Gestational Diabetes and the Gut Microbiota: Fibre and Polyphenol Supplementation as a Therapeutic Strategy
Abstract
1. Introduction
2. The Gut Microbiome
3. The Gut Microbiome during Pregnancy
4. The Gut Microbiome during GDM
5. Targeting the Gut Microbiome to Decrease GDM Risk
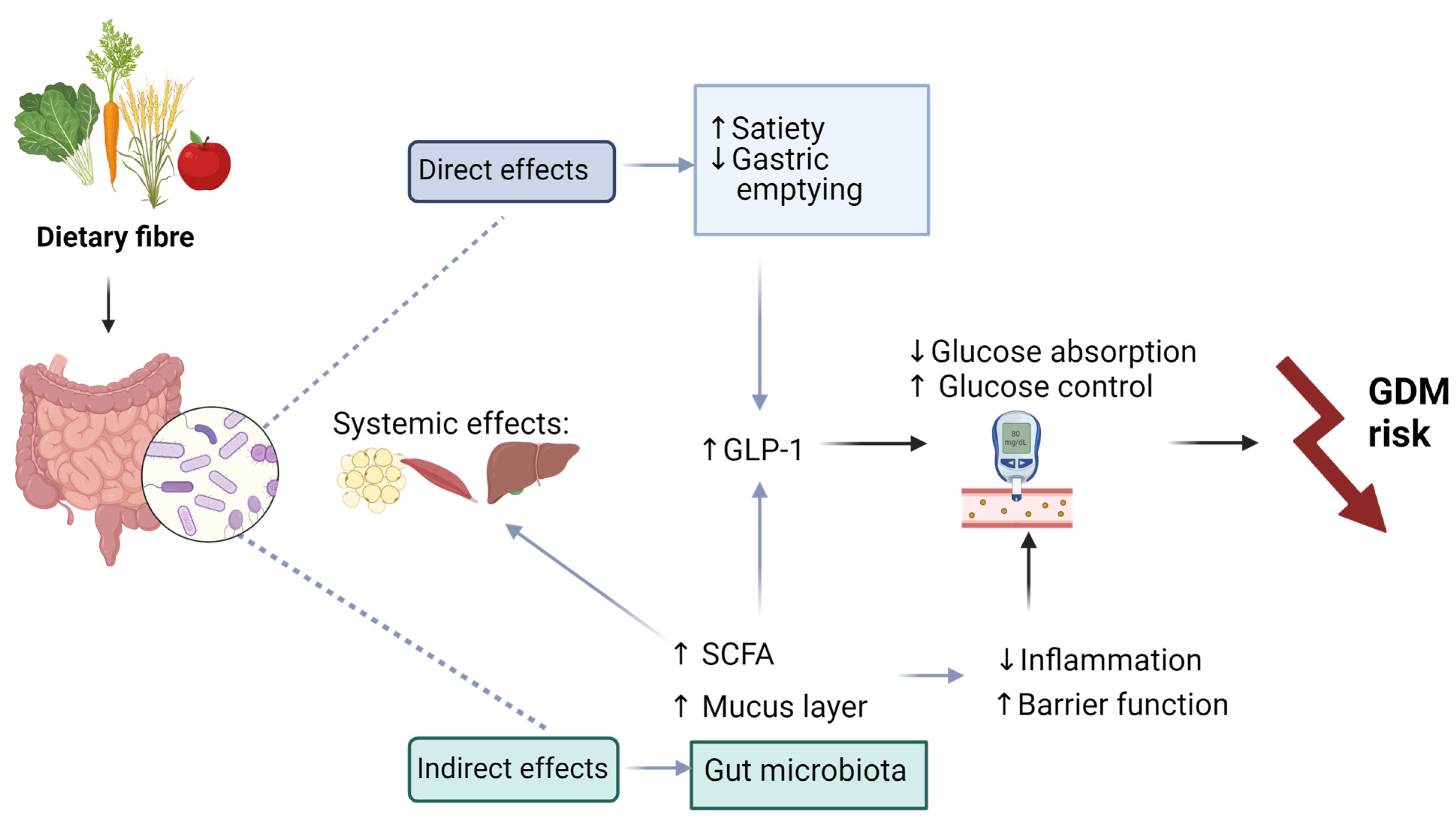
5.1. Fibre
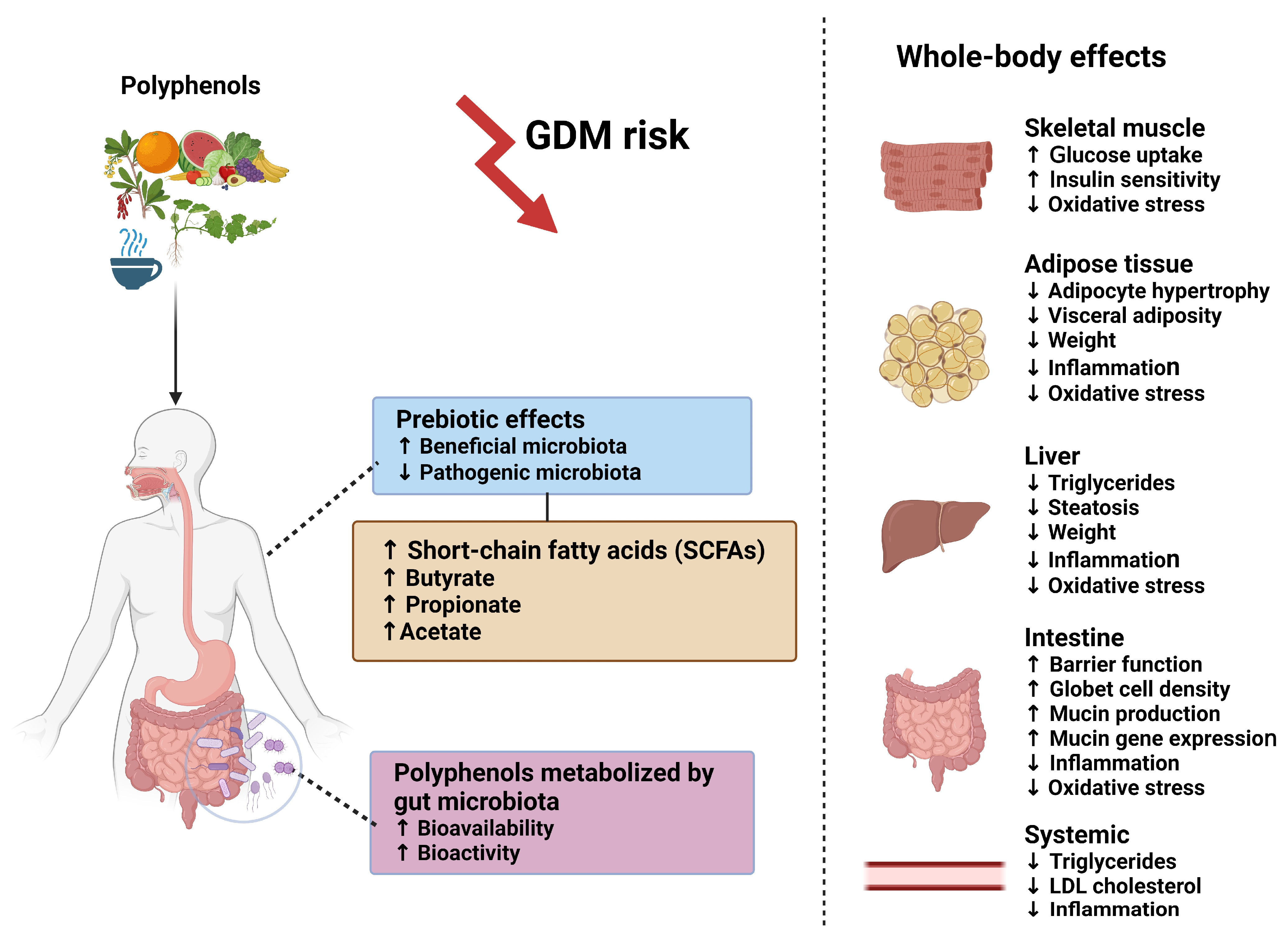
5.2. Polyphenols
6. Limitations
7. Future Perspectives
- Accumulating studies have highlighted potential links between the composition of the gut microbiome and the development of GDM; however, the existing evidence remains inconclusive [15,16,31,37]. Further research is warranted to delineate the gut microbiome profiles in both normal pregnancies and those complicated by GDM. These studies should be conducted in diverse geographical populations, ensuring an adequate sample size to account for lifestyle-associated factors such as diet, physical activity and antibiotic use. Additionally, it is crucial to consider technological factors such as sample processing and sequencing platform, as they are known to influence the identification of bacterial taxa in the GIT [42].
