Abstract
Microbial resistance to antibiotics poses a significant threat to both human and animal health, necessitating international efforts to mitigate this issue. This study aimed to assess the resistance profiles of Salmonella sp. isolates and identify the presence of intl1, sul1, and blaTEM resistance genes within antigenically characterized isolates, including Agona, Livingstone, Cerro, Schwarzengrund, Salmonella enterica subsp. enterica serotype O:4.5, Anatum, Enteritidis, Johannesburg, Corvallis, and Senftenberg. These isolates underwent susceptibility testing against 14 antibiotics. The highest resistance percentages were noted for sulfamethoxazole (91%), sulfonamides (51%), and ceftiofur (28.9%), while no resistance was observed for ciprofloxacin. Salmonella Johannesburg and Salmonella Corvallis showed resistance to one antibiotic, whereas other serovars were resistant to at least two. Salmonella Schwarzengrund exhibited resistance to 13 antibiotics. The intl1 gene was detected in six out of the ten serovars, and the sul1 gene in three, always co-occurring with intl1. The blaTEM gene was not identified. Our findings highlight the risk posed by the detected multiple resistances and genes to animal, human, and environmental health. The multidrug resistance, especially to third-generation cephalosporins and fluoroquinolones, highlights the need for stringent monitoring of Salmonella in laying hens. The potential of the environment, humans, eggs, and their products to act as vectors for antibiotic resistance represents a significant concern for One Health.
1. Introduction
Salmonellosis is a globally widespread foodborne illness [1] with significant implications for public health worldwide, as humans often contract multidrug-resistant (MDR) Salmonella infections through the consumption of contaminated animal products [2,3].
MDR Salmonella is characterized by its resistance to conventional first-line antibiotics such as ampicillin, chloramphenicol, and trimethoprim-sulfamethoxazole [4,5,6]. The global rise in the isolation of Salmonella serotypes resistant to one or more antibiotics [7] is largely attributed to the inappropriate use, overuse, and easy accessibility of antibiotics across various countries [2]. In South Africa, Salmonella has been frequently isolated from animal feeds, livestock, and their environments [8]. The European Union has reported MDR Salmonella in poultry meat samples [9]. In New South Wales and South Australia, Salmonella spp. isolated from commercial caged layer flocks exhibited the highest resistance to amoxicillin and ampicillin, followed by tetracycline, cephalothin, and trimethoprim. In Brazil, a significant portion (80.9%) of Salmonella isolates from different stages of swine production were deemed multidrug-resistant (resistant to ≥3 antibiotic classes), showing the greatest resistance to streptomycin (90.5%), tetracycline (88.1%), ampicillin (81.0%), chloramphenicol (71.4%), and ciprofloxacin (50.0%) [10]. In China, Salmonella strains isolated from broilers and poultry workers displayed MDR phenotypes, resisting up to 16 commonly used antibiotics such as ampicillin, streptomycin, kanamycin, amikacin, tetracycline, nalidixic acid, ciprofloxacin, trimethoprim/sulfamethoxazole, and chloramphenicol [11].
A global review on antibiotic resistance in Salmonella sp. isolated from poultry [12] revealed that the median prevalence rates in broilers, raw chicken meat, eggs, and laying hens were 40.5%, 30%, and 40%, respectively. The study highlighted that the highest antibiotic resistance levels within the poultry production chain were observed for nalidixic acid and ampicillin (Figure 1). MDR Salmonella presence has also been reported in the layer farm environment, suggesting public health risks associated with egg consumption [13,14] and representing a significant risk factor for salmonellosis [15]. Another study focusing on egg farm environments in Western Australia reported a high incidence of Salmonella [14].
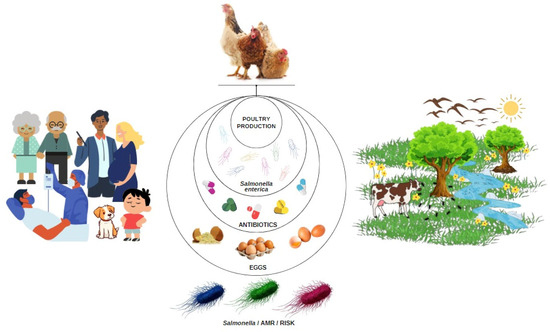
Figure 1.
Representation of interactions between layer production system, presence of antibiotic-resistant Salmonella enterica, environment, consumers, and consumption of eggs and by-products as a scenario of the risk of antibiotic resistance (AMR) for One Health.
While antibiotic resistance can occur naturally, the emergence of most resistant organisms is attributed to the acquisition of resistance genes through mutations or the transfer of genetic material, followed by selective pressure [16,17,18,19,20,21].
Food should contribute positively to human health, and over time, the concept of functional foods has evolved, emphasizing foods that lower the risk of various health issues [22,23]. However, the food chain is also a critical vector for the spread of antibiotic resistance from animals to humans. The rise of antibiotic resistance in non-typhoidal Salmonella strains poses a significant public health risk [24,25,26], underscoring the importance of monitoring resistance profiles in Salmonella Typhimurium and Salmonella Enteritidis. This not only aids in disease management but also helps track the development of potential multidrug-resistant strains. Resistance mechanisms can be shared among bacteria within the same genus or across different bacterial groups [27,28].
Genotypic characterization techniques such as quantitative PCR (qPCR) offer a more precise identification of Salmonella serovars by detecting specific DNA sequences. These methods surpass phenotypic characterization in discriminatory capability [29,30,31]. A characterization of Salmonella incorporating multi-locus sequence typing (MLST), genotype antibiotic resistance (AMR) profiles, plasmid incompatibility types, phylogeny, and serotype can elucidate the clonal populations. The diversity of AMR genes in Salmonella from various sources suggests genome alterations due to the acquisition of new AMR genes through mutations, plasmid transfers, or existing natural resistance genes [32].
Genotypic methods that analyze chromosomal or plasmid DNA enable the identification of bacteria through unique or species-specific genes, as well as the detection of particular genes [33,34]. These techniques can help to understand the development and transferability of mobile resistance-encoding elements in bacterial pathogens, driven by interactions between specific chromosomal fragments and plasmids [35]. As the prevalence of transferable virulence and antibiotic resistance increases, the role of antibiotic-resistant plasmids in Salmonella becomes a growing concern for both animal and public health [36].
This study aimed to determine the antibiotic resistance profiles of Salmonella serovars from commercial egg and laying hen farms in the Center-West region of Brazil and to identify the presence of intl1, sul1, and blaTEM resistance genes. This research represents the first phenotypic and genotypic mapping of Salmonella isolates conducted over a year in an egg and by-product production region in Brazil.
