Psychobiotics and the Microbiota–Gut–Brain Axis: Where Do We Go from Here?
Abstract
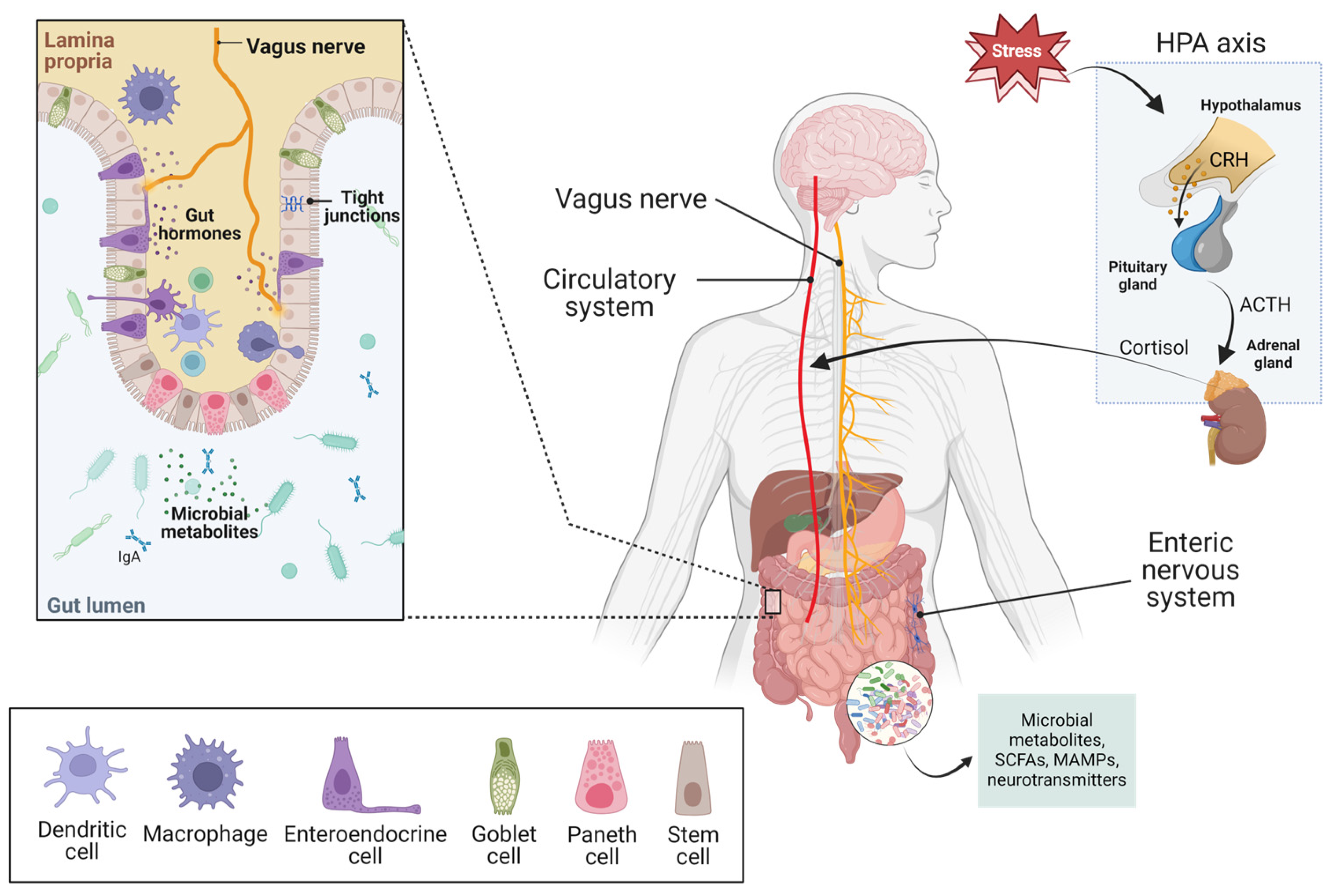
1. Introduction
2. Gut Microbiota and Neurodegenerative Diseases: Crosstalk and Potential Mechanisms
3. Psychobiotics and Neuropsychiatric Disorders (NPDs)
- High variability in strain, dose, and duration of supplementation.
- Use as an adjuvant to pharmacologic treatments or alone, or with nutraceuticals without a corresponding nutraceutical control arm.
- Heterogeneity in the prior or co-administered pharmacologic treatments.
- Heterogeneity in outcome measures and outcome assessment tools.
- Lack of patient-centered outcomes, such as social functioning.
