Abstract
Acute respiratory viruses (ARVs) are the leading cause of diseases in humans worldwide. High-risk individuals, including children and the elderly, could potentially develop severe illnesses that could result in hospitalization or death in the worst case. The most common ARVs are the Human respiratory syncytial virus, Human Metapneumovirus, Human Parainfluenza Virus, rhinovirus, coronaviruses (including SARS and MERS CoV), adenoviruses, Human Bocavirus, enterovirus (-D68 and 71), and influenza viruses. The olfactory deficits due to ARV infection are a common symptom among patients. This review provides an overview of the role of SARS-CoV-2 and other common ARVs in the development of human olfactory pathophysiology. We highlight the critical need to understand the signaling underlying the olfactory dysfunction and the development of therapeutics for this wide-ranging category of AVRs to restore the altered or loss of smell in affected patients.
1. Introduction
Respiratory viral infections are very common and constitute a serious health concern around the world, with new infectious diseases continuing to emerge [1,2]. Such pathologies could lead to death, particularly in the elderly, and increase the expenses of the health care system worldwide. Respiratory viruses have the propensity to infect and trigger diseases through the human lower and upper respiratory tracts. We are more interested in viral upper respiratory infections (URI) as they are considered to be one of the most common causes of olfactory dysfunction, accounting for up to 45% of all cases [3,4]. Although the alteration of smell following viral URI is noticed in several cases, little treatment is currently available. The occurrence of this alteration is termed post-viral olfactory dysfunction (PVOD) [5,6,7]. The most common viruses implicated in PVOD include parainfluenza virus, rhinoviruses (RV), respiratory syncytial virus (RSV), and coronaviruses (CoV) [4,8,9,10,11]. In 1956, the RSV was found and isolated from chimpanzees and infants suffering severe lower respiratory tract illness a year later. In older children and healthy adults, the RSV causing repeated URI is common and can lead to symptomatic UR tract diseases [12]. According to Heikkinen and colleagues, 5% to 10% of URI are attributed to RSV. Studies performed in mice have shown that RSV infections are associated with damage to olfactory receptor neurons [13]. Despite these findings, the occurrence of olfactory loss associated with RSV infections seems low. It needs further investigations in broader regions of the world and during cold and warm periods to establish a clearer picture of this virus-induced olfactory dysfunction [11,14,15]. Like the RSV, the PIV was discovered in the 1950s and is associated with lower respiratory tract infections and URIs in children. Young children infected by this virus are often diagnosed with respiratory irritants, vitamin A deficiency, or malnutrition [16,17]. Although the PIV is found in respiratory secretions, the major diagnosis is observed from pulmonary secretions and confirmed by chest x-rays [18]. The RV that targets humans is considered among the most infectious agents worldwide and is associated with mild upper respiratory tract infections in people [19,20,21]. The CoV was identified in the 1960s. Little attention was given to this family of viruses until both outbreaks of the severe acute respiratory syndrome (SARS)-CoV and the Middle East respiratory syndrome (MERS)-CoV were identified in 2003 and 2012, respectively [22,23,24]. The novel coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, has spread fast all over the world [1,2]. Both MERS-CoV and SARS-CoV-2 are highly pathogenic coronaviruses and have a substantial spatial range of epidemics areas globally, but regarding MERS-CoV, the vast majority of cases are confined to the Middle East [23,24]. A key factor in the transmissibility of these viruses is the active virus replication in upper respiratory tract (URT) tissues and, therefore, its massive excretion [25,26]. Since the beginning of the pandemic, growing reports have shown the issues of partial to complete loss of smell in patients who contracted COVID-19 [27,28,29,30] and have brought new focus to PVOD. This review mainly focuses on understanding the molecular signaling underlying the olfactory pathophysiology in COVID-19-infected human patients and common viruses that induce URT infection. The importance of using such mechanisms in order to find potential targets to overcome the loss of smell will also be discussed.
2. Sources and Selection Criteria
The present study intends to synthesize the current knowledge regarding the relationship between the respiratory viral pathogenesis of the olfactory system and the mechanisms underlying the loss of smell in patients infected by respiratory viruses including COVID-19. We performed a literature search using mostly databases like PubMed Central (PMC), Google Scholar, and ScienceDirect to parse original articles, meta-analyses, and systematic reviews dealing with the animal and human respiratory viruses that have a negative impact on the olfactory system functionality. For our search, we used the combination of the following keywords: respiratory virus, coronavirus, rhinovirus, parainfluenza viruses and respiratory syncytial virus, anosmia, parosmia, hyposmia, olfactory epithelium, human, mouse, hamster, loss of smell, or olfactory dysfunction were considered in this review. Only the papers that met the keyword criteria listed above were considered in this work.
3. Olfactory Receptor and Odorant Detection
In most animals, the functional olfactory system detects and discriminates among diverse chemical stimuli. Odors are important for behaviors such as eating, mating, and avoiding dangerous smells, including smoke, leaking propane gas, and spoiled food [31,32,33]. The importance of the behaviors leads to a strong belief that the loss of olfactory function is indirectly life-threatening [34,35]. Two different olfactory systems have been developed in mammals such as rodents: the main olfactory epithelium (MOE), also called olfactory mucosa, connected to the main olfactory bulb, and the accessory system called the vomeronasal organ (VNO) connected to the accessory olfactory bulb [36,37,38,39,40]. Here, the VNO will not be discussed. The configuration of the olfactory epithelium (OE) presents unique cytological characteristics as it contains different cell types, such as the ciliated olfactory receptor neurons (ORNs), the sustentacular supporting cells, and the cells of Bowman’s glands. The olfactory mucosa hosts many different types of cells, including ORNs in the intermediate layer, the sustentacular cells on the apical and basal sides, and the sensory cilia present at the apical pole where the dendrites of olfactory neurons are extended [41,42].
A deep understanding of the molecular signaling of smelling recognition is required to understand the basis of the olfactory system and, consequently, the loss of olfactory function. Starting from the beginning of the 1990s, pioneers have developed and studied the physiology of the olfactory system based on molecular biology, biochemistry, anatomy, and bioinformatics [31,43]. At first glance, getting insight into the molecular mechanisms of the perception of odors has emerged from several disciplines such as chemistry, biology, and professional odor detectors [31,43].
The detection occurs when the odorants penetrate into the nasal cavity and reach the olfactory mucosa. The odorants then interact with specific ORNs in the olfactory mucosa. Once an ORN is activated by an odorant, a nervous influx is sent to the cortex via the olfactory bulb. Readers interested in the mammalian olfactory epithelium and the perception of odor coding are invited to view an excellent review by Kurian and colleagues published in 2020 [44].
4. Viral Infection Causing Olfactory Dysfunction
The fact that the olfactory receptor neurons (ORNs) are found in the nasal cavity and expressed in the OE makes them directly exposed to all kinds of air-bound and air-way pathogens that make the ORNs vulnerable. Whether the cause is physiological or pathological, the lifespan of ORNs is relatively short with a few weeks in the OE. Moreover, stem cell reprogramming ensures the continuous regeneration of new ORNs from OE basal cells either in a physiological turnover of ORNs or in response to inflammation and OE severe damage mediated by neural injury [45,46,47]. Several airway pathogens, such as viruses, are causing damage to OE, particularly through the sustentacular cells, triggering anosmia, hyposmia, phantosmia, or parmosmia in mammals [7,9,42,48,49]. Many respiratory tract infections due to viruses like RV, PIV, RSV, CoV, and Epstein-Barr viruses (EBV) [4,8,9,10,11] have been involved in the development of olfactory disorders such as partial or total loss of smell. Doty and others have termed this pathology as virus-induced olfactory dysfunction (PVOD) [3,5,6,50]. Viral infection destroys many cells within the apical layer of the OE, which could lead to ORN functional impairment in the nose. Interestingly, the OE basal cells can constantly replace damaged ORNs with new olfactory neurons, allowing patients to recover functional olfactory responses [46,47]. In the following sections, the common viral infection of the URT leading to olfactory dysfunction like anosmia, hyposmia, phantosmia, and parmosmia [4] in animal models and humans will be discussed.
5. Viruses Impacting Respiratory System
The respiratory system is exposed to the environment and is in permanent contact with air-way pathogens like viruses. A recent investigation in humans has identified 18 viruses in patients with PVOD. Several known viruses are associated with olfactory impairment, and it is crucial to investigate the mechanisms of infection as well as the specific receptors each virus targets (Table 1).
5.1. Case of the Parainfluenza Viruses
The presence of parainfluenza type 3 (PIV3) was observed in human nasal epithelial cells (HNECs) from 88% of patients with PVOD as compared to 9% of control patients [51]. This data suggests the potential involvement of PIV3 infection in the upper airway pathology. PIV3 in the turbinate epithelial cells of PVOD is responsible for 60% of hyposmia and 40% of anosmia in patients [51]. PIV3 has been shown to infect the HNECs and, therefore, exacerbate the production of IFN-γ and pro-inflammatory cytokines [52]. This data is in line with a previous study suggesting that PIV3 may cause olfactory dysfunction through mechanisms other than nasal obstruction in patients [9].
5.2. Case of the Sendai Virus (SeV) and Possible Interaction with PIV
The SeV, the murine counterpart of the human PIV, has been shown to directly infect the mouse brain via the olfactory neurons [53]. Another investigation demonstrated that SeV infection led to impairing mouse olfaction. Interestingly, the virus persists in OE and OB tissues for over two months and reduces the regenerative power and functionality of the ORNs [54]. Very recent findings have shown that the depletion of nasal cilia via the silencing of CEP83, a protein critical for motile cilia formation in all ciliated cells, would impede the PIV infection. Indeed, the PIV receptor CX3CR1 colocalizes to motile cilia and is a plausible viral entry mechanism into the cells [55]. An additional study showed that the intercellular adhesion molecule-1 (ICAM-1) and related cytokine molecules are induced by PIV3, and it is thought that this activation participates in the inflammation during infection by viruses [56,57] (Figure 1). Further research using the power of the transcriptomic analysis is necessary to help delineate the role of the PIV and implicated mechanisms in the development of the broad range of olfactory dysfunctions in patients and to find host-response transcript signatures for possible treatments. The study of the entire RNA transcripts in a biological sample is referred to as transcriptomics, technically available as microarrays and RNA sequencing (RNA-seq) [58]. Only recently, some precision medicine trials using transcriptome analysis have been applied in the field of cancer [59,60,61,62,63]. Data from the study of Rodon and colleagues suggest that clinical trials using transcriptomics analysis can increase the number of patients matched to drugs [62]. Recent findings on the molecular basis of neuroimmune responses revealed that the transcriptomic results are in line with a few previously reported studies of respiratory viral infections like Influenza A virus, RV, and RSV. Moreover, these data highlighted putative biomarkers of interest as a direct reflection of each virus infection and deserve further investigation for the evaluation/prediction of future innovative treatments [64,65,66].