- As outlined in this review, additional investigation is necessary to explore the potential of dietary supplementation with fibre and polyphenols to shape the maternal microbiome as a nutritional intervention strategy in women with GDM. The response to food intake is influenced by genetic, epigenetic and microbial factors, underscoring the need for a patient-centred, personalized approach in the nutritional therapy of GDM [125]. RCTs to evaluate the effect of dietary modulation on shaping the gut microbiota during pregnancy and its potential to prevent or control GDM are required.
- The mother’s microbiota has the potential for vertical transmission to the offspring. Further investigations are essential to explore the influence of the maternal microbiome on foetal programming and understand how the infant microbiome might affect the physiology and long-term health of newborns [17,29,31].
- Microbiota in different locations, such as the gut, oral cavity and vagina, have been linked to GDM [38,128,129]. For example, a dysbiotic vaginal microbiome is associated with increased inflammatory cytokine expression, while increased periodontal bacteria in the oral microbiome is associated with GDM risk. Therefore, integrative studies across these body sites are required to provide a better understanding of microbial crosstalk during GDM.
- The maternal microbiome plays an important role in producing metabolites that impact both health and disease. Throughout pregnancy, the gut microbiota undergoes profound changes, resulting in an increase of pathogenic lactic acid-producing bacteria and a reduction in beneficial butyrate-producing bacteria [29]. Research to examine the relationship between metabolomics and microbial diversity is required.
- Dysbiosis in gut microbiota may serve as a potential indicator for the development of T2DM post-pregnancy [130], suggesting that modifying the gut microbiota through dietary interventions could improve diabetes-related outcomes. Subsequent research should investigate ways to ameliorate gut bacterial dysbiosis and assess the effectiveness of potential interventions, including supplementation with fibre and polyphenols, particularly among pregnant women.
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Dias, S.; Adam, S.; Rheeder, P.; Pheiffer, C. Prevalence of and Risk Factors for Gestational Diabetes Mellitus in South Africa. S. Afr. Med. J. 2019, 109, 463–467. [Google Scholar] [CrossRef]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- Chen, Y.; Quick, W.W.; Yang, W.; Zhang, Y.; Baldwin, A.; Moran, J.; Moore, V.; Sahai, N.; Dall, T.M. Cost of Gestational Diabetes Mellitus in the United States in 2007. Popul. Health Manag. 2009, 12, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Kolu, P.; Raitanen, J.; Rissanen, P.; Luoto, R. Health Care Costs Associated with Gestational Diabetes Mellitus among High-Risk Women—Results from a Randomised Trial. BMC Pregnancy Childbirth 2012, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Malaza, N.; Masete, M.; Adam, S.; Dias, S.; Nyawo, T.; Pheiffer, C. Systematic Review to Compare Adverse Pregnancy Outcomes in Women with Pregestational Diabetes and Gestational Diabetes. Int. J. Environ. Res. Public. Health 2022, 19, 10846. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D. Type 2 Diabetes Mellitus after Gestational Diabetes: A Systematic Review and Meta-Analysis. Lancet Lond. Engl. 2009, 373, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Harreiter, J.; Dovjak, G.; Kautzky-Willer, A. Gestational Diabetes Mellitus and Cardiovascular Risk after Pregnancy. Womens Health 2014, 10, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Mitanchez, D.; Yzydorczyk, C.; Siddeek, B.; Boubred, F.; Benahmed, M.; Simeoni, U. The Offspring of the Diabetic Mother--Short- and Long-Term Implications. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Farahvar, S.; Walfisch, A.; Sheiner, E. Gestational Diabetes Risk Factors and Long-Term Consequences for Both Mother and Offspring: A Literature Review. Expert Rev. Endocrinol. Metab. 2019, 14, 63–74. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee 15. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45, S232–S243. [Google Scholar] [CrossRef]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. Encinitas Calif. 2014, 13, 17–22. [Google Scholar]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Vijay, A.; Valdes, A.M. Role of the Gut Microbiome in Chronic Diseases: A Narrative Review. Eur. J. Clin. Nutr. 2022, 76, 489–501. [Google Scholar] [CrossRef]
- Hasain, Z.; Mokhtar, N.M.; Kamaruddin, N.A.; Mohamed Ismail, N.A.; Razalli, N.H.; Gnanou, J.V.; Raja Ali, R.A. Gut Microbiota and Gestational Diabetes Mellitus: A Review of Host-Gut Microbiota Interactions and Their Therapeutic Potential. Front. Cell. Infect. Microbiol. 2020, 10, 188. [Google Scholar] [CrossRef]
- Kunasegaran, T.; Balasubramaniam, V.R.M.T.; Arasoo, V.J.T.; Palanisamy, U.D.; Ramadas, A. The Modulation of Gut Microbiota Composition in the Pathophysiology of Gestational Diabetes Mellitus: A Systematic Review. Biology 2021, 10, 1027. [Google Scholar] [CrossRef] [PubMed]
- Neri, C.; Serafino, E.; Morlando, M.; Familiari, A. Microbiome and Gestational Diabetes: Interactions with Pregnancy Outcome and Long-Term Infant Health. J. Diabetes Res. 2021, 2021, 9994734. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Everard, A. Talking Microbes: When Gut Bacteria Interact with Diet and Host Organs. Mol. Nutr. Food Res. 2016, 60, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Suriano, F.; Nyström, E.E.L.; Sergi, D.; Gustafsson, J.K. Diet, Microbiota, and the Mucus Layer: The Guardians of Our Health. Front. Immunol. 2022, 13, 953196. [Google Scholar] [CrossRef] [PubMed]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Chen, Y. Polyphenol Supplementation Benefits Human Health via Gut Microbiota: A Systematic Review via Meta-Analysis. J. Funct. Foods 2020, 66, 103829. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef] [PubMed]
- May, K.S.; den Hartigh, L.J. Gut Microbial-Derived Short Chain Fatty Acids: Impact on Adipose Tissue Physiology. Nutrients 2023, 15, 272. [Google Scholar] [CrossRef]
