Interruption after Short-Term Nitrogen Additions Improves Ecological Stability of Larix olgensis Forest Soil by Affecting Bacterial Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Area and Design
2.2. Soil Index Determination Method
2.2.1. Chemical Test Methods of Soil Properties
2.2.2. Determination of Soil Microbial Community Structure
2.3. Statistical Analysis
3. Results
3.1. Effects of Interruption after Short-Term Nitrogen Additions on Soil Properties
3.2. Effect of Interruption after Short-Term Nitrogen Additions on Soil Bacterial Diversity
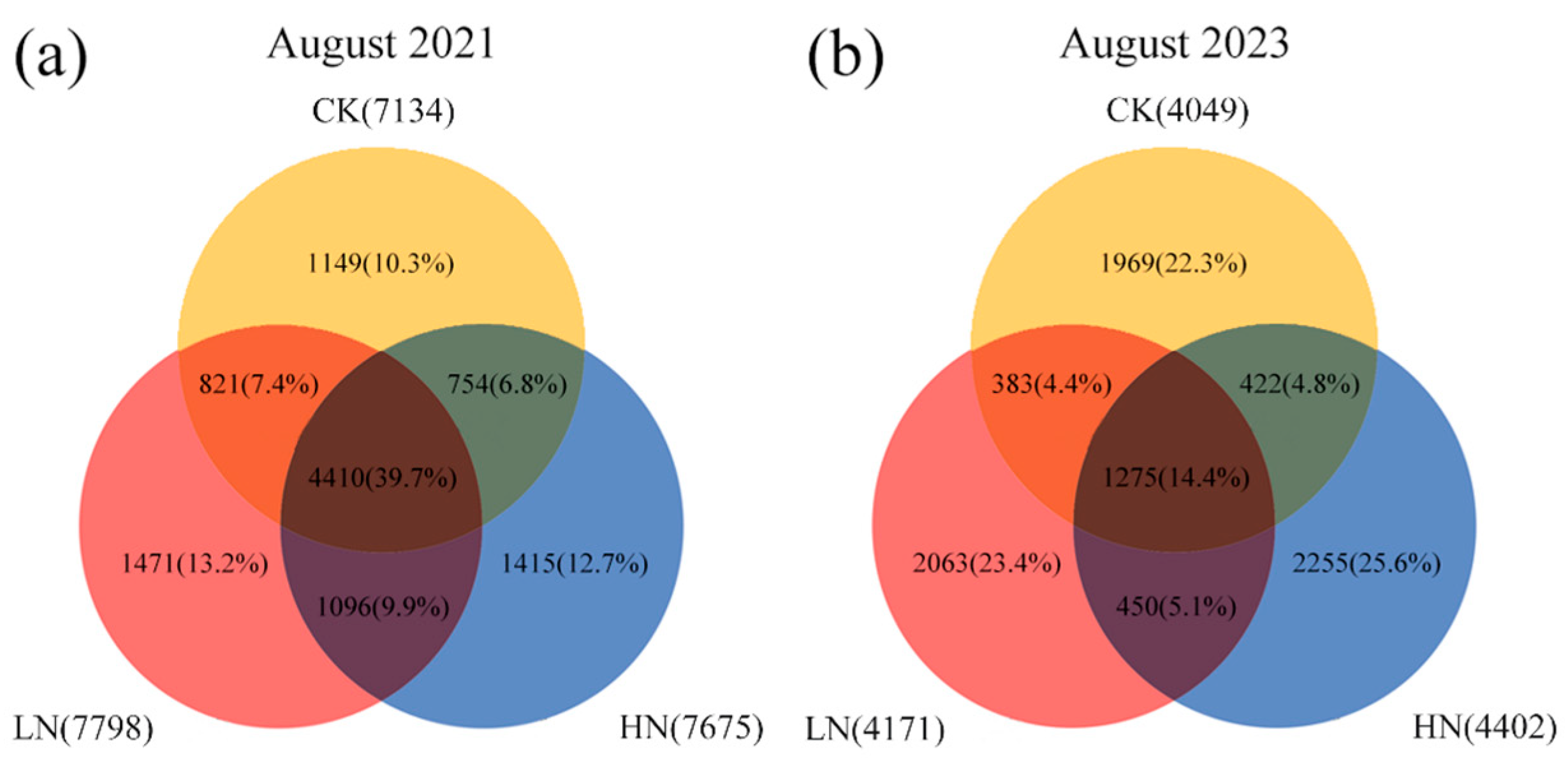
3.3. Effect of Interruption after Short-Term Nitrogen Additions on Soil Bacterial Structure
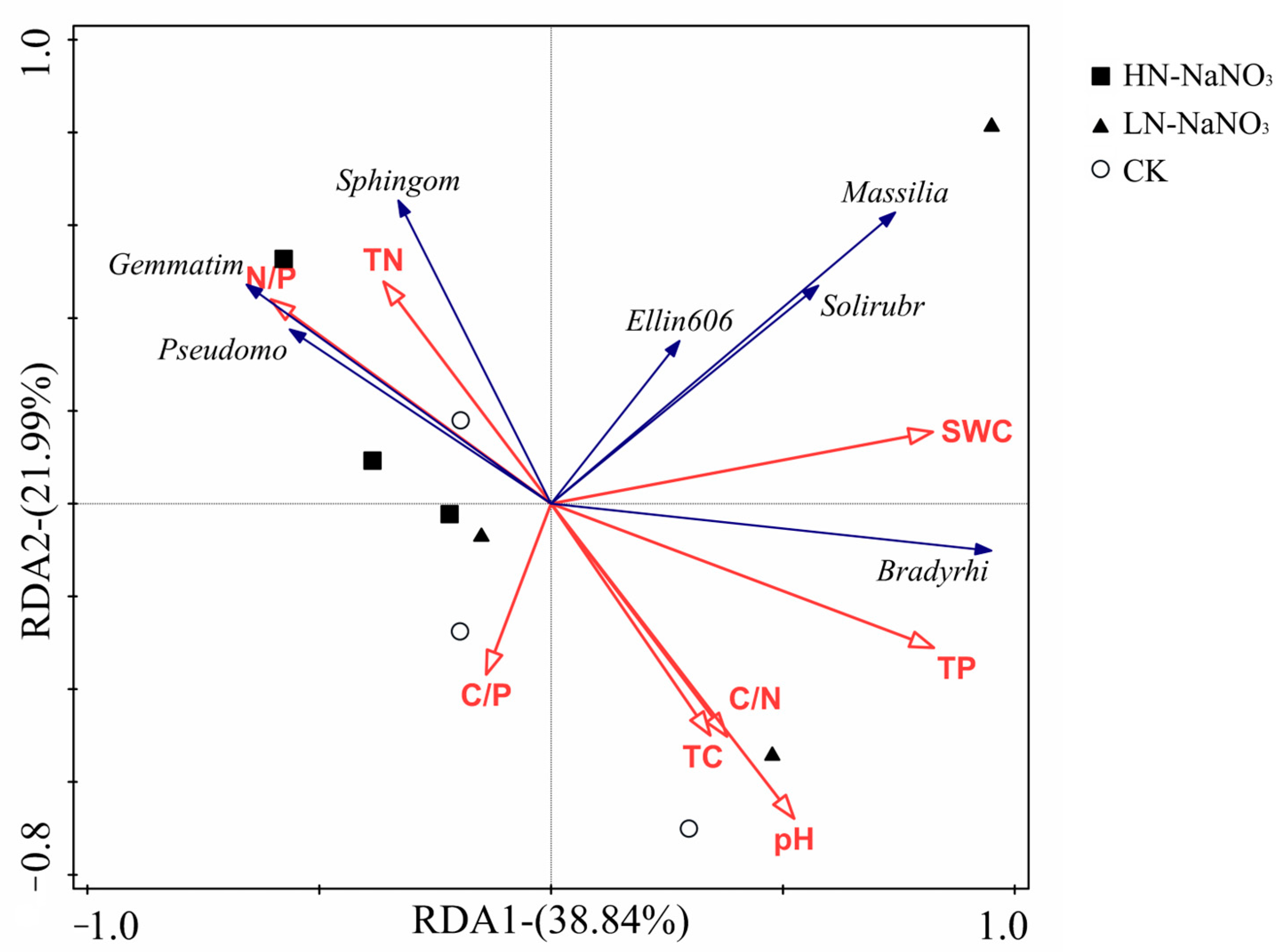
3.4. Relationships between Interruption Microbial Communities and Soil Properties after Short-Term Nitrogen Additions
4. Discussion
4.1. Changes in Soil Properties before and after Interruption of Short-Term Nitrogen Additions
4.2. Changes in Soil Bacterial Diversity before and after Interruption of Short-Term Nitrogen Additions
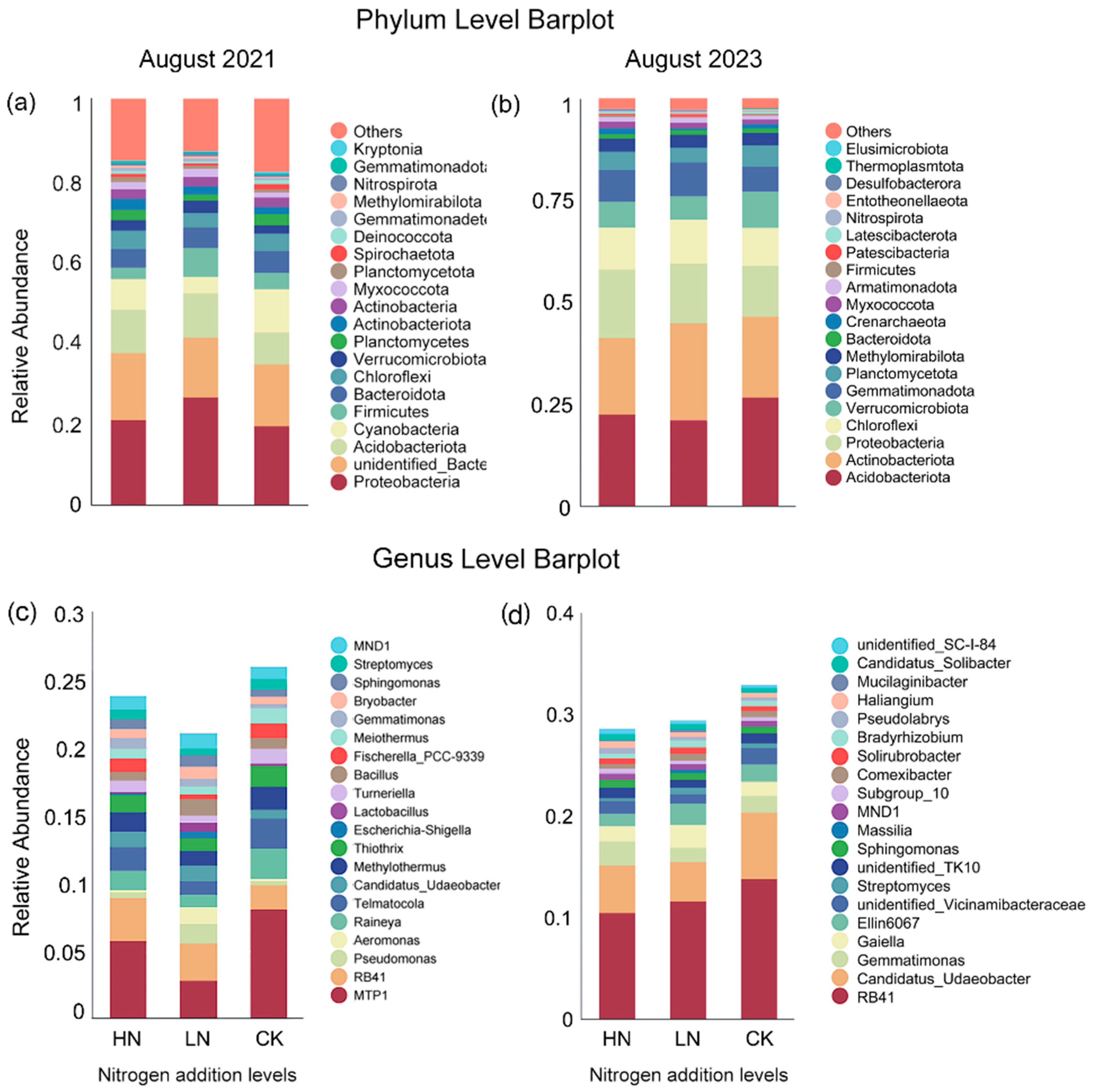
4.3. Changes in Soil Bacterial Structure at the Phylum Level before and after Interruption of Short-Term Nitrogen Additions
4.4. Changes in Soil Bacterial Structure at the Genus Level before and after Interruption of Short-Term Nitrogen Additions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ackerman, D.; Millet, D.B.; Chen, X. Global estimates of inorganic nitrogen deposition across four decades. Glob. Biogeochem. Cycles 2019, 33, 100–107. [Google Scholar] [CrossRef]
- Walker, J.T.; Beachley, G.; Amos, H.M.; Baron, J.S.; Bash, J.; Baumgardner, R.; Bell, M.D.; Benedict, K.B.; Chen, X.; Clow, D.W.; et al. Toward the improvement of total nitrogen deposition budgets in the united states. Sci. Total Environ. 2019, 691, 1328–1352. [Google Scholar] [CrossRef] [PubMed]
- Contosta, A.R.; Frey, S.D.; Cooper, A.B. Soil microbial communities vary as much over time as with chronic warming and nitrogen additions. Soil Biol. Biochem. 2015, 88, 19–24. [Google Scholar] [CrossRef]
