RNA Interference Therapeutics for Chronic Hepatitis B: Progress, Challenges, and Future Prospects
Abstract
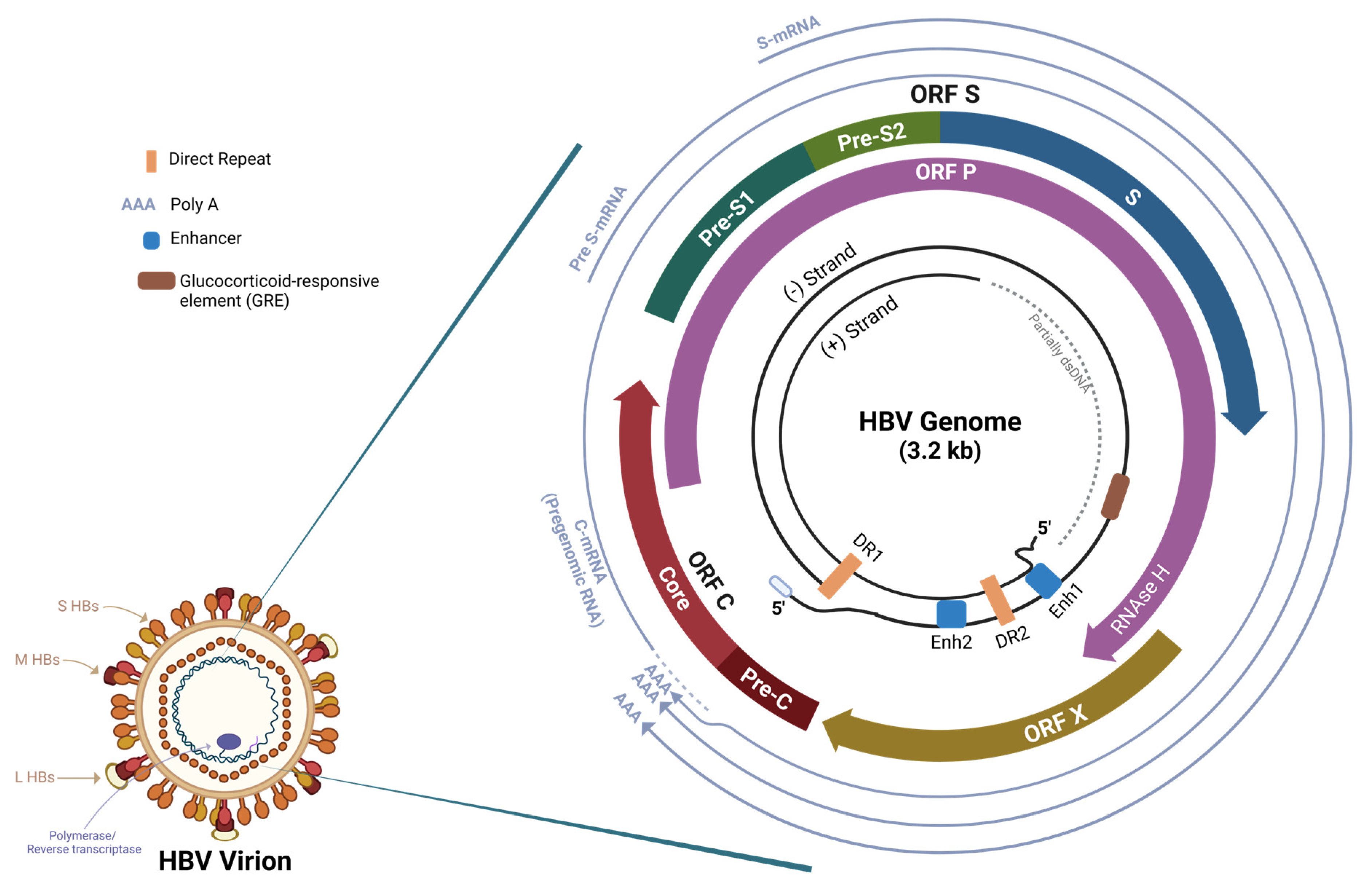
1. Introduction
2. Hepatitis B RNA Interference
3. Delivery
3.1. Non-Viral Vectors
3.2. Viral Vectors
4. Current RNAi Research
4.1. ARB-1467
4.2. ARC-520
4.3. ALG-125755
4.4. RG-6346
4.5. AB-729
4.6. VIR-2218
4.7. JNJ-3989
5. Combination Therapy
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Harris, A. Hepatitis B; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2023. [Google Scholar]
- Yeo, Y.H.; Ho, H.J.; Yang, H.-I.; Tseng, T.-C.; Hosaka, T.; Trinh, H.N.; Kwak, M.-S.; Park, Y.M.; Fung, J.Y.Y.; Buti, M.; et al. Factors Associated with Rates of HBsAg Seroclearance in Adults with Chronic HBV Infection: A Systematic Review and Meta-analysis. Gastroenterology 2019, 156, 635–646.e9. [Google Scholar] [CrossRef] [PubMed]
- Seto, W.-K.; Lo, Y.-R.; Pawlotsky, J.-M.; Yuen, M.-F. Chronic hepatitis B virus infection. Lancet 2018, 392, 2313–2324. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.J. Hepatitis B: The virus and disease. Hepatology 2009, 49, S13–S21. [Google Scholar] [CrossRef] [PubMed]
- Rinker, F.; Bremer, C.M.; Schröder, K.; Wiegand, S.B.; Bremer, B.; Manns, M.P.; Kraft, A.R.; Wedemeyer, H.; Yang, L.; Pavlovic, V.; et al. Quantitation of large, middle and small hepatitis B surface proteins in HBeAg-positive patients treated with peginterferon alfa-2a. Liver Int. 2020, 40, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Karra, V.K.; Chowdhury, S.J.; Ruttala, R.; Polipalli, S.K.; Kar, P. Clinical Significance of Quantitative HBsAg Titres and its Correlation with HBV DNA Levels in the Natural History of Hepatitis B Virus Infection. J. Clin. Exp. Hepatol. 2021, 6, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wen, J.; Xiao, W.; Zhang, B. Pregenomic RNA: How to assist the management of chronic hepatitis B? Rev. Med. Virol. 2019, 29, e2051. [Google Scholar] [CrossRef]
- Seeger, C.; Mason, W.S. Molecular biology of hepatitis B virus infection. Virology 2015, 479–480, 672–686. [Google Scholar] [CrossRef]
- Mak, L.-Y.; Seto, W.-K.; Yuen, M.-F. Novel Antivirals in Clinical Development for Chronic Hepatitis B Infection. Viruses 2021, 13, 1169. [Google Scholar] [CrossRef]
- Dana, H.; Chalbatani, G.M.; Mahmoodzadeh, H.; Karimloo, R.; Rezaiean, O.; Moradzadeh, A.; Mehmandoost, N.; Moazzen, F.; Mazraeh, A.; Marmari, V.; et al. Molecular Mechanisms and Biological Functions of siRNA. Int. J. Biomed. Sci. IJBS 2017, 13, 48–57. [Google Scholar] [CrossRef]
- Han, H. RNA Interference to Knock Down Gene Expression. In Disease Gene Identification: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2018; pp. 293–302. [Google Scholar] [CrossRef]
- Anderson, R.T.; Choi, H.S.J.; Lenz, O.; Peters, M.G.; Janssen, H.L.A.; Mishra, P.; Donaldson, E.; Westman, G.; Buchholz, S.; Miller, V.; et al. Association Between Seroclearance of Hepatitis B Surface Antigen and Long-term Clinical Outcomes of Patients with Chronic Hepatitis B Virus Infection: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 463–472. [Google Scholar] [CrossRef]