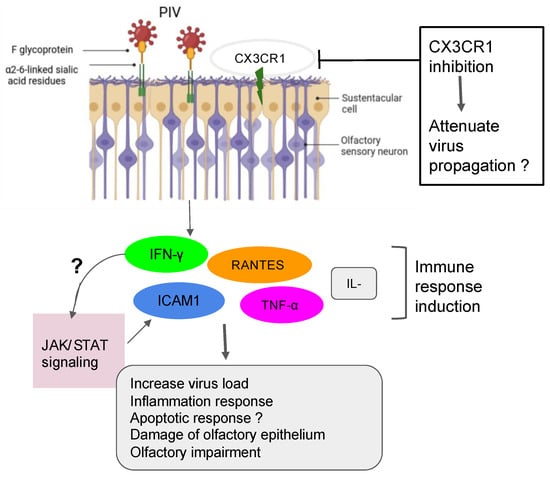
Figure 1.
Action of PIV in the olfactory impairment. PIV induced the inflammation response with damage to olfactory epithelium and the impairment of olfactory response, leading to more virus susceptibility.
5.3. Case of the Respiratory Syncytial Virus (RSV)
Recent findings have demonstrated that the RSV can infect olfactory sensory neurons (OSNs) in the nasal cavity of the mouse before accessing the central nervous system of the animal [67]. As a PIV, the RSV targets the cilia of the epithelial cells in the airways by fusionning its F-glycoprotein to the cellular receptor human nucleolin (NCL). RSV also uses another mechanism that activates protein kinases like IGF1R to get into the cells [56,68,69]. Furthermore, recent studies have shown the essential role of the nucleolin RNA binding domain RBD1,2 in allowing the infection of RSV [70]. Previous works have pointed out the importance of ORN progenitors in the turnover. They showed that RSV infection causes SOX2+ ORN progenitors damage prior to the manifestation of ORN impairment. Unfortunately, the airway allergy seems to amplify this damage induced by the RSV infection, leading to a possible loss of OMP+ ORNs [13]. Transcriptomic analysis demonstrated that olfactory signaling is among the altered pathways in patients suffering from RSV infection. This finding further supports previous work that described RSV as a causative agent of post-viral olfactory dysfunction. Interestingly, the authors highlighted that this molecular signaling could be a promising future route to investigate drug targets against RSV infection [3,34]. Sourimant et al. have recently shown that 4′-fluorouridine, a ribonucleoside analog, inhibits RSV in a selective manner in cells and human airway epithelia organoids. Although this oral therapeutic drug is efficient in small animal models, several years of investigations are needed before predicting/evaluating the outcome of applying such a type of molecule in humans [71] (Figure 2). Furthermore, many efforts are underway in the phase of clinical trials when mRNA vaccines are combined with antigens to fight multiple respiratory viruses, including the RSV [72,73].

Figure 2.
Role of RSV in olfactory impairment. RSV induced the inflammation response related to the age group with damage of olfactory epithelium and OSN progenitors and the impairment of olfactory response, leading to more virus susceptibility.

Table 1.
Summary of virus-implication in PVOD.
Table 1.
Summary of virus-implication in PVOD.
| Viruses | Animal or Cell Model | Effect of Respiratory Viruses on the Nasal Epithelial System | Comments | References |
|---|---|---|---|---|
| Parainfluenza virus | HNECs | PIV3 infection enhances the production of IFN-γ and generation of RANTES |
| [52] |
| ALI-cultured NHE cells | Inhibition of cilia or microvilli may prevent airway entry and PIV spread throughout the viral-CX3CR1 receptor interactions in the ciliated HNEs |
| [55] | |
| Sendai virus (SeV) | C57BL/6 mice and primary OSNs cultures | SeV prevents the primary cells from taking up the Ca2+ in the presence of odorants therefore altering directly the function of the OSN, |
| [54] |
| HT1080 cells | ICAM-1 is activated by PIV3 throughout the induction of the JAK/STAT signaling pathways. |
| [57] | |
| Respiratory syncytial virus (RSV) | Mice and airway organoid cultures | Prefusion RSV-F glycoprotein is required to bind with the IGF-1 receptor. Such association triggers the activation of PKCζ. |
| [68,69,70] |
| Female BALB/c mice | Reduced SOX2+ ORN progenitors was enhanced and prolonged in allergic mice infected by RSV. |
| [13] | |
| Rhinoviruses | Sinus epithelial tissue, HeLa R-19, mouse L cells | The following three major types of cellular membrane glycoproteins ICAM-1, LDLR and CDHR3 are targeted by RV to gain entry into the host cell. |
| [74,75,76,77] |
| Sinonasal epithelial cells | H2O2 significantly reduced the production of (IFN-β) and type III (IFN- λ1 and λ2) interferons that was upregulated in cells infected with RV. |
| [78] | |
| SARS-CoV-2 | ciliated HNE cells | SARS-CoV-2 establishes a link with angiotensin-converting enzyme 2 (ACE2) receptor within the airway multicilia to traverse the mucus- mucin protective barrier. |
| [55] |
| K18-hACE2 mice, golden Syrian hamsters, cellular models | SARS-CoV-2 targets the sustentacular and Bowman gland cells by binding to their ACE2 and TMPRSS2 proteins. |
| [79,80,81,82,83,84,85,86] | |
| K18-hACE2 mice, golden Syrian hamsters, cellular models | Supporting cells infected by SARS-CoV-2 affect the expression of the GLUT1/GLUT3 that interrupt the glucose trafficking to the cilia of the ORN in the mucus. |
| [87,88,89,90,91] | |
| ALI and iALI cells | SARS-CoV-2 activates key molecular mechanisms after infecting ALI and iALI cellular models. |
| [92] |
Recently, RVs were shown to be among the more predominant causative agents of PVOD in patients. The study showed that patients with anosmia were higher than those with hyposmia (58.8% vs. 19.0%, p = 0.018) [8]. In line with these findings, it is tempting to suggest that the persistence of the virus could be one factor that governs a more severe injury to the olfactory system. In fact, RVs primarily invade the ciliated respiratory epithelial cells via the glycoprotein members such as the intercellular adhesion molecule 1 (ICAM-1), the low-density lipoprotein receptor (LDLR) family members, and the cadherin-related family member 3 (CDHR3) [74,75,76,77]. RV infection induces Toll-like receptor 7 (TLR7) and retinoic acid-inducible gene I (RIG-1) that trigger the activation of cytokine expression (type I and type III IFNs) [93,94,95]. Understanding the mechanisms of RV viral-induced asthma for new therapeutic directions has gained more attention in the recent past [96,97,98]. Papi et al. have investigated the role of reducing agents such as DMSO on RV infection in the nasal epithelium. They showed that rhinovirus-induced ICAM-1 mRNA expression was inhibited by reducing agents in a dose-dependent manner. Interestingly, NF-κB and TNF-α activation, which is necessary for the ICAM-1 promoter, was completely abolished in those treated epithelial cells [99]. Moreover, it has been shown that CDHR3 genetic variants impact on the severity of RV-related pediatric respiratory tract infections by upregulating the epithelial expression of RV receptors, thus helping clinicians predict the susceptibility and severity of RV infection [100]. Complementary recent studies have demonstrated that both vitamin D and hydrogen peroxide play a critical role in attenuating the RV, mediating the ICAM-1 activation and the production of type I (IFN-β) and type III (IFN- λ1 and λ2) interferons respectively [78,101] (Figure 3). To further support these collective findings, it would be interesting to document transcriptomic profiles from animal and cellular models infected by RVs and treated by those molecules. These works would shed light on the importance of genes, including the oxidant biomarkers to be considered for the future in the development of the treatment against RV infection.
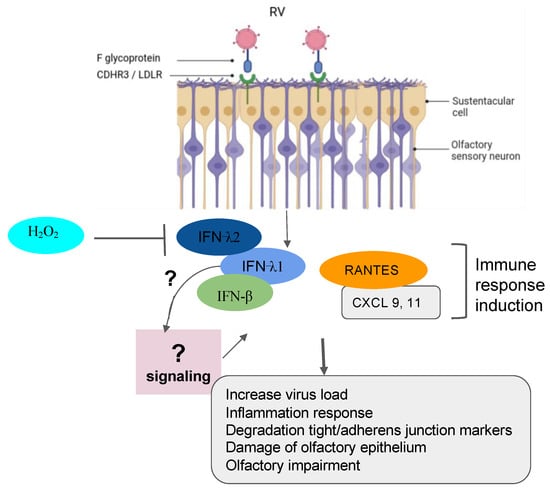
Figure 3.
Implication of RV in olfactory impairment. RV induced the inflammation response with damage to the olfactory epithelium, the degradation of the tight junction and adherent junction markers, and the impairment of olfactory response leading to more virus susceptibility.
