Arbuscular Mycorrhizal Fungi and Rhizobium Improve Nutrient Uptake and Microbial Diversity Relative to Dryland Site-Specific Soil Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and Soil Characterization
2.2. Microbial Inoculants
2.3. Dryland Field Experiment
2.4. Plant Nutrient Uptake, Yield, and Microbial Dependency
2.5. Microbial Community Analysis
3. Results
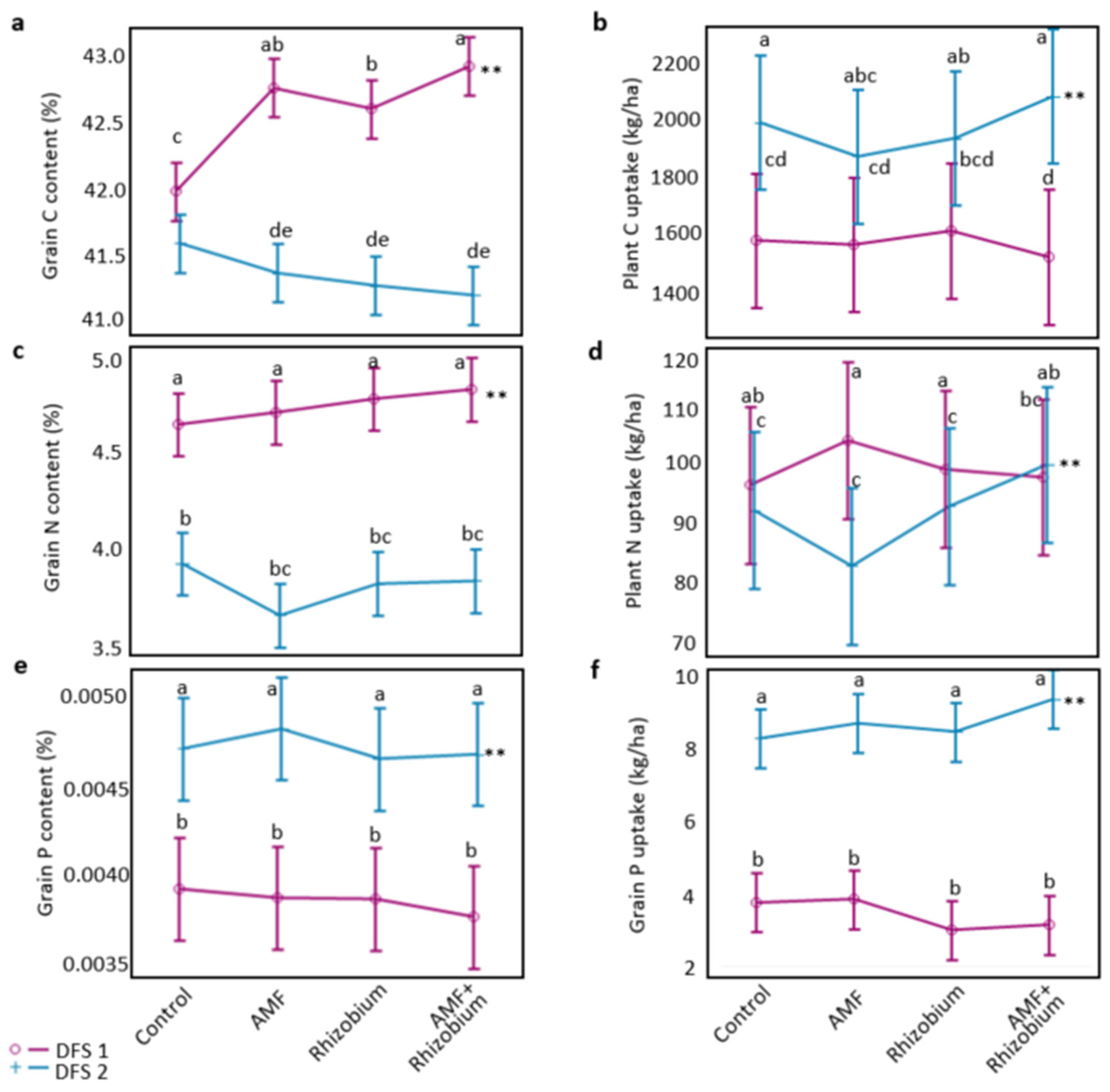
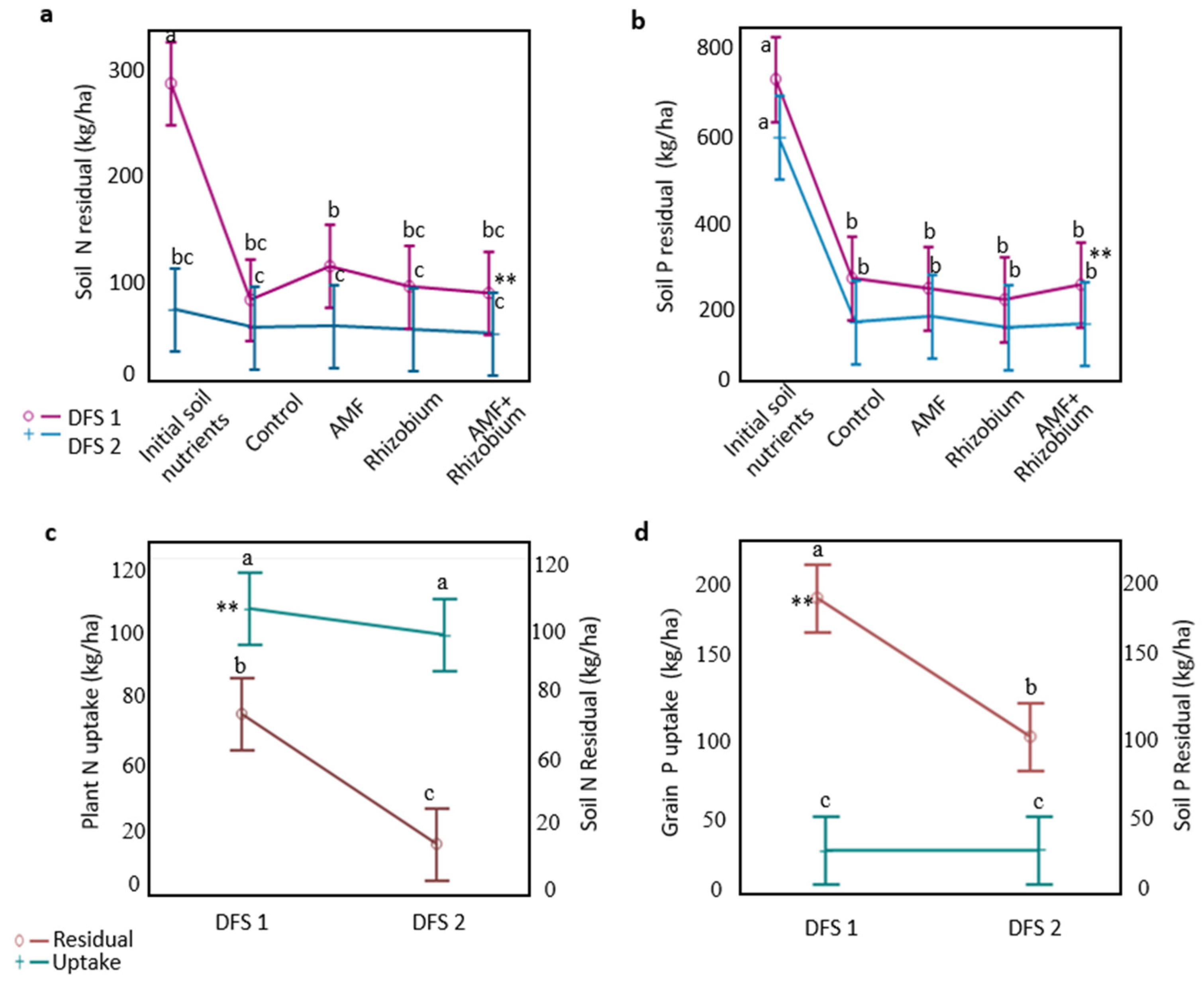
3.1. Influence of Microbial Inoculants on Plant Agronomic Performance and Nutrient Dynamics
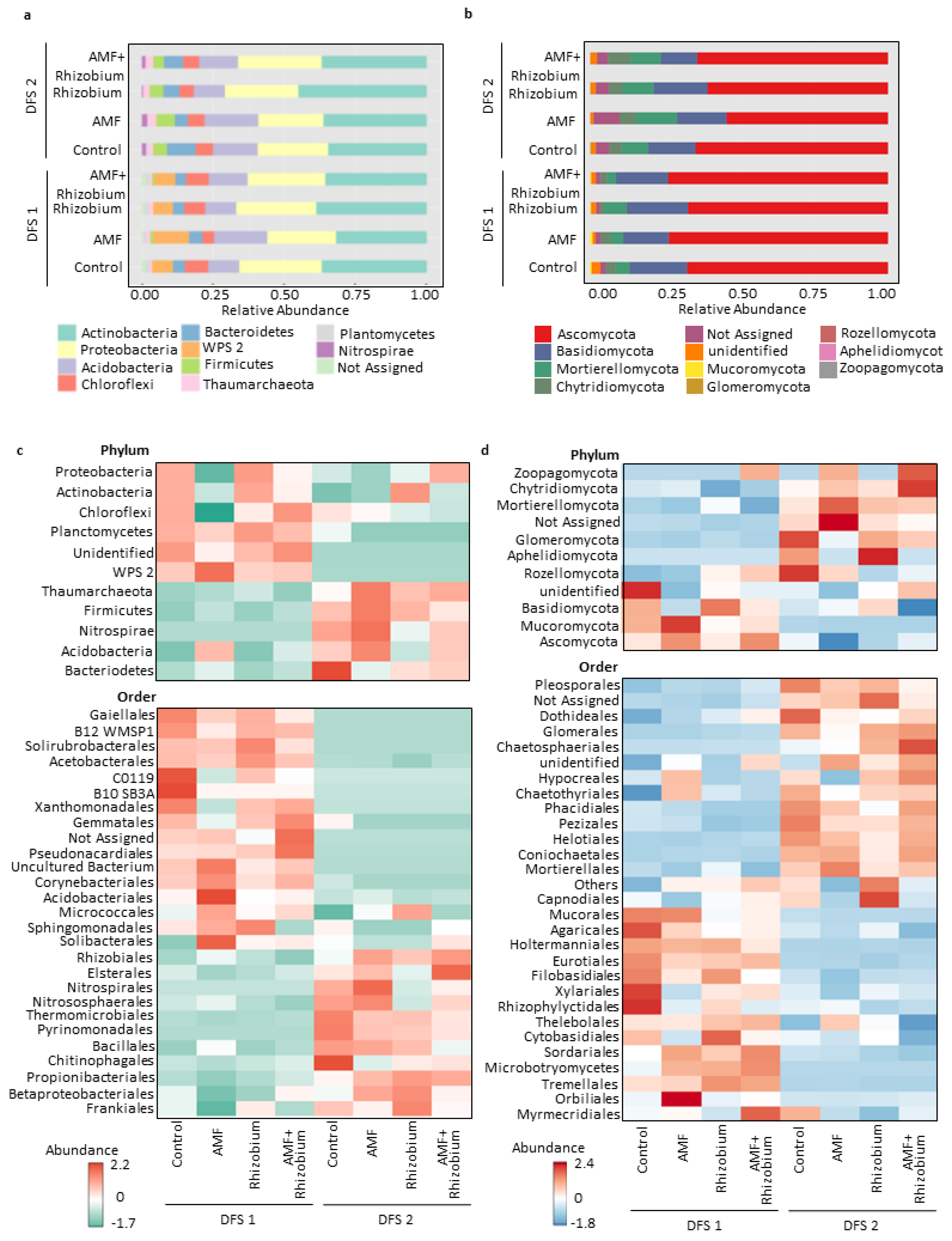