- van den Berg, F.; Limani, S.W.; Mnyandu, N.; Maepa, M.B.; Ely, A.; Arbuthnot, P. Advances with RNAi-Based Therapy for Hepatitis B Virus Infection. Viruses 2020, 12, 851. [Google Scholar] [CrossRef]
- Locarnini, S. Therapies for hepatitis B: Where to from here? Gastroenterology 2005, 128, 789–792. [Google Scholar] [CrossRef]
- Stark, G.R.; Kerr, I.M.; Williams BR, G.; Silverman, R.H.; Schreiber, R.D. How cells respond to interferons. Annu. Rev. Biochem. 1998, 67, 227–264. [Google Scholar] [CrossRef]
- Logan, G.J.; Dane, A.P.; Hallwirth, C.V.; Smyth, C.M.; Wilkie, E.E.; Amaya, A.K.; Zhu, E.; Khandekar, N.; Ginn, S.L.; Liao, S.H.Y.; et al. Identification of liver-specific enhancer–promoter activity in the 3′ untranslated region of the wild-type AAV2 genome. Nat. Genet. 2017, 49, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Sunbul, M. Hepatitis B virus genotypes: Global distribution and clinical importance. World J. Gastroenterol. 2014, 20, 5427. [Google Scholar] [CrossRef]
- Wu, H.-L.; Huang, L.-R.; Huang, C.-C.; Lai, H.-L.; Liu, C.-J.; Huang, Y.-T.; Hsu, Y.-W.; Lu, C.-Y.; Chen, D.-S.; Chen, P.-J. RNA interference-mediated control of hepatitis B virus and emergence of resistant mutant. Gastroenterology 2005, 128, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Maepa, M.; Roelofse, I.; Ely, A.; Arbuthnot, P. Progress and Prospects of Anti-HBV Gene Therapy Development. Int. J. Mol. Sci. 2015, 16, 17589–17610. [Google Scholar] [CrossRef]
- Hickerson, R.P.; Vlassov, A.V.; Wang, Q.; Leake, D.; Ilves, H.; Gonzalez-Gonzalez, E.; Contag, C.H.; Johnston, B.H.; Kaspar, R.L. Stability Study of Unmodified siRNA and Relevance to Clinical Use. Oligonucleotides 2008, 18, 345–354. [Google Scholar] [CrossRef]
- Czauderna, F. Structural variations and stabilising modifications of synthetic siRNAs in mammalian cells. Nucleic Acids Res. 2003, 31, 2705–2716. [Google Scholar] [CrossRef]
- Morrissey, D.V.; Blanchard, K.; Shaw, L.; Jensen, K.; Lockridge, J.A.; Dickinson, B.; McSwiggen, J.A.; Vargeese, C.; Bowman, K.; Shaffer, C.S.; et al. Activity of stabilized short interfering RNA in a mouse model of hepatitis B virus replication. Hepatology 2005, 41, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, D.V.; Lockridge, J.A.; Shaw, L.; Blanchard, K.; Jensen, K.; Breen, W.; Hartsough, K.; Machemer, L.; Radka, S.; Jadhav, V.; et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotechnol. 2005, 23, 1002–1007. [Google Scholar] [CrossRef]
- Hean, J.; Crowther, C.; Ely, A.; ul Islam, R.; Barichievy, S.; Bloom, K.; Weinberg, M.S.; van Otterlo, W.A.L.; de Koning, C.B.; Salazar, F.; et al. Inhibition of hepatitis B virus replication in vivo using lipoplexes containing altritol-modified antiviral siRNAs. Artif. DNA PNA XNA 2010, 1, 17–26. [Google Scholar] [CrossRef]
- Marimani, M.D.; Ely, A.; Buff, M.C.R.; Bernhardt, S.; Engels, J.W.; Scherman, D.; Escriou, V.; Arbuthnot, P. Inhibition of replication of hepatitis B virus in transgenic mice following administration of hepatotropic lipoplexes containing guanidinopropyl-modified siRNAs. J. Control. Release 2015, 209, 198–206. [Google Scholar] [CrossRef]