2. Materials and Methods
For this study, Salmonella serovars preserved in the bacteriology laboratory were utilized. The research involved two main assessments: (1) antibiotic susceptibility profiling, in which the Salmonella serovars were subjected to various classes of antibiotics; and (2) gene detection, employing qPCR assays to identify antibiotic resistance genes across all Salmonella serovars.
2.1. Study Location
The project was carried out in Goiânia, GO, Brazil (−16.67926° N −49.25629° W).
2.2. Sampling
The study analyzed 45 Salmonella isolates retrieved from samples collected over a year from commercial egg and layer farms. Laboratory identification of the Salmonella genus was conducted through biochemical testing and further verified using a polyvalent antiserum “O” agglutination test for Salmonella (Probac, São Paulo, Brazil). Serotyping of the isolates was performed at the National Reference Laboratory for Enteric Diseases at the Oswaldo Cruz Institute—FIOCRUZ (NRL).
These serovars, preserved on nutrient agar, were contributed to this research by the Laboratory of Bacteriology at the Faculty of Veterinary Medicine, Federal University of Goiás. The serovars used in this study were Agona (14/45), Livingstone (8/45), Cerro (6/45), Schwarzengrund (5/45), Enterica O:4.5 (4/45), Anatum (3/45), Enteritidis (2/45), Johannesburg (1/45), Corvallis (1/45), and Senftenberg (1/45).
2.3. Antibiotic Susceptibility Profiling
A total of 45 Salmonella isolates cultured on nutrient agar were further plated on bright green agar and incubated at 37 °C for 24 h. Following incubation, colony-forming units exhibiting typical Salmonella morphology traits were identified and selected. Two colony-forming units from each plate were then transferred to tubes containing Triple Sugar Iron (TSI) agar and incubated at 37 °C for an additional 24 h.
The antibiotic susceptibility profile of 45 isolates was assessed using the disk diffusion method, adhering to the performance standards for antibiotic susceptibility testing [37]. The antibiotics tested, along with their respective classes and concentrations, are presented in Table 1.

Table 1.
Antibiotic classes, antibiotics tested, and their respective concentrations used in antibiotic susceptibility profiling.
Isolates confirmed to display Salmonella characteristics were then transferred to 5 mL of Casoy broth and incubated until a turbidity of 0.5 on the McFarland scale was achieved. Subsequently, a swab immersed in the broth was drained by pressing it against the walls of the tube to eliminate excess fluid, and then streaked across a Mueller-Hinton agar plate to ensure a homogeneous distribution of the inoculum. The inoculated plates were left undisturbed for 15 min to allow the inoculum to be adequately absorbed by the agar. Sterilized tweezers were then used to carefully place antibiotic discs onto the inoculated surface.
Each disc was gently pressed down to enhance adherence and spaced approximately 3 cm apart. The plates were then incubated upside down at 37 °C for 24 h. Post-incubation, the zones of inhibition were measured with a ruler, and the results were interpreted based on standardized tables that account for the concentration of the discs used. Antibiotic resistance prevalence data were summarized using the median and interquartile range (IQR) across studies, with the analysis conducted using EpiInfo2000 version 3.3.1 (CDC—Atlanta, GA, USA, 2005). The resistance frequency to each specific antibiotic was tabulated, and isolates were classified as MDR if they exhibited resistance to three or more classes of antibiotics.
2.4. Gene Detection
Total DNA extraction was conducted using the boiling lysis method [38]. For each sample, 400 μL was transferred to a 1.5-mL DNA- and RNA-free polypropylene tube (Axygen, Union City, CA, USA) and centrifuged at 2000× g for 4 min. The supernatant was discarded, and the pellet was resuspended in 1 mL TE buffer (100 mL Tris/HCl 1 m, 20 μL EDTA 0.5 m, and 9.880 μL H2O), vortexed for 10 s, and centrifuged again at 2000× g for 8 min. Once again, the supernatant was discarded, and the pellet was resuspended in 100 μL TE buffer, vortexed for 10 s, and placed on a hot plate at 95 °C for 20 min. The lysate was then aliquoted and stored in a freezer at −20 °C for subsequent analysis.
qPCR assays to detect intl1, sul1, and blaTEM genes in Salmonella sp. were conducted following the protocol described by Bugarel et al. [39]. The reaction mixture for qPCR, using the TaqMan system, comprised 20 μL per sample: 4.6 μL of milli-Q water, 10 μL of master mix (1×), 2 μL of IPC mix (10×), 0.4 μL of IPC DNA (50×), and 1 μL of oligonucleotide primers (30 mM concentration) and probe (10 mM concentration), with 2 μL of the DNA extract added. An internal control was included in one of the wells of a 96-well plate using IPC DNA with and without IPC blocker reagent (Life®, Thermo Fischer Scientific, Waltham, MA, USA) as the negative control. Samples were tested for presence/absence in the StepOnePlus™ qPCR system (Applied Biosystems, Waltham, MA, USA) under the following PCR conditions: an initial hold at 60 °C for 30 s, a denaturation step at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min and 30 s for the extension step.
The real-time PCR detection of genes employed the TaqMan system with specific oligonucleotide primers for intl1 (f 5′-TGGGCAGCAGCGAAGTC-3′, r 5′-TGCGTGGAGACCGAAACC-3′, probe FAM-AGGCATTTCTGTCCTGGCTGGCG-BHQ), sul1 (f 5′-TCCTGACCCTGCGCTCTATC-3′, r 5′-TGCGCTGAGTGCATAACCA-3′, probe ROX-ATTGCTGAGGCGGACTGCAGGC-BHQ), and blaTEM (f 5′-CTGGATCTCAACAGCGG-3′, r 5′-CAACACGGGATAATACCGC, probe FAM-AGATCCTTGAGAGTTTTCGCCCCG-BHQ).
Adjustments to the apparatus were made to accommodate available filters, replacing ROX with FAM and BHQ with TAMRA as needed.
Statistical analysis to determine significant differences between gene frequencies utilized Pearson’s chi-squared test or Fisher’s exact test, as appropriate, with p ≤ 0.05 denoting statistical significance. All statistical analyses were executed using EpiInfo2000 version 3.3.1 (CDC—Atlanta, 2005).