- Yue, X.; Wen, S.; Long-Kun, D.; Man, Y.; Chang, S.; Min, Z.; Shuang-Yu, L.; Xin, Q.; Jie, M.; Liang, W. Three Important Short-Chain Fatty Acids (SCFAs) Attenuate the Inflammatory Response Induced by 5-FU and Maintain the Integrity of Intestinal Mucosal Tight Junction. BMC Immunol. 2022, 23, 19. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of Colonic Regulatory T Cells by Indigenous Clostridium Species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cai, X.; Fei, W.; Ye, Y.; Zhao, M.; Zheng, C. The Role of Short-Chain Fatty Acids in Immunity, Inflammation and Metabolism. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Di Simone, N.; Santamaria Ortiz, A.; Specchia, M.; Tersigni, C.; Villa, P.; Gasbarrini, A.; Scambia, G.; D’Ippolito, S. Recent Insights on the Maternal Microbiota: Impact on Pregnancy Outcomes. Front. Immunol. 2020, 11, 528202. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Ellberg, C.C.; Olomu, I.N.; Vyas, A.K. Gestational Microbiome: Metabolic Perturbations and Developmental Programming. Reprod. Camb. Engl. 2021, 162, R85–R98. [Google Scholar] [CrossRef] [PubMed]
- Turjeman, S.; Collado, M.C.; Koren, O. The Gut Microbiome in Pregnancy and Pregnancy Complications. Curr. Opin. Endocr. Metab. Res. 2021, 18, 133–138. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host Remodeling of the Gut Microbiome and Metabolic Changes during Pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]
- Nuriel-Ohayon, M.; Neuman, H.; Ziv, O.; Belogolovski, A.; Barsheshet, Y.; Bloch, N.; Uzan, A.; Lahav, R.; Peretz, A.; Frishman, S.; et al. Progesterone Increases Bifidobacterium Relative Abundance during Late Pregnancy. Cell Rep. 2019, 27, 730–736.e3. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Xiao, J.-Z.; Satoh, T.; Odamaki, T.; Takahashi, S.; Sugahara, H.; Yaeshima, T.; Iwatsuki, K.; Kamei, A.; Abe, K. Antiobesity Effects of Bifidobacterium Breve Strain B-3 Supplementation in a Mouse Model with High-Fat Diet-Induced Obesity. Biosci. Biotechnol. Biochem. 2010, 74, 1656–1661. [Google Scholar] [CrossRef]
- Dahl, C.; Stanislawski, M.; Iszatt, N.; Mandal, S.; Lozupone, C.; Clemente, J.C.; Knight, R.; Stigum, H.; Eggesbø, M. Gut Microbiome of Mothers Delivering Prematurely Shows Reduced Diversity and Lower Relative Abundance of Bifidobacterium and Streptococcus. PLoS ONE 2017, 12, e0184336. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Guo, R.; Li, S.; Liang, F.; Tian, C.; Zhao, X.; Long, Y.; Liu, F.; Jiang, M.; Zhang, Y.; et al. Systematic Analysis of Gut Microbiota in Pregnant Women and Its Correlations with Individual Heterogeneity. Npj Biofilms Microbiomes 2020, 6, 32. [Google Scholar] [CrossRef]
- Medici Dualib, P.; Ogassavara, J.; Mattar, R.; Mariko Koga da Silva, E.; Atala Dib, S.; de Almeida Pititto, B. Gut Microbiota and Gestational Diabetes Mellitus: A Systematic Review. Diabetes Res. Clin. Pract. 2021, 180, 109078. [Google Scholar] [CrossRef]
- Cortez, R.V.; Taddei, C.R.; Sparvoli, L.G.; Ângelo, A.G.S.; Padilha, M.; Mattar, R.; Daher, S. Microbiome and Its Relation to Gestational Diabetes. Endocrine 2019, 64, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Han, L.; Duan, T.; Lin, S.; Li, J.; Liu, X. Integrated Microbiome–Metabolome Analysis Reveals Novel Associations between Fecal Microbiota and Hyperglycemia-Related Changes of Plasma Metabolome in Gestational Diabetes Mellitus. RSC Adv. 2020, 10, 2027–2036. [Google Scholar] [CrossRef]
- Pinto, Y.; Frishman, S.; Turjeman, S.; Eshel, A.; Nuriel-Ohayon, M.; Shrossel, O.; Ziv, O.; Walters, W.; Parsonnet, J.; Ley, C.; et al. Gestational Diabetes Is Driven by Microbiota-Induced Inflammation Months before Diagnosis. Gut 2023, 72, 918–928. [Google Scholar] [CrossRef]
- Caricilli, A.M.; Saad, M.J.A. The Role of Gut Microbiota on Insulin Resistance. Nutrients 2013, 5, 829–851. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.H.J. Gut Bacteroides Species in Health and Disease. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef]
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermúdez-Humarán, L.G.; Smirnova, N.; Bergé, M.; Sulpice, T.; Lahtinen, S.; et al. Intestinal Mucosal Adherence and Translocation of Commensal Bacteria at the Early Onset of Type 2 Diabetes: Molecular Mechanisms and Probiotic Treatment. EMBO Mol. Med. 2011, 3, 559–572. [Google Scholar] [CrossRef]
- Riedel, S.; Pheiffer, C.; Johnson, R.; Louw, J.; Muller, C.J.F. Intestinal Barrier Function and Immune Homeostasis Are Missing Links in Obesity and Type 2 Diabetes Development. Front. Endocrinol. 2021, 12, 833544. [Google Scholar] [CrossRef]
- Liu, H.; Pan, L.-L.; Lv, S.; Yang, Q.; Zhang, H.; Chen, W.; Lv, Z.; Sun, J. Alterations of Gut Microbiota and Blood Lipidome in Gestational Diabetes Mellitus With Hyperlipidemia. Front. Physiol. 2019, 10, 1015. [Google Scholar] [CrossRef] [PubMed]
- Marette, A.; Jobin, C. SCFAs Take a Toll En Route to Metabolic Syndrome. Cell Metab. 2015, 22, 954–956. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kühl, C. Insulin Secretion and Insulin Resistance in Pregnancy and GDM. Implications for Diagnosis and Management. Diabetes 1991, 40 (Suppl. S2), 18–24. [Google Scholar] [CrossRef] [PubMed]
- Sonagra, A.D.; Biradar, S.M.; Murthy, D.S.J. Normal Pregnancy—A State of Insulin Resistance. J. Clin. Diagn. Res. JCDR 2014, 8, CC01–CC3. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.K.; Mukherjee, S. Evolving Interplay Between Dietary Polyphenols and Gut Microbiota—An Emerging Importance in Healthcare. Front. Nutr. 2021, 8, 634944. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, K.L.; Brennan, L.; McAuliffe, F.M. Acceptability of and Compliance with a Probiotic Capsule Intervention in Pregnancy. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2014, 125, 279–280. [Google Scholar] [CrossRef]
- Luoto, R.; Laitinen, K.; Nermes, M.; Isolauri, E. Impact of Maternal Probiotic-Supplemented Dietary Counselling on Pregnancy Outcome and Prenatal and Postnatal Growth: A Double-Blind, Placebo-Controlled Study. Br. J. Nutr. 2010, 103, 1792–1799. [Google Scholar] [CrossRef]
- Wickens, K.L.; Barthow, C.A.; Murphy, R.; Abels, P.R.; Maude, R.M.; Stone, P.R.; Mitchell, E.A.; Stanley, T.V.; Purdie, G.L.; Kang, J.M.; et al. Early Pregnancy Probiotic Supplementation with Lactobacillus Rhamnosus HN001 May Reduce the Prevalence of Gestational Diabetes Mellitus: A Randomised Controlled Trial. Br. J. Nutr. 2017, 117, 804–813. [Google Scholar] [CrossRef]
- Kamińska, K.; Stenclik, D.; Błażejewska, W.; Bogdański, P.; Moszak, M. Probiotics in the Prevention and Treatment of Gestational Diabetes Mellitus (GDM): A Review. Nutrients 2022, 14, 4303. [Google Scholar] [CrossRef]
- Yefet, E.; Bar, L.; Izhaki, I.; Iskander, R.; Massalha, M.; Younis, J.S.; Nachum, Z. Effects of Probiotics on Glycemic Control and Metabolic Parameters in Gestational Diabetes Mellitus: Systematic Review and Meta-Analysis. Nutrients 2023, 15, 1633. [Google Scholar] [CrossRef] [PubMed]