- Chen, X.; Qian, Y.; Yan, F.; Tu, J.; Yang, X.; Xing, Y.; Chen, Z. 5′-Triphosphate-siRNA activates RIG-I-dependent type I interferon production and enhances inhibition of hepatitis B virus replication in HepG2.2.15 cells. Eur. J. Pharmacol. 2013, 721, 86–95. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; He, H.; Zhou, J.; Li, X.; Ma, H.; Li, Z.; Zeng, Y.; Shao, R.; Cen, S.; et al. Structure-Based Design of Novel Chemical Modification of the 3′-Overhang for Optimization of Short Interfering RNA Performance. Biochemistry 2015, 54, 1268–1277. [Google Scholar] [CrossRef]
- Valenzuela, R.A.P.; Suter, S.R.; Ball-Jones, A.A.; Ibarra-Soza, J.M.; Zheng, Y.; Beal, P.A. Base Modification Strategies to Modulate Immune Stimulation by an siRNA. ChemBioChem 2015, 16, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Pandey, K.; Nakai, H.; Storm, T.A.; Kay, M.A. Liver Transduction with Recombinant Adeno-Associated Virus Is Primarily Restricted by Capsid Serotype Not Vector Genotype. J. Virol. 2006, 80, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, M.; Campbell, R.A.; Yanez Arteta, M.; Lawrence, M.J.; Sebastiani, F. Review of structural design guiding the development of lipid nanoparticles for nucleic acid delivery. Curr. Opin. Colloid Interface Sci. 2023, 66, 101705. [Google Scholar] [CrossRef]
- Carmona, S.; Jorgensen, M.R.; Kolli, S.; Crowther, C.; Salazar, F.H.; Marion, P.L.; Fujino, M.; Natori, Y.; Thanou, M.; Arbuthnot, P.; et al. Controlling HBV Replication in Vivo by Intravenous Administration of Triggered PEGylated siRNA-Nanoparticles. Mol. Pharm. 2009, 6, 706–717. [Google Scholar] [CrossRef]
- Zinn, E.; Pacouret, S.; Khaychuk, V.; Turunen, H.T.; Carvalho, L.S.; Andres-Mateos, E.; Shah, S.; Shelke, R.; Maurer, A.C.; Plovie, E.; et al. In Silico Reconstruction of the Viral Evolutionary Lineage Yields a Potent Gene Therapy Vector. Cell Rep. 2015, 12, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Kassner, U.; Hollstein, T.; Grenkowitz, T.; Wühle-Demuth, M.; Salewsky, B.; Demuth, I.; Dippel, M.; Steinhagen-Thiessen, E. Gene Therapy in Lipoprotein Lipase Deficiency: Case Report on the First Patient Treated with Alipogene Tiparvovec Under Daily Practice Conditions. Hum. Gene Ther. 2018, 29, 520–527. [Google Scholar] [CrossRef]
- Rensen, P.C.N.; van Leeuwen, S.H.; Sliedregt, L.A.J.M.; van Berkel, T.J.C.; Biessen, E.A.L. Design and Synthesis of Novel N -Acetylgalactosamine-Terminated Glycolipids for Targeting of Lipoproteins to the Hepatic Asialoglycoprotein Receptor. J. Med. Chem. 2004, 47, 5798–5808. [Google Scholar] [CrossRef]
- Wooddell, C.I.; Rozema, D.B.; Hossbach, M.; John, M.; Hamilton, H.L.; Chu, Q.; Hegge, J.O.; Klein, J.J.; Wakefield, D.H.; Oropeza, C.E.; et al. Hepatocyte-targeted RNAi Therapeutics for the Treatment of Chronic Hepatitis B Virus Infection. Mol. Ther. 2013, 21, 973–985. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, X.; Steer, C.J.; Song, G.; Niu, J. Efficient silencing of hepatitis B virus S gene through CRISPR-mediated base editing. Hepatol. Commun. 2022, 6, 1652–1663. [Google Scholar] [CrossRef]