- Pierik, M.; van Ruijven, J.; Bezemer, T.M.; Geerts, R.H.E.M.; Berendse, F. Recovery of plant species richness during long-term fertilization of a species-rich grassland. Ecology 2011, 92, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J. Reactive nitrogen and the world: 200 years of change. AMBIO A J. Hum. Environ. 2002, 31, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Taboada, A.; Marcos, E.; Calvo, L. Disruption of trophic interactions involving the heather beetle by atmospheric nitrogen deposition. Environ. Pollut. 2016, 218, 436–445. [Google Scholar] [CrossRef]
- Pugnaire, F.I.; Morillo, J.A.; Peñuelas, J.; Reich, P.B.; Bardgett, R.D.; Gaxiola, A.; Wardle, D.A.; van der Putten, W.H. Climate change effects on plant-soil feedbacks and consequences for biodiversity and functioning of terrestrial ecosystems. Sci. Adv. 2019, 5, eaaz1834. [Google Scholar] [CrossRef]
- Bowden, R.D.; Davidson, E.; Savage, K.; Arabia, C.; Steudler, P. Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperate forest soils at the harvard forest. For. Ecol. Manag. 2004, 196, 43–56. [Google Scholar] [CrossRef]
- Fernández-Guisuraga, J.M.; Ansola, G.; Pinto, R.; Marcos, E.; Calvo, L.; Sáenz De Miera, L.E. Resistance of soil bacterial communities from montane heathland ecosystems in the cantabrian mountains (nw spain) to a gradient of experimental nitrogen deposition. Sci. Total Environ. 2024, 920, 171079. [Google Scholar] [CrossRef]
- Cao, M.; Zheng, X.; Cui, L.; Wu, F.; Gao, H.; Jiang, J. Soil bacterial communities are more sensitive to short-term nitrogen deposition than fungal communities in subtropical chinese fir forests. For. Ecol. Manag. 2023, 549, 121490. [Google Scholar] [CrossRef]
- Ngaba, M.J.Y.; Uwiragiye, Y.; Bol, R.; de Vries, W.; Zhou, J. Low-level nitrogen and short-term addition increase soil carbon sequestration in chinese forest ecosystems. Catena 2022, 215, 106333. [Google Scholar] [CrossRef]
- Ma, X.; Song, Y.; Song, C.; Wang, X.; Wang, N.; Gao, S.; Cheng, X.; Liu, Z.; Gao, J.; Du, Y. Effect of nitrogen addition on soil microbial functional gene abundance and community diversity in permafrost peatland. Microorganisms 2021, 9, 2498. [Google Scholar] [CrossRef] [PubMed]
- Rasse, D.P. Nitrogen deposition and atmospheric CO2 interactions on fine root dynamics in temperate forests: A theoretical model analysis. Glob. Chang. Biol. 2002, 8, 486–503. [Google Scholar] [CrossRef]