| Strain/Formulation | Depressive Symptoms | Anxiety Symptoms | Sleep Quality | GI Symptoms | Refs | |
|---|---|---|---|---|---|---|
| L. paracasei Shirota (8 × 1010 CFU) | HDRS | BDI | STAI | PSQI | GSRS | [69] |
| B. coagulans MTCC 5856 (2 × 109 CFUs) | CES-D, CGI, HDRS, MADRS | Not tested | Not tested | IBS-QOL | [65] | |
| L. plantarum PS128 (6 × 1010 CFUs) | HDRS, DSSS | Not tested | Not tested | Not tested | [70] | |
| C. butyricum MIYAIRI 588 © (60 mg) | BDI, HDRS | BAI | Not tested | Not tested | [71] | |
| L. helveticus R0052, B. longum R0175 (Cerebiome®; 10% and 90% of the composition respectively, 3 × 109 CFUs total) | BDI, MADRS, QIDS-SR16, SHAPS | GAD-7, STAI | PSQI | Not tested | [66,67,68,72] | |
| S. thermophilus, B. breve, B. longum, B. infantis, L. acidophilus, L. plantarum, L. paracasei, L. delbrueckii (9 × 1011 CFUs total) | HDRS | BDI | STAI | Not tested | GSRS | [73] |
| B. breve, B. longum, P. acidilactici (4 × 109 CFUs/g; 4 g) | HDRS, MADRS | Not tested | Not tested | GSRS | [74] | |
| L. acidophilus, L. casei, B. bifidum (2 × 109 CFUs each) | BDI | Not tested | Not tested | Not tested | [64] | |
4. Psychobiotics and Microbiota in Sleep Quality, Stress and Mental Health
- High heterogeneity in the ELS stressors studied, with some stressors assumed (i.e., effect of long-term institutionalized care on microbiome in children) but not confirmed (i.e., no emotional state measure reported).
- Few studies used adult biomarkers of stress to complement/confirm self-reported or interview-reported ELS.
- Heterogeneity in the collection, processing, and analyses of stool samples.
- The impact of medications is difficult to estimate (e.g., antiretroviral therapy, antidepressants, antibiotics, etc.).
- Diet not monitored.
- Participants age range very large (from newborns to adults with PTSD ‡)
5. The Immune System and NDDs
6. Eating Behaviors, Metabolic Health, and NDDs
7. Psychobiotics as Prevention versus Treatment
8. Towards Mechanisms of Action: New Technologies for Preclinical and Clinical Investigations
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Etzel, L.; Garrett-Petters, P.; Shalev, I. Early origins of health and disease risk: The case for investigating adverse exposures and biological aging in utero, across childhood, and into adolescence. Child Dev. Perspect. 2023, 17, 149–156. [Google Scholar] [CrossRef]
- Ji, J.; Jin, W.; Liu, S.J.; Jiao, Z.; Li, X. Probiotics, prebiotics, and postbiotics in health and disease. MedComm 2023, 4, e420. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Neshat, M.; Pourjafar, H.; Jafari, S.M.; Samakkhah, S.A.; Mirzakhani, E. The role of probiotics and prebiotics in modulating of the gut-brain axis. Front. Nutr. 2023, 10, 1173660. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Młynarska, E.; Gadzinowska, J.; Tokarek, J.; Forycka, J.; Szuman, A.; Franczyk, B.; Rysz, J. The Role of the Microbiome-Brain-Gut Axis in the Pathogenesis of Depressive Disorder. Nutrients 2022, 14, 1921. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Molotla-Torres, D.E.; Guzmán-Mejía, F.; Godínez-Victoria, M.; Drago-Serrano, M.E. Role of Stress on Driving the Intestinal Paracellular Permeability. Curr. Issues Mol. Biol. 2023, 45, 9284–9305. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhao, J.; Wang, G.; Chen, W. Bifidobacterium breve HNXY26M4 Attenuates Cognitive Deficits and Neuroinflammation by Regulating the Gut-Brain Axis in APP/PS1 Mice. J. Agric. Food Chem. 2023, 71, 4646–4655. [Google Scholar] [CrossRef]
- Naomi, R.; Embong, H.; Othman, F.; Ghazi, H.F.; Maruthey, N.; Bahari, H. Probiotics for Alzheimer’s Disease: A Systematic Review. Nutrients 2021, 14, 20. [Google Scholar] [CrossRef]
- Kim, C.S.; Cha, L.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.M. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 32–40. [Google Scholar] [CrossRef]
- Mirzaei, H.; Sedighi, S.; Kouchaki, E.; Barati, E.; Dadgostar, E.; Aschner, M.; Tamtaji, O.R. Probiotics and the Treatment of Parkinson’s Disease: An Update. Cell. Mol. Neurobiol. 2022, 42, 2449–2457. [Google Scholar] [CrossRef] [PubMed]
- Megur, A.; Baltriukienė, D.; Bukelskienė, V.; Burokas, A. The Microbiota-Gut-Brain Axis and Alzheimer’s Disease: Neuroinflammation Is to Blame? Nutrients 2020, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Cheng, L.H.; Liu, Y.W.; Wu, C.C.; Wang, S.; Tsai, Y.C. Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J. Food Drug Anal. 2019, 27, 632–648. [Google Scholar] [CrossRef]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Gupta, D.; Mehrotra, R.; Mago, P. Psychobiotics: The Next-Generation Probiotics for the Brain. Curr. Microbiol. 2021, 78, 449–463. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Keller, J.; Gomez, R.; Williams, G.; Lembke, A.; Lazzeroni, L.; Murphy, G.M., Jr.; Schatzberg, A.F. HPA axis in major depression: Cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatry 2017, 22, 527–536. [Google Scholar] [CrossRef]