- Nair, J.K.; Attarwala, H.; Sehgal, A.; Wang, Q.; Aluri, K.; Zhang, X.; Gao, M.; Liu, J.; Indrakanti, R.; Schofield, S.; et al. Impact of enhanced metabolic stability on pharmacokinetics and pharmacodynamics of GalNAc–siRNA conjugates. Nucleic Acids Res. 2017, 45, 10969–10977. [Google Scholar] [CrossRef] [PubMed]
- Jenke, A.C.W.; Wilhelm, A.D.; Orth, V.; Lipps, H.J.; Protzer, U.; Wirth, S. Long-Term Suppression of Hepatitis B Virus Replication by Short Hairpin RNA Expression Using the Scaffold/Matrix Attachment Region-Based Replicating Vector System pEPI-1. Antimicrob. Agents Chemother. 2008, 52, 2355–2359. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Snyder, L.L.; Esser, J.M.; Pachuk, C.J.; Steel, L.F. Vector design for liver-specific expression of multiple interfering RNAs that target hepatitis B virus transcripts. Antivir. Res. 2008, 80, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Warnock, J.N.; Daigre, C.; Al-Rubeai, M. Introduction to Viral Vectors. In Viral Vectors for Gene Therapy: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–25. [Google Scholar] [CrossRef]
- Maepa, M.B.; Ely, A.; Grayson, W.; Arbuthnot, P. Sustained Inhibition of HBV Replication In Vivo after Systemic Injection of AAVs Encoding Artificial Antiviral Primary MicroRNAs. Mol. Ther. Nucleic Acids 2017, 7, 190–199. [Google Scholar] [CrossRef]
- Ivacik, D.; Ely, A.; Ferry, N.; Arbuthnot, P. Sustained inhibition of hepatitis B virus replication in vivo using RNAi-activating lentiviruses. Gene Ther. 2015, 22, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Uprichard, S.L.; Boyd, B.; Althage, A.; Chisari, F.V. Clearance of hepatitis B virus from the liver of transgenic mice by short hairpin RNAs. Proc. Natl. Acad. Sci. USA 2005, 102, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Carmona, S.; Ely, A.; Crowther, C.; Moolla, N.; Salazar, F.H.; Marion, P.L.; Ferry, N.; Weinberg, M.S.; Arbuthnot, P. Effective Inhibition of HBV Replication in Vivo by Anti-HBx Short Hairpin RNAs. Mol. Ther. 2006, 13, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, J.C.; Kowalski, P.S.; Anderson, D.G. Advances in the delivery of RNA therapeutics: From concept to clinical reality. Genome Med. 2017, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, G.; Xi, L.; Yin, A.; Gao, Y.; You, W.; Wang, X.; Sun, B. Hepatitis B virus inhibition in mice by lentiviral vector mediated short hairpin RNA. BMC Gastroenterol. 2009, 9, 73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, J.-W.; Lee, S.H.; Park, Y.S.; Jeong, S.-H.; Kim, N.; Lee, D.H. Inhibition of in vitro hepatitis B virus replication by lentivirus-mediated short-hairpin RNA against HBx. Korean J. Hepatol. 2009, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Munis, A.M. Gene Therapy Applications of Non-Human Lentiviral Vectors. Viruses 2020, 12, 1106. [Google Scholar] [CrossRef] [PubMed]
- Mowa, M.B.; Crowther, C.; Ely, A.; Arbuthnot, P. Inhibition of Hepatitis B Virus Replication by Helper Dependent Adenoviral Vectors Expressing Artificial Anti-HBV Pri-miRs from a Liver-Specific Promoter. BioMed Res. Int. 2014, 2014, 718743. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Ko, T.-M.; Ma, H.-I.; Wu, H.-L.; Xiao, X.; Li, J.; Chang, C.-M.; Wu, P.-Y.; Chen, C.-H.; Han, J.-M.; et al. Long-term inhibition of hepatitis B virus in transgenic mice by double-stranded adeno-associated virus 8-delivered short hairpin, R.N.A. Gene Ther. 2007, 14, 11–19. [Google Scholar] [CrossRef]