- Callaway, L.K.; McIntyre, H.D.; Barrett, H.L.; Foxcroft, K.; Tremellen, A.; Lingwood, B.E.; Tobin, J.M.; Wilkinson, S.; Kothari, A.; Morrison, M.; et al. Probiotics for the Prevention of Gestational Diabetes Mellitus in Overweight and Obese Women: Findings From the SPRING Double-Blind Randomized Controlled Trial. Diabetes Care 2019, 42, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Nachum, Z.; Perlitz, Y.; Shavit, L.Y.; Magril, G.; Vitner, D.; Zipori, Y.; Weiner, E.; Alon, A.S.; Ganor-Paz, Y.; Nezer, M.; et al. The Effect of Oral Probiotics on Glycemic Control of Women with Gestational Diabetes Mellitus-a Multicenter, Randomized, Double-Blind, Placebo-Controlled. Trial. Am. J. Obstet. Gynecol. MFM 2024, 6, 101224. [Google Scholar] [CrossRef]
- Davidson, S.J.; Barrett, H.L.; Price, S.A.; Callaway, L.K.; Dekker Nitert, M. Probiotics for Preventing Gestational Diabetes. Cochrane Database Syst. Rev. 2021, 4, CD009951. [Google Scholar] [CrossRef] [PubMed]
- Mokkala, K.; Röytiö, H.; Munukka, E.; Pietilä, S.; Ekblad, U.; Rönnemaa, T.; Eerola, E.; Laiho, A.; Laitinen, K. Gut Microbiota Richness and Composition and Dietary Intake of Overweight Pregnant Women Are Related to Serum Zonulin Concentration, a Marker for Intestinal Permeability. J. Nutr. 2016, 146, 1694–1700. [Google Scholar] [CrossRef]
- Röytiö, H.; Mokkala, K.; Vahlberg, T.; Laitinen, K. Dietary Intake of Fat and Fibre According to Reference Values Relates to Higher Gut Microbiota Richness in Overweight Pregnant Women—CORRIGENDUM. Br. J. Nutr. 2018, 120, 599–600. [Google Scholar] [CrossRef]
- Salinas-Roca, B.; Rubió-Piqué, L.; Montull-López, A. Polyphenol Intake in Pregnant Women on Gestational Diabetes Risk and Neurodevelopmental Disorders in Offspring: A Systematic Review. Nutrients 2022, 14, 3753. [Google Scholar] [CrossRef]
- Viana, L.V.; Gross, J.L.; Azevedo, M.J. Dietary Intervention in Patients with Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Clinical Trials on Maternal and Newborn Outcomes. Diabetes Care 2014, 37, 3345–3355. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary Fibre in Gastrointestinal Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary Fibre and Whole Grains in Diabetes Management: Systematic Review and Meta-Analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Deehan, E.C.; Duar, R.M.; Armet, A.M.; Perez-Muñoz, M.E.; Jin, M.; Walter, J. Modulation of the Gastrointestinal Microbiome with Nondigestible Fermentable Carbohydrates To Improve Human Health. Microbiol. Spectr. 2017, 5, 1110–1128. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.A.; Mikkelsen, D.; Flanagan, B.M.; Gidley, M.J. “Dietary Fibre”: Moving beyond the “Soluble/Insoluble” Classification for Monogastric Nutrition, with an Emphasis on Humans and Pigs. J. Anim. Sci. Biotechnol. 2019, 10, 45. [Google Scholar] [CrossRef]
- Capuano, E. The Behavior of Dietary Fiber in the Gastrointestinal Tract Determines Its Physiological Effect. Crit. Rev. Food Sci. Nutr. 2017, 57, 3543–3564. [Google Scholar] [CrossRef]
- Gasaly, N.; de Vos, P.; Hermoso, M.A. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef]
- Song, X.; Zhong, L.; Lyu, N.; Liu, F.; Li, B.; Hao, Y.; Xue, Y.; Li, J.; Feng, Y.; Ma, Y.; et al. Inulin Can Alleviate Metabolism Disorders in Ob/Ob Mice by Partially Restoring Leptin-Related Pathways Mediated by Gut Microbiota. Genom. Proteom. Bioinform. 2019, 17, 64–75. [Google Scholar] [CrossRef]
- Reece, E.A.; Hagay, Z.; Caseria, D.; Gay, L.J.; DeGennaro, N. Do Fiber-Enriched Diabetic Diets Have Glucose-Lowering Effects in Pregnancy? Am. J. Perinatol. 1993, 10, 272–274. [Google Scholar] [CrossRef]
- Barati, Z.; Iravani, M.; Karandish, M.; Haghighizadeh, M.H.; Masihi, S. The Effect of Oat Bran Consumption on Gestational Diabetes: A Randomized Controlled Clinical Trial. BMC Endocr. Disord. 2021, 21, 67. [Google Scholar] [CrossRef]
- Basu, A.; Feng, D.; Planinic, P.; Ebersole, J.L.; Lyons, T.J.; Alexander, J.M. Dietary Blueberry and Soluble Fiber Supplementation Reduces Risk of Gestational Diabetes in Women with Obesity in a Randomized Controlled Trial. J. Nutr. 2021, 151, 1128–1138. [Google Scholar] [CrossRef]
- Basu, A.; Crew, J.; Ebersole, J.L.; Kinney, J.W.; Salazar, A.M.; Planinic, P.; Alexander, J.M. Dietary Blueberry and Soluble Fiber Improve Serum Antioxidant and Adipokine Biomarkers and Lipid Peroxidation in Pregnant Women with Obesity and at Risk for Gestational Diabetes. Antioxidants 2021, 10, 1318. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-K.; Cheng, D.-C.; Yang, Y.-M.; Wang, X.-H.; Chen, Y.; Zhang, L.; Xiu, L.; Xu, X.-M. The Role of High-Content Complex Dietary Fiber in Medical Nutrition Therapy for Gestational Diabetes Mellitus. Front. Pharmacol. 2021, 12, 684898. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, J.; Ma, W.; Miao, M.; Sun, G. Effects of Additional Dietary Fiber Supplements on Pregnant Women with Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Studies. Nutrients 2022, 14, 4626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-Y.; Cheng, D.-C.; Cao, Y.-N.; Su, Y.; Chen, L.; Liu, W.-Y.; Yu, Y.-X.; Xu, X.-M. The Effect of Dietary Fiber Supplement on Prevention of Gestational Diabetes Mellitus in Women with Pre-Pregnancy Overweight/Obesity: A Randomized Controlled Trial. Front. Pharmacol. 2022, 13, 922015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, S.; Solomon, C.G.; Hu, F.B. Dietary Fiber Intake, Dietary Glycemic Load, and the Risk for Gestational Diabetes Mellitus. Diabetes Care 2006, 29, 2223–2230. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, Y.; Della Corte, K.; Yu, D.; Xue, H.; Shan, S.; Tian, G.; Liang, Y.; Zhang, J.; He, F.; et al. Relevance of Dietary Glycemic Index, Glycemic Load and Fiber Intake before and during Pregnancy for the Risk of Gestational Diabetes Mellitus and Maternal Glucose Homeostasis. Clin. Nutr. Edinb. Scotl. 2021, 40, 2791–2799. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Tao, Y.; Zhang, Y.; Zhang, X.; Xue, C.; Liu, Y. Dietary Fiber Intake, Dietary Glycemic Load, and the Risk of Gestational Diabetes Mellitus during the Second Trimester: A Nested Case-Control Study. Asia Pac. J. Clin. Nutr. 2021, 30, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Pajunen, L.; Korkalo, L.; Koivuniemi, E.; Houttu, N.; Pellonperä, O.; Mokkala, K.; Shivappa, N.; Hébert, J.R.; Vahlberg, T.; Tertti, K.; et al. A Healthy Dietary Pattern with a Low Inflammatory Potential Reduces the Risk of Gestational Diabetes Mellitus. Eur. J. Nutr. 2022, 61, 1477–1490. [Google Scholar] [CrossRef]
- Wan, J.; An, L.; Ren, Z.; Wang, S.; Yang, H.; Ma, J. Effects of Galactooligosaccharides on Maternal Gut Microbiota, Glucose Metabolism, Lipid Metabolism and Inflammation in Pregnancy: A Randomized Controlled Pilot Study. Front. Endocrinol. 2023, 14, 1034266. [Google Scholar] [CrossRef]