- Weng, X.; Wang, M.; Sui, X.; Frey, B.; Liu, Y.; Zhang, R.; Ni, H.; Li, M. High ammonium addition changes the diversity and structure of bacterial communities in temperate wetland soils of northeastern china. Microorganisms 2023, 11, 2033. [Google Scholar] [CrossRef] [PubMed]
- Treseder, K.K. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol. Lett. 2008, 11, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, D.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Berthrong, S.T.; Yeager, C.M.; Gallegos-Graves, L.; Steven, B.; Eichorst, S.A.; Jackson, R.B.; Kuske, C.R. Nitrogen fertilization has a stronger effect on soil nitrogen-fixing bacterial communities than elevated atmospheric CO2. Appl. Environ. Microbiol. 2014, 80, 3103–3112. [Google Scholar] [CrossRef]
- Isbell, F.; Tilman, D.; Polasky, S.; Binder, S.; Hawthorne, P. Low biodiversity state persists two decades after cessation of nutrient enrichment. Ecol. Lett. 2013, 16, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.M.; Hobbie, S.E.; Venterea, R.; Tilman, D. Long-lasting effects on nitrogen cycling 12 years after treatments cease despite minimal long-term nitrogen retention. Glob. Chang. Biol. 2009, 15, 1755–1766. [Google Scholar] [CrossRef]
- Tian, D.; Jiang, L.; Ma, S.; Fang, W.; Schmid, B.; Xu, L.; Zhu, J.; Li, P.; Losapio, G.; Jing, X.; et al. Effects of nitrogen deposition on soil microbial communities in temperate and subtropical forests in china. Sci. Total Environ. 2017, 607–608, 1367–1375. [Google Scholar] [CrossRef]
- Alvarez-Clare, S.; Mack, M.C.; Brooks, M.; Yavitt, J.B. A direct test of nitrogen and phosphorus limitation to net primary productivity in a lowland tropical wet forest. Ecology 2013, 94, 1540–1551. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, X.; Cao, N.; Duan, C.; Ding, C.; Huang, Y.; Wang, J. Biodegradable microplastics enhance soil microbial network complexity and ecological stochasticity. J. Hazard. Mater. 2022, 439, 129610. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Yang, L. A long-term effect of Larix monocultures on soil physicochemical properties and microbes in northeast China. Eur. J. Soil Biol. 2020, 96, 103149. [Google Scholar] [CrossRef]