6. Mechanisms of SARS-CoV-2 Mediating the Loss of Smell
The post-COVID and the long-term-COVID have both tremendously triggered a lot of complications in different human systems. The loss or reduction of smell, among other complications of the nervous system, is an associated symptom for patients affected by different variants of COVID-19, including the omicron variant [102,103,104,105,106]. Moreover, studies reported that the prevalence of olfactory dysfunction differs greatly between populations and approaches [106,107,108]. Currently, many COVID-19 vaccines are authorized to help protect and eliminate the virus. The COVID-19 pathology and the cellular mechanism by which the olfactory dysfunction occurs have gained a lot of attention since the pandemic, and researchers are still investigating underlying signaling and complications (Table 1) [55,91,92,106,109,110,111,112]. Earlier in the pandemic, reports hypothesized that five potential mechanisms were considered to get insights into the olfactory dysfunction in COVID-19 patients: (1) obstruction/congestion and rhinorrhea of the nasal airway, (2) damage and loss of ORNs, (3) Olfactory center damage in the brain, (4) damage of the olfactory supporting cells in the OE, and (5) Inflammation-related olfactory epithelium dysfunction [107,113]. Butowt et al. have recently reviewed that at least the following hypotheses (1)–(3) turned out to be implausible in explaining the olfactory dysfunction in patients [113]. This allegation is further confirmed by very recent studies showing that SARS-CoV-2 infection significantly increased the expression of interferon-stimulated and inflammatory genes. Alteration of extracellular matrix genes was also observed in ALI and iALI-infected cells [92]. Here, we will particularly review the mechanisms related to the second, the fourth, and the fifth scenarios according to the available findings. Healthy sensory cilia of ORNs in the olfactory epithelium are crucial in perceiving odorant molecules before sending the information to the olfactory bulbs and then to the upper parts of the brain [45]. It has been reported in humans that SARS-CoV-2 may indirectly affect the olfactory cilia, hindering the smelling system’s efficacy [114]. Reports suggested that ORNs lack the ability to express the entry proteins of SARS-CoV-2 in the OE. The virus seems to establish first contact in human nasal epithelia by binding its spike S protein to specific cells in the OE [115]. These reports are confirmed by a study based on in silico data, predicting that mature ORNs do not express the virus entry protein, the angiotensin-converting enzyme 2 (ACE2), and therefore are not likely to be infected by SARS-CoV-2 [81]. Furthermore, supporting data by Bryche et al. showed that SARS-CoV-2 was not detected in the ORNs of golden Siryan hamsters [85]. However, in a few cases, authors suggested that SARS-CoV-2 could infect ORNs in hamsters [111]. Based on the fact that COVID-19-related loss of smell disappeared within 1–2 weeks, while the regeneration of dead ORNs needs more than two weeks, many data tend to conclude that COVID-19-related olfactory dysfunction (OD) is not directly associated with the impairment of the ORNs [107,113,115,116,117]. Consequently, studying the entry protein expression within the cells in the OE will help to understand the sensitivity of the OE to SARS-CoV-2 infection related to the high prevalence of ODs in patients. Many groups are now interested in the organization of the sustentacular cells in the OE and thought that they might play a central role in leading to OD. A high level of expression of ACE2 and the transmembrane serine protease 2 (TMPRSS2) is particularly found on the sustentacular cells, suggesting a path to the neurotropism of SARS-CoV-2 in the OE. The ACE2 and TMPRSS2 are respectively known as the SARS-CoV-2 receptor and the SARS-CoV-2 cell entry-priming protease. ACE2 is found mainly on different parts of the sustentacular cells, both in humans and mice. The ACE2 and TMPRSS2 genes tend to be co-regulated [113,118,119,120,121,122]. Different approaches using tissues, cells, and organ systems in humans, golden Syrian hamsters, and hACE2 transgenic mice have been employed to study the pathological impact of the SARS-CoV-2. Here, we discussed findings related particularly to the OE in inducing ODs in humans. The spike protein (S protein) of SARS-CoV-2 mediates the passage of the virus into the host cell by fusing the viral and host cell membranes. In fact, via his spike S, SARS-CoV-2 employs ACE2 as the host functional receptor and TMPRSS2 as the cellular priming protease facilitating viral uptake, both signalings being confirmed by Single-cell RNA sequencing (scRNA-seq) datasets from the Human Cell Atlas consortium [123,124,125]. Another study showed that SARS-CoV-2 Nucleocapsid protein (NP) was observed in human OE through the neuronal marker Tuj1 9 h post-infection. This data further supported the enrichment of ACE2 in human olfactory sustentacular cells [119,126]. Earlier in the pandemic, the golden Syrian hamster was used as a model to document the pathology of SARS-CoV-2 in the OE post-infection. Reports showed that the sustentacular cells are rapidly infected by SARS-CoV-2. This viral neurotropism is associated with a massive recruitment of immune cells in the OE and lamina propria, which could drive the disorganization of the OE structure [85]. This study is consistent with a high level of Tumor Necrosis Factor α (TNF α) observed in OE samples from COVID-19-suffering patients and in ALI and iALI-infected cells [79,92]. Furthermore, the inflammation induced by SARS-CoV-2 infected supporting cells may play an important role in the onset and persistence of loss of smell in patients. This SARS-CoV-2-associated inflammation status was confirmed by the transcriptome of the in vitro human airway epithelium and by analyzing the expression of selected targets in the olfactory bulb using RNA-seq and RT-qPCR tools. Interestingly, this study showed that the proinflammatory markers, including NFKBIA, CSF1, FOSL1, Cxcl10, Il-1β, Ccl5, and Irf7 overexpression continued up to 14 dpi when animals had recovered from ageusia/anosmia [92,127]. These findings are in line with a very recent study showing the implication of immune cell infiltration and altered gene expression in OE in driving persistent smell loss in a subset of patients with SARS-CoV-2. Moreover, this study particularly demonstrates that T cell-mediated inflammation lasts longer in the OE after the acute SARS-CoV-2 infection has been eliminated from the tissue, suggesting a mechanistic insight into the long-term post-COVID-19 smell loss [105]. The OE disorganization is followed by a drastic deterioration of the cilia layer of the ORNs that leads to the impairment of the olfactory capacity of the animal [85]. Investigations in humans and hamsters using, respectively, Transmission Electron Microscopy (TEM) studies and Scanning Electron Microscopy (SEM) analysis showed various levels of cilia height that undergo regeneration in the course of patient recovery, including smell restoration. Data using the golden Syrian hamster showed that the regenerated cilia in the epithelium are accompanied by a decreased expression of FOXJ1+, highlighting the importance of this marker in respiratory ciliogenesis. This later finding by Schreiner et al., could in part shed light on the inquiry of how could we regenerate cilia during patient recovery, although a lot needs to be done in the roadmap of treating loss of smell related to nasal cilia deterioration by SARS-CoV-2 [55,104,128,129] (Figure 4; Table 1).

Figure 4.
Role of SARS-CoV-2 in olfactory impairment. SARS-CoV-2 induced disruption of the nasal epithelium, with loss/damage of olfactory sensory neurons, sustentacular cells, Bowman’s gland, and supporting cells. All figures were created with BioRender.com (accessed on 7 October 2023).
According to the literature, different variants of SARS-CoV-2 do not directly target the ORNs in the OE; instead, they are found in the majority expressed in the sustentacular cells [42]. A recent study by Seehusen et al. showed that K18-hACE2 transgenic mouse expressing the human ACE2 is highly sensitive to at least five variants of SARS-CoV-2 that infected not only the supportive cells in OE and the respiratory epithelium but invaded the CNS of the animal five days post-infection. Interestingly, the expression of hACE2 seems to convey higher binding affinity when compared to the wild-type mouse [130]. Using this transgenic mouse is revealed to be a serious option for therapy development against loss of smell as these animals exhibited low mortality when treated with COVID-19 convalescent antisera [80,130]. For instance, the miRNA is shown to play a crucial role in the regulation of immune genes deregulated, and the development of miRNA antagonists or mimics seems to be a promising new therapeutic strategy for the treatment of patients with COVID-19 on other respiratory viruses-induced PVOD [72,73,92,131,132,133].
It is now accepted that ACE2 is not the only obligate entry for SARS-CoV-2 as it has been suggested that molecules including PIKfyve or neuropilin-1 (NRP-1) may participate in SARS-CoV-2 entry [82,83,84]. Like ACE2, NRP-1 is highly expressed in the respiratory and olfactory epithelium, which further supports the infectivity and entry of SARS-CoV-2 in the human OE. NRP-1 is not only found in supportive cells but is expressed in nearly every cell type in the nasal passages, including the ORN, therefore giving SARS-CoV-2 a route to access those cells and impair the olfactory response. Interestingly, Daly et al. demonstrated that the selective inhibition of the S1-NRP-1 interaction reduces SARS-CoV-2 infection [84,86,87].
Taken together, the high expression of ACE2, TMPRSS2, and NRP-1 in supportive and other olfactory cells and their impact on olfactory neurophysiology maintenance and in the development of human olfactory pathophysiology supports them as potential targets for signaling-based therapeutics of olfactory dysfunction.
7. Pathological Implications of ARV Co-Infections
Coinfections with SARS CoV-2 and other ARVs may have tremendous pathological implications with potentially fatal outcomes [134,135,136,137]. The proportion of coinfections in SARS-CoV-2-infected patients and the types of viruses involved vary in different regions of the world depending on the sensitivity of the diagnostic tests used, population studied, climate, sampling period, and temporal variations in viral epidemiology. Some studies report proportions that vary from 1.7 to 20% [134,138,139,140,141,142,143]. Authors hypothesize that competitive advantage may play a role in SARS-CoV-2 interaction with other respiratory viruses during coinfection, and this is one reason why the coinfection rate in SARS-CoV-2 patients is much lower [142]. In many cases, the detection of RSV, influenza A, and other coinfections led to changes in clinical management in admitted patients to the medical intensive care unit [136,137].
Picornaviruses, influenza A and B, RSV, and parainfluenza are among the most detected viruses in COPD exacerbations [135]. Increased susceptibility to viral respiratory infections such as SARS-CoV-2 has been reported in chronic obstructive pulmonary disease (COPD), often worsened by bacterial co-infections and leading to serious clinical outcomes [137].
Trifonova et al. reported a more intensive replication of SARS CoV-2 and influenza viruses compared to that of other respiratory viruses involved in coinfections. The level of the viral load involved in mixed infections depends largely on the time of exposure to one virus relative to the other. These authors explain their findings by the fact of positive or negative interactions between SARS-CoV-2 and other respiratory viruses via interferon-mediated or other immunological mechanisms [134].