- Misiak, B.; Łoniewski, I.; Marlicz, W.; Frydecka, D.; Szulc, A.; Rudzki, L.; Samochowiec, J. The HPA axis dysregulation in severe mental illness: Can we shift the blame to gut microbiota? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 102, 109951. [Google Scholar] [CrossRef]
- Rosin, S.; Xia, K.; Azcarate-Peril, M.A.; Carlson, A.L.; Propper, C.B.; Thompson, A.L.; Grewen, K.; Knickmeyer, R.C. A preliminary study of gut microbiome variation and HPA axis reactivity in healthy infants. Psychoneuroendocrinology 2021, 124, 105046. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.G.; Schloesser, D.; Arensdorf, A.M.; Simmons, J.M.; Cui, C.; Valentino, R.; Gnadt, J.W.; Nielsen, L.; Hillaire-Clarke, C.S.; Spruance, V.; et al. The Emerging Science of Interoception: Sensing, Integrating, Interpreting, and Regulating Signals within the Self. Trends Neurosci. 2021, 44, 3–16. [Google Scholar] [CrossRef]
- Craig, A.D. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002, 3, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Knight, R.; Mazmanian, S.K.; Cryan, J.F.; Tillisch, K. Gut microbes and the brain: Paradigm shift in neuroscience. J. Neurosci. 2014, 34, 15490–15496. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]
- Intili, G.; Paladino, L.; Rappa, F.; Alberti, G.; Plicato, A.; Calabrò, F.; Fucarino, A.; Cappello, F.; Bucchieri, F.; Tomasello, G.; et al. From Dysbiosis to Neurodegenerative Diseases through Different Communication Pathways: An Overview. Biology 2023, 12, 195. [Google Scholar] [CrossRef]
- Pavan, S.; Gorthi, S.P.; Prabhu, A.N.; Das, B.; Mutreja, A.; Vasudevan, K.; Shetty, V.; Ramamurthy, T.; Ballal, M. Dysbiosis of the Beneficial Gut Bacteria in Patients with Parkinson’s Disease from India. Ann. Indian Acad. Neurol. 2023, 26, 908–916. [Google Scholar] [CrossRef]
- Sheng, C.; Lin, L.; Lin, H.; Wang, X.; Han, Y.; Liu, S.L. Altered Gut Microbiota in Adults with Subjective Cognitive Decline: The SILCODE Study. J. Alzheimers Dis. 2021, 82, 513–526. [Google Scholar] [CrossRef]
- Sheng, C.; Du, W.; Liang, Y.; Xu, P.; Ding, Q.; Chen, X.; Jia, S.; Wang, X. An integrated neuroimaging-omics approach for the gut-brain communication pathways in Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1211979. [Google Scholar] [CrossRef]
- Hochuli, N.; Kadyan, S.; Park, G.; Patoine, C.; Nagpal, R. Pathways linking microbiota-gut-brain axis with neuroinflammatory mechanisms in Alzheimer’s pathophysiology. Microbiome Res. Rep. 2024, 3, 9. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Hendriksen, H.M.A.; de Leeuw, F.A.; Doorduijn, A.S.; van Leeuwenstijn, M.; Teunissen, C.E.; Barkhof, F.; Scheltens, P.; Kraaij, R.; van Duijn, C.M.; et al. Gut Microbiota Composition Is Related to AD Pathology. Front. Immunol. 2021, 12, 794519. [Google Scholar] [CrossRef]
- Haran, J.P.; Bhattarai, S.K.; Foley, S.E.; Dutta, P.; Ward, D.V.; Bucci, V.; McCormick, B.A. Alzheimer’s Disease Microbiome Is Associated with Dysregulation of the Anti-Inflammatory P-Glycoprotein Pathway. mBio 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Oleksak, I.; Leśniewski, M.; Welian-Polus, I.; Maliszewska, K.; Ziółkowska, J. Does gut microbiota have an impact on the origin of Alzheimer’s and Parkinson’s disease?—literature review. J. Educ. Health Sport 2024, 57, 70–85. [Google Scholar] [CrossRef]
- Zhan, Y.; Al-Nusaif, M.; Ding, C.; Zhao, L.; Dong, C. The potential of the gut microbiome for identifying Alzheimer’s disease diagnostic biomarkers and future therapies. Front. Neurosci. 2023, 17, 1130730. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yi, J.; Zhang, Y.G.; Zhou, J.; Sun, J. Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol. Rep. 2015, 3, e12356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Unnikrishnan, A.; Deepa, S.S.; Liu, Y.; Li, Y.; Ikeno, Y.; Sosnowska, D.; Van Remmen, H.; Richardson, A. A new role for oxidative stress in aging: The accelerated aging phenotype in Sod1(-/)(-) mice is correlated to increased cellular senescence. Redox Biol. 2017, 11, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; van Esch, B.; Henricks, P.A.J.; Folkerts, G.; Garssen, J. The Anti-inflammatory Effects of Short Chain Fatty Acids on Lipopolysaccharide- or Tumor Necrosis Factor α-Stimulated Endothelial Cells via Activation of GPR41/43 and Inhibition of HDACs. Front. Pharm. 2018, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Fock, E.; Parnova, R. Mechanisms of Blood-Brain Barrier Protection by Microbiota-Derived Short-Chain Fatty Acids. Cells 2023, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- González-Sanmiguel, J.; Schuh, C.; Muñoz-Montesino, C.; Contreras-Kallens, P.; Aguayo, L.G.; Aguayo, S. Complex Interaction between Resident Microbiota and Misfolded Proteins: Role in Neuroinflammation and Neurodegeneration. Cells 2020, 9, 2476. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Shannon, K.M.; Kordower, J.H.; Voigt, R.M.; Shaikh, M.; Jaglin, J.A.; Estes, J.D.; Dodiya, H.B.; Keshavarzian, A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE 2011, 6, e28032. [Google Scholar] [CrossRef]