3. Results
The highest resistance percentages observed were to sulfamethoxazole (91%) followed by sulfonamides (51%) and ceftiofur (28.9%). Only ciprofloxacin showed no resistance among the tested serovars (Table 2). Resistance to just one antibiotic, sulfamethoxazole, was noted exclusively in Salmonella Johannesburg and Salmonella Corvallis, whereas all other examined serovars displayed resistance to at least two antibiotics (Table 3). Salmonella Schwarzengrund showed resistance to thirteen antibiotics, followed by Salmonella Enteritidis and Salmonella enterica subsp. enterica serotype O:4.5, each resistant to five antibiotics. Salmonella Agona exhibited resistance to four antibiotics, while both Salmonella Livingstone and Salmonella Cerro were resistant to three. Salmonella Senftenberg and another unidentified Salmonella serovar showed resistance to two antibiotics. Serovars such as Agona, Salmonella enterica subsp. enterica serotype O:4.5, and Schwarzengrund were identified as MDR, demonstrating resistance to three or more classes of antibiotics (Figure 2).

Table 2.
Antibiotic susceptibility profile of 45 Salmonella isolates.

Table 3.
Antibiotic resistance of Salmonella sp. serovars.
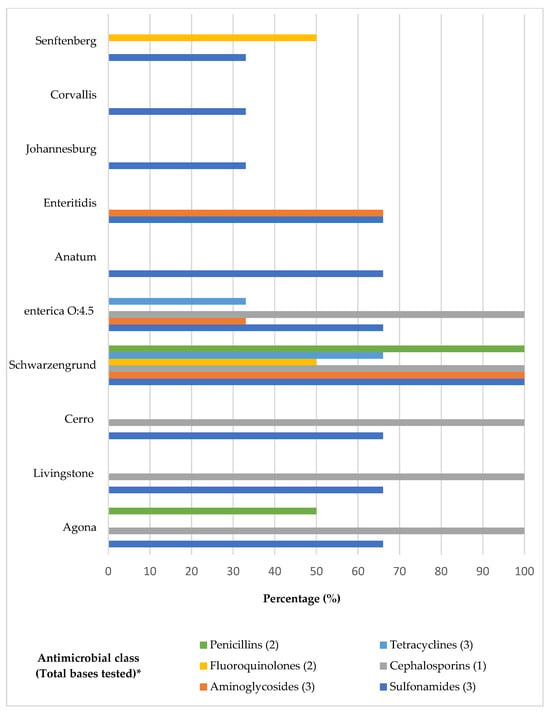
Figure 2.
Distribution of Salmonella serovars resistant to antibiotic classes. * Tested bases of each antibiotic class: Penicillins (Amoxicillin clavulanic acid, Ampicillin); Fluoroquinolones (Enrofloxacin; Ciprofloxacin); Aminoglycosides (Gentamicin, Neomycin, Apramycin); Tetracyclines (Tetracycline, Oxytetracycline, Doxycycline); Cephalosporins (Ceftiofur); Sulfonamides (Sulfamethoxazole, Trimethoprim-sulfamethoxazole, Sulfonamides).
The presence of resistance genes sul1 and intl1 was confirmed (Table 4). Class 1 integrons were found in serovars Cerro, Johannesburg, and Salmonella enterica subsp. enterica O:4.5 and was associated with the sul1 gene in serovars Agona, Livingstone, and Schwarzengrund.

Table 4.
Resistance to ampicillin (AMP), amoxicillin with clavulanic acid (AMC), and sulfonamides (SUL) and qPCR for intl1, sul1, and blaTEM resistance genes in different serovars.
Ampicillin resistance was detected in 1/5 (20%) of the Schwarzengrund isolates. Resistance to amoxicillin-clavulanic acid was observed in 1/14 (7.1%) of Agona isolates and 1/5 (20%) of Schwarzengrund isolates. The blaTEM gene, however, was not detected in any of the tested isolates.
4. Discussion
The indiscriminate use of antibiotics in human and animal healthcare, as well as in food production, coupled with their subsequent release into the environment, contribute to the emergence of antibiotic-resistant Salmonella strains [40]. Understanding the link between antibiotic resistance and the presence of resistance genes in Salmonella strains is crucial and warrants continuous investigation [17,19,22,26,41]. Contaminated poultry products represent the primary transmission vector for Salmonella. Therefore, monitoring antibiotic resistance in Salmonella originating from poultry is essential [42]. The assessment of both phenotypic and genotypic resistance in Salmonella isolated from commercial eggs in Brazil reveals a high prevalence within the egg production sector, highlighting the need for effective management and biosecurity measures. Research focusing on Salmonella in eggs has become crucial in preventing foodborne diseases, with various serovars being isolated annually from both eggs and environmental samples such as laying hen farms [43].
Tajbakhsh et al. [44] reported a human Salmonella isolate in Iran showing resistance to sulfamethoxazole (30%), trimethoprim-sulfamethoxazole (15%), and ampicillin (14%), which differs from the findings of this study possibly due to variations in the sources of Salmonella isolation. This discrepancy underscores the presence of resistant bacteria across food, animals, and humans. Lozano-Villegas et al. [45] suggested that the variation in Salmonella strains isolated from broilers and humans could be attributed to differences in pathogenic potential, influenced by the type and number of detected virulence genes, including those linked to antibiotic resistance.
Hu et al. [46] observed that among 143 Salmonella isolates from eggs and chicken sources across different countries, there was a high prevalence of genes conferring resistance to aminoglycosides (70.63%), tetracyclines (26.57%), and β-lactamases (15.38%), indicating higher resistance levels than those reported in this study. Barlow et al. [24] highlighted an annual 30% increase in the likelihood of infections caused by bacteria resistant to quinolones and trimethoprim-sulfamethoxazole, emphasizing the public health significance of salmonellosis and the critical need for judicious antibiotic use in both human and veterinary medicine.
In contrast, Galdino et al. [47], who analyzed the susceptibility profiles of 18 avian Salmonella sp. isolates from poultry fecal samples, found no resistance to neomycin or tetracycline. This is in contrast to our findings, which indicate 75.6% intermediate resistance to neomycin and 15.5% resistance to tetracycline. Additionally, the resistance to sulfonamides was reported at 51% in this study, whereas the aforementioned researchers reported a significantly lower rate of 11%. This variance could be attributed to the different sources of Salmonella isolation, with [47] focusing on environmental samples rather than eggs.
A study on food poisoning within the European Union focusing on the susceptibility profile of Salmonella sp. indicated resistance levels of 21–35% to ampicillin, 36–52% to sulfonamides, and 38–59% to tetracycline. These figures for ampicillin and tetracycline are considerably higher than those observed in this study, which were 4.4% and 2.2%, respectively. Moreover, Salmonella strains isolated from chicken sources have exhibited high levels of MDR. Among these, ampicillin resistance was most prevalent, with resistance genes such as blaTEM, sul1, and tetA being detected and linked to MDR [48]. MDR Salmonella isolates from fecal swabs showed a similar trend, with 100% harboring the bla gene and 77% possessing the tetA extended-spectrum beta-lactamase (ESBL) gene [49].