- Hernandez, T.L.; Van Pelt, R.E.; Anderson, M.A.; Daniels, L.J.; West, N.A.; Donahoo, W.T.; Friedman, J.E.; Barbour, L.A. A Higher-Complex Carbohydrate Diet in Gestational Diabetes Mellitus Achieves Glucose Targets and Lowers Postprandial Lipids: A Randomized Crossover Study. Diabetes Care 2014, 37, 1254–1262. [Google Scholar] [CrossRef]
- Tsitsou, S.; Athanasaki, C.; Dimitriadis, G.; Papakonstantinou, E. Acute Effects of Dietary Fiber in Starchy Foods on Glycemic and Insulinemic Responses: A Systematic Review of Randomized Controlled Crossover Trials. Nutrients 2023, 15, 2383. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Hu, T.; Xu, C.; Xie, N.; Chen, D. Interventional Effect of Dietary Fiber on Blood Glucose and Pregnancy Outcomes in Patients with Gestational Diabetes Mellitus. Zhejiang Xue Xue Bao Yi Xue Ban J. Zhejiang Univ. Med. Sci. 2021, 50, 305–312. [Google Scholar] [CrossRef]
- Hernandez, T.L.; Mande, A.; Barbour, L.A. Nutrition Therapy within and beyond Gestational Diabetes. Diabetes Res. Clin. Pract. 2018, 145, 39–50. [Google Scholar] [CrossRef]
- Paone, P.; Suriano, F.; Jian, C.; Korpela, K.; Delzenne, N.M.; Van Hul, M.; Salonen, A.; Cani, P.D. Prebiotic Oligofructose Protects against High-Fat Diet-Induced Obesity by Changing the Gut Microbiota, Intestinal Mucus Production, Glycosylation and Secretion. Gut Microbes 2022, 14, 2152307. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Chassaing, B.; Singh, V.; Pellizzon, M.; Ricci, M.; Fythe, M.D.; Kumar, M.V.; Gewirtz, A.T. Fiber-Mediated Nourishment of Gut Microbiota Protects against Diet-Induced Obesity by Restoring IL-22-Mediated Colonic Health. Cell Host Microbe 2018, 23, 41–53.e4. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O.; Birchenough, G.M.H.; Ståhlman, M.; Arike, L.; Johansson, M.E.V.; Hansson, G.C.; Bäckhed, F. Bifidobacteria or Fiber Protects against Diet-Induced Microbiota-Mediated Colonic Mucus Deterioration. Cell Host Microbe 2018, 23, 27–40.e7. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.M.; da Silva, N.B.M.; de Freitas, R.M.P.; de Freitas, M.B.D.; Chaves, J.B.P.; Oliveira, L.L.; Martino, H.S.D.; de Cássia Gonçalves Alfenas, R. Effects of Yacon Flour Associated with an Energy Restricted Diet on Intestinal Permeability, Fecal Short Chain Fatty Acids, Oxidative Stress and Inflammation Markers Levels in Adults with Obesity or Overweight: A Randomized, Double Blind, Placebo Controlled Clinical Trial. Arch. Endocrinol. Metab. 2021, 64, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Deehan, E.C.; Zhang, Z.; Riva, A.; Armet, A.M.; Perez-Muñoz, M.E.; Nguyen, N.K.; Krysa, J.A.; Seethaler, B.; Zhao, Y.-Y.; Cole, J.; et al. Elucidating the Role of the Gut Microbiota in the Physiological Effects of Dietary Fiber. Microbiome 2022, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, A.; Sandhu, A.K.; Edirisinghe, I.; Burton-Freeman, B.M. Red Raspberry and Fructo-Oligosaccharide Supplementation, Metabolic Biomarkers, and the Gut Microbiota in Adults with Prediabetes: A Randomized Crossover Clinical Trial. J. Nutr. 2022, 152, 1438–1449. [Google Scholar] [CrossRef]
- Roy, R.; Nguyen-Ngo, C.; Lappas, M. Short-Chain Fatty Acids as Novel Therapeutics for Gestational Diabetes. J. Mol. Endocrinol. 2020, 65, 21–34. [Google Scholar] [CrossRef]
- Kim, Y.A.; Keogh, J.B.; Clifton, P.M. Probiotics, Prebiotics, Synbiotics and Insulin Sensitivity. Nutr. Res. Rev. 2018, 31, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arango, L.F.; Barrett, H.L.; Wilkinson, S.A.; Callaway, L.K.; McIntyre, H.D.; Morrison, M.; Dekker Nitert, M. Low Dietary Fiber Intake Increases Collinsella Abundance in the Gut Microbiota of Overweight and Obese Pregnant Women. Gut Microbes 2018, 9, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Ferrocino, I.; Ponzo, V.; Gambino, R.; Zarovska, A.; Leone, F.; Monzeglio, C.; Goitre, I.; Rosato, R.; Romano, A.; Grassi, G.; et al. Changes in the Gut Microbiota Composition during Pregnancy in Patients with Gestational Diabetes Mellitus (GDM). Sci. Rep. 2018, 8, 12216. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Wang, Q.; Wang, X.; Fan, C.; Luan, T.; Yan, L.; Zhang, Y.; Zeng, X.; Dai, Y.; Li, P. The Protective Effects of Inulin-Type Fructans against High-Fat/Sucrose Diet-Induced Gestational Diabetes Mice in Association with Gut Microbiota Regulation. Front. Microbiol. 2022, 13, 832151. [Google Scholar] [CrossRef] [PubMed]
- Sugino, K.Y.; Hernandez, T.L.; Barbour, L.A.; Kofonow, J.M.; Frank, D.N.; Friedman, J.E. A Maternal Higher-Complex Carbohydrate Diet Increases Bifidobacteria and Alters Early Life Acquisition of the Infant Microbiome in Women with Gestational Diabetes Mellitus. Front. Endocrinol. 2022, 13, 921464. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Guo, X.; Zhou, Y.; Cao, G. The Effects of Probiotics/Synbiotics on Glucose and Lipid Metabolism in Women with Gestational Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2023, 15, 1375. [Google Scholar] [CrossRef] [PubMed]
- Tomsett, K.I.; Barrett, H.L.; Dekker, E.E.; Callaway, L.K.; McIntyre, D.H.; Dekker Nitert, M. Dietary Fiber Intake Alters Gut Microbiota Composition but Does Not Improve Gut Wall Barrier Function in Women with Future Hypertensive Disorders of Pregnancy. Nutrients 2020, 12, 3862. [Google Scholar] [CrossRef]
- Shabalala, S.; Muller, C.J.F.; Louw, J.; Johnson, R. Polyphenols, Autophagy and Doxorubicin-Induced Cardiotoxicity. Life Sci. 2017, 180, 160–170. [Google Scholar] [CrossRef]
- Johnson, R.; de Beer, D.; Dludla, P.V.; Ferreira, D.; Muller, C.J.F.; Joubert, E. Aspalathin from Rooibos (Aspalathus linearis): A Bioactive C-Glucosyl Dihydrochalcone with Potential to Target the Metabolic Syndrome. Planta Med. 2018, 84, 568–583. [Google Scholar] [CrossRef]
- Jack, B.U.; Malherbe, C.J.; Mamushi, M.; Muller, C.J.F.; Joubert, E.; Louw, J.; Pheiffer, C. Adipose Tissue as a Possible Therapeutic Target for Polyphenols: A Case for Cyclopia Extracts as Anti-Obesity Nutraceuticals. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 120, 109439. [Google Scholar] [CrossRef]
- Samodien, E.; Johnson, R.; Pheiffer, C.; Mabasa, L.; Erasmus, M.; Louw, J.; Chellan, N. Diet-Induced Hypothalamic Dysfunction and Metabolic Disease, and the Therapeutic Potential of Polyphenols. Mol. Metab. 2019, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.M.; Do, V.V.; Lee, A.H. Polyphenol-Rich Foods and Risk of Gestational Diabetes: A Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2019, 73, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Jorquera, G.; Fornes, R.; Cruz, G.; Thomas-Valdés, S. Association of Polyphenols Consumption with Risk for Gestational Diabetes Mellitus and Preeclampsia: A Systematic Review and Meta-Analysis. Antioxidants 2022, 11, 2294. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A Polyphenol-Rich Cranberry Extract Protects from Diet-Induced Obesity, Insulin Resistance and Intestinal Inflammation in Association with Increased Akkermansia spp. Population in the Gut Microbiota of Mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia Muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Henning, S.M.; Lee, R.-P.; Lu, Q.-Y.; Summanen, P.H.; Thames, G.; Corbett, K.; Downes, J.; Tseng, C.-H.; Finegold, S.M.; et al. Pomegranate Extract Induces Ellagitannin Metabolite Formation and Changes Stool Microbiota in Healthy Volunteers. Food Funct. 2015, 6, 2487–2495. [Google Scholar] [CrossRef]
