Using Fungi in Artificial Microbial Consortia to Solve Bioremediation Problems
Abstract
1. Introduction
- -
- easy-to-use reproducibility of the compositions of the consortia;
- -
- the possibility of introducing maximum targeted metabolic activity into the consortia cells, which are improved, including through the genetic modification of cells;
- -
- targeted variation of the ratios of cell concentrations in the consortium, to regulate the rates of associated biochemical processes catalyzed by cells;
- -
2. Main Targets for Bioremediation Based on Various Consortia Containing Fungi
2.1. Removal of Heavy Metals
2.2. Decolorization of Dyes
2.3. Destruction of Synthetic Polymers
2.4. Degradation of Pesticides
2.5. Degradation of Polycyclic Aromatic Hydrocarbons
2.6. Degradation of Pharmaceutical Pollutants
2.7. Elimination of Pollutant Mixtures
3. Analysis of Current Trends in the Development of Fungal-Containing Consortia
3.1. Genetically Modified Microorganisms in Artificial Consortia with Fungi
3.2. Role of Composition in Artificial Consortia with Fungal Cells
- -
- microbial consortium containing T. versicolor, P. ostreatus, Phanerochaete sp., Pseudomonas fluorescens and B. subtilis cells was applied for the treatment of non-domestic wastewater. This fungal/bacterial consortium was prepared by mixing fungal biomass pellets with suspensions of bacterial cells. The removal of colored substances (2700 Color Units550nm), COD (1.75 g/L) and nitrate (3 mg/L) was 91 ± 2%, 90 ± 4% and 17 ± 2%, respectively, after 15 days of water treatment at a pilot plant [131];
- -
- consortium of A. niger, Mucor hiemalis and Galactomyces geotrichum, has been tested for the treatment of real wastewater from industry at a pilot scale station (110 L) and industrial wastewater treatment plant (1000 L). The efficiency of COD removal in the industrial reactor was 50% under the influence of this consortium [132];
- -
- consortium containing Acinetobacter oleivorans, Corynebacterium sp., Pseudomonas sp, Rhodococcus sp., Micrococcus sp. and yeast Yarrowia sp. was tested by Ecophile Co., Ltd. (Korea) in the biodegradation of hydrocarbons in soil (2300 mg/kg) contaminated with diesel fuel. This large-scale experiment involved two samples of 100 metric tons of contaminated soil, both without (control) and with consortium treatment (109 cells/kg of soil). The introduction of consortium reduced pollution by 57.7% within 2 weeks, whereas in the control (without the consortium), degradation was only 10.1% [133].
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bala, S.; Garg, D.; Thirumalesh, B.V.; Sharma, M.; Sridhar, K.; Inbaraj, B.S.; Tripathi, M. Recent strategies for bioremediation of emerging pollutants: A review for a green and sustainable environment. Toxics 2022, 10, 484. [Google Scholar] [CrossRef] [PubMed]
- Maslova, O.; Senko, O.; Gladchenko, M.A.; Gaydamaka, S.N.; Efremenko, E. Prospects for combined applications of nanostructured catalysts and biocatalysts for elimination of hydrocarbon pollutants. Appl. Sci. 2023, 13, 5815. [Google Scholar] [CrossRef]
- Demarco, C.F.; Quadro, M.S.; Selau Carlos, F.; Pieniz, S.; Morselli, L.B.G.A.; Andreazza, R. Bioremediation of aquatic environments contaminated with heavy metals: A review of mechanisms, solutions and perspectives. Sustainability 2023, 15, 1411. [Google Scholar] [CrossRef]
- Thacharodi, A.; Hassan, S.; Singh, T.; Mandal, R.; Khan, H.A.; Hussain, M.A.; Pugazhendhi, A. Bioremediation of polycyclic aromatic hydrocarbons: An updated microbiological review. Chemosphere 2023, 328, 138498. [Google Scholar] [CrossRef] [PubMed]