However, the resistance to sulfonamides in this study was found to be similar (51%). In terms of ciprofloxacin resistance, no resistant isolate was identified, and a 4.4% resistance rate was observed for enrofloxacin. These findings are closely aligned with those reported in Italy and Denmark, where resistance rates of 1% to ciprofloxacin and 0.6% to enrofloxacin were recorded [50].
In a distinct study conducted in Goiás, Brazil, among 32 Salmonella isolates obtained from drag swabs collected from 23 chicken farms, 29 (90.6%) exhibited resistance to ceftiofur [49]. Nonetheless, the level of resistance to ceftiofur noted in this investigation was 28.9%, and intermediate resistance levels to ciprofloxacin and enrofloxacin were observed. Given that fluoroquinolones and third- and fourth-generation cephalosporins are classified by the World Health Organization (WHO) as “critically important” antibiotics—used in treating serious human infections and diseases caused by organisms transmitted from non-human sources or capable of acquiring resistance genes—the resistance levels noted in this study, particularly to ceftiofur and intermediate resistance to ciprofloxacin and enrofloxacin, raise concerns [51].
In this study, Enteritidis isolates showed no resistance to ampicillin, doxycycline, or tetracycline, contrasting with findings by Lu et al. [31] who observed resistance to the above three antibiotics in avian Salmonella Enteritidis samples collected from fecal samples (chicken hatcheries, farms, and slaughterhouses) in China. Similarly, Ribeiro et al. [52] reported a 67.1% resistance rate to tetracycline and a 22.8% resistance rate to gentamicin in Salmonella Enteritidis isolates of avian origin (clinical and environmental poultry samples) from southern Brazil. These data differ from the findings in this study, where no Salmonella Enteritidis isolate showed resistance to tetracycline, and 50% exhibited resistance to gentamicin. The discrepancy, especially in tetracycline resistance, may be attributed to the ban on tetracycline use in veterinary medicine for production animals, which likely contributed to the reduced prevalence of the tetA gene [53,54,55]. Furthermore, Salmonella Enteritidis isolates from eggs demonstrated high resistance gene prevalence rates, including the spvC gene, primarily located on the Salmonella virulence plasmid, detected in 50.8% of Salmonella Enteritidis samples, with 27% of this serovar being MDR [56].
Cui et al. [57], in their analysis conducted in China, found that among 25 Agona isolates, 40% were resistant to ampicillin, 32% to trimethoprim-sulfamethoxazole, and 28% to gentamicin. These results are akin to resistance observed in other β-lactams tested in Salmonella isolates from fecal swabs in Brazil, including a 34% resistance rate to amoxicillin-clavulanic acid. Moreover, 81% of these isolates were also resistant to non-beta-lactam antibiotics such as ciprofloxacin and enrofloxacin, 72% to tetracycline, and 97% to trimethoprim-sulfamethoxazole [49]. This variation in resistance among Salmonella isolates from different regions may be due to regional peculiarities in antibiotic usage [58].
According to Hsu et al. [59], the detection of the intl1 gene is closely related to the presence of Salmonella Genomic Island 1 (SGI-1) and indicates the presence of a class 1 integron. This structure houses a cassette of genes that promote antibiotic resistance, including the sul1 gene, which is responsible for resistance to sulfonamides.
The class 1 integron is commonly found in Salmonella Typhimurium [60,61,62,63,64], but in this study, it was identified solely in serovars Cerro, Salmonella enterica subsp. enterica O: 4.5, and Johannesburg and was associated with the sul1 gene in the serovars Agona, Livingstone, and Schwarzengrund (Table 3). It is likely that these serovars, sharing the same habitat, have acquired resistance genes on plasmids from other bacteria. This hypothesis is supported by Bloomfield et al. [65], who described plasmids as the primary vehicles for the acquisition of resistance genes in the genus Salmonella.
The detection and frequency of the intl1 gene in isolates of serovars Cerro, Schwarzengrund, Salmonella enterica subsp. enterica O: 4.5, and Johannesburg, all from the same environment, echo the findings of Alam et al. [66], who reported the intl1 presence across various serovars, likely acquired through horizontal gene transfer.
Table 3 reveals the absence of the intl1 gene in serovars Anatum, Enteritidis, Corvallis, and Senftenberg. Moreover, the sul1 gene was not detected in these serovars, nor in isolates from Johannesburg, Cerro, or Salmonella enterica subsp. enterica O:4.5, being found exclusively alongside the intl1 gene.
Hsu et al. [59] noted that multidrug resistance is closely related to the presence of class 1 integrons. In this study, serovar Corvallis did not exhibit the intl1 gene or MDR, while serovars Anatum and Senftenberg showed resistance to two antibiotics, and Enteritidis to five, without the detection of the intl1 gene among them. It is plausible that the observed MDR in serovars Anatum, Senftenberg, and Enteritidis might be associated with class 2 or 3 integrons, which, as Yang et al. [67] suggest, can confer resistance to numerous antibiotics. Firoozeh et al. [68] and Ramatla et al. [69] identified class 1 integrons predominantly in plasmids and transposons, whereas class 2 and 3 integrons are usually found in transposons, with class 1 being more prevalent than class 2. Notably, class 3 integrons have not been reported in Salmonella species, a finding consistent with this research.
Table 3 indicates that all three Livingstone isolates exhibited phenotypic resistance to sulfonamides and harbored the sul1 gene. However, among the sulfonamide-resistant isolates from Cerro, Schwarzengrund, and Salmonella enterica subsp. enterica O:4.5, the sul1 gene was detected in only one Cerro isolate, two Schwarzengrund isolates, and one Salmonella enterica subsp. enterica O:4.5 isolate. This observation aligns with Wannaprasat et al. [70], who identified the sul3 gene, which confers sulfonamide resistance and is associated with class 1 integrons, in the absence of sul1.
Our ampicillin data differ from those reported by Hawkey et al. [58], who detected the blaTEM gene in 18% of human-derived Salmonella isolates resistant to this antibiotic, our study found no blaTEM gene presence. Similarly, Salmonella isolates resistant to cefotaxime from broiler litter samples were blaTEM-negative [71]. Nonetheless, the blaTEM gene was present in 79.2% and the tetA gene in 75% of Salmonella isolates from sulfonamide-treated diarrheic calves [72].