- Qu, T.; Li, M.; Zhao, X.; Luo, H.; Zhao, L. Nitrogen deposition may benefit to larix olgensis root soils. Forests 2023, 14, 1014. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Townsend, A.R. Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc. Natl. Acad. Sci. USA 2006, 103, 10316–10321. [Google Scholar] [CrossRef]
- Jian, S.; Li, J.; Chen, J.; Wang, G.; Mayes, M.A.; Dzantor, K.E.; Hui, D.; Luo, Y. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef]
- Högberg, P.; Lucas, R.W.; Högberg, M.N.; Skyllberg, U.; Egnell, G.; Larson, J.; Binkley, D. What happens to trees and soils during five decades of experimental nitrogen loading? For. Ecol. Manag. 2023, 553, 121644. [Google Scholar] [CrossRef]
- Aber, J.D.; Nadelhoffer, K.J.; Steudler, P.; Melillo, J.M. Nitrogen saturation in northern forest ecosystems. Bioscience 1989, 39, 378–386. [Google Scholar] [CrossRef]
- de Vries, W.; Du, E.; Butterbach-Bahl, K. Short and long-term impacts of nitrogen deposition on carbon sequestration by forest ecosystems. Curr. Opin. Environ. Sustain. 2014, 9–10, 90–104. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Q.; Sun, Q.; Mao, B.; Zeng, D. Understory vegetation interacts with nitrogen addition to affect soil phosphorus transformations in a nutrient-poor pinus sylvestris var. Mongolica plantation. For. Ecol. Manag. 2022, 507, 120026. [Google Scholar] [CrossRef]
- Scott, J.T.; Lambie, S.M.; Stevenson, B.A.; Schipper, L.A.; Parfitt, R.L.; Mcgill, A.C. Carbon and nitrogen leaching under high and low phosphate fertility pasture with increasing nitrogen inputs. Agric. Ecosyst. Environ. 2015, 202, 139–147. [Google Scholar] [CrossRef]
- Shutenko, G.S.; Andriuzzi, W.S.; Dyckmans, J.; Luo, Y.; Wilkinson, T.L.; Schmidt, O. Rapid transfer of c and n excreted by decomposer soil animals to plants and above-ground herbivores. Soil Biol. Biochem. 2022, 166, 108582. [Google Scholar] [CrossRef]
- Nobile, C.M.; Bravin, M.N.; Becquer, T.; Paillat, J.M. Phosphorus sorption and availability in an andosol after a decade of organic or mineral fertilizer applications: Importance of ph and organic carbon modifications in soil as compared to phosphorus accumulation. Chemosphere 2020, 239, 124709. [Google Scholar] [CrossRef]