- Saleh, I.A.; Zouari, N.; Al-Ghouti, M.A. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environ. Technol. Innov. 2020, 19, 101026. [Google Scholar] [CrossRef]
- Efremenko, E.; Stepanov, N.; Senko, O.; Maslova, O.; Lyagin, I.; Aslanli, A. Progressive biocatalysts for the treatment of aqueous systems containing pharmaceutical pollutants. Life 2023, 13, 841. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Lyagin, I.V.; Maslova, O.V.; Senko, O.V.; Stepanov, N.A.; Aslanli, A.G.G. Catalytic degradation of microplastics. Russ. Chem. Rev. 2023, 92, 1–48. [Google Scholar] [CrossRef]
- Lobo-Moreira, A.B.; Xavier-Santos, S.; Damacena-Silva, L.; Caramori, S.S. Trends on microalgae-fungi consortia research: An alternative for biofuel production? Front. Microbiol. 2022, 13, 903737. [Google Scholar] [CrossRef]
- Peng, X.; Wilken, S.E.; Lankiewicz, T.S.; Gilmore, S.P.; Brown, J.L.; Henske, J.K.; Swift, C.L.; Salamov, A.; Barry, K.; Grigoriev, I.V.; et al. Genomic and functional analyses of fungal and bacterial consortia that enable lignocellulose breakdown in goat gut microbiomes. Nat. Microbiol. 2021, 6, 499–511. [Google Scholar] [CrossRef]
- Otaiku, A. Fungi consortia in situ biodegradation of xenobiotic, military shooting range, Kachia, Kaduna, Nigeria. Appl. Biotechnol. Bioeng. 2020, 7, 246–274. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, H.; Li, P.; Jin, J.; Li, Z. The bacterial consortia promote plant growth and secondary metabolite accumulation in Astragalus mongholicus under drought stress. BMC Plant Biol. 2022, 22, 475. [Google Scholar] [CrossRef]
- Vaksmaa, A.; Guerrero-Cruz, S.; Ghosh, P.; Zeghal, E.; Hernando-Morales, V.; Niemann, H. Role of fungi in bioremediation of emerging pollutants. Front. Mar. Sci. 2023, 10, 1070905. [Google Scholar] [CrossRef]
- Mittermeier, F.; Bäumler, M.; Arulrajah, P.; García Lima, J.J.; Hauke, S.; Stock, A.; Weuster-Botz, D. Artificial microbial consortia for bioproduction processes. Eng. Life Sci. 2022, 23, e2100152. [Google Scholar] [CrossRef]
- Johns, N.I.; Blazejewski, T.; Gomes, A.L.; Wang, H.H. Principles for designing synthetic microbial communities. Curr. Opin. Microbiol. 2016, 31, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wu, R.; Zhang, W.; Xin, F.; Jiang, M. Construction of stable microbial consortia for effective biochemical synthesis. Trends Biotechnol. 2023, 41, 1430–1441. [Google Scholar] [CrossRef]
- Efremenko, E.; Senko, O.; Stepanov, N.; Aslanli, A.; Maslova, O.; Lyagin, I. Quorum sensing as a trigger that improves characteristics of microbial biocatalysts. Microorganisms 2023, 11, 1395. [Google Scholar] [CrossRef] [PubMed]
- Akerman-Sanchez, G.; Rojas-Jimenez, K. Fungi for the bioremediation of pharmaceutical-derived pollutants: A bioengineering approach to water treatment. Environ. Adv. 2021, 4, 100071. [Google Scholar] [CrossRef]
- Che, S.; Men, Y. Synthetic microbial consortia for biosynthesis and biodegradation: Promises and challenges. J. Ind. Microbiol. Biotechnol. 2019, 46, 1343–1358. [Google Scholar] [CrossRef] [PubMed]
- Lashani, E.; Amoozegar, M.A.; Turner, R.J.; Moghimi, H. Use of microbial consortia in bioremediation of metalloid polluted environments. Microorganisms 2023, 11, 891. [Google Scholar] [CrossRef]
- Massot, F.; Bernard, N.; Alvarez, L.M.M.; Martorell, M.M.; Mac Cormack, W.P.; Ruberto, L.A.M. Microbial associations for bioremediation. What does “microbial consortia” mean? Appl. Microbiol. Biotechnol. 2022, 106, 2283–2297. [Google Scholar] [CrossRef]
- Efremenko, E.; Senko, O.; Maslova, O.; Lyagin, I.; Aslanli, A.; Stepanov, N. Destruction of mycotoxins in poultry waste under anaerobic conditions within methanogenesis catalyzed by artificial microbial consortia. Toxins 2023, 15, 205. [Google Scholar] [CrossRef]