- Uemura, N.; Yagi, H.; Uemura, M.T.; Hatanaka, Y.; Yamakado, H.; Takahashi, R. Inoculation of α-synuclein preformed fibrils into the mouse gastrointestinal tract induces Lewy body-like aggregates in the brainstem via the vagus nerve. Mol Neurodegener 2018, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, S.; Nalbantoğlu Ö, U.; Bayraktar, A.; Ercan, F.B.; Gündoğdu, A.; Velioğlu, H.A.; Göl, M.F.; Soylu, A.E.; Koç, F.; Gülpınar, E.A.; et al. Stratification of the Gut Microbiota Composition Landscape across the Alzheimer’s Disease Continuum in a Turkish Cohort. Msystems 2022, 7, e0000422. [Google Scholar] [CrossRef]
- Koukoulis, T.F.; Beauchamp, L.C.; Kaparakis-Liaskos, M.; McQuade, R.M.; Purnianto, A.; Finkelstein, D.I.; Barnham, K.J.; Vella, L.J. Do Bacterial Outer Membrane Vesicles Contribute to Chronic Inflammation in Parkinson’s Disease? J. Parkinsons Dis. 2024, 14, 227–244. [Google Scholar] [CrossRef]
- Cárdenas, V.M.; Boller, F.; Román, G.C. Helicobacter pylori, Vascular Risk Factors and Cognition in U.S. Older Adults. Brain Sci. 2019, 9, 370. [Google Scholar] [CrossRef]
- Khatoon, S.; Kalam, N.; Rashid, S.; Bano, G. Effects of gut microbiota on neurodegenerative diseases. Front. Aging Neurosci. 2023, 15, 1145241. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef]
- Widner, B.; Leblhuber, F.; Walli, J.; Tilz, G.P.; Demel, U.; Fuchs, D. Tryptophan degradation and immune activation in Alzheimer’s disease. J. Neural. Transm. 2000, 107, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Iwaniak, P.; Owe-Larsson, M.; Urbańska, E.M. Microbiota, Tryptophan and Aryl Hydrocarbon Receptors as the Target Triad in Parkinson’s Disease-A Narrative Review. Int. J. Mol. Sci. 2024, 25, 2915. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Zimetti, F.; Caffarra, P.; Tassotti, M.; Bernini, F.; Brighenti, F.; Zini, A.; Zanotti, I. The Gut Microbial Metabolite Trimethylamine-N-Oxide Is Present in Human Cerebrospinal Fluid. Nutrients 2017, 9, 1053. [Google Scholar] [CrossRef] [PubMed]
- Ide, M.; Harris, M.; Stevens, A.; Sussams, R.; Hopkins, V.; Culliford, D.; Fuller, J.; Ibbett, P.; Raybould, R.; Thomas, R.; et al. Periodontitis and Cognitive Decline in Alzheimer’s Disease. PLoS ONE 2016, 11, e0151081. [Google Scholar] [CrossRef]
- Poole, S.; Singhrao, S.K.; Kesavalu, L.; Curtis, M.A.; Crean, S. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J. Alzheimers Dis. 2013, 36, 665–677. [Google Scholar] [CrossRef]
- Ilievski, V.; Zuchowska, P.K.; Green, S.J.; Toth, P.T.; Ragozzino, M.E.; Le, K.; Aljewari, H.W.; O’Brien-Simpson, N.M.; Reynolds, E.C.; Watanabe, K. Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration and amyloid beta production in wild type mice. PLoS ONE 2018, 13, e0204941. [Google Scholar] [CrossRef]
- Nicholson, J.S.; Landry, K.S. Oral Dysbiosis and Neurodegenerative Diseases: Correlations and Potential Causations. Microorganisms 2022, 10, 1326. [Google Scholar] [CrossRef]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’Anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef]
- Shir, D.; Graff-Radford, J.; Hofrenning, E.I.; Lesnick, T.G.; Przybelski, S.A.; Lowe, V.J.; Knopman, D.S.; Petersen, R.C.; Jack, C.R., Jr.; Vemuri, P.; et al. Association of plasma glial fibrillary acidic protein (GFAP) with neuroimaging of Alzheimer’s disease and vascular pathology. Alzheimers Dement (Amst) 2022, 14, e12291. [Google Scholar] [CrossRef]
- Guo, Y.; You, J.; Zhang, Y.; Liu, W.-S.; Huang, Y.-Y.; Zhang, Y.-R.; Zhang, W.; Dong, Q.; Feng, J.-F.; Cheng, W.; et al. Plasma proteomic profiles predict future dementia in healthy adults. Nat. Aging 2024, 4, 1–14. [Google Scholar] [CrossRef]
- Castelli, V.; d’Angelo, M.; Lombardi, F.; Alfonsetti, M.; Antonosante, A.; Catanesi, M.; Benedetti, E.; Palumbo, P.; Cifone, M.G.; Giordano, A.; et al. Effects of the probiotic formulation SLAB51 in in vitro and in vivo Parkinson’s disease models. Aging 2020, 12, 4641–4659. [Google Scholar] [CrossRef]
- Ribera, C.; Sánchez-Ortí, J.V.; Clarke, G.; Marx, W.; Mörkl, S.; Balanzá-Martínez, V. Probiotic, prebiotic, synbiotic and fermented food supplementation in psychiatric disorders: A systematic review of clinical trials. Neurosci. Biobehav. Rev. 2024, 158, 105561. [Google Scholar] [CrossRef] [PubMed]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Nagabhushanam, K.; Arumugam, S.; Majeed, S.; Ali, F. Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: A randomised, doubleblind, placebo controlled, multi-centre, pilot clinical study. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Djafarian, K. Effect of probiotic and prebiotic versus placebo on appetite in patients with major depressive disorder: Post hoc analysis of a randomised clinical trial. J. Hum. Nutr. Diet 2020, 33, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Heidarzadeh-Rad, N.; Gökmen-Özel, H.; Kazemi, A.; Almasi, N.; Djafarian, K. Effects of a Psychobiotic Supplement on Serum Brain-derived Neurotrophic Factor Levels in Depressive Patients: A Post Hoc Analysis of a Randomized Clinical Trial. J. Neurogastroenterol. Motil. 2020, 26, 486–495. [Google Scholar] [CrossRef]