The results from this study, focusing on Salmonella isolates from commercial egg and laying hen farms, reveal a resistance pattern to sulfamethoxazole, sulfonamides, and ceftiofur. This resistance profile may stem from continuous exposure to these antibiotics and subsequent selective pressure, leading to the transfer of resistance genes such as intl1, sul1, and blaTEM between strains. Understanding antibiotic resistance profiles in Salmonella from chickens, laying hens, and eggs is crucial for reassessing regulations and formulating more effective resistance prevention strategies, as MDR Salmonella with zoonotic potential can transfer resistance genes from animals to humans [40].
In layer farms and commercial eggs from Central-Western Brazil, the occurrence of MDR Salmonella serovars such as Agona, Salmonella enterica subspecies enterica O:4.5, and Schwarzengrund may pose a public health risk [42,73]. Our study contributes valuable data on resistance profiles and MDR Salmonella in chickens, identifying multiple resistances to third-generation cephalosporins and fluoroquinolones, deemed “critically important antibiotics” (CIA).
The National Plan for Residue and Contaminant Control [74] has identified antibiotics such as ciprofloxacin, enrofloxacin, and florfenicol in high concentrations in commercial eggs. Given that the central-western region of Brazil accounts for 13.06% of the country’s chick production and is a major exporter of fresh eggs [75], this research offers significant insights into the potential risks for layer farms and commercial egg production. Therefore, the phenotypic and genotypic resistance of Salmonella to various antibiotics demands attention concerning environmental, human, and animal health.
5. Conclusions
Salmonella isolates exhibited elevated resistance rates to sulfamethoxazole, sulfonamides, and ceftiofur, while no resistance to ciprofloxacin was detected. Salmonella Johannesburg and Salmonella Corvallis were resistant to a single antibiotic, sulfamethoxazole. In contrast, the other serovars demonstrated resistance to two or more antibiotics, with Salmonella Schwarzengrund showing resistance to thirteen antibiotics. The detection of the spvC gene in Salmonella Enteritidis highlights the urgency of controlling this serovar due to its virulence-associated structures. The identification of the SGI-1 genomic island, indicated by the presence of the intl1 gene in six out of the ten serovars studied and the sul1 gene in three, suggests that Salmonella strains in the commercial egg production sector harbor genes facilitating host invasion and survival. The monitoring and control of Salmonella sp., through the identification of virulence and antibiotic resistance genes, are critical for the safety of commercial egg production. Government bodies, researchers, and poultry producers have a responsibility to address antibiotic resistance through judicious antibiotic use, active surveillance of MDR strains, and exploration of alternative disease control and prevention measures, reflecting the critical importance of these efforts in maintaining food chain integrity. Additionally, the mapping of phenotypic and genotypic antibiotic resistance in Salmonella serovars aids in informing governmental strategies to ensure the effectiveness of surveillance systems. Given the classification of Salmonella by the World Health Organization (WHO) as a high-priority pathogen in the context of antibiotic resistance, this issue constitutes a global challenge with wide-ranging implications across the One Health spectrum.
Author Contributions
Conceptualization, D.M.C.M.; methodology, D.M.C.M.; software, I.A.N.; validation, V.D.S.J.; formal analysis, D.M.C.M. and S.C.D.; investigation, D.M.C.M.; resources, D.M.C.M.; data curation, M.A.A. and C.M.; writing—original draft preparation, D.M.C.M. and E.d.P.N.; writing—review and editing, A.M.D.S.A.; E.d.P.N. and C.M.; visualization, A.M.D.S.A.; supervision, M.A.A. and C.M.; project administration, M.A.A., C.M.; funding acquisition, M.A.A. and C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author (privacy).
Acknowledgments
The authors would like to express their gratitude to the farms: CNPq-Brasil, Laboratório de Enterobactérias, Fundação Oswaldo Cruz, EMBRAPA Swine and Poultry, Centro de Pesquisa em Alimentos, and the Veterinary and Animal Science School at the Federal University of Goiás.
Conflicts of Interest
Author Sabrina Castilho Duarte was employed by the company Brazilian Agricultural Research Corporation (Embrapa). The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zhu, A.; Zhi, W.; Qiu, Y.; Wei, L.; Tian, J.; Pan, Z.; Duan, L. Surveillance study of the prevalence and antimicrobial resistance of Salmonella in pork from open markets in Xuzhou, China. Food Control 2019, 98, 474–480. [Google Scholar] [CrossRef]
- Jajere, S.M. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet. World 2019, 12, 504–521. [Google Scholar] [CrossRef] [PubMed]
- Manafi, L.; Aliakbarlu, J.; DastmalchiSaei, H. Antibiotic resistance and biofilm formation ability of Salmonella serotypes isolated from beef, mutton, and meat contact surfaces at retail. J. Food Sci. 2020, 85, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Marchello, C.S.; Carr, S.D.; Crump, J.A. A Systematic Review on Antimicrobial Resistance among Salmonella Typhi Worldwide. Am. J. Trop. Med. Hyg. 2020, 103, 2518–2527. [Google Scholar] [CrossRef]
- Khan, M.; Shamim, S. Understanding the Mechanism of Antimicrobial Resistance and Pathogenesis of Salmonella enterica Serovar Typhi. Microorganisms 2022, 10, 2006. [Google Scholar] [CrossRef] [PubMed]
- Ayoola, M.B.; Das, A.R.; Krishnan, B.S.; Smith, D.R.; Nanduri, B.; Ramkumar, M. Predicting Salmonella MIC and Deciphering Genomic Determinants of Antibiotic Resistance and Susceptibility. Microorganisms 2024, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, D. Determination of Antimicrobial Resistance of Salmonella in Pork. Methods Mol. Biol. 2021, 21, 179–186. [Google Scholar]