- Michler, T.; Große, S.; Mockenhaupt, S.; Röder, N.; Stückler, F.; Knapp, B.; Ko, C.; Heikenwalder, M.; Protzer, U.; Grimm, D. Blocking sense-strand activity improves potency, safety and specificity of anti-hepatitis B virus short hairpin RNA. EMBO Mol. Med. 2016, 8, 1082–1098. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, X.; Hu, W.; Li, A.; Li, Y.; Huang, H.; Yan, R.; Zhang, Y.; Li, J.; Li, H.; et al. Long-term and efficient inhibition of hepatitis B virus replication by AAV8-delivered artificial microRNAs. Antivir. Res. 2022, 204, 105366. [Google Scholar] [CrossRef]
- Chen, C.-C.; Sun, C.-P.; Ma, H.-I.; Fang, C.-C.; Wu, P.-Y.; Xiao, X.; Tao, M.-H. Comparative Study of Anti-hepatitis B Virus RNA Interference by Double-stranded Adeno-associated Virus Serotypes 7, 8, and 9. Mol. Ther. 2009, 17, 352–359. [Google Scholar] [CrossRef][Green Version]
- Santiago-Ortiz, J.; Ojala, D.S.; Westesson, O.; Weinstein, J.R.; Wong, S.Y.; Steinsapir, A.; Kumar, S.; Holmes, I.; Schaffer, D.V. AAV ancestral reconstruction library enables selection of broadly infectious viral variants. Gene Ther. 2015, 22, 934–946. [Google Scholar] [CrossRef]
- Yuen, M.-F.; Wong, D.K.-H.; Schluep, T.; Lai, C.-L.; Ferrari, C.; Locarnini, S.; Lo, R.C.-L.; Gish, R.G.; Hamilton, J.; Wooddell, C.I.; et al. Long-term serological, virological and histological responses to RNA inhibition by ARC-520 in Chinese chronic hepatitis B patients on entecavir treatment. Gut 2022, 71, 789–797. [Google Scholar] [CrossRef]
- Yuen, M.; Schiefke, I.; Yoon, J.; Ahn, S.H.; Heo, J.; Kim, J.H.; Lik Yuen Chan, H.; Yoon, K.T.; Klinker, H.; Manns, M.; et al. RNA Interference Therapy With ARC-520 Results in Prolonged Hepatitis B Surface Antigen Response in Patients with Chronic Hepatitis B Infection. Hepatology 2020, 72, 19–31. [Google Scholar] [CrossRef]
- Wooddell, C.I.; Yuen, M.-F.; Chan, H.L.-Y.; Gish, R.G.; Locarnini, S.A.; Chavez, D.; Ferrari, C.; Given, B.D.; Hamilton, J.; Kanner, S.B.; et al. RNAi-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg. Sci. Transl. Med. 2017, 9, eaan0241. [Google Scholar] [CrossRef]
- Schluep, T.; Lickliter, J.; Hamilton, J.; Lewis, D.L.; Lai, C.; Lau, J.Y.; Locarnini, S.A.; Gish, R.G.; Given, B.D. Safety, Tolerability, and Pharmacokinetics of ARC-520 Injection, an RNA Interference-Based Therapeutic for the Treatment of Chronic Hepatitis B Virus Infection, in Healthy Volunteers. Clin. Pharmacol. Drug Dev. 2017, 6, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Arbutus Biopharma Corporation. Arbutus Reports Fourth Quarter and Year-end 2018 Financial Results and Describes Recent Clinical Accomplishments and Key 2019 Objectives. 7 March 2019. Available online: https://investor.arbutusbio.com/news-releases/news-release-details/arbutus-reports-fourth-quarter-and-year-end-2018-financial (accessed on 13 December 2023).