- Otaka, M.; Kikuchi-Hayakawa, H.; Ogura, J.; Ishikawa, H.; Yomogida, Y.; Ota, M.; Hidese, S.; Ishida, I.; Aida, M.; Matsuda, K.; et al. Effect of Lacticaseibacillus paracasei Strain Shirota on Improvement in Depressive Symptoms, and Its Association with Abundance of Actinobacteria in Gut Microbiota. Microorganisms 2021, 9, 1026. [Google Scholar] [CrossRef]
- Chen, H.-M.; Kuo, P.-H.; Hsu, C.-Y.; Chiu, Y.-H.; Liu, Y.-W.; Lu, M.-L.; Chen, C.-H. Psychophysiological Effects of Lactobacillus plantarum PS128 in Patients with Major Depressive Disorder: A Preliminary 8-Week Open Trial. Nutrients 2021, 13, 3731. [Google Scholar] [CrossRef]
- Miyaoka, T.; Kanayama, M.; Wake, R.; Hashioka, S.; Hayashida, M.; Nagahama, M.; Okazaki, S.; Yamashita, S.; Miura, S.; Miki, H.; et al. Clostridium butyricum MIYAIRI 588 as Adjunctive Therapy for Treatment-Resistant Major Depressive Disorder: A Prospective Open-Label Trial. Clin. Neuropharmacol. 2018, 41, 151–155. [Google Scholar] [CrossRef]
- Wallace, C.J.K.; Milev, R.V. The Efficacy, Safety, and Tolerability of Probiotics on Depression: Clinical Results From an Open-Label Pilot Study. Front. Psychiatry 2021, 12, 618279. [Google Scholar] [CrossRef]
- Schaub, A.-C.; Schneider, E.; Vazquez-Castellanos, J.F.; Schweinfurth, N.; Kettelhack, C.; Doll, J.P.K.; Yamanbaeva, G.; Mählmann, L.; Brand, S.; Beglinger, C.; et al. Clinical, gut microbial and neural effects of a probiotic add-on therapy in depressed patients: A randomized controlled trial. Transl. Psychiatry 2022, 12, 227. [Google Scholar] [CrossRef]
- Tian, P.; Zou, R.; Wang, L.; Chen, Y.; Qian, X.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; Wang, G.; et al. Multi-Probiotics ameliorate Major depressive disorder and accompanying gastrointestinal syndromes via serotonergic system regulation. J. Adv. Res. 2023, 45, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.J.; Webb, T.L.; Martyn-St James, M.; Rowse, G.; Weich, S. Improving sleep quality leads to better mental health: A meta-analysis of randomised controlled trials. Sleep Med. Rev. 2021, 60, 101556. [Google Scholar] [CrossRef] [PubMed]
- Staines, A.C.; Broomfield, N.; Pass, L.; Orchard, F.; Bridges, J. Do non-pharmacological sleep interventions affect anxiety symptoms? A meta-analysis. J. Sleep Res. 2022, 31, e13451. [Google Scholar] [CrossRef] [PubMed]
- Gardani, M.; Bradford, D.R.R.; Russell, K.; Allan, S.; Beattie, L.; Ellis, J.G.; Akram, U. A systematic review and meta-analysis of poor sleep, insomnia symptoms and stress in undergraduate students. Sleep Med. Rev. 2022, 61, 101565. [Google Scholar] [CrossRef]
- Bruce-Keller, A.J.; Salbaum, J.M.; Berthoud, H.R. Harnessing Gut Microbes for Mental Health: Getting From Here to There. Biol. Psychiatry 2018, 83, 214–223. [Google Scholar] [CrossRef]
- Murack, M.; Chandrasegaram, R.; Smith, K.B.; Ah-Yen, E.G.; Rheaume, É.; Malette-Guyon, É.; Nanji, Z.; Semchishen, S.N.; Latus, O.; Messier, C.; et al. Chronic sleep disruption induces depression-like behavior in adolescent male and female mice and sensitization of the hypothalamic-pituitary-adrenal axis in adolescent female mice. Behav. Brain Res. 2021, 399, 113001. [Google Scholar] [CrossRef]
- Murray, E.; Sharma, R.; Smith, K.B.; Mar, K.D.; Barve, R.; Lukasik, M.; Pirwani, A.F.; Malette-Guyon, E.; Lamba, S.; Thomas, B.J.; et al. Probiotic consumption during puberty mitigates LPS-induced immune responses and protects against stress-induced depression- and anxiety-like behaviors in adulthood in a sex-specific manner. Brain Behav. Immun. 2019, 81, 198–212. [Google Scholar] [CrossRef]
- Tremblay, A.; Lingrand, L.; Maillard, M.; Feuz, B.; Tompkins, T.A. The effects of psychobiotics on the microbiota-gut-brain axis in early-life stress and neuropsychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 105, 110142. [Google Scholar] [CrossRef] [PubMed]
- Gröger, N.; Matas, E.; Gos, T.; Lesse, A.; Poeggel, G.; Braun, K.; Bock, J. The transgenerational transmission of childhood adversity: Behavioral, cellular, and epigenetic correlates. J. Neural. Transm. 2016, 123, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, M.C.; Verosky, B.; Chen, H.J.; Davis, O.; Gur, T.L. The Maternal Microbiome as a Map to Understanding the Impact of Prenatal Stress on Offspring Psychiatric Health. Biol. Psychiatry 2024, 95, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.I.; Kumari, A.; Kapila, S.; Kapila, R. Probiotic lactobacilli mediated changes in global epigenetic signatures of human intestinal epithelial cells during Escherichia coli challenge. Ann. Microbiol. 2019, 69, 603–612. [Google Scholar] [CrossRef]
- National Institutes of Mental Health. Mental Illness. Available online: https://www.nimh.nih.gov/health/statistics/mental-illness (accessed on 24 January 2024).
