Steinman, M.; Meares, K.; Donahoe, J.; Kelly, N.; Locke, S.; Smith, J.L.; Carter, C.N. A diagnostic evaluation of real-time PCR, fluorescent antibody and microscopic agglutination tests in cases of equine leptospiral abortion. Equine Vet. J. 2015, 47, 171–174. [Google Scholar] [CrossRef]
- Brandes, K.; Wollanke, B.; Niedermaier, G.; Brem, S.; Gerhards, H. Recurrent uveitis in horses: Vitreal examinations with ultrastructural detection of leptospires. J. Vet. Medicine. A Physiol. Pathol. Clin. Med. 2007, 54, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Hartskeerl, R.A.; Goris, M.G.; Brem, S.; Meyer, P.; Kopp, H.; Gerhards, H.; Wollanke, B. Classification of leptospira from the eyes of horses suffering from recurrent uveitis. J. Vet. Medicine. B Infect. Dis. Vet. Public Health 2004, 51, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Donahue, J.M.; Smith, B.J.; Poonacha, K.B.; Donahoe, J.K.; Rigsby, C.L. Prevalence and serovars of leptospira involved in equine abortions in central Kentucky during the foaling seasons. J. Veter. Diagn. Investig. 1995, 7, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Poonacha, K.B.; Donahue, J.M.; Giles, R.C.; Hong, C.B.; Petrites-Murphy, M.B.; Smith, B.J.; Swerczek, T.W.; Tramontin, R.R.; Tuttle, P.A. Leptospirosis in equine fetuses, stillborn foals, and placentas. Vet. Pathol. 1993, 30, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Timoney, J.F.; Kalimuthusamy, N.; Velineni, S.; Donahue, J.M.; Artiushin, S.C.; Fettinger, M. A unique genotype of Leptospira interrogans serovar Pomona type kennewicki is associated with equine abortion. Vet. Microbiol. 2011, 150, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Ellis, W.A.; Bryson, D.G.; O’Brien, J.J.; Neill, S.D. Leptospiral infection in aborted equine foetuses. Equine Vet. J. 1983, 15, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Turk, N.; Milas, Z.; Habuš, J.; Štritof Majetić, Z.; Mojčec Perko, V.; Barbić, L.; Stevanović, V.; Perharić, M.; Starešina, V. Equine leptospirosis in Croatia-occurrence of subclinical infections Equine leptospirosis in Croatia-occurrence of subclinical infections and abortions and abortions STAREŠINA: Equine Equine leptospirosis in Croatia-occurrence of subclinical infections and abortions. Leptospirosis in Croatia-occurrence of subclinical infections and abortions. Vet. Arh. 2013, 83, 253–262. [Google Scholar]
- Dikken, H.; Kmety, E. Serological typing methods of leptospires. Methods Microbiol. 1978, 11, 260–295. [Google Scholar]
- Hartskeerl, R.A.; Smits, H.L.; Korver, H.; Goris, M.G.A.; Terpstra, W.J. Manual International Course on Laboratory Methods for the Diagnosis of Leptospirosis; KIT: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Ahmed, N.; Manjulata, S.D.; De Los Á Valverde, M.; Vijayachari, P.; Machang’u, R.S.; Ellis, W.A.; Hartskeerl, R.A. Multilocus sequence typing method for identification and genotypic classification of pathogenic Leptospira species. Ann. Clin. Microbiol. Antimicrob. 2006, 5, 28. [Google Scholar]
- Guglielmini, J.; Bourhy, P.; Schiettekatte, O.; Zinini, F.; Brisse, S.; Picardeau, M. Genus-wide Leptospira core genome multilocus sequence typing for strain taxonomy and global surveillance. PLoS Neglected Trop. Dis. 2019, 13, e0007374. [Google Scholar] [CrossRef]
- Benvin, I.; Perko, V.M.; Maljković, M.M.; Habuš, J.; Štritof, Z.; Hađina, S.; Perharić, M.; Zečević, I.; Cvetnić, M.; Turk, N. Serological Surveillance of Equine Leptospirosis in Croatia in the Period from 2012 to 2022: A Key Insight into the Changing Epizootiology. J. Equine Vet. Sci. 2023, 127, 104844. [Google Scholar] [CrossRef] [PubMed]
- Pappas, G.; Papadimitriou, P.; Siozopoulou, V.; Christou, L.; Akritidis, N. The globalization of leptospirosis: Worldwide incidence trends. Int. J. Infect. Dis. 2008, 12, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Arent, Z.J.; Kedzierska-Mieszkowska, S. Seroprevalence study of leptospirosis in horses in northern Poland. Vet. Rec. 2013, 172, 269. [Google Scholar] [CrossRef] [PubMed]