- Senko, O.; Maslova, O.; Aslanli, A.; Efremenko, E. Impact of perfluorocarbons with gas transport function on growth of phototrophic microorganisms in a free and immobilized state and in consortia with bacteria. Appl. Sci. 2023, 13, 1868. [Google Scholar] [CrossRef]
- Maslova, O.; Senko, O.; Stepanov, N.; Gladchenko, M.; Gaydamaka, S.; Akopyan, A.; Eseva, E.; Anisimov, A.; Efremenko, E. Sulfur containing mixed wastes in anaerobic processing by new immobilized synthetic consortia. Bioresour. Technol. 2022, 362, 127794. [Google Scholar] [CrossRef]
- Efremenko, E.; Stepanov, N.; Maslova, O.; Senko, O.; Aslanli, A.; Lyagin, I. “Unity and struggle of opposites” as a basis for the functioning of synthetic bacterial immobilized consortium that continuously degrades organophosphorus pesticides. Microorganisms 2022, 10, 1394. [Google Scholar] [CrossRef]
- Maslova, O.; Senko, O.; Stepanov, N.; Gladchenko, M.; Gaydamaka, S.; Akopyan, A.; Polikarpova, P.; Lysenko, S.; Anisimov, A.; Efremenko, E. Formation and use of anaerobic consortia for the biotransformation of sulfur-containing extracts from pre-oxidized crude oil and oil fractions. Bioresour. Technol. 2021, 319, 124248. [Google Scholar] [CrossRef]
- Senko, O.; Stepanov, N.; Maslova, O.; Efremenko, E. “Nature-like” cryoimmobilization of phototrophic microorganisms: New opportunities for their long-term storage and sustainable use. Sustainability 2022, 14, 661. [Google Scholar] [CrossRef]
- Jiang, J.; Jin, W.; Tu, R.; Han, S.; Ji, Y.; Zhou, X. Harvesting of microalgae Chlorella pyrenoidosa by bio-flocculation with bacteria and filamentous fungi. Waste Biomass Valor. 2021, 12, 145–154. [Google Scholar] [CrossRef]
- Nazari, M.T.; Freitag, J.F.; Cavanhi, V.A.F.; Colla, L.M. Microalgae harvesting by fungal-assisted bioflocculation. Rev. Environ. Sci. Biotechnol. 2020, 19, 369–388. [Google Scholar] [CrossRef]
- Kregiel, D.; James, S.A.; Rygala, A.; Berlowska, J.; Antolak, H.; Pawlikowska, E. Consortia formed by yeasts and acetic acid bacteria Asaia spp. in soft drinks. Antonie Leeuwenhoek 2018, 111, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Rusyn, I.; Dmytruk, O.V.; Dmytruk, K.V.; Onyeaka, H.; Gryzenhout, M.; Gafforov, Y. Filamentous fungi for sustainable remediation of pharmaceutical compounds, heavy metal and oil hydrocarbons. Front. Bioeng. Biotechnol. 2023, 11, 1106973. [Google Scholar] [CrossRef] [PubMed]
- Maslova, O.; Stepanov, N.; Senko, O.; Efremenko, E. Production of various organic acids from different renewable sources by immobilized cells in the regimes of separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SSF). Bioresour. Technol. 2019, 272, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chib, S.; Jamwal, V.L.; Kumar, V.; Gandhi, S.G.; Saran, S. Fungal production of kojic acid and its industrial applications. Appl. Microbiol. Biotechnol. 2023, 107, 2111–2130. [Google Scholar] [CrossRef] [PubMed]
- Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; Ismail, A.; El Khoury, A. Mycotoxins: Factors influencing production and control strategies. AIMS Agric. Food 2021, 6, 416–447. [Google Scholar] [CrossRef]
- Hennig, S.; Wenzel, M.; Haas, C.; Hoffmann, A.; Weber, J.; Rödel, G.; Ostermann, K. New approaches in bioprocess-control: Consortium guidance by synthetic cell-cell communication based on fungal pheromones. Eng. Life Sci. 2018, 18, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Meldgin, D.R.; Collins, J.J.; Lu, T. Designing microbial consortia with defined social interactions. Nat. Chem. Biol. 2018, 14, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Yan, W.; Ding, M.; Yuan, Y. Construction of microbial consortia for microbial degradation of complex compounds. Front. Bioeng. Biotechnol. 2022, 10, 1051233. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Kumar, P.S.; Hemavathy, R.V.; Jeevanantham, S.; Harikumar, P.; Priyanka, G.; Devakirubai, D.R.A. A comprehensive review on sources, analysis and toxicity of environmental pollutants and its removal methods from water environment. Sci. Total Environ. 2022, 812, 152456. [Google Scholar] [CrossRef]