3.2. Variation in the Soil Microbial Communities Associated with Increased Plant Yield in Two Contrasting Dryland Sites
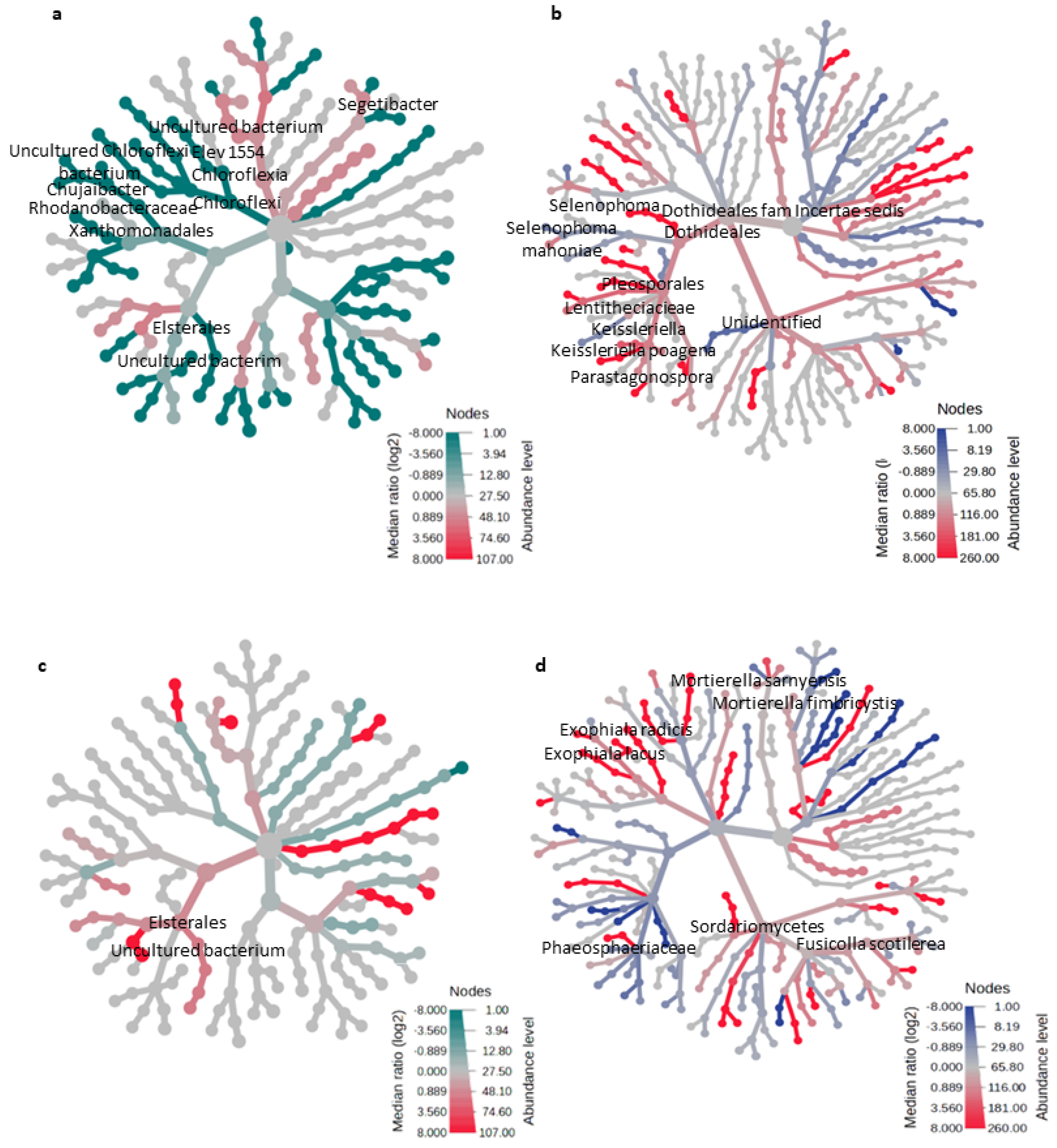
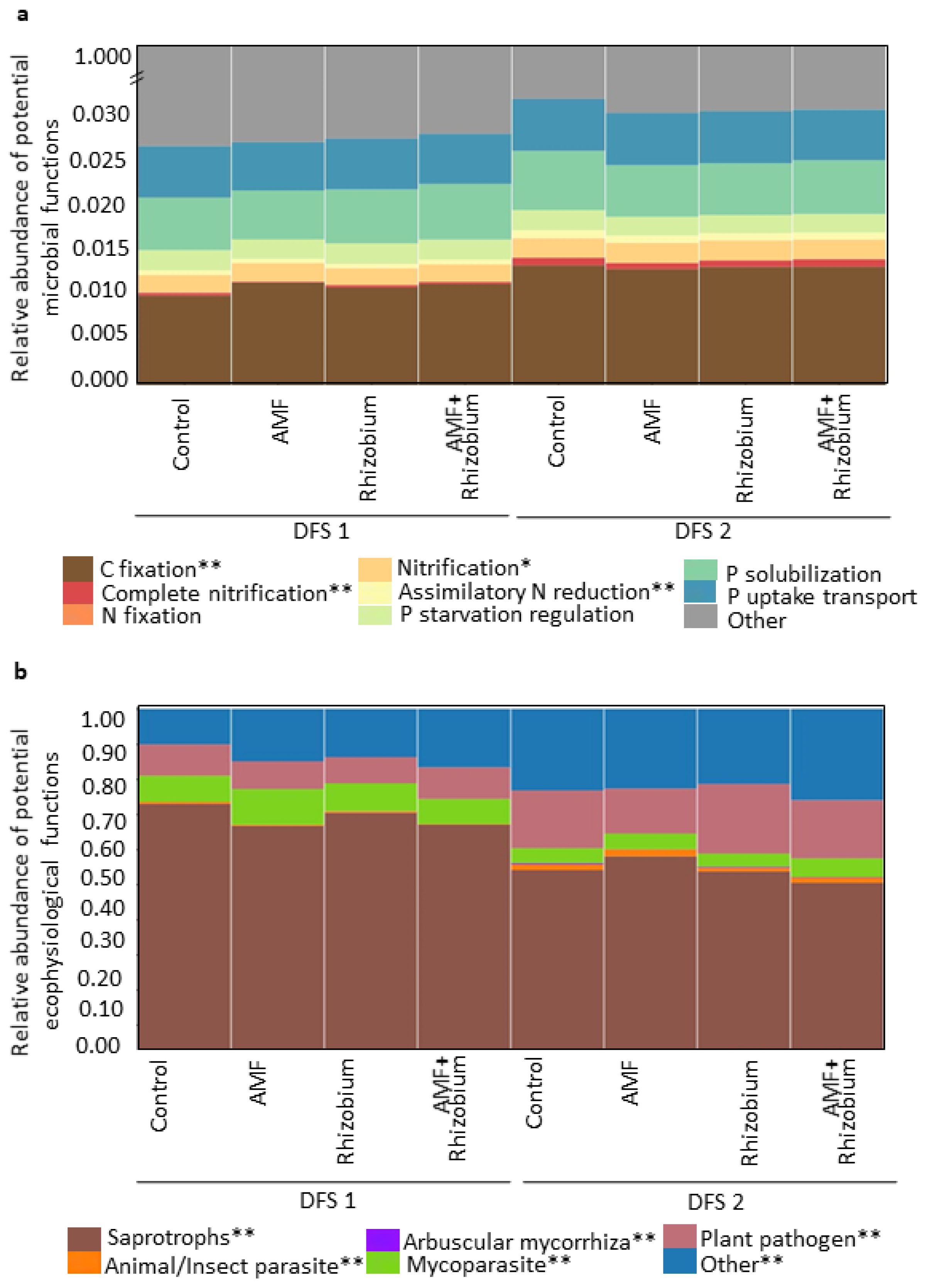
3.3. Effect of Microbial Inoculants on the Potential Functions of the Microbial Community Relative to Nutrient Cycling and Soil Health
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nation. CropWat|Land & Water. 2018. Available online: http://www.fao.org/land-water/databases-and-software/crop-information/soybean/en/ (accessed on 5 January 2019).