- StatCan. The Social and Economic Impact of COVID-19: A Six-Month Update. Available online: https://www150.statcan.gc.ca/n1/pub/11-631-x/2020004/pdf/s3-eng.pdf (accessed on 24 January 2024).
- Alving-Jessep, E.; Botchway, E.; Wood, A.G.; Hilton, A.C.; Blissett, J.M. The development of the gut microbiome and temperament during infancy and early childhood: A systematic review. Dev. Psychobiol. 2022, 64, e22306. [Google Scholar] [CrossRef]
- Agusti, A.; Lamers, F.; Tamayo, M.; Benito-Amat, C.; Molina-Mendoza, G.V.; Penninx, B.; Sanz, Y. The Gut Microbiome in Early Life Stress: A Systematic Review. Nutrients 2023, 15, 2566. [Google Scholar] [CrossRef]
- Graham, A.M.; Doyle, O.; Tilden, E.L.; Sullivan, E.L.; Gustafsson, H.C.; Marr, M.; Allen, M.; Mackiewicz Seghete, K.L. Effects of Maternal Psychological Stress During Pregnancy on Offspring Brain Development: Considering the Role of Inflammation and Potential for Preventive Intervention. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2022, 7, 461–470. [Google Scholar] [CrossRef]
- Liu, Y.W.; Liu, W.H.; Wu, C.C.; Juan, Y.C.; Wu, Y.C.; Tsai, H.P.; Wang, S.; Tsai, Y.C. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Res. 2016, 1631, 1–12. [Google Scholar] [CrossRef]
- Cowan, C.S.M.; Stylianakis, A.A.; Richardson, R. Early-life stress, microbiota, and brain development: Probiotics reverse the effects of maternal separation on neural circuits underpinning fear expression and extinction in infant rats. Dev. Cogn. Neurosci. 2019, 37, 100627. [Google Scholar] [CrossRef] [PubMed]
- Natale, N.R.; Kent, M.; Fox, N.; Vavra, D.; Lambert, K. Neurobiological effects of a probiotic-supplemented diet in chronically stressed male Long-Evans rats: Evidence of enhanced resilience. IBRO Neurosci. Rep. 2021, 11, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Bairamian, D.; Sha, S.; Rolhion, N.; Sokol, H.; Dorothée, G.; Lemere, C.A.; Krantic, S. Microbiota in neuroinflammation and synaptic dysfunction: A focus on Alzheimer’s disease. Mol. Neurodegener. 2022, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Liu, Y.; Hou, Y.; Cao, S.; Chen, Z.; Zhang, Y.; Cai, X.; Yan, Q.; Li, Z.; Yuan, Y.; et al. Psychological stress-induced microbial metabolite indole-3-acetate disrupts intestinal cell lineage commitment. Cell Metab. 2024, 36, 466–483. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014, 14, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.C.; Chen, S. Translational Neurodegeneration in the era of fast growing international brain research. Transl. Neurodegener. 2022, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Marogianni, C.; Sokratous, M.; Dardiotis, E.; Hadjigeorgiou, G.M.; Bogdanos, D.; Xiromerisiou, G. Neurodegeneration and Inflammation-An Interesting Interplay in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8421. [Google Scholar] [CrossRef] [PubMed]
- McCauley, M.E.; Baloh, R.H. Inflammation in ALS/FTD pathogenesis. Acta. Neuropathol. 2019, 137, 715–730. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Postler, T.S.; Ghosh, S. Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab. 2017, 26, 110–130. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Solanki, R.; Karande, A.; Ranganathan, P. Emerging role of gut microbiota dysbiosis in neuroinflammation and neurodegeneration. Front. Neurol. 2023, 14, 1149618. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e1412. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.J.; Chen, J.L.; Liao, J.F.; Chen, Y.H.; Chieu, M.W.; Ke, Y.Y.; Hsu, C.C.; Tsai, Y.C.; Hsieh-Li, H.M. Lactobacillus plantarum PS128 prevents cognitive dysfunction in Alzheimer’s disease mice by modulating propionic acid levels, glycogen synthase kinase 3 beta activity, and gliosis. BMC Complement Med. 2021, 21, 259. [Google Scholar] [CrossRef]
- Borzabadi, S.; Oryan, S.; Eidi, A.; Aghadavod, E.; Daneshvar Kakhaki, R.; Tamtaji, O.R.; Taghizadeh, M.; Asemi, Z. The Effects of Probiotic Supplementation on Gene Expression Related to Inflammation, Insulin and Lipid in Patients with Parkinson’s Disease: A Randomized, Double-blind, PlaceboControlled Trial. Arch. Iran. Med. 2018, 21, 289–295. [Google Scholar]