8. Clinical Trials for Respiratory Virus Infection
The protection against viral infection affecting human respiratory tracts constitutes a huge challenge and, therefore, forces clinicians and scientists to develop effective antivirals or vaccines to alleviate the death rate in patients [144]. Although mRNA COVID-19 vaccines are being authorized and administrated since 2020, for most of the respiratory viruses, there is no vaccine currently available. Moreover, in general, vaccination is efficient before the onset of the viral season infection. For example, the RSV seasonality was affected by the COVID-19 pandemic [145]. Currently, two molecules are being used against RSV: ribavirin for treatment and palivizumab for prevention. Many other antivirals to prevent RSV are in experimental stages as well as the use of anti-inflammatory drugs is to be considered, although the efficiency is to be verified [146]. To date, several recombinant RSV subunit vaccines are in different clinical phases 1 and 2 trials [147,148], which is in line with the undergoing clinical development of new vaccines for protecting the children and the elderly against RSV infection [149,150,151,152,153]. Very recently, the Food and Drug Administration (FDA) has approved the first vaccines for the prevention of RSV-associated low respiratory tract (LRT) in adults 60 years old and over. Both the GSK RSV vaccine and the Pfizer RSV vaccine were evaluated for their efficacy and safety. The efficacy of 1 dose of the GSK and Pfizer vaccine in preventing medically attended RSV-associated LRT was 77.5% and 81.0%, respectively, and both were safe according to the low severe reactogenicity events (group participants vs. control group participants) [145]. For instance, it is to be elucidated whether those new RSV vaccines will help to prevent the loss of smell within the URT in adults. The inhalation of ALX-0171 to prevent RSV infection in infants and toddlers is at Phase IIb clinical trial and is still under investigation for approval by the US FDA [154]. Regarding the PIV, the treatment is still for symptoms, although several investigations are being conducted to prepare a viable vaccine [155,156]. A study in 2019 has shown that DAS181, a sialidase fusion protein, may have clinical activity, particularly in immunocompromised patients with PIV, based on the data from the clinical phase 3 trials [157]. Karron and colleagues have recently conducted a phase I clinical trial of the live-attenuated recombinant human PIV2 in adults, as well as in children and seronegative children. In fact, rHPIV2-15C/948L/∆1724 was appropriately restricted in replication in adults and HPIV2-seropositive children but was overattenuated for HPIV2-seronegative children. Their evaluation showed that the rHPIV2-15C/948L/∆1724 represents less attenuated alternatives for pediatric vaccine development [158]. Further testing and clinical trials are required in the fight against the human PIV, especially PIV type 3, as it is considered to be the most virulent form of human PIV [159]. To our knowledge, there are no available vaccines or drugs against rhinovirus infections. Physical distancing seems to be not effective in reducing the transmission of this virus. But, it is essential to notice that rhinovirus infection is not associated with a significant rate of hospitalization or death [160]. Several researchers have demonstrated that daily dosing of 1 million IU of intranasal IFN-alpha gave 75–87% protection against rhinovirus infection [161,162]. The use of zinc gluconate lozenges has proven to be an effective treatment in reducing symptoms of rhinovirus infection [163]. For instance, if the vaccine against rhinovirus is not available, one may consider taking an antiviral with other antimediators for better protection from infection by the rhinovirus [164]. According to the WHO, COVID-19 treatment guidelines are evolving and fortunately several COVID-19 vaccines have been approved to actively immunize the general population. One can track the COVID-19 vaccines following this documentation [152].
9. Conclusions and Perspective
Our literature review further confirms the previous extended investigations showing that loss of smell and taste are among the key associated symptoms with most COVID-19 variants, including the omicron variant, which causes runny nose, headache, fatigue, sneezing, and sore throat [165]. The last three years have been an important rush towards deciphering the underlying mechanisms the SARS-CoV-2 deploys to impair the olfaction in infected patients. Furthermore, it is interesting to delineate the similarities and differences between the molecular mechanisms of SARS-CoV-2 and the other respiratory viruses induced olfactory dysfunction. Both SARS-CoV-2 and non-SARS-CoV-2 attach to the cilia during the initial stages of infection to later enter the nasal epithelium [55]. Interestingly, all these findings underline the importance of the immune-mediated inflammatory injury to the olfactory neuroepithelium that is now accepted as a consequence of those URT virus infections. However, the molecular signature related to the type of olfactory dysfunction, according to Table 1, seems particular for each respiratory virus. The animal or cellular models being used and the seasonal periods could add more complexity to the putative mechanisms for viral infection-induced olfactory dysfunction. Investigative literature on the COVID-19 mechanistic route has made clear that this virus seems to attach to ACE2-TMPRSS2 complex and/or NRP-1 on the host cell prior to infection and later triggers intrinsic immune responses. In the nasal mucosal microenvironment, those markers play a crucial role in inflammatory response mechanisms and are confirmed by several recent studies on understanding SARS-CoV-2 invasion [80,82,83,84,86,130,166]. Currently, the mechanism of how SARS-CoV-2 causes smell loss is widely documented, and more investigations are needed on the non-SARS-CoV-2 to complete the picture of comparing the particularity of each respiratory virus causing URT-related PVOD. For instance, this work emphasizes the urgency and necessity of finding an adequate therapeutic solution against COVID-19 and other respiratory viral pathogens-induced olfactory dysfunction. In addition, the mechanisms of taste dysfunction due to COVID-19 infection are not discussed in this review. But, it would be interesting to decipher the possible pathogenesis between ageusia and anosmia and other types of PVOD in COVID-19 and other non-COVID-19 patients in the future.
Author Contributions
Writing—original draft preparation, S.F.W. and A.A.M.D.; Writing—review and editing, S.F.W., A.A.M.D., B.N., F.T. and N.D.; Conceptualization and supervision, S.F.W. and A.A.M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, J.T.; Leung, K.; Leung, G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: A modelling study. Lancet 2020, 395, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.S.; Azhar, E.I.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; McHugh, T.D.; Memish, Z.A.; Drosten, C.; et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Seiden, A.M. Postviral olfactory loss. Otolaryngol. Clin. N. Am. 2004, 37, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Zhen Yu, L.; Luigi Angelo, V.; Paolo, B.-R.; Abigail, W.; Claire, H. Post-viral olfactory loss and parosmia. BMJ Med. 2023, 2, e000382. [Google Scholar] [CrossRef]
- Moran, D.T.; Jafek, B.W.; Eller, P.M.; Rowley, J.C., 3rd. Ultrastructural histopathology of human olfactory dysfunction. Microsc. Res. Tech. 1992, 23, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Welge-Lussen, A.; Wolfensberger, M. Olfactory disorders following upper respiratory tract infections. Adv. Otorhinolaryngol. 2006, 63, 125–132. [Google Scholar] [CrossRef]
- Dicpinigaitis, P.V. Post-viral Anosmia (Loss of Sensation of Smell) Did Not Begin with COVID-19! Lung 2021, 199, 237–238. [Google Scholar] [CrossRef]
- Tian, J.; Pinto, J.M.; Li, L.; Zhang, S.; Sun, Z.; Wei, Y. Identification of Viruses in Patients with Postviral Olfactory Dysfunction by Multiplex Reverse-Transcription Polymerase Chain Reaction. Laryngoscope 2021, 131, 158–164. [Google Scholar] [CrossRef]
- Suzuki, M.; Saito, K.; Min, W.P.; Vladau, C.; Toida, K.; Itoh, H.; Murakami, S. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope 2007, 117, 272–277. [Google Scholar] [CrossRef]
- Imam, S.A.; Lao, W.P.; Reddy, P.; Nguyen, S.A.; Schlosser, R.J. Is SARS-CoV-2 (COVID-19) postviral olfactory dysfunction (PVOD) different from other PVOD? World J. Otorhinolaryngol. Head. Neck Surg. 2020, 6 (Suppl. S1), S26–S32. [Google Scholar] [CrossRef]
- Sugiura, M.; Aiba, T.; Mori, J.; Nakai, Y. An epidemiological study of postviral olfactory disorder. Acta Otolaryngol. Suppl. 1998, 538, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Chiguer, D.L.; Tirado-Mendoza, R.; Marquez-Navarro, A.; Ambrosio-Hernandez, J.R.; Ruiz-Fraga, I.; Aguilar-Vargas, R.E.; Lira-Martinez, J.M.; Lopez-Valdes, J.C. Detection and molecular characterization of respiratory viruses that cause acute respiratory infection in the adult population. Gac. Med. Mex. 2019, 155 (Suppl. S1), S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Ueha, R.; Mukherjee, S.; Ueha, S.; de Almeida Nagata, D.E.; Sakamoto, T.; Kondo, K.; Yamasoba, T.; Lukacs, N.W.; Kunkel, S.L. Viral disruption of olfactory progenitors is exacerbated in allergic mice. Int. Immunopharmacol. 2014, 22, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, T.; Jarvinen, A. The common cold. Lancet 2003, 361, 51–59. [Google Scholar] [CrossRef]
- Potter, M.R.; Chen, J.H.; Lobban, N.S.; Doty, R.L. Olfactory dysfunction from acute upper respiratory infections: Relationship to season of onset. Int. Forum Allergy Rhinol. 2020, 10, 706–712. [Google Scholar] [CrossRef]
- Henrickson, K.J. Parainfluenza viruses. Clin. Microbiol. Rev. 2003, 16, 242–264. [Google Scholar] [CrossRef]
- Rafeek, R.A.M.; Divarathna, M.V.M.; Noordeen, F. A review on disease burden and epidemiology of childhood parainfluenza virus infections in Asian countries. Rev. Med. Virol. 2021, 31, e2164. [Google Scholar] [CrossRef]
- Weston, S.; Frieman, M.B. Respiratory Viruses. In Encyclopedia of Microbiology (Fourth Edition); Schmidt, T.M., Ed.; Academic Press: Oxford, UK, 2019; pp. 85–101. [Google Scholar] [CrossRef]
- van Kempen, M.; Bachert, C.; Van Cauwenberge, P. An update on the pathophysiology of rhinovirus upper respiratory tract infections. Rhinology 1999, 37, 97–103. [Google Scholar]
- Xatzipsalti, M.; Kyrana, S.; Tsolia, M.; Psarras, S.; Bossios, A.; Laza-Stanca, V.; Johnston, S.L.; Papadopoulos, N.G. Rhinovirus viremia in children with respiratory infections. Am. J. Respir. Crit. Care Med. 2005, 172, 1037–1040. [Google Scholar] [CrossRef]
- Loeffelholz, M.J.; Trujillo, R.; Pyles, R.B.; Miller, A.L.; Alvarez-Fernandez, P.; Pong, D.L.; Chonmaitree, T. Duration of rhinovirus shedding in the upper respiratory tract in the first year of life. Pediatrics 2014, 134, 1144–1150. [Google Scholar] [CrossRef]
- WHO. Severe Acute Respiratory Syndrome (SARS). Available online: https://www.who.int/health-topics/severe-acute-respiratory-syndrome#tab=tab_3 (accessed on 3 July 2023).