- Song, H.; Chu, Q.; Yan, F.; Yang, Y.; Han, W.; Zheng, X. Red Pitaya Betacyanins Protects from Diet-Induced Obesity, Liver Steatosis and Insulin Resistance in Association with Modulation of Gut Microbiota in Mice. J. Gastroenterol. Hepatol. 2016, 31, 1462–1469. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, X.; Cao, S.; Wang, L.; Wang, D.; Yang, H.; Feng, Y.; Wang, S.; Li, L. Caffeic Acid Ameliorates Colitis in Association with Increased Akkermansia Population in the Gut Microbiota of Mice. Oncotarget 2016, 7, 31790–31799. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Etxeberria, U.; Taminiau, B.; Daube, G.; Van Hul, M.; Everard, A.; Cani, P.D.; Bindels, L.B.; Delzenne, N.M. Rhubarb Extract Prevents Hepatic Inflammation Induced by Acute Alcohol Intake, an Effect Related to the Modulation of the Gut Microbiota. Mol. Nutr. Food Res. 2017, 61, 1500899. [Google Scholar] [CrossRef]
- Heyman-Lindén, L.; Kotowska, D.; Sand, E.; Bjursell, M.; Plaza, M.; Turner, C.; Holm, C.; Fåk, F.; Berger, K. Lingonberries Alter the Gut Microbiota and Prevent Low-Grade Inflammation in High-Fat Diet Fed Mice. Food Nutr. Res. 2016, 60, 29993. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Interactions of Gut Microbiota with Dietary Polyphenols and Consequences to Human Health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef]
- Power, K.A.; Lepp, D.; Zarepoor, L.; Monk, J.M.; Wu, W.; Tsao, R.; Liu, R. Dietary Flaxseed Modulates the Colonic Microenvironment in Healthy C57Bl/6 Male Mice which May Alter Susceptibility to Gut-Associated Diseases. J. Nutr. Biochem. 2016, 28, 61–69. [Google Scholar] [CrossRef]
- Yuan, X.; Long, Y.; Ji, Z.; Gao, J.; Fu, T.; Yan, M.; Zhang, L.; Su, H.; Zhang, W.; Wen, X.; et al. Green Tea Liquid Consumption Alters the Human Intestinal and Oral Microbiome. Mol. Nutr. Food Res. 2018, 62, 1800178. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.C.D.; Cecatti, C.; Fidélix, M.P.; Adorno, M.A.T.; Sakamoto, I.K.; Cesar, T.B.; Sivieri, K. Effect of Daily Consumption of Orange Juice on the Levels of Blood Glucose, Lipids, and Gut Microbiota Metabolites: Controlled Clinical Trials. J. Med. Food 2019, 22, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, Q.; Ma, W.; Tian, F.; Shen, H.; Zhou, M. A Combination of Quercetin and Resveratrol Reduces Obesity in High-Fat Diet-Fed Rats by Modulation of Gut Microbiota. Food Funct. 2017, 8, 4644–4656. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhu, H.; Liu, J.; Kwek, E.; Ma, K.Y.; Chen, Z.-Y. Mangiferin Alleviates Trimethylamine-N-Oxide (TMAO)-Induced Atherogenesis and Modulates Gut Microbiota in Mice. Food Funct. 2023, 14, 9212–9225. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary Polyphenol Impact on Gut Health and Microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef] [PubMed]
- Lippolis, T.; Cofano, M.; Caponio, G.R.; De Nunzio, V.; Notarnicola, M. Bioaccessibility and Bioavailability of Diet Polyphenols and Their Modulation of Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 3813. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.; Hughes, R.E. Quercetin, Flavonoids and the Life-Span of Mice. Exp. Gerontol. 1982, 17, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Are Polyphenols Antioxidants or Pro-Oxidants? What Do We Learn from Cell Culture and in Vivo Studies? Highlight Issue Polyphen. Health 2008, 476, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Nacka-Aleksić, M.; Pirković, A.; Vilotić, A.; Bojić-Trbojević, Ž.; Jovanović Krivokuća, M.; Giampieri, F.; Battino, M.; Dekanski, D. The Role of Dietary Polyphenols in Pregnancy and Pregnancy-Related Disorders. Nutrients 2022, 14, 5246. [Google Scholar] [CrossRef]
- Bashiardes, S.; Godneva, A.; Elinav, E.; Segal, E. Towards Utilization of the Human Genome and Microbiome for Personalized Nutrition. Curr. Opin. Biotechnol. 2018, 51, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Shenhav, L.; Furman, O.; Briscoe, L.; Thompson, M.; Silverman, J.D.; Mizrahi, I.; Halperin, E. Modeling the Temporal Dynamics of the Gut Microbial Community in Adults and Infants. PLOS Comput. Biol. 2019, 15, e1006960. [Google Scholar] [CrossRef]
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From Theory to Practice. Foods 2021, 10, 2595. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, J.; Shi, W.; Du, N.; Xu, X.; Zhang, Y.; Ji, P.; Zhang, F.; Jia, Z.; Wang, Y.; et al. Dysbiosis of Maternal and Neonatal Microbiota Associated with Gestational Diabetes Mellitus. Gut 2018, 67, 1614. [Google Scholar] [CrossRef]
- Dias, S.; Pheiffer, C.; Adam, S. The Maternal Microbiome and Gestational Diabetes Mellitus: Cause and Effect. Microorganisms 2023, 11, 2217. [Google Scholar] [CrossRef]
- Kuang, Y.-S.; Lu, J.-H.; Li, S.-H.; Li, J.-H.; Yuan, M.-Y.; He, J.-R.; Chen, N.-N.; Xiao, W.-Q.; Shen, S.-Y.; Qiu, L.; et al. Connections between the Human Gut Microbiome and Gestational Diabetes Mellitus. GigaScience 2017, 6, 1–12. [Google Scholar] [CrossRef]

| Reference | Fibre | Study Design | Effect on Gut Microbiota | Study Findings |
|---|---|---|---|---|
| Schroeder et al., 2018 [89] | Inulin | Male C57BL/J6 mice were fed a western style diet (WSD) and 1% inulin or Bifidobacterium longum or a combination of inulin and B. longum for 4 weeks. | Effects on microbiota composition were not measured. | None of the treatments affected metabolic parameters, however, both treatments improved mucus function in WDS-fed mice. Inulin treatment prevented the penetrability of the intestinal mucus layer and B. longum treatment restored mucus growth. |
| Zou et al., 2018 [88] | Inulin | Male C57BL/6 mice were fed a high fat diet (HFD) containing 20% cellulose (non-digestible fibre control) or HFD containing 20% inulin fibre (soluble fibre) for 4 weeks. Gut microbiota was assessed by 16S rRNA sequencing of fecal samples. | Inulin treatment increased gut bacterial load and the abundance of Bifidobacteriaceae. Inulin reduced the Firmicutes/Bacteroidetes ratio and the abundance of Proteobacteria, Streptococcus, Clostridium, and Enterococcaceae. | Inulin reduced body weight gain, dysglycemia, hepatic steatosis and adiposity (adipocyte size). Treatment had no effect on serum triglycerides but decreased cholesterol levels were reported. |