- Magwedere, K.; Rauff, D.; De Klerk, G.; Keddy, K.H.; Dziva, F. Incidence of Nontyphoidal Salmonella in Food-Producing Animals, Animal Feed, and the Associated Environment in South Africa, 2012–2014. Clin. Infect Dis. 2015, 61, S283–S289. [Google Scholar] [CrossRef]
- Campos, J.; Mourão, J.; Silveira, L.; Saraiva, M.; Correia, C.B.; Maçãs, A.P.; Peixe, L.; Antunes, P. Imported poultry meat as a source of extended-spectrum cephalosporin-resistant CMY-2-producing Salmonella Heidelberg and Salmonella Minnesota in the European Union, 2014–2015. Int. J. Antimicrob. Agents 2018, 51, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Viana, C.; Sereno, M.J.; Pegoraro, K.; Yamatogi, R.S.; Call, D.R.; Dos Santos Bersot, L.; Nero, L.A. Distribution, diversity, virulence genotypes and antibiotic resistance for Salmonella isolated from a Brazilian pork production chain. Int. J. Food Microbiol. 2019, 310, 108310. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zeng, X.; Zhang, P.; Zhang, D.; Wang, C.; Lin, J. Characterization of the emerging multidrug-resistant Salmonella enterica serovar Indiana strains in China. Emerg. Microbes Infect. 2019, 8, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Castro-Vargas, R.E.; Herrera-Sánchez, M.P.; Rodríguez-Hernández, R.; Rondón-Barragán, I.S. Antibiotic resistance in Salmonella sp. isolated from poultry: A global overview. Vet. World 2020, 13, 2070–2084. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salazar, E.; Gudiño, M.E.; Sevillano, G.; Zurita, J.; Guerrero-López, R.; Jaramillo, K.; Calero-Cáceres, W. Antibiotic resistance of Salmonella strains from layer poultry farms in central Ecuador. J. Appl. Microbiol. 2020, 128, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Sodagari, H.R.; Habib, I.; Whiddon, S.; Wang, P.; Mohammed, A.B.; Robertson, I.; Goodchild, S. Occurrence and Characterization of Salmonella Isolated from Table Egg Layer Farming Environments in Western Australia and Insights into Biosecurity and Egg Handling Practices. Pathogens 2020, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, W.; Li, W.; Ding, Y.; Zhang, Y.; Yang, Q.; Wang, J.; Wang, X. A broad-spectrum phage controls multidrug-resistant Salmonella in liquid eggs. Food Res. Int. 2020, 132, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Q&A: Antibiotic resistance: Where does it come from and what can we do about it? BMC Biol. 2010, 8, 123. [Google Scholar]
- Torres, C.; Alonso, C.A.; Ruiz-Ripa, L.; León-Sampedro, R.; Del Campo, R.; Coque, T.M. Antimicrobial Resistance in Enterococcus spp. of animal origin. Microbiol. Spectr. 2018, 6, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Paveenkittiporn, W.; Kamjumphol, W.; Kerdsin, A. Draft Genome Sequence of Invasive Salmonella enterica Serovar Cannstatt Harboring mcr-1.1, Isolated from a Fatal Sepsis Case. Microbiol. Resour. Announc. 2021, 10, e01270-20. [Google Scholar] [CrossRef] [PubMed]
- Gudipati, S.; Zervos, M.; Herc, E. Can the One Health Approach Save Us from the Emergence and Reemergence of Infectious Pathogens in the Era of Climate Change: Implications for Antimicrobial Resistance? Antibiotics 2020, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.E.; Amosun, E.A.; Opebiyi, O.O.; Oyekunle, M.A.; Dipeolu, M.A.; Otesile, E.B. Multidrug resistant enterohaemorrhagic Escherichia coli serogroups in the faeces of hunted Wildlife, Abeokuta, Nigeria. Vet. Ital. 2022, 58, 173–179. [Google Scholar]
- Song, H.; Zou, S.; Huang, Y.; Jian, C.; Liu, W.; Tian, L.; Gong, L.; Chen, Z.; Sun, Z.; Wang, Y. Salmonella Typhimurium with Eight Tandem Copies of blaNDM-1 on a HI2 Plasmid. Microorganisms 2024, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Bonciu, E. Aspects of the involvement of biotechnology in functional food and nutraceuticals. University of Craiova, Faculty of Agronomy. Sci. Pap.-Ser. A Agron. 2020, 2, 261–266. [Google Scholar]
- Iannetti, S.; Scarpone, R.; Dall’Acqua, F.; Rosato, R.; Chiumiento, F.; Cioci, D.; Migliorati, G.; Morelli, D.; Calistri, P. Estimation of the risk of fipronil ingestionthrough the consumption of contaminated table eggs for the Italian consumer. Vet. Ital. 2022, 58, 181–188. [Google Scholar]
- Barlow, R.S.; Debess, E.E.; Winthrop, K.L.; Lapidus, J.A.; Vega, R.; Cieslak, P.R. Travel-associated antimicrobial drug-resistant nontyphoidal Salmonellae, 2004–2009. Emerg. Infect Dis. 2014, 20, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Linke, L.; Doster, E.; Hyatt, D.; Burgess, B.A.; Magnuson, R.; Pabilonia, K.L.; Morley, P.S. Genomic diversity of class I integrons from antimicrobial resistant strains of Salmonella Typhimurium isolated from livestock, poultry and humans. PLoS ONE 2020, 15, e0243477. [Google Scholar] [CrossRef] [PubMed]
- Katzouraki, G.; Vasiliadis, E.S.; Marougklianis, V.; Evangelopoulos, D.S.; Pneumaticos, S.G. A Systematic Review of the Diagnosis and Treatment of Non-Typhoid Salmonella Spondylodiscitis in Immunocompetent Children. Children 2022, 9, 1852. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.J.I.; Islam, M.; Sikder, T.; Rubaya, R.; Halder, J.; Alam, J. Multidrug-resistant Escherichia coli and Salmonella spp. isolated from pigeons. Vet. World 2020, 13, 2156–2165. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2020/2021. EFSA J. 2023, 21, e07867. [Google Scholar]
- Velilla, A.V.; Terzolo, H.R. Biología molecular aplicada a La detección y caracterización de Salmonella enterica. In Proceedings of the XXI Congresso Latinoamericano de Avicultura, La Habana, Cuba, 6–9 October 2009; Volume 1, pp. 328–334. [Google Scholar]
- Heymans, R.; Vila, A.; Heerwaarden, C.A.M.; Jansen, C.C.C.; Castelijn, G.A.A.; Voort, M.; Biesta-Peters, E.G. Rapid detection and differentiation of Salmonella species, Salmonella Typhimurium and Salmonella Enteritidis by multiplex quantitative PCR. PLoS ONE 2018, 13, e0206316. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, C.; Wu, G.; Zhao, H.; He, T.; Cao, X.; Dai, L.; Xia, L.; Qin, S.; Shen, J. Prevalence of antimicrobial resistance among Salmonella isolates from chicken in China. Foodborne Pathog. Dis. 2011, 8, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Hernandez, A.; Carrascal-Camacho, A.K.; Varón-García, A.; Brashears, M.M.; Sanchez-Plata, M.X. Genotypic Characterization of Antimicrobial Resistant Salmonella spp. Strains from Three Poultry Processing Plants in Colombia. Foods 2021, 25, 491. [Google Scholar] [CrossRef] [PubMed]