- de Groot, R.J.; Baker, S.C.; Baric, R.S.; Brown, C.S.; Drosten, C.; Enjuanes, L.; Fouchier, R.A.; Galiano, M.; Gorbalenya, A.E.; Memish, Z.A.; et al. Middle East respiratory syndrome coronavirus (MERS-CoV): Announcement of the Coronavirus Study Group. J. Virol. 2013, 87, 7790–7792. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Khalafalla, A.I.; Lu, X.; Al-Mubarak, A.I.; Dalab, A.H.; Al-Busadah, K.A.; Erdman, D.D. MERS-CoV in Upper Respiratory Tract and Lungs of Dromedary Camels, Saudi Arabia, 2013–2014. Emerg. Infect. Dis. 2015, 21, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Wolfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Muller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef]
- de Melo, G.D.; Lazarini, F.; Levallois, S.; Hautefort, C.; Michel, V.; Larrous, F.; Verillaud, B.; Aparicio, C.; Wagner, S.; Gheusi, G.; et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci. Transl. Med. 2021, 13, 596. [Google Scholar] [CrossRef] [PubMed]
- Zazhytska, M.; Kodra, A.; Hoagland, D.A.; Frere, J.; Fullard, J.F.; Shayya, H.; McArthur, N.G.; Moeller, R.; Uhl, S.; Omer, A.D.; et al. Non-cell-autonomous disruption of nuclear architecture as a potential cause of COVID-19-induced anosmia. Cell 2022, 185, 1052–1064.e12. [Google Scholar] [CrossRef] [PubMed]
- Bilinska, K.; Butowt, R. Anosmia in COVID-19: A Bumpy Road to Establishing a Cellular Mechanism. ACS Chem. Neurosci. 2020, 11, 2152–2155. [Google Scholar] [CrossRef]
- Samaranayake, L.P.; Fakhruddin, K.S.; Panduwawala, C. Sudden onset, acute loss of taste and smell in coronavirus disease 2019 (COVID-19): A systematic review. Acta Odontol. Scand. 2020, 78, 467–473. [Google Scholar] [CrossRef]
- Dammalli, M.; Dey, G.; Madugundu, A.K.; Kumar, M.; Rodrigues, B.; Gowda, H.; Siddaiah, B.G.; Mahadevan, A.; Shankar, S.K.; Prasad, T.S.K. Proteomic Analysis of the Human Olfactory Bulb. OMICS 2017, 21, 440–453. [Google Scholar] [CrossRef]
- Dammalli, M.; Dey, G.; Kumar, M.; Madugundu, A.K.; Gopalakrishnan, L.; Gowrishankar, B.S.; Mahadevan, A.; Shankar, S.K.; Prasad, T.S.K. Proteomics of the Human Olfactory Tract. OMICS 2018, 22, 77–87. [Google Scholar] [CrossRef]
- Oboti, L.; Peretto, P.; Marchis, S.D.; Fasolo, A. From chemical neuroanatomy to an understanding of the olfactory system. Eur. J. Histochem. 2011, 55, e35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barral-Arca, R.; Gomez-Carballa, A.; Cebey-Lopez, M.; Bello, X.; Martinon-Torres, F.; Salas, A. A Meta-Analysis of Multiple Whole Blood Gene Expression Data Unveils a Diagnostic Host-Response Transcript Signature for Respiratory Syncytial Virus. Int. J. Mol. Sci. 2020, 21, 1831. [Google Scholar] [CrossRef] [PubMed]
- Boesveldt, S.; Postma, E.M.; Boak, D.; Welge-Luessen, A.; Schopf, V.; Mainland, J.D.; Martens, J.; Ngai, J.; Duffy, V.B. Anosmia-A Clinical Review. Chem. Senses 2017, 42, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Firestein, S. How the olfactory system makes sense of scents. Nature 2001, 413, 211–218. [Google Scholar] [CrossRef]
- Brennan, P.A.; Keverne, E.B. Something in the air? New insights into mammalian pheromones. Curr. Biol. 2004, 14, R81–R89. [Google Scholar] [CrossRef]
- Buck, L.B. The molecular architecture of odor and pheromone sensing in mammals. Cell 2000, 100, 611–618. [Google Scholar] [CrossRef]
- Mombaerts, P. Genes and ligands for odorant, vomeronasal and taste receptors. Nat. Rev. Neurosci. 2004, 5, 263–278. [Google Scholar] [CrossRef]
- Restrepo, D.; Arellano, J.; Oliva, A.M.; Schaefer, M.L.; Lin, W. Emerging views on the distinct but related roles of the main and accessory olfactory systems in responsiveness to chemosensory signals in mice. Horm. Behav. 2004, 46, 247–256. [Google Scholar] [CrossRef]
- Lavoie, J.; Gasso Astorga, P.; Segal-Gavish, H.; Wu, Y.C.; Chung, Y.; Cascella, N.G.; Sawa, A.; Ishizuka, K. The Olfactory Neural Epithelium As a Tool in Neuroscience. Trends Mol. Med. 2017, 23, 100–103. [Google Scholar] [CrossRef]
- Liang, F.; Wang, Y. COVID-19 Anosmia: High Prevalence, Plural Neuropathogenic Mechanisms, and Scarce Neurotropism of SARS-CoV-2? Viruses 2021, 13, 2225. [Google Scholar] [CrossRef]
- Olender, T.; Lancet, D.; Nebert, D.W. Update on the olfactory receptor (OR) gene superfamily. Hum. Genom. 2008, 3, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Kurian, S.M.; Gordon, S.; Barrick, B.; Dadlani, M.N.; Fanelli, B.; Cornell, J.B.; Head, S.R.; Marsh, C.L.; Case, J. Feasibility and Comparison Study of Fecal Sample Collection Methods in Healthy Volunteers and Solid Organ Transplant Recipients Using 16S rRNA and Metagenomics Approaches. Biopreserv. Biobank. 2020, 18, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Glezer, I.; Malnic, B. Olfactory receptor function. Handb. Clin. Neurol. 2019, 164, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Schwob, J.E. Neural regeneration and the peripheral olfactory system. Anat. Rec. 2002, 269, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Graziadei, P.P.; Graziadei, G.A. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J. Neurocytol. 1979, 8, 1–18. [Google Scholar] [CrossRef]
- Urata, S.; Maruyama, J.; Kishimoto-Urata, M.; Sattler, R.A.; Cook, R.; Lin, N.; Yamasoba, T.; Makishima, T.; Paessler, S. Regeneration Profiles of Olfactory Epithelium after SARS-CoV-2 Infection in Golden Syrian Hamsters. ACS Chem. Neurosci. 2021, 12, 589–595. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, W.H.; Wee, J.H.; Kim, J.W. Prognosis of postviral olfactory loss: Follow-up study for longer than one year. Am. J. Rhinol. Allergy 2014, 28, 419–422. [Google Scholar] [CrossRef]
- Doty, R.L.; Hawkes, C.H. Chemosensory dysfunction in neurodegenerative diseases. Handb. Clin. Neurol. 2019, 164, 325–360. [Google Scholar] [CrossRef]
- Wang, J.H.; Kwon, H.J.; Jang, Y.J. Detection of parainfluenza virus 3 in turbinate epithelial cells of postviral olfactory dysfunction patients. Laryngoscope 2007, 117, 1445–1449. [Google Scholar] [CrossRef]
- Lewandowska-Polak, A.; Brauncajs, M.; Paradowska, E.; Jarzebska, M.; Kurowski, M.; Moskwa, S.; Lesnikowski, Z.J.; Kowalski, M.L. Human parainfluenza virus type 3 (HPIV3) induces production of IFNgamma and RANTES in human nasal epithelial cells (HNECs). J. Inflamm. 2015, 12, 16. [Google Scholar] [CrossRef]
- Mori, I.; Komatsu, T.; Takeuchi, K.; Nakakuki, K.; Sudo, M.; Kimura, Y. Parainfluenza virus type 1 infects olfactory neurons and establishes long-term persistence in the nerve tissue. J. Gen. Virol. 1995, 76 Pt 5, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Pinto, J.M.; Cui, X.; Zhang, H.; Li, L.; Liu, Y.; Wu, C.; Wei, Y. Sendai Virus Induces Persistent Olfactory Dysfunction in a Murine Model of PVOD via Effects on Apoptosis, Cell Proliferation, and Response to Odorants. PLoS ONE 2016, 11, e0159033. [Google Scholar] [CrossRef]
- Wu, C.T.; Lidsky, P.V.; Xiao, Y.; Cheng, R.; Lee, I.T.; Nakayama, T.; Jiang, S.; He, W.; Demeter, J.; Knight, M.G.; et al. SARS-CoV-2 replication in airway epithelia requires motile cilia and microvillar reprogramming. Cell 2023, 186, 112–130 e120. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Volpe, S.J.; Chang, E.H. The Role of Viruses in the Inception of Chronic Rhinosinusitis. Clin. Exp. Otorhinolaryngol. 2022, 15, 310–318. [Google Scholar] [CrossRef]
- Gao, J.; Choudhary, S.; Banerjee, A.K.; De, B.P. Human parainfluenza virus type 3 upregulates ICAM-1 (CD54) expression in a cytokine-independent manner. Gene Expr. 2000, 9, 115–121. [Google Scholar] [CrossRef][Green Version]
- Cieslik, M.; Chinnaiyan, A.M. Cancer transcriptome profiling at the juncture of clinical translation. Nat. Rev. Genet. 2018, 19, 93–109. [Google Scholar] [CrossRef]
- Worst, B.C.; van Tilburg, C.M.; Balasubramanian, G.P.; Fiesel, P.; Witt, R.; Freitag, A.; Boudalil, M.; Previti, C.; Wolf, S.; Schmidt, S.; et al. Next-generation personalised medicine for high-risk paediatric cancer patients—The INFORM pilot study. Eur. J. Cancer 2016, 65, 91–101. [Google Scholar] [CrossRef]
- Weidenbusch, B.; Richter, G.H.S.; Kesper, M.S.; Guggemoos, M.; Gall, K.; Prexler, C.; Kazantsev, I.; Sipol, A.; Lindner, L.; Nathrath, M.; et al. Transcriptome based individualized therapy of refractory pediatric sarcomas: Feasibility, tolerability and efficacy. Oncotarget 2018, 9, 20747–20760. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.M.; Fountzilas, E.; Bleris, L.; Kurzrock, R. Transcriptomics and solid tumors: The next frontier in precision cancer medicine. Semin. Cancer Biol. 2022, 84, 50–59. [Google Scholar] [CrossRef]