- Efremenko, E.; Senko, O.; Maslova, O.; Stepanov, N.; Aslanli, A.; Lyagin, I. Biocatalysts in synthesis of microbial polysaccharides: Properties and development trends. Catalysts 2022, 12, 1377. [Google Scholar] [CrossRef]
- Chaudhary, P.; Beniwal, V.; Sharma, P.; Goyal, S.; Kumar, R.; Alkhanjaf, A.A.M.; Umar, A. Unloading of hazardous Cr and tannic acid from real and synthetic waste water by novel fungal consortia. Environ. Technol. Innov. 2022, 26, 102230. [Google Scholar] [CrossRef]
- Talukdar, D.; Jasrotia, T.; Sharma, R.; Jaglan, S.; Kumar, R.; Vats, R.; Umar, A. Evaluation of novel indigenous fungal consortium for enhanced bioremediation of heavy metals from contaminated sites. Environ. Technol. Innov. 2020, 20, 101050. [Google Scholar] [CrossRef]
- Hassan, A.; Pariatamby, A.; Ossai, I.C.; Hamid, F.S. Bioaugmentation assisted mycoremediation of heavy metal and/metalloid landfill contaminated soil using consortia of filamentous fungi. Biochem. Eng. J. 2020, 157, 107550. [Google Scholar] [CrossRef]
- Hassan, A.; Periathamby, A.; Ahmed, A.; Innocent, O.; Hamid, F.S. Effective bioremediation of heavy metal–contaminated landfill soil through bioaugmentation using consortia of fungi. J. Soils Sediments 2020, 20, 66–80. [Google Scholar] [CrossRef]
- Zango, U.U.; Ahluwalia, S.S.; Sharma, A.K. Microbial consortium of Aspergillus fumigatus, Aspergillus terreus and Paenibacillus dendritiformis in the bioremoval of cadmium. Int. J. Pharm. Res. 2018, 10, 230–238. [Google Scholar] [CrossRef]
- Sharma, R.; Jasrotia, T.; Kumar, R.; Kumar, R.; Alothman, A.A.; mana AL-Anazy, M.; Algahtani, K.N.; Umar, A. Multi-biological combined system: A mechanistic approach for removal of multiple heavy metals. Chemosphere 2021, 276, 130018. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhang, T.; Wang, F. Microbial-based heavy metal bioremediation: Toxicity and eco-friendly approaches to heavy metal decontamination. Appl. Sci. 2023, 13, 8439. [Google Scholar] [CrossRef]
- Patel, H.; Yadav, V.K.; Yadav, K.K.; Choudhary, N.; Kalasariya, H.; Alam, M.M.; Gacem, A.; Amanullah, M.; Ibrahium, H.A.; Park, J.-W.; et al. A Recent and systemic approach towards microbial biodegradation of dyes from textile industries. Water 2022, 14, 3163. [Google Scholar] [CrossRef]
- Dhir, B. Degradation of dyes using filamentous fungi. In Dye Biodegradation, Mechanisms and Techniques. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry, 1st ed.; Muthu, S.S., Khadir, A., Eds.; Springer: Singapore, 2022; pp. 51–66. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, G.; Dwivedi, S.K. Dye degradation by fungi. In Dye Biodegradation, Mechanisms and Techniques. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry; Muthu, S.S., Khadir, A., Eds.; Springer: Singapore, 2022; pp. 113–140. [Google Scholar] [CrossRef]
- Ali, S.S.; Al-Tohamy, R.; Koutra, E.; El-Naggar, A.H.; Kornaros, M.; Sun, J. Valorizing lignin-like dyes and textile dyeing wastewater by a newly constructed lipid-producing and lignin modifying oleaginous yeast consortium valued for biodiesel and bioremediation. J. Hazard. Mater. 2021, 403, 123575. [Google Scholar] [CrossRef] [PubMed]