- Agarwal, K.; Gane, E.; Cheng, W.; Sievert, W.; Roberts, S.; Ahn, S.H.; Kim, Y.J.; Streinu-Cercel, A.; Denning, J.; Symonds, W.; et al. Bi-weekly Dosing of ARB-1467 LNP siRNA in HBeAgNegative, Virally Suppressed Patients with Chronic HBV Infection Leads to Deeper Declines in HBsAg and Potential Association with IL28b. In Proceedings of the American Association for the Study of Liver Diseases’ Liver Meeting, Washington, DC, USA, 20–24 October 2017. [Google Scholar]
- Yuen, R.; Berliba, E.; Kim, Y.; Holmes, J.; Lim, Y.; Strasser, S.; Schwabe, C.; Jucov, A.; Lee, A.; Thi, E.; et al. Safety and pharmacodynamics of the GALNAC-siRNA AB-729 in subjects with chronic hepatitis B infection. Hepatology 2020, 72, 62A–63A. [Google Scholar]
- Paratala, B.; Park, J.-J.; Ganchua, S.; Gane, E.; Yuen, R.; Lee, A.; Moore, C.; Lam, A.; Sevinsky, H.; Sims, K.; et al. Inhibition of hepatitis B surface antigen in chronic hepatitis B subjects by RNA interference therapeutic AB-729 is accompanied by upregulation of HBV-specific T cell activation markers. J. Hepatol. 2021, 75, S761. [Google Scholar]
- Thi, E.; Yuen, R.; Gane, E.; Sevinsky, H.; Sims, K.; Anderson, M.; Lam, A.; Sofia, M.; Cloherty, G.; Picchio, G. Inhibition of hepatitis B surface antigen by RNA interference therapeutic AB-729 in chronic hepatitis B patients correlates with suppression of all HBsAg isoforms, H.B.V.R.N.A. J. Hepatol. 2021, 75, S760–S761. [Google Scholar] [CrossRef]
- Yuen, M.; Berliba, E.; Sukeepaisarnjaroen, W.; Tangkijvanich, P.; Leerapun, A.; Holmes, J.; Gane, E.; Jucov, A.; Thi, E.; Sofia, M.; et al. Low HBsAg Levels Maintained following Cessation of the Galnac-Sirna, Ab-729, in Chronic Hepatitis B Subjects on Nucleos(T)Ide Analogue Therapy. In Proceedings of the American Association for the Study of Liver Diseases’ Liver Meeting, Digital, 13–15 November 2021. [Google Scholar]
- Lim, T.H.; Yoon, J.-H.; Schwabe, C.; Thompson, A.; Brown, B.; Gane, E. LO9—HBV RNAi Inhibitor RG6346 in Phase 1b-2a TRIAL Was Safe, Well-Tolerated, and Resulted in Substantial and Durable Reductions in Serum HBsAg Levels. In Proceedings of the American Association for the Study of Liver Diseases’ Liver Meeting, Digital, 13–16 November 2020. [Google Scholar]
- Schlegel, M.K.; Janas, M.M.; Jiang, Y.; Barry, J.D.; Davis, W.; Agarwal, S.; Berman, D.; Brown, C.R.; Castoreno, A.; LeBlanc, S.; et al. From bench to bedside: Improving the clinical safety of GalNAc–siRNA conjugates using seed-pairing destabilization. Nucleic Acids Res. 2022, 50, 6656–6670. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.K.; Willoughby, J.L.S.; Chan, A.; Charisse, K.; Alam Md, R.; Wang, Q.; Hoekstra, M.; Kandasamy, P.; Kel’in, A.V.; Milstein, S.; et al. Multivalent N -Acetylgalactosamine-Conjugated siRNA Localizes in Hepatocytes and Elicits Robust RNAi-Mediated Gene Silencing. J. Am. Chem. Soc. 2014, 136, 16958–16961. [Google Scholar] [CrossRef] [PubMed]
- Janas, M.M.; Schlegel, M.K.; Harbison, C.E.; Yilmaz, V.O.; Jiang, Y.; Parmar, R.; Zlatev, I.; Castoreno, A.; Xu, H.; Shulga-Morskaya, S.; et al. Selection of GalNAc-conjugated siRNAs with limited off-target-driven rat hepatotoxicity. Nat. Commun. 2018, 9, 723. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.V.; Fanget, M.C.; MacLauchlin, C.; Clausen, V.A.; Li, J.; Cloutier, D.; Shen, L.; Robbie, G.J.; Mogalian, E. Clinical and Preclinical Single-Dose Pharmacokinetics of VIR-2218, an RNAi Therapeutic Targeting HBV Infection. Drugs R&D 2021, 21, 455–465. [Google Scholar] [CrossRef]
- Gane, E.; Lim, Y.; Cloutier, D.; Shen, L.; Cathcart, A.; Ding, X.; Pang, P.; Huang, S.; Yuen, R. Safety and antiviral activity of VIR-2218, an X-targeting RNAi therapeutic, in participants with chronic hepatitis B infection: Week 48 follow-up results. Hepatology 2021, 75, S287–S288. [Google Scholar]