- Doublet, B.; Douard, G.; Targant, H.; Meunier, D.; Madec, J.; Cloeckaert, A. Antibiotic marker modifications of λ Red and FLP helper plasmids, pKD46 and pCP20, for inactivation of chromosomal genes using PCR products in multidrug-resistant strains. J. Microbiol. Methods 2008, 75, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.M.; Mohler, V.L.; Gunn, A.A.; House, J.K. Development of a qPCR for the detection and quantification of Salmonella spp. in sheep feces and tissues. J. Vet. Diagn. Investig. 2020, 32, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, K.; Chan, E.W.; Yao, W.; Chen, S. Transmission of Chromosomal MDR DNA Fragment Encoding Ciprofloxacin Resistance by a Conjugative Helper Plasmid in Salmonella. Front Microbiol. 2020, 11, 556227. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Su, L.H.; Janapatla, R.P.; Lin, C.Y.; Chiu, C.H. Genetic analysis of virulence and antimicrobial-resistant plasmid pOU7519 in Salmonella enterica serovar Choleraesuis. J. Microbiol. Immunol. Infect. 2020, 53, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Clinical Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; CLSI supplement M100; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2023. [Google Scholar]
- Santos, L.R.; Nascimento, V.P.; de Oliveira, S.D.; Flores, M.L.; Pontes, A.P.; Ribeiro, A.R.; Salle, C.T.; Lopes, R.F. Polymerase chain reaction (PCR) for the detection of Salmonella in artificially inoculated chicken meat. Rev. Inst. Med. Trop. São Paulo 2001, 43, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Bugarel, M.; Granier, S.A.; Weill, F.X.; Fach, P.; Brisabois, A. A multiplex real-time PCR assay targeting virulence and resistance genes in Salmonella enterica serotype Typhimurium. BMC Microbiol. 2011, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Arkali, A.; Çetinkaya, B. Molecular identification and antibiotic resistance profiling of Salmonella species isolated from chickens in eastern Turkey. BMC Vet. Res. 2020, 19, 205. [Google Scholar] [CrossRef] [PubMed]
- Tawyabur, M.; Islam, M.S.; Sobur, M.A.; Hossain, M.J.; Mahmud, M.M.; Paul, S.; Hossain, M.T.; Ashour, H.M.; Rahman, M.T. Isolation and Characterization of Multidrug-Resistant Escherichia coli and Salmonella spp. from Healthy and Diseased Turkeys. Antibiotics 2020, 9, 770. [Google Scholar] [CrossRef]
- Ince, S.S.; Akan, M. Phenotypic and genotypic characterization of antimicrobial resistance in commonly isolated Salmonella serovars from chickens. Turk. J. Vet. Anim. Sci. 2023, 47, 19–25. [Google Scholar] [CrossRef]
- Garcia, J.S.; Gast, R.K.; Guard, J.Y.; Karcher, D.M.; Jones, D. Tissue Colonization and Egg and Environmental Contamination Associated with the Experimental Infection of Cage-Free Laying Hens with Salmonella Braenderup. Avian Dis. 2022, 66, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, M.; Hendriksen, R.S.; Nochi, Z.; Zali, M.R.; Aarestrup, F.M.; Garcia-Migura, L. Antimicrobial resistance in Salmonella spp. recovered from patients admitted to six different hospitals in Tehran, Iran from 2007 to 2008. Folia Microbiol. 2012, 57, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Villegas, K.J.; Herrera-Sánchez, M.P.; Beltrán-Martínez, M.A.; Cárdenas-Moscoso, S.; Rondón-Barragán, I.S. Molecular Detection of Virulence Factors in Salmonella serovars Isolated from Poultry and Human Samples. Vet. Med. Int. 2023, 2, 1875253. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Cao, G.; Brown, E.W.; Allard, M.W.; Ma, L.M.; Khan, A.A.; Zhang, G. Antimicrobial resistance and related gene analysis of Salmonella from egg and chicken sources by whole-genome sequencing. Poult. Sci. 2020, 99, 7076–7083. [Google Scholar] [CrossRef] [PubMed]
- Galdino, V.M.C.; Melo, R.T.; Oliveira, R.P.; Mendonça, E.P.; Nalevaiko, P.C.; Rossi, D.A. Virulência de Salmonella spp. de origem avícola e resistência a antimicrobianos. Biosci. J. 2013, 29, 932–939. [Google Scholar]
- Khoo, E.; Roslee, R.; Zakaria, Z.; Ahmad, N.I. Virulence gene profiles and antimicrobial susceptibility of Salmonella Brancaster from chicken. J. Vet. Sci. 2023, 24, e82. [Google Scholar] [CrossRef]
- Penha-Filho, R.A.C.; Ferreira, J.C.; Galetti, R.; Kanashiro, A.M.I.; Berchieri, A.J.; Costa Darini, A.L. The rise of multidrug resistant Salmonella isolates in healthy chickens in Brazil by successful establishment of plasmid IncHI2A carrying several antibiotic resistance genes. Braz. J. Microbiol. 2023, 54, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Aarestrup, F.M.; Norrung, B. Scientific Opinion of the Panel on Biological Hazards on a request from the European Food Safety Authority on foodborne antimicrobial resistance as a biological hazard in Question No EFSA-Q-2007-089. Eur. Food Saf. Auth. 2008, 765, 1–84. [Google Scholar]
- WHO—World Healthy Organization. Critically Important Antibacterial Agents for Human Medicine for Risk Management Strategies of Non-Human Use: Report of a WHO Working Group Consultation. 6th Revision 2018; World Health Organization: Genebra, Switzerland, 2019; pp. 1–52. [Google Scholar]
- Ribeiro, A.R.; Kellermann, A.; Santos, L.R.; Nascimento, V.P. Resistência antimicrobiana em Salmonella Enteritidis isoladas de amostras clínicas e ambientais de frangos de corte e matrizes pesadas. Arq. Bras. Med. Veterinária Zootec. 2008, 60, 1259–1262. [Google Scholar] [CrossRef]
- Alcântara, J.B.; Martins, P.C.; Nascente, E.P.; Café, M.B.; Pascoal, L.M.; Teles, A.V.; Jayme, V.S.; Andrade, M.A. Salmonella enterica diversity and antimicrobial resistance profile in broiler slaughterhouse by-products. Vet. Ital. 2022, 58, 209–213. [Google Scholar]