- Liebig, M.A.; Acosta-Martinez, V.; Archer, D.W.; Halvorson, J.J.; Hendrickson, J.R.; Kronberg, S.L.; Samson-Liebig, S.E.; Vetter, J.M. Conservation practices induce tradeoffs in soil function: Observations from the northern Great Plains. Soil Sci. Soc. Am. J. 2022, 86, 1413–1430. [Google Scholar] [CrossRef]
- Peterson, G.A. Dryland Farming. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- FAOSTAT. 2022. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 15 February 2024).
- State Agriculture Overview. USDA/NASS 2022 State Agriculture Overview for Montana. 2022. Available online: https://www.nass.usda.gov/Quick_Stats/Ag_Overview/stateOverview.php?state=MONTANA (accessed on 14 February 2022).
- Püschel, D.; Janoušková, M.; Voříšková, A.; Gryndlerová, H.; Vosátka, M.; Jansa, J. Arbuscular mycorrhiza stimulates biological nitrogen fixation in two Medicago spp. through improved phosphorus acquisition. Front. Plant Sci. 2017, 8, 390. [Google Scholar] [CrossRef] [PubMed]
- Ruisi, P.; Giambalvo, D.; Di Miceli, G.; Frenda, A.S.; Saia, S.; Amato, G. Tillage effects on yield and nitrogen fixation of legumes in mediterranean conditions. Agron. J. 2012, 104, 1459–1466. [Google Scholar] [CrossRef]
- Jones, M.D.; Durall, D.M.; Tinker, P.B. A comparison of arbuscular and ectomycorrhizal Eucalyptus coccifera: Growth response, phosphorus uptake efficiency and external hyphal production. New Phytol. 1998, 140, 125–134. [Google Scholar] [CrossRef]
- Goltapeh, E.M.; Danesh, Y.R.; Prasad, R.; Varma, A. Mycorrhizal fungi: What we know and what should we know? In Mycorrhiza; Springer: Berlin/Heidelberg, Germany, 2008; pp. 3–27. [Google Scholar]
- Řezáčová, V.; Konvalinková, T.; Jansa, J. Carbon fluxes in mycorrhizal plants. In Mycorrhiza-Eco-Physiology, Secondary Metabolites, Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–21. [Google Scholar]
- Yang, L.; Luo, Y.; Lu, B.; Zhou, G.; Chang, D.; Gao, S.; Zhang, J.; Che, Z.; Cao, W. Long-term maize and pea intercropping improved subsoil carbon storage while reduced greenhouse gas emissions. Agric. Ecosyst. Environ. 2023, 349, 108444. [Google Scholar] [CrossRef]
- Kumari, M.; Swarupa, P.; Kesari, K.K.; Kumar, A. Microbial inoculants as plant biostimulants: A review on risk status. Life 2022, 13, 12. [Google Scholar] [CrossRef]
- Khangura, R.; Ferris, D.; Wagg, C.; Bowyer, J. Regenerative Agriculture—A Literature Review on the Practices and Mechanisms Used to Improve Soil Health. Sustainability 2023, 15, 2338. [Google Scholar] [CrossRef]
- Xavier, G.R.; da Jesusn, E.C.; Dias, A.; Coelho, M.R.R.; Molina, Y.C.; Rumjanek, N.G. Contribution of Biofertilizers to Pulse Crops: From Single-Strain Inoculants to New Technologies Based on Microbiomes Strategies. Plants 2023, 12, 954. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, G.K.; Verma, J.P.; Jaiswal, D.K.; Mukherjee, A.; Singh, S.; de Araujo Pereira, A.P.; Liu, H.; Abd_Allah, E.F.; Singh, B.K. Phytomicrobiome for promoting sustainable agriculture and food security: Opportunities, challenges, and solutions. Microbiol. Res. 2021, 248, 126763. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Li, Y.; Laterrière, M.; Lay, C.Y.; Klabi, R.; Masse, J.; St-Arnaud, M.; Yergeau, É.; Lupwayi, N.Z.; Gan, Y.; Hamel, C. Effects of arbuscular mycorrhizal fungi inoculation and crop sequence on root-associated microbiome, crop productivity and nutrient uptake in wheat-based and flax-based cropping systems. Appl. Soil Ecol. 2021, 168, 104136. [Google Scholar] [CrossRef]