- Yuen, M.-F.; Locarnini, S.; Given, B.; Schluep, T.; Hamilton, J.; Biermer, M.; Kalmeijer, R.; Beumont, M.; Lenz, O.; Cloherty, G.; et al. First Clinical Experience with RNA Interference-based Triple Combination Therapy in Chronic Hepatitis B: JNJ-3989, JNJ-6379 and a Nucleos(t)ide Analogue. In Proceedings of the American Association for the Study of Liver Diseases’ Liver Meeting, Boston, MA, USA, 8–12 November 2019. [Google Scholar]
- Yuen, M.-F.; Asselah, T.; Jacobson, I.M.; Brunetto, M.R.; Janssen, H.L.A.; Takehara, T.; Hou, J.L.; Kakuda, T.N.; Lambrecht, T.; Beumont, M.; et al. Efficacy and safety of the siRNA JNJ-73763989 and the capsid assembly modulator JNJ-56136379 (bersacapavir) with nucleos(t)ide analogues for the treatment of chronic hepatitis B virus infection (REEF-1): A multicentre, double-blind, active-controlled, randomised, phase 2b trial. Lancet Gastroenterol. Hepatol. 2023, 8, 790–802. [Google Scholar] [CrossRef]
- Yuen, M.-F.; Locarnini, S.; Lim, T.H.; Strasser, S.I.; Sievert, W.; Cheng, W.; Thompson, A.J.; Given, B.D.; Schluep, T.; Hamilton, J.; et al. Combination treatments including the small-interfering RNAJNJ-3989 induce rapid sometimes prolonged viral responses in patients with, C.H.B. J. Hepatol. 2022, 77, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Jucov, A.; Gane, E.; Fitzgerald, M.; Le, K.; Wang, S.; Ammar, L.; Burnett, C.; Haceatrean, A.; Gupta, K.; Clark, D.; et al. Safety pharmacokinetics antiviral activity of single ascending doses of, A.L.G.-1.2.5.7.5.5.; a GalNAc-conjugated small interfering, R.N.A.; in subjects with chronic hepatitis, B. J. Hepatol. 2023, 78, S1162–S1163. [Google Scholar] [CrossRef]
- Hong, J.; Montero, S.M.; Tan, H.; de Costa, T.; Nie, Y.; Pandey, R.; Rajwanshi, V.; Bhattacharya, A.; Kao, C.; Kang, H.; et al. ALG-125755, a Small Interfering RNA (siRNA) Against Hepatitis B Virus (HBV) Effectively Inhibits Hepatitis B Surface Antigen (HBsAg) Secretion in HBV Cell Models and the AAV-HBV Mouse Model. In Proceedings of the American Association for the Study of Liver Diseases’ Liver Meeting, Washington, DC, USA, 20–24 October 2017; Volume 2021, pp. 12–15. [Google Scholar]
- Sepp-Lorenzino, L.; Abrams, M.; Carayannopoulos, L.; Koser, M.; Ludmerer, S.; Charisse, K.B.; Freedman, D.; Jadhav, V.; Rajeev, K.G.; Hinkle, G.; et al. ALN-HBV, a GalNAc-siRNA Enhanced Stabilization Chemistry RNAi Therapeutic for the Treatment of Chronic Hepatitis B Virus Infection: 1857. Hepatology 2014, 60, 1091A. [Google Scholar]
- Yuen, M.-F.; Locarnini, S.; Lim, T.H.; Strasser, S.; Sievert, W.; Cheng, W.; Thompson, A.; Given, B.; Schluep, T.; Hamilton, J.; et al. PS-080-Short term RNA interference therapy in chronic hepatitis B using JNJ-3989 brings majority of patients to HBsAg < 100 IU/mL threshold. J. Hepatol. 2019, 70, e51–e52. [Google Scholar] [CrossRef]
- Yuen, R.; Lim, Y.-S.; Cloutier, D.; Thanawala, V.; Shen, L.; Arizpe, A.; Tay, C.; Gupta, S.V.; Cathcart, A.; Hwang, C.; et al. Preliminary results from a phase 2 study evaluating vir-2218 alone and in combination with pegylated interferon alfa-2a in participants with chronic hepatitis B infection. In Proceedings of the American Association for the Study of Liver Diseases’ Liver Meeting, Digital Experience, Online, 13–15 November 2021; Volume 74, p. 63A. [Google Scholar]
- Hoffmann-La Roche. A Trial to Evaluate the Efficacy and Safety of Multiple Combination Therapies in Participants with Chronic Hepatitis B (Piranga); NCT04225715; Hoffmann-La Roche: Basel, Switzerland, 2020. [Google Scholar]
- Mani, N.; Cole, A.G.; Phelps, J.R.; Ardzinski, A.; Cobarrubias, K.D.; Cuconati, A.; Dorsey, B.D.; Evangelista, E.; Fan, K.; Guo, F.; et al. Preclinical Profile of AB-423, an Inhibitor of Hepatitis B Virus Pregenomic RNA Encapsidation. Antimicrob. Agents Chemother. 2018, 62, e00082-18. [Google Scholar] [CrossRef]
- Li, G.-Q. Combination of small interfering RNA and lamivudine on inhibition of human B virus replication in HepG2.2.15 cells. World J. Gastroenterol. 2007, 13, 2324. [Google Scholar] [CrossRef]