- Mohammed, Y.; Dubie, T. Isolation, identification and antimicrobial susceptibility profile of Salmonella isolated from poultry farms in Addis Ababa, Ethiopia. Vet. Med. Sci. 2022, 8, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria-A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef] [PubMed]
- Guillén, S.; Marcén, M.; Álvarez, I.; Mañas, P.; Cebrián, G. Stress resistance of emerging poultry-associated Salmonella serovars. Int. J. Food Microbiol. 2020, 16, 108884. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Li, J.; Sun, Z.; Hu, C.; Jin, S.; Li, F.; Guo, Y.; Ran, L.; Ma, Y. Characterization of Salmonella enterica isolates from infants and toddlers in Wuhan, China. J. Antimicrob. Chemother. 2009, 63, 87–94. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hawkey, J.; Le Hello, S.; Doublet, B.; Granier, S.A.; Hendriksen, R.S.; Fricke, W.F.; Ceyssens, P.J.; Gomart, C.; Billman-Jacobe, H.; Holt, K.E.; et al. Global phylogenomics of multidrug-resistant Salmonella enterica serotype Kentucky ST198. Microb. Genom. 2019, 5, e000269. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.; Tang, C.; Lin, H.; Chen, Y.; Chen, Y.; Su, Y.; Chen, D.S.; Lin, J.; Chang, C. Comparative study of class 1 integron, ampicillin, chloramphenicol, streptomycin, sulfamethoxazole, tetracycline (ACSSuT) and fluoroquinolone resistance in various Salmonella serovars from humans and animals. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Chauhan, S.L.; Kumar, S.; Jindal, N.; Mahajan, N.K.; Joshi, V.G. Carriage of Class 1 integrons and molecular characterization of intI1 gene in multidrug-resistant Salmonella spp. isolates from broilers. Vet. World 2019, 12, 609–613. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Su, J.; Xu, Z. Antibiotic Susceptibility, Biofilm-Forming Ability, and Incidence of Class 1 Integron of Salmonella spp., Escherichia coli, and Staphylococcus aureus isolated from various foods in a school canteen in China. Foodborne Pathog. Dis. 2020, 17, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Monte, D.F.M.; Sellera, F.P.; Lopes, R.; Keelara, S.; Landgraf, M.; Greene, S.; Fedorka-Cray, P.J.; Thakur, S. Class 1 integron-borne cassettes harboring blaCARB-2 gene in multidrug-resistant and virulent Salmonella Typhimurium ST19 strains recovered from clinical human stool samples, United States. PLoS ONE 2020, 30, e0240978. [Google Scholar] [CrossRef] [PubMed]
- Eshaghi-Zadeh, S.H.; Fahimi, H.; Fardsanei, F.; Soltan-Dallal, M.M. Antimicrobial Resistance and Presence of Class 1 Integrons Among Different Serotypes of Salmonella spp. Recovered from Children with Diarrhea in Tehran, Iran. Infect. Disord. Drug Targets 2020, 20, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Curraize, C.; Siebor, E.; Neuwirth, C.; Hall, R.M. SGI0, a relative of Salmonella genomic islands SGI1 and SGI2, lacking a class 1 integron, found in Proteus mirabilis. Plasmid 2020, 107, 102453. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, S.; Duong, V.T.; Tuyen, H.T.; Campbell, J.I.; Thomson, N.R.; Parkhill, J.; Le Phuc, H.; Chau, T.T.H.; Maskell, D.J.; Perron, G.G.; et al. Mobility of antimicrobial resistance across serovars and disease presentations in non-typhoidal Salmonella from animals and humans in Vietnam. Microb. Genom. 2022, 8, 000798. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.B.; Mahmud, M.; Akter, R.; Hasan, M.; Sobur, A.; Nazir, K.N.H.; Noreddin, A.; Rahman, T.; El Zowalaty, M.E.; Rahman, M. Molecular Detection of Multidrug Resistant Salmonella Species Isolated from Broiler Farm in Bangladesh. Pathogens 2020, 9, 201. [Google Scholar] [CrossRef]
- Yang, C.M.; Lin, M.F.; Lin, C.H.; Huang, Y.T.; Hsu, C.T.; Liou, M.L. Characterization of antimicrobial resistance patterns and integrons in human fecal Escherichia coli in Taiwan. Jpn. J. Infect. Dis. 2009, 62, 177–181. [Google Scholar] [CrossRef]
- Firoozeh, F.; Zahraei-Salehi, T.; Shahcheraghi, F.; Karimi, V.; Aslani, M.M. Characterization of class I integrons among Salmonella enterica serovar Enteritidis isolated from humans and poultry. FEMS Immunol. Med. Microbiol. 2012, 64, 237–243. [Google Scholar] [CrossRef]
- Ramatla, T.; Mileng, K.; Ndou, R.; Mphuti, N.; Syakalima, M.; Lekota, K.E.; Thekisoe, O.M.M. Molecular Detection of Integrons, Colistin and β-lactamase Resistant Genes in Salmonella enterica Serovars Enteritidis and Typhimurium Isolated from Chickens and Rats Inhabiting Poultry Farms. Microorganisms 2022, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Wannaprasat, W.; Padungtod, P.; Chuanchuen, R. Class 1 integrons and virulence genes in Salmonella enterica isolates from pork and humans. Int. J. Antimicrob. Agents 2011, 37, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Caballero, M.A.; Lozano-Puentes, M.P.; Ospina, M.A.; Varón-López, M. First report of multidrug-resistant Salmonella Infantis in broiler litter in Tolima, Colombia. Vet. World 2022, 15, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Hossain, M.T.; Islam, M.S.; Islam, M.Z.; Islam, P.; Shaha, S.N.; Sikder, M.H.; Rafiq, K. Isolation of multidrug-resistant Escherichia coli and Salmonella spp. from sulfonamide-treated diarrheic calves. Vet. World 2022, 15, 2870–2876. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Sidjabat, H.E.; Davis, S.; Vong da Silva, P.G.; Alves, A.; Dos Santos, C.; Jong, J.B.D.C.; da Conceição, F.; Felipe, N.D.J.; Ximenes, A.; et al. Prevalence of Antimicrobial Resistance in Escherichia coli and Salmonella Species Isolates from Chickens in Live Bird Markets and Boot Swabs from Layer Farms in Timor-Leste. Antibiotics 2024, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Agricultura Pecuária e Abastecimento. Resultados do Plano Nacional de Controle de Resíduos e Contaminantes—PNCRC 2022; MAPA: Brasília, Brasil, 2023; pp. 1–13. [Google Scholar]
- ABPA. Associação Brasileira de Proteína Animal; Relatório Anual ABPA 2023; ABPA: São Paulo, Brazil, 2023; pp. 1–75. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).