- Achat, D.L.; Bakker, M.R.; Augusto, L.; Derrien, D.; Gallegos, N.; Lashchinskiy, N.; Milin, S.; Nikitich, P.; Raudina, T.; Rusalimova, O.; et al. Phosphorus status of soils from contrasting forested ecosystems in southwestern siberia: Effects of microbiological and physicochemical properties. Biogeosciences 2013, 10, 733–752. [Google Scholar] [CrossRef]
- Spohn, M. Element cycling as driven by stoichiometric homeostasis of soil microorganisms. Basic Appl. Ecol. 2016, 17, 471–478. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:n:p stoichiometry in soil: Is there a “redfield ratio” for the microbial biomass. Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Reich, P.B.; Khachane, A.N.; Campbell, C.D.; Thomas, N.; Freitag, T.E.; Abu Al-Soud, W.; Sørensen, S.; Bardgett, R.D.; Singh, B.K. It is elemental: Soil nutrient stoichiometry drives bacterial diversity. Environ. Microbiol. 2017, 19, 1176–1188. [Google Scholar] [CrossRef]
- Mchugh, T.A.; Morrissey, E.M.; Mueller, R.C.; Gallegos Graves, L.V.; Kuske, C.R.; Reed, S.C. Bacterial, fungal, and plant communities exhibit no biomass or compositional response to two years of simulated nitrogen deposition in a semiarid grassland. Environ. Microbiol. 2017, 19, 1600–1611. [Google Scholar] [CrossRef]
- Baath, E.; Kritzberg, E. Ph tolerance in freshwater bacterioplankton: Trait variation of the community as measured by leucine incorporation. Appl. Environ. Microbiol. 2015, 81, 7411–7419. [Google Scholar] [CrossRef]
- Huang, L.; Bai, J.; Wen, X.; Zhang, G.; Zhang, C.; Cui, B.; Liu, X. Microbial resistance and resilience in response to environmental changes under the higher intensity of human activities than global average level. Glob. Chang. Biol. 2020, 26, 2377–2389. [Google Scholar] [CrossRef]
- Louca, S.; Polz, M.F.; Mazel, F.; Albright, M.B.N.; Huber, J.A.; O’Connor, M.I.; Ackermann, M.; Hahn, A.S.; Srivastava, D.S.; Crowe, S.A.; et al. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2018, 2, 936–943. [Google Scholar] [CrossRef]
- de Menezes, A.B.; Prendergast Miller, M.T.; Richardson, A.E.; Toscas, P.; Farrell, M.; Macdonald, L.M.; Baker, G.; Wark, T.; Thrall, P.H. Network analysis reveals that bacteria and fungi form modules that correlate independently with soil parameters. Environ. Microbiol. 2015, 17, 2677–2689. [Google Scholar] [CrossRef]