- Chen, Y.; Du, D.; Wu, J.; Chan, C.-P.; Tan, Y.; Kung, H.; He, M.-L. Inhibition of hepatitis B virus replication by stably expressed, s.h.R.N.A. Biochem. Biophys. Res. Commun. 2003, 311, 398–404. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, Z.; Ye, J.; Yao, H.; Zheng, S.; Ding, J. Combination of small interfering RNAs mediates greater inhibition of human hepatitis B virus replication and antigen expression. J. Zhejiang Univ. Sci. 2005, 6B, 236–241. [Google Scholar] [CrossRef]
- Wooddell, C.I.; Gehring, A.J.; Yuen, M.-F.; Given, B.D. RNA Interference Therapy for Chronic Hepatitis B Predicts the Importance of Addressing Viral Integration When Developing Novel Cure Strategies. Viruses 2021, 13, 581. [Google Scholar] [CrossRef]
- Bunse, T.; Kosinska, A.D.; Michler, T.; Protzer, U. PD-L1 Silencing in Liver Using siRNAs Enhances Efficacy of Therapeutic Vaccination for Chronic Hepatitis, B. Biomolecules 2022, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.-F.; Heo, J.; Jang, J.-W.; Yoon, J.-H.; Kweon, Y.-O.; Park, S.-J.; Tami, Y.; You, S.; Yates, P.; Tao, Y.; et al. Safety, tolerability and antiviral activity of the antisense oligonucleotide bepirovirsen in patients with chronic hepatitis B: A phase 2 randomized controlled trial. Nat. Med. 2021, 27, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Kang, H.; Luo, M.; Nie, Y.; Pandey, R.; Montero, S.M.; de Costa, T.; Cortez, J.; Rajwanshi, V.; Smith, D.; et al. Combination drug interactions of hepatitis B virus (HBV) small interfering RNA (siRNA) and antisense oligonucleotides (ASO) in vitro and in vivo. J. Hepatol. 2021, 75, S720. [Google Scholar]
- Grimm, D.; Streetz, K.L.; Jopling, C.L.; Storm, T.A.; Pandey, K.; Davis, C.R.; Marion, P.; Salazar, F.; Kay, M.A. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 2006, 441, 537–541. [Google Scholar] [CrossRef] [PubMed]

| Drug | Number of Triggers | siRNA Target | Delivery | Phase | Status | References |
|---|---|---|---|---|---|---|
| ARC-520 | 2 | ORF X | Cholesterol | 2 | Completed/Terminated | [55,56,57,58] |
| ARB-1467 | 3 | ORF S and X | LNP | 2a | Completed | [59,60] |
| AB-729 | 1 | ORF X | GalNAc | 2 | Completed | [61,62,63,64] |
| RG-6346 | 1 | ORF S | GalNAc | 2a | Completed | [65] |
| VIR-2218 | 1 | ORF X | GalNAc | 1/2 | Completed | [66,67,68,69,70] |
| JNJ-3839 | 2 | ORF S and X | GalNAc | 1/2a | Completed/Terminated | [71,72,73] |
| ALG-125755 | 1 | ORF S | GalNAc | 1a/1b | Completed | [74] |
| Drug | Phase of Development | Total Number of Participants Receiving Treatment | Maximum Reduction in HBsAg (log10) | Number of Days Suppression Was Maintained | References |
|---|---|---|---|---|---|
| ARC-520 | 2 | 58 | 1.4 | >85 days | [56] |
| ARB-1467 | 2a | 12 | 2.7 | 70 days | [60] |
| AB-729 | 2 | 34 | 2.16 | 336 days | [64] |
| RG-6346 | 2a | 16 | 1.91 | 448 days | [65] |
| VIR-2218 | 2 | 24 | 1.65 | 336 days | [70] |
| JNJ-3839 | 2a | 40 | >2.5 | 113 days | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sneller, L.; Lin, C.; Price, A.; Kottilil, S.; Chua, J.V. RNA Interference Therapeutics for Chronic Hepatitis B: Progress, Challenges, and Future Prospects. Microorganisms 2024, 12, 599. https://doi.org/10.3390/microorganisms12030599
Sneller L, Lin C, Price A, Kottilil S, Chua JV. RNA Interference Therapeutics for Chronic Hepatitis B: Progress, Challenges, and Future Prospects. Microorganisms. 2024; 12(3):599. https://doi.org/10.3390/microorganisms12030599
Chicago/Turabian StyleSneller, Laura, Christine Lin, Angie Price, Shyam Kottilil, and Joel V. Chua. 2024. "RNA Interference Therapeutics for Chronic Hepatitis B: Progress, Challenges, and Future Prospects" Microorganisms 12, no. 3: 599. https://doi.org/10.3390/microorganisms12030599
APA StyleSneller, L., Lin, C., Price, A., Kottilil, S., & Chua, J. V. (2024). RNA Interference Therapeutics for Chronic Hepatitis B: Progress, Challenges, and Future Prospects. Microorganisms, 12(3), 599. https://doi.org/10.3390/microorganisms12030599