- Bankole, P.O.; Adekunle, A.A.; Govindwar, S.P. Biodegradation of a monochlorotriazine dye, cibacron brilliant red 3B-A in solid state fermentation by wood-rot fungal consortium, Daldinia concentrica and Xylaria polymorpha: Co-biomass decolorization of cibacron brilliant red 3B-A dye. Int. J. Biol. Macromol. 2018, 120, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Eltarahony, M.; El-Fakharany, E.; Abu-Serie, M.; ElKady, M.; Ibrahim, A. Statistical modeling of methylene blue degradation by yeast-bacteria consortium; optimization via agro-industrial waste, immobilization and application in real effluents. Microb. Cell Fact. 2021, 20, 234. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Rahim, W.M.; Moawad, H.; Azeiz, A.Z.A.; Sadowsky, M.J. Biodegradation of azo dyes by bacterial or fungal consortium and identification of the biodegradation products. Egypt. J. Aquat. Res. 2021, 47, 269–276. [Google Scholar] [CrossRef]
- Tang, W.; Xu, X.; Ye, B.C.; Cao, P.; Ali, A. Decolorization and degradation analysis of Disperse Red 3B by a consortium of the fungus Aspergillus sp. XJ-2 and the microalgae Chlorella sorokiniana XJK. RSC Adv. 2019, 9, 14558–14566. [Google Scholar] [CrossRef]
- Purnomo, A.S.; Mawaddah, M.O. Biodecolorization of methyl orange by mixed cultures of brown-rot fungus Daedalea dickinsii and bacterium Pseudomonas aeruginosa. Biodiversitas 2020, 21, 2297–2302. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Xie, R.; Schagerl, M.; Khalil, M.A.; Sun, J. Decolorization of reactive azo dye using novel halotolerant yeast consortium HYC and proposed degradation pathway. Ecotoxicol. Environ. Saf. 2023, 263, 115258. [Google Scholar] [CrossRef]
- Thakor, R.; Mistry, H.; Tapodhan, K.; Bariya, H. Efficient biodegradation of Congo red dye using fungal consortium incorporated with Penicillium oxalicum and Aspergillus tubingensis. Folia Microbiol. 2022, 67, 33–43. [Google Scholar] [CrossRef]
- Siracusa, V. Microbial degradation of synthetic biopolymers waste. Polymers 2019, 11, 1066. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.; Xing, B. Environmental source, fate, and toxicity of microplastics. J. Hazard. Mater. 2021, 407, 124357. [Google Scholar] [CrossRef]
- Yang, W.; Cheng, P.; Adams, C.A.; Zhang, S.; Sun, Y.; Yu, H.; Wang, F. Effects of microplastics on plant growth and arbuscular mycorrhizal fungal communities in a soil spiked with ZnO nanoparticles. Soil Biol. Biochem. 2021, 155, 108179. [Google Scholar] [CrossRef]
- Elsamahy, T.; Sun, J.; Elsilk, S.E.; Ali, S.S. Biodegradation of low-density polyethylene plastic waste by a constructed tri-culture yeast consortium from wood-feeding termite: Degradation mechanism and pathway. J. Hazard. Mater. 2023, 448, 130944. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.P.; Barbosa, M.; Amaral, J.S.; Pinto, V.; Rodrigues, J.L.; Ferreira, M.J.; Barreiro, M.F. Biobased additives as biodegradability enhancers with application in TPU-based footwear components. J. Renew. Mater. 2016, 4, 47. [Google Scholar] [CrossRef]
- Sowmya, H.V.; Ramalingappa, B.; Nayanashree, G.; Thippeswamy, B.; Krishnappa, M. Polyethylene degradation by fungal consortium. Int. J. Environ. Res. 2015, 9, 823–830. [Google Scholar] [CrossRef]
- DSouza, G.C.; Sheriff, R.S.; Ullanat, V.; Shrikrishna, A.; Joshi, A.V.; Hiremath, L.; Entoori, K. Fungal biodegradation of low-density polyethylene using consortium of Aspergillus species under controlled conditions. Heliyon 2021, 7, 51–66. [Google Scholar] [CrossRef]
- Kučić Grgić, D.; Miloloža, M.; Ocelić Bulatović, V.; Ukić, Š.; Slouf, M.; Gajdosova, V. Screening the efficacy of a microbial consortium of bacteria and fungi isolated from different environmental samples for the degradation of LDPE/TPS films. Separations 2023, 10, 79. [Google Scholar] [CrossRef]