- Ryan, M.H.; Graham, J.H. Little evidence that farmers should consider abundance or diversity of arbuscular mycorrhizal fungi when managing crops. New Phytol. 2018, 220, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Egidi, E.; Liu, H.; Kaur, S.; Singh, B.K. New frontiers in agriculture productivity: Optimised microbial inoculants and in situ microbiome engineering. Biotechnol. Adv. 2019, 37, 107371. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Tiwari, G.; Duraivadivel, P.; Sharma, S.P.H. 1-Aminocyclopropane-1-carboxylic acid deaminase producing beneficial rhizobacteria ameliorate the biomass characters of Panicum maximum Jacq. by mitigating drought and salt stress. Sci. Rep. 2018, 8, 17513. [Google Scholar] [CrossRef] [PubMed]
- Masrahi, A.S.; Alasmari, A.; Shahin, M.G.; Qumsani, A.T.; Oraby, H.F.; Awad-Allah, M.M.A. Role of arbuscular mycorrhizal fungi and phosphate solubilizing bacteria in improving yield, yield components, and nutrients uptake of barley under salinity soil. Agriculture 2023, 13, 537. [Google Scholar] [CrossRef]
- Cozzolino, V.; Monda, H.; Savy, D.; Di Meo, V.; Vinci, G.; Smalla, K. Cooperation among phosphate-solubilizing bacteria, humic acids and arbuscular mycorrhizal fungi induces soil microbiome shifts and enhances plant nutrient uptake. Chem. Biol. Technol. Agric. 2021, 8, 31. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Vivanco, J.M. Manipulating the soil microbiome to increase plant health and productivity. Biol. Fertil. Soils 2012, 287. [Google Scholar]
- Calderon, R.B.; Jeong, C.; Ku, H.H.; Coghill, L.M.; Ju, Y.J.; Kim, N.; Ham, J.H. Changes in the microbial community in soybean plots treated with biochar and poultry litter. Agronomy 2021, 11, 1428. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G.; Tumpa, F.H.; Sultana, A.; Akter, M.A.; Chakraborty, A. Plant microbiome–an account of the factors that shape community composition and diversity. Curr. Plant Biol. 2020, 23, 100161. [Google Scholar] [CrossRef]
- Fitzpatrick, C.R.; Schneider, A.C. Unique bacterial assembly, composition, and interactions in a parasitic plant and its host. J. Exp. Bot. 2020, 71, 2198–2209. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Gu, Y.; Friman, V.P.; Kowalchuk, G.A.; Xu, Y.; Shen, Q.; Jousset, A. Initial soil microbiome composition and functioning predetermine future plant health. Sci. Adv. 2019, 5, eaaw0759. [Google Scholar] [CrossRef] [PubMed]
- Sainju, U.M.; Liptzin, D.; Stevens, W.B. How soil carbon fractions relate to soil properties and crop yields in dryland cropping systems? Soil Sci. Soc. Am. J. 2022, 86, 795–809. [Google Scholar] [CrossRef]
- Gelderman, R.H.; Beegle, D. Nitrate-nitrogen. In Recommended Chemical Soil Test Procedures for the North Central Region; U.S. Department of Agriculture: Washington, DC, USA, 1998; Volume 221, pp. 17–20. [Google Scholar]
- Warncke, D.; Brown, J.R. Potassium and other basic cations. In Recommended Chemical Soil Test Procedures for the North Central Region; U.S. Department of Agriculture: Washington, DC, USA, 1998; Volume 1001, p. 31. [Google Scholar]
- Combs, S.M.; Nathan, M.V. Soil organic matter. In Recommended Chemical Soil Test Procedures for the North Central Region; U.S. Department of Agriculture: Washington, DC, USA, 1998; Volume 221, pp. 53–58. [Google Scholar]
- AGTIV. Active Ingredients. 2023. Available online: https://www.ptagtiv.com/en/active-ingredients/#mycorrhizae (accessed on 7 August 2023).