- Javed, Z.; Tripathi, G.D.; Mishra, M.; Dashora, K. Actinomycetes—the microbial machinery for the organic-cycling, plant growth, and sustainable soil health. Biocatal. Agric. Biotechnol. 2021, 31, 101893. [Google Scholar] [CrossRef]
- Conradie, T.A.; Jacobs, K. Distribution patterns of acidobacteriota in different fynbos soils. PLoS ONE 2021, 16, e248913. [Google Scholar] [CrossRef]
- Lu, G.; Xie, B.; Cagle, G.A.; Wang, X.; Han, G.; Wang, X.; Hou, A.; Guan, B. Effects of simulated nitrogen deposition on soil microbial community diversity in coastal wetland of the yellow river delta. Sci. Total Environ. 2021, 757, 143825. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Lueders, T.; Kindler, R.; Miltner, A.; Friedrich, M.W.; Kaestner, M. Identification of bacterial micropredators distinctively active in a soil microbial food web. Appl. Environ. Microbiol. 2006, 72, 5342–5348. [Google Scholar] [CrossRef]
- Li, C.; Li, H.; Yao, T.; Su, M.; Ran, F.; Li, J.; He, L.; Chen, X.; Zhang, C.; Qiu, H. Effects of swine manure composting by microbial inoculation: Heavy metal fractions, humic substances, and bacterial community metabolism. J. Hazard. Mater. 2021, 415, 125559. [Google Scholar] [CrossRef]
- Jonasson, S.; Castro, J.; Michelsen, A. Interactions between plants, litter and microbes in cycling of nitrogen and phosphorus in the arctic. Soil Biol. Biochem. 2006, 38, 526–532. [Google Scholar] [CrossRef]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete nitrification by nitrospira bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef]
- Banerjee, S.; Kirkby, C.A.; Schmutter, D.; Bissett, A.; Kirkegaard, J.A.; Richardson, A.E. Network analysis reveals functional redundancy and keystone taxa amongst bacterial and fungal communities during organic matter decomposition in an arable soil. Soil Biol. Biochem. 2016, 97, 188–198. [Google Scholar] [CrossRef]
- Debruyn, J.M.; Nixon, L.T.; Fawaz, M.N.; Johnson, A.M.; Radosevich, M. Global biogeography and quantitative seasonal dynamics of gemmatimonadetes in soil. Appl. Environ. Microbiol. 2011, 77, 6295–6300. [Google Scholar] [CrossRef]