- Chigwada, A.D.; Ogola, H.J.O.; Tekere, M. Multivariate analysis of enriched landfill soil consortia provide insight on the community structural perturbation and functioning during low-density polyethylene degradation. Microbiol. Res. 2023, 274, 127425. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, T.A.; Barbosa, R.; Mesquita, A.B.; Ferreira, J.H.; de Carvalho, L.H.; Alves, T.S. Fungal degradation of reprocessed PP/PBAT/thermoplastic starch blends. J. Mater. Res. Technol. 2020, 9, 2338–2349. [Google Scholar] [CrossRef]
- Rajan, P.; Rangasamy, M.; Sundaram, S.K. Bioremediation of preprocessed plastic wastes through microbial consortium. J. Adv. Sci. Res. 2020, 11, 106–113. [Google Scholar]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and enzymatic degradation of synthetic plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef]
- Srikanth, M.; Sandeep, T.S.R.S.; Sucharitha, K.; Godi, S. Biodegradation of plastic polymers by fungi: A brief review. Bioresour. Bioprocess. 2022, 9, 42. [Google Scholar] [CrossRef]
- Yasin, N.M.; Akkermans, S.; Van Impe, J.F. Enhancing the biodegradation of (bio) plastic through pretreatments: A critical review. Waste Manag. 2022, 150, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bokade, P.; Purohit, H.J.; Bajaj, A. Myco-remediation of chlorinated pesticides: Insights into fungal metabolic system. Indian J. Microbiol. 2021, 61, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Jesionowski, T.; Pinelo, M.; Meyer, A.S.; Iqbal, H.M.; Bilal, M.; Nquyen, L.N.; Nghiem, L.D. Free and immobilized biocatalysts for removing micropollutants from water and wastewater: Recent progress and challenges. Bioresour. Technol. 2022, 344, 126201. [Google Scholar] [CrossRef]
- Rodríguez-Castillo, G.; Molina-Rodríguez, M.; Cambronero-Heinrichs, J.C.; Quirós-Fournier, J.P.; Lizano-Fallas, V.; Jiménez-Rojas, C.; .Masis-Mora, M.; Castro-Gutierrez, V.; Mata-Araya, I.; Rodríguez-Rodríguez, C.E. Simultaneous removal of neonicotinoid insecticides by a microbial degrading consortium: Detoxification at reactor scale. Chemosphere 2019, 235, 1097–1106. [Google Scholar] [CrossRef]
- Sariwati, A.; Purnomo, A.S.; Kamei, I. Abilities of co-cultures of brown-rot fungus Fomitopsis pinicola and Bacillus subtilis on biodegradation of DDT. Curr. Microbiol. 2017, 74, 1068–1075. [Google Scholar] [CrossRef]
- Purnomo, A.S.; Ashari, K.; Hermansyah, F.T. Evaluation of the synergistic effect of mixed cultures of white-rot fungus Pleurotus ostreatus and biosurfactant-producing bacteria on DDT biodegradation. J. Microbiol. Biotechnol. 2017, 27, 1306–1315. [Google Scholar] [CrossRef]
- Hultberg, M.; Bodin, H. Effects of fungal-assisted algal harvesting through biopellet formation on pesticides in water. Biodegradation 2018, 29, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Levio-Raiman, M.; Briceño, G.; Leiva, B.; López, S.; Schalchli, H.; Lamilla, C.; Bornhardt, C.; Diez, M.C. Treatment of pesticide-contaminated water using a selected fungal consortium: Study in a batch and packed-bed bioreactor. Agronomy 2021, 11, 743. [Google Scholar] [CrossRef]
- Castro-Gutiérrez, V.; Masís-Mora, M.; Carazo-Rojas, E.; Mora-López, M.; Rodríguez-Rodríguez, C.E. Fungal and bacterial co-bioaugmentation of a pesticide-degrading biomixture: Pesticide removal and community structure variations during different treatments. Water Air Soil Pollut. 2019, 230, 247. [Google Scholar] [CrossRef]
- Purnomo, A.S.; Sariwati, A.; Kamei, I. Synergistic interaction of a consortium of the brown-rot fungus Fomitopsis pinicola and the bacterium Ralstonia pickettii for DDT biodegradation. Heliyon 2020, 6, e04027. [Google Scholar] [CrossRef]