- Blatti, S.; Overesch, G.; Gerber, V.; Frey, J.; Hüssy, D. Seroprävalenz von Leptospira spp. bei klinisch gesunden Pferden in der Schweiz. Schweiz. Arch. Tierheilkd. 2011, 153, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Vera, E.; Taddei, S.; Cavirani, S.; Schiavi, J.; Angelone, M.; Cabassi, C.S.; Schiano, E.; Quintavalla, F. Leptospira seroprevalence in bardigiano horses in northern Italy. Animals 2020, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Agerholm, J.S.; Klas, E.M.; Damborg, P.; Borel, N.; Pedersen, H.G.; Christoffersen, M. A Diagnostic Survey of Aborted Equine Fetuses and Stillborn Premature Foals in Denmark. Front. Vet. Sci. 2021, 8, 740621. [Google Scholar] [CrossRef] [PubMed]
- Marenzoni, M.L.; Lepri, E.; Proietti, P.C.; Bietta, A.; Coletti, M.; Timoney, P.J.; Passamonti, F. Causes of equine abortion, stillbirth and neonatal death in central Italy. Vet. Rec. 2012, 170, 262. [Google Scholar] [CrossRef]
- Habus, J.; Persic, Z.; Spicic, S.; Vince, S.; Stritof, Z.; Milas, Z.; Cvetnic, Z.; Perharic, M.; Turk, N. New trends in human and animal leptospirosis in Croatia, 2009–2014. Acta Trop. 2017, 168, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Malalana, F. Leptospirosis in horses: A European perspective. Equine Vet. J. 2019, 51, 285–286. [Google Scholar] [CrossRef]
- Stritof Majetic, Z.; Galloway, R.; Ruzic Sabljic, E.; Milas, Z.; Mojcec Perko, V.; Habus, J.; Margaletic, J.; Pernar, R.; Turk, N. Epizootiological survey of small mammals as Leptospira spp. reservoirs in Eastern Croatia. Acta Trop. 2014, 131, 111–116. [Google Scholar] [CrossRef]
- Varni, V.; Chiani, Y.; Nagel, A.; Ruybal, P.; Vanasco, N.B.; Caimi, K. Simplified MLST scheme for direct typing of Leptospira human clinical samples. Pathog. Glob. Health 2018, 112, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Grillova, L.; Cokelaer, T.; Mariet, J.F.; da Fonseca, J.P.; Picardeau, M. Core genome sequencing and genotyping of Leptospira interrogans in clinical samples by target capture sequencing. BMC Infect. Dis. 2023, 23, 157. [Google Scholar] [CrossRef] [PubMed]

| No. | Serogroup | Serovar | Strain | Genomic Species |
|---|---|---|---|---|
| 1 | Grippotyphosa | Grippotyphosa | Moskva V | L. kirschneri |
| 2 | Sejroe | Sejroe | M4 | L. borgpetersenii |
| 3 | Australis | Australis | Ballico | L. interrogans |
| 4 | Australis | Bratislava | Jež Bratislava | L. interrogans |
| 5 | Pomona | Pomona | Pomona | L. interrogans |
| 6 | Pomona | Mozdok | 5621 | L. kirschneri |
| 7 | Canicola | Canicola | Hond Utrecht IV | L. interrogans |
| 8 | Icterohaemmorhagiae | Icterohaemmorhagiae | RGA | L. interrogans |
| 9 | Tarassovi | Tarassovi | Peterpelitsin | L. borgpetersenii |
| 10 | Sejroe | Saxkoebing | Mus 24 | L. interrogans |
| 11 | Ballum | Ballum | Mus 127 | L. borgpetersenii |
| 12 | Batavie | Batavie | Swart | L. interrogans |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zečević, I.; Picardeau, M.; Vince, S.; Hađina, S.; Perharić, M.; Štritof, Z.; Stevanović, V.; Benvin, I.; Turk, N.; Lohman Janković, I.; et al. Association between Exposure to Leptospira spp. and Abortion in Mares in Croatia. Microorganisms 2024, 12, 1039. https://doi.org/10.3390/microorganisms12061039
Zečević I, Picardeau M, Vince S, Hađina S, Perharić M, Štritof Z, Stevanović V, Benvin I, Turk N, Lohman Janković I, et al. Association between Exposure to Leptospira spp. and Abortion in Mares in Croatia. Microorganisms. 2024; 12(6):1039. https://doi.org/10.3390/microorganisms12061039
Chicago/Turabian StyleZečević, Iva, Mathieu Picardeau, Silvijo Vince, Suzana Hađina, Matko Perharić, Zrinka Štritof, Vladimir Stevanović, Iva Benvin, Nenad Turk, Ivana Lohman Janković, and et al. 2024. "Association between Exposure to Leptospira spp. and Abortion in Mares in Croatia" Microorganisms 12, no. 6: 1039. https://doi.org/10.3390/microorganisms12061039
APA StyleZečević, I., Picardeau, M., Vince, S., Hađina, S., Perharić, M., Štritof, Z., Stevanović, V., Benvin, I., Turk, N., Lohman Janković, I., & Habuš, J. (2024). Association between Exposure to Leptospira spp. and Abortion in Mares in Croatia. Microorganisms, 12(6), 1039. https://doi.org/10.3390/microorganisms12061039