- Rodon, J.; Soria, J.C.; Berger, R.; Miller, W.H.; Rubin, E.; Kugel, A.; Tsimberidou, A.; Saintigny, P.; Ackerstein, A.; Brana, I.; et al. Genomic and transcriptomic profiling expands precision cancer medicine: The WINTHER trial. Nat. Med. 2019, 25, 751–758. [Google Scholar] [CrossRef]
- Oberg, J.A.; Glade Bender, J.L.; Sulis, M.L.; Pendrick, D.; Sireci, A.N.; Hsiao, S.J.; Turk, A.T.; Dela Cruz, F.S.; Hibshoosh, H.; Remotti, H.; et al. Implementation of next generation sequencing into pediatric hematology-oncology practice: Moving beyond actionable alterations. Genome Med. 2016, 8, 133. [Google Scholar] [CrossRef]
- Nicolas De Lamballerie, C.; Pizzorno, A.; Dubois, J.; Padey, B.; Julien, T.; Traversier, A.; Carbonneau, J.; Orcel, E.; Lina, B.; Hamelin, M.E.; et al. Human Respiratory Syncytial Virus-Induced Immune Signature of Infection Revealed by Transcriptome Analysis of Clinical Pediatric Nasopharyngeal Swab Samples. J. Infect. Dis. 2021, 223, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, T.K.; Schauble, S.; Mirhakkak, M.H.; Wu, W.L.; Ng, A.C.; Yip, C.C.Y.; Lopez, A.G.; Wolf, T.; Yeung, M.L.; Chan, K.H.; et al. Comparative Transcriptomic Analysis of Rhinovirus and Influenza Virus Infection. Front. Microbiol. 2020, 11, 1580. [Google Scholar] [CrossRef] [PubMed]
- Al-Shalan, H.A.M.; Hu, D.; Wang, P.; Uddin, J.; Chopra, A.; Greene, W.K.; Ma, B. Transcriptomic Profiling of Influenza A Virus-Infected Mouse Lung at Recovery Stage Using RNA Sequencing. Viruses 2023, 15, 2198. [Google Scholar] [CrossRef]
- Bryche, B.; Fretaud, M.; Saint-Albin Deliot, A.; Galloux, M.; Sedano, L.; Langevin, C.; Descamps, D.; Rameix-Welti, M.A.; Eleouet, J.F.; Le Goffic, R.; et al. Respiratory syncytial virus tropism for olfactory sensory neurons in mice. J. Neurochem. 2020, 155, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.D.; Bilawchuk, L.M.; McDonough, J.E.; Jamieson, K.C.; Elawar, F.; Cen, Y.; Duan, W.; Lin, C.; Song, H.; Casanova, J.L.; et al. IGF1R is an entry receptor for respiratory syncytial virus. Nature 2020, 583, 615–619. [Google Scholar] [CrossRef]
- Tayyari, F.; Marchant, D.; Moraes, T.J.; Duan, W.; Mastrangelo, P.; Hegele, R.G. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat. Med. 2011, 17, 1132–1135. [Google Scholar] [CrossRef]
- Mastrangelo, P.; Chin, A.A.; Tan, S.; Jeon, A.H.; Ackerley, C.A.; Siu, K.K.; Lee, J.E.; Hegele, R.G. Identification of RSV Fusion Protein Interaction Domains on the Virus Receptor, Nucleolin. Viruses 2021, 13, 261. [Google Scholar] [CrossRef]
- Sourimant, J.; Lieber, C.M.; Aggarwal, M.; Cox, R.M.; Wolf, J.D.; Yoon, J.J.; Toots, M.; Ye, C.; Sticher, Z.; Kolykhalov, A.A.; et al. 4′-Fluorouridine is an oral antiviral that blocks respiratory syncytial virus and SARS-CoV-2 replication. Science 2022, 375, 161–167. [Google Scholar] [CrossRef]
- Li, H.H.; Xu, J.; He, L.; Denny, L.I.; Rustandi, R.R.; Dornadula, G.; Fiorito, B.; Zhang, Z.Q. Development and qualification of cell-based relative potency assay for a human respiratory syncytial virus (RSV) mRNA vaccine. J. Pharm. Biomed. Anal. 2023, 234, 115523. [Google Scholar] [CrossRef]
- Whitaker, J.A.; Sahly, H.M.E.; Healy, C.M. mRNA vaccines against respiratory viruses. Curr. Opin. Infect. Dis. 2023, 36, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, Y.A.; Watters, K.; Ashraf, S.; Griggs, T.F.; Devries, M.K.; Jackson, D.J.; Palmenberg, A.C.; Gern, J.E. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc. Natl. Acad. Sci. USA 2015, 112, 5485–5490. [Google Scholar] [CrossRef] [PubMed]
- Staunton, D.E.; Merluzzi, V.J.; Rothlein, R.; Barton, R.; Marlin, S.D.; Springer, T.A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell 1989, 56, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Hofer, F.; Gruenberger, M.; Kowalski, H.; Machat, H.; Huettinger, M.; Kuechler, E.; Blaas, D. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc. Natl. Acad. Sci. USA 1994, 91, 1839–1842. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, Y.A.; Gern, J.E. Rhinoviruses and Their Receptors: Implications for Allergic Disease. Curr. Allergy Asthma Rep. 2016, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Han, M.S.; Lee, T.H.; Lee, D.B.; Park, J.H.; Lee, S.H.; Kim, T.H. Hydrogen peroxide attenuates rhinovirus-induced anti-viral interferon secretion in sinonasal epithelial cells. Front. Immunol. 2023, 14, 1086381. [Google Scholar] [CrossRef]
- Torabi, A.; Mohammadbagheri, E.; Akbari Dilmaghani, N.; Bayat, A.H.; Fathi, M.; Vakili, K.; Alizadeh, R.; Rezaeimirghaed, O.; Hajiesmaeili, M.; Ramezani, M.; et al. Proinflammatory Cytokines in the Olfactory Mucosa Result in COVID-19 Induced Anosmia. ACS Chem. Neurosci. 2020, 11, 1909–1913. [Google Scholar] [CrossRef]
- Zheng, J.; Wong, L.R.; Li, K.; Verma, A.K.; Ortiz, M.E.; Wohlford-Lenane, C.; Leidinger, M.R.; Knudson, C.M.; Meyerholz, D.K.; McCray, P.B., Jr.; et al. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature 2021, 589, 603–607. [Google Scholar] [CrossRef]
- Bilinska, K.; Jakubowska, P.; Von Bartheld, C.S.; Butowt, R. Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age. ACS Chem. Neurosci. 2020, 11, 1555–1562. [Google Scholar] [CrossRef]
- Davies, J.; Randeva, H.S.; Chatha, K.; Hall, M.; Spandidos, D.A.; Karteris, E.; Kyrou, I. Neuropilin-1 as a new potential SARS-CoV-2 infection mediator implicated in the neurologic features and central nervous system involvement of COVID-19. Mol. Med. Rep. 2020, 22, 4221–4226. [Google Scholar] [CrossRef]
- Kang, Y.L.; Chou, Y.Y.; Rothlauf, P.W.; Liu, Z.; Soh, T.K.; Cureton, D.; Case, J.B.; Chen, R.E.; Diamond, M.S.; Whelan, S.P.J.; et al. Inhibition of PIKfyve kinase prevents infection by Zaire ebolavirus and SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 20803–20813. [Google Scholar] [CrossRef] [PubMed]
- Mayi, B.S.; Leibowitz, J.A.; Woods, A.T.; Ammon, K.A.; Liu, A.E.; Raja, A. The role of Neuropilin-1 in COVID-19. PLoS Pathog. 2021, 17, e1009153. [Google Scholar] [CrossRef] [PubMed]
- Bryche, B.; St Albin, A.; Murri, S.; Lacote, S.; Pulido, C.; Ar Gouilh, M.; Lesellier, S.; Servat, A.; Wasniewski, M.; Picard-Meyer, E.; et al. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav. Immun. 2020, 89, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.L.; Simonetti, B.; Klein, K.; Chen, K.E.; Williamson, M.K.; Anton-Plagaro, C.; Shoemark, D.K.; Simon-Gracia, L.; Bauer, M.; Hollandi, R.; et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 2020, 370, 861–865. [Google Scholar] [CrossRef]
- Acevedo, C.; Blanchard, K.; Bacigalupo, J.; Vergara, C. Possible ATP trafficking by ATP-shuttles in the olfactory cilia and glucose transfer across the olfactory mucosa. FEBS Lett. 2019, 593, 601–610. [Google Scholar] [CrossRef]
- Villar, P.S.; Vergara, C.; Bacigalupo, J. Energy sources that fuel metabolic processes in protruding finger-like organelles. FEBS J. 2021, 288, 3799–3812. [Google Scholar] [CrossRef]
- Krishnan, S.; Nordqvist, H.; Ambikan, A.T.; Gupta, S.; Sperk, M.; Svensson-Akusjarvi, S.; Mikaeloff, F.; Benfeitas, R.; Saccon, E.; Ponnan, S.M.; et al. Metabolic Perturbation Associated With COVID-19 Disease Severity and SARS-CoV-2 Replication. Mol. Cell Proteom. 2021, 20, 100159. [Google Scholar] [CrossRef]
- Khan, M.; Yoo, S.J.; Clijsters, M.; Backaert, W.; Vanstapel, A.; Speleman, K.; Lietaer, C.; Choi, S.; Hether, T.D.; Marcelis, L.; et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell 2021, 184, 5932–5949 e5915. [Google Scholar] [CrossRef]
- Assou, S.; Ahmed, E.; Morichon, L.; Nasri, A.; Foisset, F.; Bourdais, C.; Gros, N.; Tieo, S.; Petit, A.; Vachier, I.; et al. The Transcriptome Landscape of the In Vitro Human Airway Epithelium Response to SARS-CoV-2. Int. J. Mol. Sci. 2023, 24, 12017. [Google Scholar] [CrossRef]
- Lavoie, T.B.; Kalie, E.; Crisafulli-Cabatu, S.; Abramovich, R.; DiGioia, G.; Moolchan, K.; Pestka, S.; Schreiber, G. Binding and activity of all human alpha interferon subtypes. Cytokine 2011, 56, 282–289. [Google Scholar] [CrossRef]