- Aruotu, J.O.; Chikere, C.B.; Okafor, C.P.; Edamkue, I. Microbial consortium for polycyclic aromatic hydrocarbons degradation from petroleum hydrocarbon polluted soils in rivers state, Nigeria. Appl. Sci. 2023, 13, 9335. [Google Scholar] [CrossRef]
- Premnath, N.; Mohanrasu, K.; Rao, R.G.R.; Dinesh, G.H.; Prakash, G.S.; Ananthi, V.; Ponnuchamy, K.; Muthusomy, G.; Arun, A. A crucial review on polycyclic aromatic hydrocarbons-environmental occurrence and strategies for microbial degradation. Chemosphere 2021, 280, 130608. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Gupta, S.; Tripathi, V.; Chauhan, A.; Parashar, D.; Shankar, P.; Kashyap, V. Microbiome based approaches for the degradation of polycyclic aromatic hydrocarbons (PAHs): A current perception. Chemosphere 2023, 341, 139951. [Google Scholar] [CrossRef]
- Haiping, L.; Fanping, M. Efficiency, mechanism, influencing factors, and integrated technology of biodegradation for aromatic compounds by microalgae: A review. Environ. Pollut. 2023, 335, 122248. [Google Scholar]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): A review. Front. Microbiol. 2016, 7, 1369. [Google Scholar] [CrossRef]
- Ma, X.K.; Li, T.T.; Fam, H.; Charles Peterson, E.; Zhao, W.W.; Guo, W.; Zhou, B. The influence of heavy metals on the bioremediation of polycyclic aromatic hydrocarbons in aquatic system by a bacterial-fungal consortium. Environ. Technol. 2018, 39, 2128–2137. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Das, A.; Palaniswamy, M.; Angayarkanni, J. Degradation of benzo[a]pyrene by Pleurotus ostreatus PO-3 in the presence of defined fungal and bacterial co-cultures. J. Basic Microbiol. 2017, 57, 95–103. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Y.; Li, K.; Wang, L.; Li, D.; Liu, N.; Huang, S. Synergistic remediation of phenanthrene–cadmium co-contaminants by an immobilized acclimated bacterial–fungal consortium and its community response. Chemosphere 2023, 336, 139234. [Google Scholar] [CrossRef]
- Zafra, G.; Absalón, Á.E.; Anducho-Reyes, M.Á.; Fernandez, F.J.; Cortés-Espinosa, D.V. Construction of PAH-degrading mixed microbial consortia by induced selection in soil. Chemosphere 2017, 172, 120–126. [Google Scholar] [CrossRef]
- Khandelwal, A.; Sugavanam, R.; Ramakrishnan, B.; Dutta, A.; Varghese, E.; Banerjee, T.; Nain, L.; Singh, S.B.; Singh, N. Bio-polysaccharide composites mediated degradation of polyaromatic hydrocarbons in a sandy soil using free and immobilized consortium of Kocuria rosea and Aspergillus sydowii. Environ. Sci. Pollut. Res. 2022, 29, 80005–80020. [Google Scholar] [CrossRef]
- Kamyabi, A.; Nouri, H.; Moghimi, H. Characterization of pyrene degradation and metabolite identification by Basidioascus persicus and mineralization enhancement with bacterial-yeast co-culture. Ecotoxicol. Environ. Saf. 2018, 163, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Sandoval, O.; Gutierrez-Alcantara, E.J.; Perez-Balan, R.; Rodriguez-Vazquez, G.; Zamorategui-Molina, A.; Tirado-Torres, D. Degradation of polycyclic aromatic hydrocarbons using bacterial isolate from the contaminated soil and white rot fungus Pleurotus ostreatus. Appl. Ecol. Environ. Res. 2018, 16, 3815–3829. [Google Scholar] [CrossRef]
- Pozdnyakova, N.; Muratova, A.; Turkovskaya, O. Degradation of polycyclic aromatic hydrocarbons by co-culture of Pleurotus ostreatus Florida and Azospirillum brasilense. Appl. Microbiol. 2022, 2, 735–748. [Google Scholar] [CrossRef]
- Mahesh, N.; Balakumar, S.; Danya, U.; Shyamalagowri, S.; Babu, P.S.; Aravind, J.; Kamaraj, M.; Govarthanan, M. A review on mitigation of emerging contaminants in an aqueous environment using microbial bio-machines as sustainable tools: Progress and limitations. J. Water Process. Eng. 2022, 47, 102712. [Google Scholar] [CrossRef]