- Song, L.; Niu, X.; Zhang, N.; Li, T. Effect of biochar-immobilized sphingomonas sp. Pj2 on bioremediation of pahs and bacterial community composition in saline soil. Chemosphere 2021, 279, 130427. [Google Scholar] [CrossRef]
- Tanunchai, B.; Ji, L.; Schröder, O.; Gawol, S.J.; Geissler, A.; Wahdan, S.F.M.; Buscot, F.; Kalkhof, S.; Schulze, E.; Noll, M.; et al. Fate of a biodegradable plastic in forest soil: Dominant tree species and forest types drive changes in microbial community assembly, influence the composition of plastisphere, and affect poly(butylene succinate-co-adipate) degradation. Sci. Total Environ. 2023, 873, 162230. [Google Scholar] [CrossRef]
- Graham, E.B.; Knelman, J.E. Implications of soil microbial community assembly for ecosystem restoration: Patterns, process, and potential. Microb. Ecol. 2023, 85, 809–819. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, R.; Teng, L.; Chen, H.; Li, M.; Wang, L.; Zhran, M.; Cao, F. Genotypic variation in grain cadmium concentration in wheat: Insights into soil pollution, agronomic characteristics, and rhizosphere microbial communities. Environ. Pollut. 2024, 340, 122792. [Google Scholar] [CrossRef]
- Özbolat, O.; Sánchez-Navarro, V.; Zornoza, R.; Egea-Cortines, M.; Cuartero, J.; Ros, M.; Pascual, J.A.; Boix-Fayos, C.; Almagro, M.; de Vente, J.; et al. Long-term adoption of reduced tillage and green manure improves soil physicochemical properties and increases the abundance of beneficial bacteria in a mediterranean rainfed almond orchard. Geoderma 2023, 429, 116218. [Google Scholar] [CrossRef]
- Větrovský, T.; Steffen, K.T.; Baldrian, P.; Freitag, M. Potential of cometabolic transformation of polysaccharides and lignin in lignocellulose by soil actinobacteria. PLoS ONE 2014, 9, e89108. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, M.; Zhang, Y.; Wang, J.; Shen, H.; Lin, Y.; Tang, X.; Hui, D.; Lambers, H.; Sardans, J.; et al. Soil phosphorus availability affects diazotroph communities during vegetation succession in lowland subtropical forests. Appl. Soil Ecol. 2021, 166, 104009. [Google Scholar] [CrossRef]
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Newman, L.; Vangronsveld, J. Exploiting plant–microbe partnerships to improve biomass production and remediation. Trends Biotechnol. 2009, 27, 591–598. [Google Scholar] [CrossRef]