| Song et al., 2019 [70] | Inulin | Male C57BL/6J mice and ob/ob mice were fed a chow diet supplemented with inulin (10 g/kg body weight/day) in the drinking water for 4 weeks. Gut microbiota was assessed by 16S rRNA sequencing of cecal samples. | Inulin supplementation reduced α-diversity and decreased the Firmicutes/Bacteroidetes ratio. Inulin increased the abundance of Prevotellaceae UCG 001, Oscillibacter, Lachnospiraceae UCG 006, Lachnospiraceae UCG 008, Enterobacter, and Parvibacter. | Inulin supplementation reduced food intake and total cholesterol and improved glucose tolerance and liver steatosis. Improvement in metabolic parameters were associated with increased production of SCFAs by Prevotellaceae UCG 001. |
| Miao et al., 2022 [97] | Inulin-type fructan (ITF) | Female C57BL/6J mice were fed a high fat/high sucrose (HFHS) diet for 4 weeks prior to pregnancy and for 18 days during pregnancy. Mice received 3.33 g/kg bodyweight ITF per day by oral gavage for the duration of HFHS feeding. Gut microbiota was assessed by 16S rRNA sequencing of fecal samples. | ITF increased α-diversity and fecal SCFAs (butyrate and acetate) production. ITF further increased the abundance of Verrucomicrobia, Bifidobacterium and Akkermansia, while levels of Dubosiella were reduced. | ITF treatment reduced body weight gain and improved glucose tolerance and lipid metabolism (decreased triglycerides, total and low density lipoprotein (LDL) cholesterol) in HFHS-fed mice, which was asscoaited with increased SCFA modulating gut microbiota. |
| Paone et al., 2022 [87] | Fructo-oligosaccharides (FOS) | Male C57BL/6 J mice were fed a HFD with 10% FOS in the drinking water for 6 weeks. Gut microbiota was assessed by 16S rRNA sequencing of fecal samples. | FOS treatment increased the abundance of Odoribacter, Akkermansia, Muribaculaceae and Ruminococcaceae. | FOS treatment reduced body weight and improved glucose tolerance in HFD-fed mice. FOS increased plasma glucagon-like peptide 1 (GLP-1) levels and the number of intestinal goblet cells producing mucins. The genera increased by FOS treatment correlated negatively with glucose tolerance but were positively associated with mucus layer/mucus production. |
| Röytiö et al., 2017 [60] | No intervention | 100 women with overweight/obesity were enrolled at <17 weeks of gestation. Three-day food records were collected before the study. Gut microbiota was assessed by 16S rRNA sequencing of fecal samples. | The recommended fibre and fat intake correlated with increased microbiota richness and α-diversity. | Microbiota diversity and richness inversely correlated with inflammatory markers. |
| Ferrocino et al., 2018 [96] | Standard nutritional recommendations | 41 patients with GDM were enrolled between 24 and 28 weeks of gestation. A dietary questionnaire was conducted. Gut microbiota was assessed by 16S rRNA sequencing of fecal samples. | α-diversity significantly increased in women with GDM. The abundance of Firmicutes increased and abundance of Bacteroidetes and Actinobacteria decreased. | Adherence to nutritional recommendations decreased the abundance of Bacteroides. Faecalibacterium correlated with fasting glucose concentrations. Collinsella was positively and Blautia inversely correlated with insulin and HOMA-IR. Sutterella was associated with inflammatory marker C-reactive protein. |
| Gomez-Arango et al., 2018 [95] | No intervention | 57 women with overweight and 73 women with obesity were enrolled at 16 weeks of gestation. A dietary questionnaire was used to assess macronutrient intake in these women. Gut microbiota was assessed by 16S rRNA sequencing of fecal samples. | Low fibre intake correlated with increased levels of Collinsella and lactate producing bacteria. High fibre intake was associated with SCFA producing bacteria. | Low fibre intake may allow overgrowth of Collinsella, which was correlated with increased insulin levels. |
| Sugino et al., 2022 [98] | Meals with different types (same amount) of fibre provided | 34 women with GDM were randomised to either CHOICE diet (60% complex carbohydrates, 25% fat) or conventional diet (40% complex carbohydrates, 45% fat) from 30 weeks of gestation. Gut microbiota was assessed by shotgun metagenomic sequencing of fecal samples. | CHOICE diet increased the abundance of Bifidobacteriaceae (B. adolescentis) in women with GDM. | Maternal glucose levels did not differ between the treatment groups. The CHOICE diet increased infant α-diversity over time. |
| Wan et al., 2023 [82] | Galacto-oligosaccharides (GOS), 60 g per day | 52 women were enrolled at 6–8 weeks of gestation with follow up at 11–13 weeks and 24–28 weeks. Women received 60 g of GOS daily. Gut microbiota was assessed by 16S rRNA sequencing of fecal samples. | GOS increased the abundance of Paraprevotella and Dorea spp. and decreased the abundance of LachnospiraceaeUCG_001. | No differences in GDM incidence, fasting plasma glucose, lipids, interleukin 6 (IL-6) and neonatal outcomes were observed. |
| Reference | Polyphenol | Study Design | Effect on Gut Microbiota | Study Findings |
|---|---|---|---|---|
| Anhé et al., 2015 [107] | Cranberry extract | High-fat/high-sucrose (HFHS)-fed male C57BL/6J mice were treated with 200 mg/kg of cranberry extract daily for 8 weeks. Gut microbiota was assessed by 16S rRNA sequencing of fecal samples. | The cranberry extract increased levels of the mucin-degrading bacterium Akkermansia. | The cranberry extract protects against diet-induced obesity (decreased weight gain, visceral obesity, liver weight and triglyceride accumulation), insulin resistance and intestinal inflammation in association with increased Akkermansia spp. in the gut microbiota of HFHS-fed mice. |
| Roopchand et al., 2015 [108] | Grape extract | Male C57BL/6J mice were fed a high-fat diet (HFD) containing 1% Concord grape polyphenols for 13 weeks. Gut microbiota was assessed by 16S rRNA sequencing of cecal and fecal samples. | The grape extract increased levels of Akkermansia muciniphila and decreased the ratio of Firmicutes to Bacteroidetes. | The grape extract improved metabolic outcomes (weight gain and adiposity) and lowered intestinal and systemic inflammation in association with increased Akkermansia spp. in the gut microbiota of HFD-fed mice. |
| Heyman-Lindén et al., 2016 [113] | Lingonberries extract | Male C57BL/6J were fed HFD diet with 20% lingonberries for 11 weeks. Gut microbiota was assessed by 16S rRNA sequencing of cecal samples. | The lingonberries extract increased the abundance of Akkermansia and Faecalibacterium. | The lingonberries extract was able to prevent diet-induced low-grade inflammation, which was associated with an increase in Akkermansia and Faecalibacterium. |