- Bonfili, L.; Cecarini, V.; Berardi, S.; Scarpona, S.; Suchodolski, J.S.; Nasuti, C.; Fiorini, D.; Boarelli, M.C.; Rossi, G.; Eleuteri, A.M. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci. Rep. 2017, 7, 2426. [Google Scholar] [CrossRef]
- Mohammadi, G.; Dargahi, L.; Peymani, A.; Mirzanejad, Y.; Alizadeh, S.A.; Naserpour, T.; Nassiri-Asl, M. The Effects of Probiotic Formulation Pretreatment (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) on a Lipopolysaccharide Rat Model. J. Am. Coll. Nutr. 2019, 38, 209–217. [Google Scholar] [CrossRef]
- Konturek, S.J.; Konturek, J.W.; Pawlik, T.; Brzozowski, T. Brain-gut axis and its role in the control of food intake. J. Physiol. Pharm. 2004, 55, 137–154. [Google Scholar]
- Zhang, Y.; Yu, W.; Zhang, L.; Wang, M.; Chang, W. The Interaction of Polyphenols and the Gut Microbiota in Neurodegenerative Diseases. Nutrients 2022, 14, 5373. [Google Scholar] [CrossRef]
- Catalkaya, G.; Venema, K.; Lucini, L.; Rocchetti, G.; Delmas, D.; Daglia, M.; De Filippis, A.; Xiao, H.; Quiles, J.L.; Xiao, J.; et al. Interaction of dietary polyphenols and gut microbiota: Microbial metabolism of polyphenols, influence on the gut microbiota, and implications on host health. Food Front. 2020, 1, 109–133. [Google Scholar] [CrossRef]
- Godet, A.; Fortier, A.; Bannier, E.; Coquery, N.; Val-Laillet, D. Interactions between emotions and eating behaviors: Main issues, neuroimaging contributions, and innovative preventive or corrective strategies. Rev. Endocr. Metab. Disord. 2022, 23, 807–831. [Google Scholar] [CrossRef]
- Leyrolle, Q.; Cserjesi, R.; Mulders, M.; Zamariola, G.; Hiel, S.; Gianfrancesco, M.A.; Rodriguez, J.; Portheault, D.; Amadieu, C.; Leclercq, S.; et al. Specific gut microbial, biological, and psychiatric profiling related to binge eating disorders: A cross-sectional study in obese patients. Clin. Nutr. 2021, 40, 2035–2044. [Google Scholar] [CrossRef]
- Lee, T.H.; Yau, S.Y. From Obesity to Hippocampal Neurodegeneration: Pathogenesis and Non-Pharmacological Interventions. Int. J. Mol. Sci. 2020, 22, 201. [Google Scholar] [CrossRef]
- de A Boleti, A.P.; de O Cardoso, P.H.; BE, F.F.; PS, E.S.; de Moraes, L.; Migliolo, L. Adipose tissue, systematic inflammation, and neurodegenerative diseases. Neural. Regen. Res. 2023, 18, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Scherer, T.; Sakamoto, K.; Buettner, C. Brain insulin signalling in metabolic homeostasis and disease. Nat. Rev. Endocrinol. 2021, 17, 468–483. [Google Scholar] [CrossRef]
- Russo, C.; Valle, M.S.; Russo, A.; Malaguarnera, L. The Interplay between Ghrelin and Microglia in Neuroinflammation: Implications for Obesity and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 13432. [Google Scholar] [CrossRef]
- Dos Santos, V.V.; Rodrigues, A.L.; De Lima, T.C.; de Barioglio, S.R.; Raisman-Vozari, R.; Prediger, R.D. Ghrelin as a neuroprotective and palliative agent in Alzheimer’s and Parkinson’s disease. Curr. Pharm. Des. 2013, 19, 6773–6790. [Google Scholar] [CrossRef]
- Coccurello, R. Anhedonia in depression symptomatology: Appetite dysregulation and defective brain reward processing. Behav. Brain Res. 2019, 372, 112041. [Google Scholar] [CrossRef]
- Mir, H.D.; Giorgini, G.; Di Marzo, V. The emerging role of the endocannabinoidome-gut microbiome axis in eating disorders. Psychoneuroendocrinology 2023, 154, 106295. [Google Scholar] [CrossRef]
- Armeli, F.; Bonucci, A.; Maggi, E.; Pinto, A.; Businaro, R. Mediterranean Diet and Neurodegenerative Diseases: The Neglected Role of Nutrition in the Modulation of the Endocannabinoid System. Biomolecules 2021, 11, 790. [Google Scholar] [CrossRef]
- Kang, E.Y.; Kim, D.Y.; Kim, H.K.; Shin, W.S.; Park, Y.S.; Kim, T.H.; Kim, W.; Cao, L.; Lee, S.G.; Gang, G.; et al. Modified Korean MIND Diet: A Nutritional Intervention for Improved Cognitive Function in Elderly Women through Mitochondrial Respiration, Inflammation Suppression, and Amino Acid Metabolism Regulation. Mol. Nutr. Food Res. 2023, 67, e2300329. [Google Scholar] [CrossRef]