| Treatments | Nitrogen Addition | Interruption | Nitrogen Addition × Interruption | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Soil pH | 18.328 | 0.001 ** | 18.328 | 0.000 ** | 1.251 | 0.310 |
| SWC (%) | 6.695 | 0.006 ** | 1.180 | 0.292 | 6.843 | 0.006 ** |
| TC (mg/kg) | 115.241 | 0.000 ** | 0.136 | 0.736 | 2.135 | 0.147 |
| TN (mg/kg) | 229.710 | 0.000 ** | 5.396 | 0.030 * | 26.614 | 0.000 ** |
| TP (mg/kg) | 20.908 | 0.001 ** | 13.428 | 0.001 ** | 13.808 | 0.000 ** |
| C:N | 287.558 | 0.000 ** | 15.851 | 0.001** | 7.392 | 0.005 ** |
| C:P | 61.143 | 0.000 ** | 436.935 | 0.000 ** | 10.140 | 0.001 ** |
| N:P | 150.122 | 0.000 ** | 26.264 | 0.000 ** | 54.458 | 0.000 ** |
| C:N:P | 209.255 | 0.000 ** | 291.396 | 0.001 ** | 12.913 | 0.000 ** |
| Chao1 | 4.768 | 0.018 * | 2049.800 | 0.001 ** | 7.026 | 0.004 ** |
| Shannon | 8.839 | 0.001 ** | 5.952 | 0.752 | 10.660 | 0.000 ** |
| Simpson | 6.484 | 0.006 ** | 16.658 | 0.000 ** | 3.213 | 0.058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, T.; Zhao, X.; Yan, S.; Liu, Y.; Ameer, M.J.; Zhao, L. Interruption after Short-Term Nitrogen Additions Improves Ecological Stability of Larix olgensis Forest Soil by Affecting Bacterial Communities. Microorganisms 2024, 12, 969. https://doi.org/10.3390/microorganisms12050969
Qu T, Zhao X, Yan S, Liu Y, Ameer MJ, Zhao L. Interruption after Short-Term Nitrogen Additions Improves Ecological Stability of Larix olgensis Forest Soil by Affecting Bacterial Communities. Microorganisms. 2024; 12(5):969. https://doi.org/10.3390/microorganisms12050969
Chicago/Turabian StyleQu, Tongbao, Xiaoting Zhao, Siyu Yan, Yushan Liu, Muhammad Jamal Ameer, and Lei Zhao. 2024. "Interruption after Short-Term Nitrogen Additions Improves Ecological Stability of Larix olgensis Forest Soil by Affecting Bacterial Communities" Microorganisms 12, no. 5: 969. https://doi.org/10.3390/microorganisms12050969
APA StyleQu, T., Zhao, X., Yan, S., Liu, Y., Ameer, M. J., & Zhao, L. (2024). Interruption after Short-Term Nitrogen Additions Improves Ecological Stability of Larix olgensis Forest Soil by Affecting Bacterial Communities. Microorganisms, 12(5), 969. https://doi.org/10.3390/microorganisms12050969