| Power et al., 2016 [115] | Flaxseed | Male C57BL/6 male mice were fed a maintenance diet supplemented with 10% flaxseed for 3 weeks. Gut microbiota was assessed by 16S rRNA sequencing of fecal samples. | The flaxseed extract increased Prevotella spp. and reduced Akkermansia muciniphila abundance. | The flaxseed extract exhibited beneficial responses contributing to an enhanced mucus barrier (increased goblet cell density, mucin production, and mucin gene expression), which was associated with a 20-fold increase in Prevotella spp. and a 30-fold reduction in Akkermansia muciniphila abundance. |
| Song et al., 2016 [110] | Red pitaya fruit extract | Male C57BL/6J mice were fed a HFD containing 200 mg/kg red pitaya extract for 14 weeks. Gut microbiota was assessed by 16S rRNA sequencing of fecal samples. | The red pitaya fruit extract decreased Firmicutes and increased Bacteroidetes and Akkermansia. | The red pitaya extract protects against diet-induced obesity and its related metabolic disorders (reduced weight gain, visceral adiposity, improved hepatic steatosis, adipose hypertrophy, insulin resistance and inflammatory status) by decreasing the ratio of Firmicutes and Bacteroidetes and increasing Akkermansia in the gut microflora. |
| Zhang et al., 2016 [111] | Caffeic acid | Female C57BL/6 mice with colitis were fed a diet with 1 mM caffeic acid. Gut microbiota was assessed by 16S rRNA sequencing of fecal samples. | Caffeic acid decreased Firmicutes and increased Bacteroidetes and the mucin-degrading bacterium Akkermansia. | The caffeic acid exerted anti-inflammatory effects which was associated with a decrease in the Firmicutes/Bacteroidetes ratio and an increase in Akkermansia in mice with colitis. |
| Neyrinck et al., 2017 [112] | Rhubarb extract | Male C57BL/6J mice were fed a control diet supplemented with 0.3% Rhubarb extract for 17 days and thereafter challenged with 30% w/v, 6 g/kg body weight alcohol. Gut microbiota was assessed by 16S rRNA sequencing of cecal samples. | The rhubarb extract increased Akkermansia muciniphila and Parabacteroides goldsteinii. | The rhubarb extract improved alcohol-induced hepatic injury and downregulated markers of inflammation and oxidative stress in the liver, which was associated with increased Akkermansia muciniphila and Parabacteroides goldsteinii. |
| Zhao et al., 2017 [118] | Resveratrol and quercetin | Male Wistar rats were fed a HFD diet with a combination of quercetin (30 mg/kg body weight) and resveratrol (15 mg/kg body weight) daily for 10 weeks. Gut microbiota was assessed by 16S rRNA sequencing of fecal samples. | Resveratrol and quercetin decreased Firmicutes and increased Bacteroidales, Christensenellaceae, Akkermansia and Ruminococcaceae. Levels of Desulfovibrionaceae, Acidaminococcaceae, Coriobacteriaceae, Bilophila and Lachnospiraceae were decreased. | Resveratrol and quercetin reduced HFD-induced weight gain, visceral adiposity, serum lipids and inflammatory markers, which was associated with microbiome modulation. |
| He et al., 2023 [119] | Mangiferin | Female ApoE−/− mice were fed a high-choline diet plus 0.5% mangiferin for 15 weeks. Gut microbiota was assessed by 16S rRNA sequencing of fecal samples. | Mangiferin increased beneficial taxa Akkermansia, Parabacteroides and Bifidobacteriaceae while reducing the pathogenic genus Helicobacter. | Mangiferin exhibited anti-inflammatory and cholesterol-lowering effects, which was associated with microbiome modulation. |
| Li et al., 2015 [109] | Pomegranate extract | 20 healthy volunteers (9 females and 11 males) received 1000 mg of pomegranate extract daily for 4 weeks. Gut microbiota was assessed by 16S rRNA sequencing of fecal samples. | The pomegranate extract increased levels of Akkermansia muciniphila and Proteobacteria and decreased Actinobacteria. | The article did not assess the health benefits of the pomegranate extract, but suggests that it may mediate beneficial effects on weight maintenance and insulin sensitivity by changing the ratio of Firmicutes to Bacteroidetes and increasing Akkermansia in the gut microflora. |
| Yuan et al., 2018 [116] | Green Tea | 12 healthy volunteers (4 females and 8 males) received 400 mL of green tea liquid daily for 2 weeks. Gut microbiota was assessed by 16S rRNA sequencing of fecal samples. | Green tea increased the Firmicutes to Bacteroidetes ratio and elevated short chain fatty acid (SCFA) producing genera Faecalibacterium, Blautia, Bifidobacterium, Roseburia, Eubacterium and Coprococcus. | The green tea increased SCFA-producing bacteria and reduced the expression of functional markers of inflammation (lipopolysaccharide (LPS) synthesis). The overall composition of the gut microbiota was influenced by age, sex, body mass index and the status of bowel movements (however, these factors did not influence baseline or green tea intervention microbiome profile). |
| Lima et al., 2019 [117] | Orange juice | 10 healthy females received 300 mL of orange juice daily for of 60 days. Gut microbiota was assessed using bacterial culture and polymerase chain denaturing gradient gel electrophoresis (DGGE) of fecal samples. | Orange juice increased the population of Bifidobacterium spp. and Lactobacillus spp. | Daily intake of orange juice improved blood biochemical parameters, such as low-density lipoprotein-cholesterol, triglycerides, glucose and insulin sensitivity, which was associated with increased Bifidobacterium spp. and Lactobacillus spp. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pheiffer, C.; Riedel, S.; Dias, S.; Adam, S. Gestational Diabetes and the Gut Microbiota: Fibre and Polyphenol Supplementation as a Therapeutic Strategy. Microorganisms 2024, 12, 633. https://doi.org/10.3390/microorganisms12040633
Pheiffer C, Riedel S, Dias S, Adam S. Gestational Diabetes and the Gut Microbiota: Fibre and Polyphenol Supplementation as a Therapeutic Strategy. Microorganisms. 2024; 12(4):633. https://doi.org/10.3390/microorganisms12040633
Chicago/Turabian StylePheiffer, Carmen, Sylvia Riedel, Stephanie Dias, and Sumaiya Adam. 2024. "Gestational Diabetes and the Gut Microbiota: Fibre and Polyphenol Supplementation as a Therapeutic Strategy" Microorganisms 12, no. 4: 633. https://doi.org/10.3390/microorganisms12040633
APA StylePheiffer, C., Riedel, S., Dias, S., & Adam, S. (2024). Gestational Diabetes and the Gut Microbiota: Fibre and Polyphenol Supplementation as a Therapeutic Strategy. Microorganisms, 12(4), 633. https://doi.org/10.3390/microorganisms12040633