- Kheirouri, S.; Alizadeh, M. MIND diet and cognitive performance in older adults: A systematic review. Crit. Rev.Food. Sci. Nutr. 2022, 62, 8059–8077. [Google Scholar] [CrossRef]
- Torabynasab, K.; Shahinfar, H.; Jazayeri, S.; Effatpanah, M.; Azadbakht, L.; Abolghasemi, J. Adherence to the MIND diet is inversely associated with odds and severity of anxiety disorders: A case–control study. BMC Psychiatry 2023, 23, 330. [Google Scholar] [CrossRef]
- Pot, B.; Vandenplas, Y. Factors that influence clinical efficacy of live biotherapeutic products. Eur. J. Med. Res. 2021, 26, 40. [Google Scholar] [CrossRef]
- Le Morvan de Sequeira, C.; Hengstberger, C.; Enck, P.; Mack, I. Effect of Probiotics on Psychiatric Symptoms and Central Nervous System Functions in Human Health and Disease: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 621. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, V.L.; Cleare, A.J.; Young, A.H.; Stone, J.M. Acceptability, Tolerability, and Estimates of Putative Treatment Effects of Probiotics as Adjunctive Treatment in Patients With Depression: A Randomized Clinical Trial. JAMA Psychiatry 2023, 80, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Spacova, I.; Binda, S.; Ter Haar, J.A.; Henoud, S.; Legrain-Raspaud, S.; Dekker, J.; Espadaler-Mazo, J.; Langella, P.; Martín, R.; Pane, M.; et al. Comparing technology and regulatory landscape of probiotics as food, dietary supplements and live biotherapeutics. Front. Microbiol. 2023, 14, 1272754. [Google Scholar] [CrossRef]
- Veiga, P.; Suez, J.; Derrien, M.; Elinav, E. Moving from probiotics to precision probiotics. Nat. Microbiol. 2020, 5, 878–880. [Google Scholar] [CrossRef]
- Huang, Y.; Sheth, R.U.; Zhao, S.; Cohen, L.A.; Dabaghi, K.; Moody, T.; Sun, Y.; Ricaurte, D.; Richardson, M.; Velez-Cortes, F.; et al. High-throughput microbial culturomics using automation and machine learning. Nat. Biotechnol. 2023, 41, 1424–1433. [Google Scholar] [CrossRef]
- Afrizal, A.; Hitch, T.C.A.; Viehof, A.; Treichel, N.; Riedel, T.; Abt, B.; Buhl, E.M.; Kohlheyer, D.; Overmann, J.; Clavel, T. Anaerobic single-cell dispensing facilitates the cultivation of human gut bacteria. Env. Microbiol. 2022, 24, 3861–3881. [Google Scholar] [CrossRef]
- Ravindra, N.G.; Espinosa, C.; Berson, E.; Phongpreecha, T.; Zhao, P.; Becker, M.; Chang, A.L.; Shome, S.; Marić, I.; De Francesco, D.; et al. Deep representation learning identifies associations between physical activity and sleep patterns during pregnancy and prematurity. NPJ Digit. Med. 2023, 6, 171. [Google Scholar] [CrossRef]
- Ge, T.J.; Rahimzadeh, V.N.; Mintz, K.; Park, W.G.; Martinez-Martin, N.; Liao, J.C.; Park, S.M. Passive monitoring by smart toilets for precision health. Sci. Transl. Med. 2023, 15, eabk3489. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.J.; Peters, S.R.; Overmyer, K.A.; Paulson, B.R.; Westphall, M.S.; Coon, J.J. Real-time health monitoring through urine metabolomics. NPJ Digit. Med. 2019, 2, 109. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binda, S.; Tremblay, A.; Iqbal, U.H.; Kassem, O.; Le Barz, M.; Thomas, V.; Bronner, S.; Perrot, T.; Ismail, N.; Parker, J.A. Psychobiotics and the Microbiota–Gut–Brain Axis: Where Do We Go from Here? Microorganisms 2024, 12, 634. https://doi.org/10.3390/microorganisms12040634
Binda S, Tremblay A, Iqbal UH, Kassem O, Le Barz M, Thomas V, Bronner S, Perrot T, Ismail N, Parker JA. Psychobiotics and the Microbiota–Gut–Brain Axis: Where Do We Go from Here? Microorganisms. 2024; 12(4):634. https://doi.org/10.3390/microorganisms12040634
Chicago/Turabian StyleBinda, Sylvie, Annie Tremblay, Umar Haris Iqbal, Ola Kassem, Mélanie Le Barz, Vincent Thomas, Stéphane Bronner, Tara Perrot, Nafissa Ismail, and J.Alex Parker. 2024. "Psychobiotics and the Microbiota–Gut–Brain Axis: Where Do We Go from Here?" Microorganisms 12, no. 4: 634. https://doi.org/10.3390/microorganisms12040634
APA StyleBinda, S., Tremblay, A., Iqbal, U. H., Kassem, O., Le Barz, M., Thomas, V., Bronner, S., Perrot, T., Ismail, N., & Parker, J. A. (2024). Psychobiotics and the Microbiota–Gut–Brain Axis: Where Do We Go from Here? Microorganisms, 12(4), 634. https://doi.org/10.3390/microorganisms12040634