- Campbell, C.R.; PCO. Reference plant analysis procedures for the southern region of the United States. South Co-Op Ser. Bull. 1992, 368, 71–73. [Google Scholar]
- Paye, W.S.; Szogi, A.A.; Shumaker, P.D.; Billman, E.D. Annual Ryegrass (Lolium multiflorum Lam.) Growth Response to Nitrogen in a Sandy Soil Amended with Acidified Manure and Municipal Sludge after “Quick Wash” Treatment. Agronomy 2023, 13, 2655. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.A. Arbuscular mycorrhizal fungi as a determinant of plant diversity: In search of underlying mechanisms and general principles. In Mycorrhizal Ecology; Springer: Berlin/Heidelberg, Germany, 2002; pp. 243–265. [Google Scholar]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Quince, C.; Lanzen, A.; Davenport, R.J.; Turnbaugh, P.J. Removing Noise From Pyrosequenced Amplicons. BMC Bioinform. 2011, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Gohl, D.M.; Vangay, P.; Garbe, J.; MacLean, A.; Hauge, A.; Becker, A.; Gould, T.J.; Clayton, J.B.; Johnson, T.J.; Hunter, R.; et al. Systematic improvement of amplicon marker gene methods for increased accuracy in microbiome studies. Nat. Biotechnol. 2016, 34, 942–949. [Google Scholar] [CrossRef]
- Estaki, M.; Jiang, L.; Bokulich, N.A.; McDonald, D.; González, A.; Kosciolek, T.; Martino, C.; Zhu, Q.; Birmingham, A.; Vázquez-Baeza, Y.; et al. QIIME 2 Enables Comprehensive End-to-End Analysis of Diverse Microbiome Data and Comparative Studies with Publicly Available Data. Curr. Protoc. Bioinforma 2020, 70, e100. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.I.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2-Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef] [PubMed]
- Gower, J.C. Principal coordinates analysis. In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 1–7. [Google Scholar]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- Mauri, M.; Elli, T.; Caviglia, G.; Uboldi, G.; Azzi, M. RAWGraphs: A visualisation platform to create open outputs. In Proceedings of the the 12th Biannual Conference on Italian SIGCHI Chapter, Cagliari, Italy, 18–20 September 2017; pp. 1–5. [Google Scholar]
- Wemheuer, F.; Taylor, J.A.; Daniel, R.; Johnston, E.; Meinicke, P.; Thomas, T.; Wemheuer, B. Tax4Fun2: Prediction of habitat-specific functional profiles and functional redundancy based on 16S rRNA gene sequences. Environ. Microbiome 2020, 15, 11. [Google Scholar] [CrossRef]
- Põlme, S.; Abarenkov, K.; Henrik Nilsson, R.; Lindahl, B.D.; Clemmensen, K.E.; Kauserud, H.; Nguyen, N.; Kjøller, R.; Bates, S.T.; Baldrian, P.; et al. FungalTraits: A user-friendly traits database of fungi and fungus-like stramenopiles. Fungal Divers. 2020, 105, 1–16. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational multivariate analysis of variance (PERMANOVA). In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 1–15. [Google Scholar]
- Foster, Z.S.L.; Sharpton, T.J.; Grünwald, N.J. Metacoder: An R package for visualization and manipulation of community taxonomic diversity data. PLoS Comput. Biol. 2017, 13, e1005404. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Yan, Y.; Cheng, L.; Lu, Y.; Chen, J.; Liu, F.; Tan, J. Arbuscular Mycorrhizal Fungi and Rhizobium Facilitate Nitrogen and Phosphate Availability in Soybean/Maize Intercropping Systems. J. Soil Sci. Plant Nutr. 2023, 23, 2723–2731. [Google Scholar] [CrossRef]
- Liu, A.; Ku, Y.S.; Contador, C.A.; Lam, H.M. The impacts of domestication and agricultural practices on legume nutrient acquisition through symbiosis with rhizobia and arbuscular mycorrhizal fungi. Front. Genet. 2020, 11, 583954. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Zhao, B.; Jia, X.; Yu, N.; Murray, J.D.; Yi, K.; Wang, E. Microbe-dependent and independent nitrogen and phosphate acquisition and regulation in plants. New Phytol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zahran, H.H. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol Biol. Rev. 1999, 63, 968–989. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.P.; Faria, J.M.S.; Dordio, A.V.; Carvalho, A.J.P. Organic Farming—A Sustainable Option to Reduce Soil Degradation. In Agroecological Approaches for Sustainable Soil Management; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 83–143. [Google Scholar]
- Jindo, K.; Audette, Y.; Olivares, F.L.; Canellas, L.P.; Smith, D.S.; Paul Voroney, R. Biotic and abiotic effects of soil organic matter on the phytoavailable phosphorus in soils: A review. Chem. Biol. Technol. Agric. 2023, 10, 29. [Google Scholar] [CrossRef]
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah, J.A.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowalchuk, G.A.; Hart, M.M.; Bago, A.; et al. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef]