- Jaks, E.; Gavutis, M.; Uze, G.; Martal, J.; Piehler, J. Differential receptor subunit affinities of type I interferons govern differential signal activation. J. Mol. Biol. 2007, 366, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.P.; Sheikh, F.; Kotenko, S.V.; Dickensheets, H. The expanded family of class II cytokines that share the IL-10 receptor-2 (IL-10R2) chain. J. Leukoc. Biol. 2004, 76, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ninaber, D.K.; van Schadewijk, A.; Hiemstra, P.S. Tiotropium and Fluticasone Inhibit Rhinovirus-Induced Mucin Production via Multiple Mechanisms in Differentiated Airway Epithelial Cells. Front. Cell Infect. Microbiol. 2020, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Lo, D.; Kennedy, J.L.; Kurten, R.C.; Panettieri, R.A., Jr.; Koziol-White, C.J. Modulation of airway hyperresponsiveness by rhinovirus exposure. Respir. Res. 2018, 19, 208. [Google Scholar] [CrossRef]
- Loxham, M.; Smart, D.E.; Bedke, N.J.; Smithers, N.P.; Filippi, I.; Blume, C.; Swindle, E.J.; Tariq, K.; Howarth, P.H.; Holgate, S.T.; et al. Allergenic proteases cleave the chemokine CX3CL1 directly from the surface of airway epithelium and augment the effect of rhinovirus. Mucosal Immunol. 2018, 11, 404–414. [Google Scholar] [CrossRef]
- Papi, A.; Papadopoulos, N.G.; Stanciu, L.A.; Bellettato, C.M.; Pinamonti, S.; Degitz, K.; Holgate, S.T.; Johnston, S.L. Reducing agents inhibit rhinovirus-induced up-regulation of the rhinovirus receptor intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells. FASEB J. 2002, 16, 1934–1936. [Google Scholar] [CrossRef]
- Song, Y.P.; Tang, M.F.; Leung, A.S.Y.; Tao, K.P.; Chan, O.M.; Wong, G.W.K.; Chan, P.K.S.; Chan, R.W.Y.; Leung, T.F. Interactive effects between CDHR3 genotype and rhinovirus species for diagnosis and severity of respiratory tract infections in hospitalized children. Microbiol. Spectr. 2023, 11, e0118123. [Google Scholar] [CrossRef]
- Greiller, C.L.; Suri, R.; Jolliffe, D.A.; Kebadze, T.; Hirsman, A.G.; Griffiths, C.J.; Johnston, S.L.; Martineau, A.R. Vitamin D attenuates rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) and platelet-activating factor receptor (PAFR) in respiratory epithelial cells. J. Steroid Biochem. Mol. Biol. 2019, 187, 152–159. [Google Scholar] [CrossRef]
- Khani, E.; Khiali, S.; Beheshtirouy, S.; Entezari-Maleki, T. Potential pharmacologic treatments for COVID-19 smell and taste loss: A comprehensive review. Eur. J. Pharmacol. 2021, 912, 174582. [Google Scholar] [CrossRef]
- Silva Andrade, B.; Siqueira, S.; de Assis Soares, W.R.; de Souza Rangel, F.; Santos, N.O.; Dos Santos Freitas, A.; Ribeiro da Silveira, P.; Tiwari, S.; Alzahrani, K.J.; Goes-Neto, A.; et al. Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses 2021, 13, 700. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-de-Las-Penas, C.; Cancela-Cilleruelo, I.; Rodriguez-Jimenez, J.; Gomez-Mayordomo, V.; Pellicer-Valero, O.J.; Martin-Guerrero, J.D.; Hernandez-Barrera, V.; Arendt-Nielsen, L.; Torres-Macho, J. Associated-Onset Symptoms and Post-COVID-19 Symptoms in Hospitalized COVID-19 Survivors Infected with Wuhan, Alpha or Delta SARS-CoV-2 Variant. Pathogens 2022, 11, 725. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Sevilla, J.J.; Guerri-Fernadez, R.; Bertran Recasens, B. Is There Less Alteration of Smell Sensation in Patients with Omicron SARS-CoV-2 Variant Infection? Front Med. 2022, 9, 852998. [Google Scholar] [CrossRef] [PubMed]
- Chee, J.; Chern, B.; Loh, W.S.; Mullol, J.; Wang, Y. Pathophysiology of SARS-CoV-2 Infection of Nasal Respiratory and Olfactory Epithelia and Its Clinical Impact. Curr. Allergy Asthma Rep. 2023, 23, 121–131. [Google Scholar] [CrossRef]
- Mutiawati, E.; Fahriani, M.; Mamada, S.S.; Fajar, J.K.; Frediansyah, A.; Maliga, H.A.; Ilmawan, M.; Emran, T.B.; Ophinni, Y.; Ichsan, I.; et al. Anosmia and dysgeusia in SARS-CoV-2 infection: Incidence and effects on COVID-19 severity and mortality, and the possible pathobiology mechanisms—A systematic review and meta-analysis. F1000Res 2021, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Butowt, R.; von Bartheld, C.S. Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection. Neuroscientist 2021, 27, 582–603. [Google Scholar] [CrossRef]
- Kalra, R.S.; Dhanjal, J.K.; Meena, A.S.; Kalel, V.C.; Dahiya, S.; Singh, B.; Dewanjee, S.; Kandimalla, R. COVID-19, Neuropathology, and Aging: SARS-CoV-2 Neurological Infection, Mechanism, and Associated Complications. Front. Aging Neurosci. 2021, 13, 662786. [Google Scholar] [CrossRef]
- Rebholz, H.; Braun, R.J.; Ladage, D.; Knoll, W.; Kleber, C.; Hassel, A.W. Loss of Olfactory Function-Early Indicator for COVID-19, Other Viral Infections and Neurodegenerative Disorders. Front. Neurol. 2020, 11, 569333. [Google Scholar] [CrossRef]
- Reyna, R.A.; Kishimoto-Urata, M.; Urata, S.; Makishima, T.; Paessler, S.; Maruyama, J. Recovery of anosmia in hamsters infected with SARS-CoV-2 is correlated with repair of the olfactory epithelium. Sci. Rep. 2022, 12, 628. [Google Scholar] [CrossRef]
- Tanzadehpanah, H.; Lotfian, E.; Avan, A.; Saki, S.; Nobari, S.; Mahmoodian, R.; Sheykhhasan, M.; Froutagh, M.H.S.; Ghotbani, F.; Jamshidi, R.; et al. Role of SARS-CoV-2 and ACE2 in the pathophysiology of peripheral vascular diseases. Biomed. Pharmacother. 2023, 166, 115321. [Google Scholar] [CrossRef]
- Butowt, R.; Bilinska, K.; von Bartheld, C.S. Olfactory dysfunction in COVID-19: New insights into the underlying mechanisms. Trends Neurosci. 2023, 46, 75–90. [Google Scholar] [CrossRef]
- Buqaileh, R.; Saternos, H.; Ley, S.; Aranda, A.; Forero, K.; AbouAlaiwi, W.A. Can cilia provide an entry gateway for SARS-CoV-2 to human ciliated cells? Physiol. Genom. 2021, 53, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Butowt, R.; Meunier, N.; Bryche, B.; von Bartheld, C.S. The olfactory nerve is not a likely route to brain infection in COVID-19: A critical review of data from humans and animal models. Acta Neuropathol. 2021, 141, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Otorhinolaryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Min, P.; Lee, S.; Kim, S.W. Prevalence and Duration of Acute Loss of Smell or Taste in COVID-19 Patients. J. Korean Med. Sci. 2020, 35, e174. [Google Scholar] [CrossRef] [PubMed]
- Fodoulian, L.; Tuberosa, J.; Rossier, D.; Boillat, M.; Kan, C.; Pauli, V.; Egervari, K.; Lobrinus, J.A.; Landis, B.N.; Carleton, A.; et al. SARS-CoV-2 Receptors and Entry Genes Are Expressed in the Human Olfactory Neuroepithelium and Brain. iScience 2020, 23, 101839. [Google Scholar] [CrossRef]
- Chen, M.; Shen, W.; Rowan, N.R.; Kulaga, H.; Hillel, A.; Ramanathan, M., Jr.; Lane, A.P. Elevated ACE-2 expression in the olfactory neuroepithelium: Implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur. Respir. J. 2020, 56, 2001948. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280 e278. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e1019. [Google Scholar] [CrossRef]
- Gkogkou, E.; Barnasas, G.; Vougas, K.; Trougakos, I.P. Expression profiling meta-analysis of ACE2 and TMPRSS2, the putative anti-inflammatory receptor and priming protease of SARS-CoV-2 in human cells, and identification of putative modulators. Redox Biol. 2020, 36, 101615. [Google Scholar] [CrossRef]
- Sungnak, W.; Huang, N.; Becavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-Lopez, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Ou, G. COVID-19, cilia, and smell. FEBS J. 2020, 287, 3672–3676. [Google Scholar] [CrossRef]
- Sternberg, A.; Naujokat, C. Structural features of coronavirus SARS-CoV-2 spike protein: Targets for vaccination. Life Sci. 2020, 257, 118056. [Google Scholar] [CrossRef]
- Chen, M.; Pekosz, A.; Villano, J.S.; Shen, W.; Zhou, R.; Kulaga, H.; Li, Z.; Beck, S.E.; Witwer, K.W.; Mankowski, J.L.; et al. Evolution of nasal and olfactory infection characteristics of SARS-CoV-2 variants. bioRxiv 2022. [Google Scholar] [CrossRef]
- von Bartheld, C.S.; Wang, L. Prevalence of Olfactory Dysfunction with the Omicron Variant of SARS-CoV-2: A Systematic Review and Meta-Analysis. Cells 2023, 12, 430. [Google Scholar] [CrossRef] [PubMed]
- von Bartheld, C.S.; Hagen, M.M.; Butowt, R. The D614G Virus Mutation Enhances Anosmia in COVID-19 Patients: Evidence from a Systematic Review and Meta-analysis of Studies from South Asia. ACS Chem. Neurosci. 2021, 12, 3535–3549. [Google Scholar] [CrossRef]