- Busby, P.E.; Soman, C.; Wagner, M.R.; Friesen, M.L.; Kremer, J.; Bennett, A.; Morsy, M.; Eisen, J.A.; Leach, J.E.; Dangl, J.L. Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol. 2017, 15, e2001793. Available online: https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.2001793 (accessed on 26 September 2022). [CrossRef]
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef]
- Dangi, S.R.; Allen, B.L.; Jabro, J.D.; Rand, T.A.; Campbell, J.W.; Calderon, R.B. The Effect of Alternative Dryland Crops on Soil Microbial Communities. Soil Syst. 2024, 8, 4. [Google Scholar] [CrossRef]
- Hu, J.; Cyle, K.T.; Miller, G.; Shi, W. Water deficits shape the microbiome of Bermudagrass roots to be Actinobacteria rich. FEMS Microbiol. Ecol. 2023, 99, fiad036. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Raaijmakers, J.M.; Carrión, V.J. Importance of Bacteroidetes in host–microbe interactions and ecosystem functioning. Trends Microbiol. 2023, 31, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, B.; Li, Y.; Jiang, D.; Zhou, Y.; Ding, A.; Zong, Y.; Ling, X.; Zhang, S.; Lu, H. Ubiquity, diversity, and activity of comammox Nitrospira in agricultural soils. Sci. Total Environ. 2020, 706, 135684. [Google Scholar] [CrossRef] [PubMed]
- Oliverio, A.M.; Bissett, A.; McGuire, K.; Saltonstall, K.; Turner, B.L.; Fierer, N. The role of phosphorus limitation in shaping soil bacterial communities and their metabolic capabilities. MBio 2020, 11, 10–1128. [Google Scholar] [CrossRef]
- Hashmi, I.; Bindschedler, S.; Junier, P. Firmicutes. In Beneficial Microbes in Agro-Ecology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 363–396. [Google Scholar]
- Wang, S.; Meade, A.; Lam, H.M.; Luo, H. Evolutionary timeline and genomic plasticity underlying the lifestyle diversity in Rhizobiales. Msystems 2020, 5, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Idbella, M.; Bonanomi, G. Uncovering the dark side of agriculture: How land use intensity shapes soil microbiome and increases potential plant pathogens. Appl. Soil Ecol. 2023, 192, 105090. [Google Scholar] [CrossRef]
- Hestrin, R.; Kan, M.; Lafler, M.; Wollard, J.; Kimbrel, J.A.; Ray, P.; Blazewicz, S.J.; Stuart, R.; Craven, K.; Firestone, M.; et al. Plant-associated fungi support bacterial resilience following water limitation. ISME J. 2022, 16, 2752–2762. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liang, Z.; Kuzyakov, Y.; Li, W.; He, Y.; Wang, C.; Xiao, Y.; Chen, K.; Sun, G.; Lei, Y. Dissolved organic matter defines microbial communities during initial soil formation after deglaciation. Sci. Total Environ. 2023, 878, 163171. [Google Scholar] [CrossRef]
- Conradie, T.; Jacobs, K. Seasonal and agricultural response of Acidobacteria present in two fynbos rhizosphere soils. Diversity 2020, 12, 277. [Google Scholar] [CrossRef]
- Bonfante, P.; Venice, F. Mucoromycota: Going to the roots of plant-interacting fungi. Fungal Biol. Rev. 2020, 34, 100–113. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, T.; Sheng, K.; Shi, W.; Han, Y.; Wang, Y.; Li, H. Continuous straw return for 8 years mitigates the negative effects of inorganic fertilisers on C-cycling soil bacteria. Eur. J. Soil Sci. 2022, 73, e13322. [Google Scholar] [CrossRef]
- Sivaram, A.K.; Abinandan, S.; Chen, C.; Venkateswartlu, K.; Megharaj, M. Microbial Inoculant Carriers: Soil Health Improvement and Moisture Retention in Sustainable Agriculture; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef]
- Gupta, R.; Anand, G.; Gaur, R.; Yadav, D. Plant–microbiome interactions for sustainable agriculture: A review. Physiol. Mol. Biol. Plants 2021, 27, 165–179. Available online: https://link.springer.com/article/10.1007/s12298-021-00927-1 (accessed on 15 February 2024). [CrossRef]
- Yates, R.J.; Abaidoo, R.; Howieson, J.G. Field Experiments with Rhizobia; Australian Centre for International Agricultural Research: Canberra, Australia, 2016. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderon, R.B.; Dangi, S.R. Arbuscular Mycorrhizal Fungi and Rhizobium Improve Nutrient Uptake and Microbial Diversity Relative to Dryland Site-Specific Soil Conditions. Microorganisms 2024, 12, 667. https://doi.org/10.3390/microorganisms12040667
Calderon RB, Dangi SR. Arbuscular Mycorrhizal Fungi and Rhizobium Improve Nutrient Uptake and Microbial Diversity Relative to Dryland Site-Specific Soil Conditions. Microorganisms. 2024; 12(4):667. https://doi.org/10.3390/microorganisms12040667
Chicago/Turabian StyleCalderon, Rosalie B., and Sadikshya R. Dangi. 2024. "Arbuscular Mycorrhizal Fungi and Rhizobium Improve Nutrient Uptake and Microbial Diversity Relative to Dryland Site-Specific Soil Conditions" Microorganisms 12, no. 4: 667. https://doi.org/10.3390/microorganisms12040667
APA StyleCalderon, R. B., & Dangi, S. R. (2024). Arbuscular Mycorrhizal Fungi and Rhizobium Improve Nutrient Uptake and Microbial Diversity Relative to Dryland Site-Specific Soil Conditions. Microorganisms, 12(4), 667. https://doi.org/10.3390/microorganisms12040667