- Schreiner, T.; Allnoch, L.; Beythien, G.; Marek, K.; Becker, K.; Schaudien, D.; Stanelle-Bertram, S.; Schaumburg, B.; Mounogou Kouassi, N.; Beck, S.; et al. SARS-CoV-2 Infection Dysregulates Cilia and Basal Cell Homeostasis in the Respiratory Epithelium of Hamsters. Int. J. Mol. Sci. 2022, 23, 5124. [Google Scholar] [CrossRef]
- Seehusen, F.; Clark, J.J.; Sharma, P.; Bentley, E.G.; Kirby, A.; Subramaniam, K.; Wunderlin-Giuliani, S.; Hughes, G.L.; Patterson, E.I.; Michael, B.D.; et al. Neuroinvasion and Neurotropism by SARS-CoV-2 Variants in the K18-hACE2 Mouse. Viruses 2022, 14, 1020. [Google Scholar] [CrossRef] [PubMed]
- Trobaugh, D.W.; Klimstra, W.B. MicroRNA Regulation of RNA Virus Replication and Pathogenesis. Trends Mol. Med. 2017, 23, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Hossain, G.S.; Kocerha, J. The Potential for microRNA Therapeutics and Clinical Research. Front. Genet. 2019, 10, 478. [Google Scholar] [CrossRef]
- Osan, J.K.; DeMontigny, B.A.; Mehedi, M. Immunohistochemistry for protein detection in PFA-fixed paraffin-embedded SARS-CoV-2-infected COPD airway epithelium. STAR Protoc. 2021, 2, 100663. [Google Scholar] [CrossRef]
- Trifonova, I.; Christova, I.; Madzharova, I.; Angelova, S.; Voleva, S.; Yordanova, R.; Tcherveniakova, T.; Krumova, S.; Korsun, N. Clinical significance and role of coinfections with respiratory pathogens among individuals with confirmed severe acute respiratory syndrome coronavirus-2 infection. Front. Public. Health 2022, 10, 959319. [Google Scholar] [CrossRef] [PubMed]
- Rohde, G.; Wiethege, A.; Borg, I.; Kauth, M.; Bauer, T.T.; Gillissen, A.; Bufe, A.; Schultze-Werninghaus, G. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: A case-control study. Thorax 2003, 58, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Choi, S.M.; Lee, J.; Park, Y.S.; Lee, C.H.; Yim, J.J.; Yoo, C.G.; Kim, Y.W.; Han, S.K.; Lee, S.M. Respiratory virus of severe pneumonia in South Korea: Prevalence and clinical implications. PLoS ONE 2018, 13, e0198902. [Google Scholar] [CrossRef] [PubMed]
- Olloquequi, J. COVID-19 Susceptibility in chronic obstructive pulmonary disease. Eur. J. Clin. Investig. 2020, 50, e13382. [Google Scholar] [CrossRef]
- Alhumaid, S.; Al Mutair, A.; Al Alawi, Z.; Alshawi, A.M.; Alomran, S.A.; Almuhanna, M.S.; Almuslim, A.A.; Bu Shafia, A.H.; Alotaibi, A.M.; Ahmed, G.Y.; et al. Coinfections with Bacteria, Fungi, and Respiratory Viruses in Patients with SARS-CoV-2: A Systematic Review and Meta-Analysis. Pathogens 2021, 10, 809. [Google Scholar] [CrossRef]
- Cooksey, G.L.S.; Morales, C.; Linde, L.; Schildhauer, S.; Guevara, H.; Chan, E.; Gibb, K.; Wong, J.; Lin, W.; Bonin, B.J.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 and Respiratory Virus Sentinel Surveillance, California, USA, May 10, 2020-June 12, 2021. Emerg. Infect. Dis. 2022, 28, 9–19. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, N.; Park, Y.; Kim, J.; Jeon, K.; Park, M.J.; Song, W. Prevalence and Clinical Impact of Coinfection in Patients with Coronavirus Disease 2019 in Korea. Viruses 2022, 14, 446. [Google Scholar] [CrossRef]
- Kim, D.; Quinn, J.; Pinsky, B.; Shah, N.H.; Brown, I. Rates of Co-infection Between SARS-CoV-2 and Other Respiratory Pathogens. JAMA 2020, 323, 2085–2086. [Google Scholar] [CrossRef]
- Nowak, M.D.; Sordillo, E.M.; Gitman, M.R.; Paniz Mondolfi, A.E. Coinfection in SARS-CoV-2 infected patients: Where are influenza virus and rhinovirus/enterovirus? J. Med. Virol. 2020, 92, 1699–1700. [Google Scholar] [CrossRef]
- Burrel, S.; Hausfater, P.; Dres, M.; Pourcher, V.; Luyt, C.E.; Teyssou, E.; Soulie, C.; Calvez, V.; Marcelin, A.G.; Boutolleau, D. Co-infection of SARS-CoV-2 with other respiratory viruses and performance of lower respiratory tract samples for the diagnosis of COVID-19. Int. J. Infect. Dis. 2021, 102, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Nainwal, N. Treatment of respiratory viral infections through inhalation therapeutics: Challenges and opportunities. Pulm. Pharmacol. Ther. 2022, 77, 102170. [Google Scholar] [CrossRef] [PubMed]
- Melgar, M.; Britton, A.; Roper, L.E.; Talbot, H.K.; Long, S.S.; Kotton, C.N.; Havers, F.P. Use of Respiratory Syncytial Virus Vaccines in Older Adults: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Tregoning, J.S.; Schwarze, J. Respiratory viral infections in infants: Causes, clinical symptoms, virology, and immunology. Clin. Microbiol. Rev. 2010, 23, 74–98. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.; Drews, S.J.; Marchant, D.J. Respiratory Syncytial Virus: Infection, Detection, and New Options for Prevention and Treatment. Clin. Microbiol. Rev. 2017, 30, 277–319. [Google Scholar] [CrossRef]
- Coultas, J.A.; Smyth, R.; Openshaw, P.J. Respiratory syncytial virus (RSV): A scourge from infancy to old age. Thorax 2019, 74, 986–993. [Google Scholar] [CrossRef]
- Graham, B.S. Vaccine development for respiratory syncytial virus. Curr. Opin. Virol. 2017, 23, 107–112. [Google Scholar] [CrossRef]
- Green, C.A.; Drysdale, S.B.; Pollard, A.J.; Sande, C.J. Vaccination against Respiratory Syncytial Virus. Interdiscip. Top. Gerontol. Geriatr. 2020, 43, 182–192. [Google Scholar] [CrossRef]
- Ruckwardt, T.J.; Morabito, K.M.; Graham, B.S. Immunological Lessons from Respiratory Syncytial Virus Vaccine Development. Immunity 2019, 51, 429–442. [Google Scholar] [CrossRef]
- COVID-19 Vaccine Tracker. Available online: https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker (accessed on 6 November 2023).
- Clinical Trials. Available online: https://www.mayo.edu/research/clinical-trials/cls-20147700#moreinfo (accessed on 6 November 2023).
- Dose Ranging Study of ALX-0171 in Infants Hospitalized for Respiratory Syncytial Virus Lower Respiratory Tract Infection (Respire). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02979431 (accessed on 6 November 2023).
- Vainionpaa, R.; Hyypia, T. Biology of parainfluenza viruses. Clin. Microbiol. Rev. 1994, 7, 265–275. [Google Scholar] [CrossRef]
- Sato, M.; Wright, P.F. Current status of vaccines for parainfluenza virus infections. Pediatr. Infect. Dis. J. 2008, 27 (Suppl. 10), S123–S125. [Google Scholar] [CrossRef] [PubMed]
- Chemaly, R.F.; Marty, F.M.; Wolfe, C.R.; Lawrence, S.J.; Dadwal, S.; Soave, R.; Farthing, J.; Hawley, S.; Montanez, P.; Hwang, J.; et al. DAS181 Treatment of Severe Lower Respiratory Tract Parainfluenza Virus Infection in Immunocompromised Patients: A Phase 2 Randomized, Placebo-Controlled Study. Clin. Infect. Dis. 2021, 73, e773–e781. [Google Scholar] [CrossRef] [PubMed]
- Karron, R.A.; Herbert, K.; Wanionek, K.; Schmidt, A.C.; Schaap-Nutt, A.; Collins, P.L.; Buchholz, U.J. Evaluation of a Live-Attenuated Human Parainfluenza Virus Type 2 Vaccine in Adults and Children. J. Pediatr. Infect. Dis. Soc. 2023, 12, 173–176. [Google Scholar] [CrossRef]
- Schmidt, A.C.; Schaap-Nutt, A.; Bartlett, E.J.; Schomacker, H.; Boonyaratanakornkit, J.; Karron, R.A.; Collins, P.L. Progress in the development of human parainfluenza virus vaccines. Expert. Rev. Respir. Med. 2011, 5, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Kasman, L.M. Engineering the common cold to be a live-attenuated SARS-CoV-2 vaccine. Front. Immunol. 2022, 13, 871463. [Google Scholar] [CrossRef]
- Sung, R.Y.; Yin, J.; Oppenheimer, S.J.; Tam, J.S.; Lau, J. Treatment of respiratory syncytial virus infection with recombinant interferon alfa-2a. Arch. Dis. Child. 1993, 69, 440–442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Douglas, R.M.; Moore, B.; Miles, H.B.; Pinnock, C.B. Could preventive intranasal interferon lower the morbidity in children prone to respiratory illness? Med. J. Aust. 1990, 152, 524–528. [Google Scholar] [CrossRef]
- Al-Nakib, W.; Higgins, P.G.; Barrow, I.; Batstone, G.; Tyrrell, D.A. Prophylaxis and treatment of rhinovirus colds with zinc gluconate lozenges. J. Antimicrob. Chemother. 1987, 20, 893–901. [Google Scholar] [CrossRef]
- Gwaltney, J.M., Jr. Combined antiviral and antimediator treatment of rhinovirus colds. J. Infect. Dis. 1992, 166, 776–782. [Google Scholar] [CrossRef]
- Iacobucci, G. COVID-19: Runny nose, headache, and fatigue are commonest symptoms of omicron, early data show. BMJ 2021, 375, n3103. [Google Scholar] [CrossRef]
- Ohkubo, K.; Lee, C.H.; Baraniuk, J.N.; Merida, M.; Hausfeld, J.N.; Kaliner, M.A. Angiotensin-converting enzyme in the human nasal mucosa. Am. J. Respir. Cell Mol. Biol. 1994, 11, 173–180. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).