- Rios-Miguel, A.B.; van Bergen, T.J.; Zillien, C.; Ragas, A.M.; van Zelm, R.; Jetten, M.S.; Hedriks, A.J.; Welte, C.U. Predicting and improving the microbial removal of organic micropollutants during wastewater treatment: A review. Chemosphere 2023, 333, 138908. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Liu, C.X.; Xu, Q.M.; Cheng, J.S.; Yuan, Y.J. Simultaneous removal of ciprofloxacin, norfloxacin, sulfamethoxazole by co-producing oxidative enzymes system of Phanerochaete chrysosporium and Pycnoporus sanguineus. Chemosphere 2018, 195, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Q.M.; Cheng, J.S.; Yuan, Y.J. Improving the bioremoval of sulfamethoxazole and alleviating cytotoxicity of its biotransformation by laccase producing system under coculture of Pycnoporus sanguineus and Alcaligenes faecalis. Bioresour. Technol. 2016, 220, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Bankole, P.O.; Adekunle, A.A.; Jeon, B.H.; Govindwar, S.P. Novel cobiomass degradation of NSAIDs by two wood rot fungi, Ganoderma applanatum and Laetiporus sulphureus: Ligninolytic enzymes induction, isotherm and kinetic studies. Ecotoxicol. Environ. Saf. 2020, 203, 110997. [Google Scholar] [CrossRef]
- Kasonga, T.K.; Coetzee, M.A.; Van Zijl, C.; Momba, M.N.B. Removal of pharmaceutical’estrogenic activity of sequencing batch reactor effluents assessed in the T47D-KBluc reporter gene assay. J. Environ. Manag. 2019, 240, 209–218. [Google Scholar] [CrossRef]
- Bodin, H.; Daneshvar, A.; Gros, M.; Hultberg, M. Effects of biopellets composed of microalgae and fungi on pharmaceuticals present at environmentally relevant levels in water. Ecol. Eng. 2016, 91, 169–172. [Google Scholar] [CrossRef]
- Angeles-de Paz, G.; Ledezma-Villanueva, A.; Robledo-Mahón, T.; Pozo, C.; Calvo, C.; Aranda, E.; Purswani, J. Assembled mixed co-cultures for emerging pollutant removal using native microorganisms from sewage sludge. Chemosphere 2023, 313, 137472. [Google Scholar] [CrossRef]
- Li, S.; Zhu, L. Copper regulates degradation of typical antibiotics by microalgal-fungal consortium in simulated swine wastewater: Insights into metabolic routes and dissolved organic matters. Water Res. 2023, 245, 120654. [Google Scholar] [CrossRef]
- Liu, X.; He, L.; Zhang, X.; Kong, D.; Chen, Z.; Lin, J.; Wang, C. Bioremediation of petroleum-contaminated saline soil by Acinetobacter baumannii and Talaromyces sp. and functional potential analysis using metagenomic sequencing. Environ. Pollut. 2022, 311, 119970. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, X.; Chen, X.; Kong, D.; Liu, X.; Shen, S. Synergistic degradation of crude oil by indigenous bacterial consortium and exogenous fungus Scedosporium boydii. Bioresour. Technol. 2018, 264, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Atakpa, E.O.; Zhou, H.; Jiang, L.; Ma, Y.; Liang, Y.; Li, Y.; Zhang, D.; Zhang, C. Improved degradation of petroleum hydrocarbons by co-culture of fungi and biosurfactant-producing bacteria. Chemosphere 2022, 290, 133337. [Google Scholar] [CrossRef] [PubMed]
- Rajpal, N.; Ratan, J.K.; Divya, N.; Hebbani, A.V. Bioremediation of greywater using a novel bacterial–fungal consortium: Optimization and validation of the operating parameters in vitro. Environ. Technol. 2021, 43, 2430–2442. [Google Scholar] [CrossRef]
- Rajpal, N.; Verma, S.; Kumar, N.; Lee, J.; Kim, K.H.; Ratan, J.K.; Divya, N. Bioremediation of carbendazim and thiamethoxam in domestic greywater using a bioaugmented microbial consortium. Environ. Technol. Innov. 2023, 30, 103087. [Google Scholar] [CrossRef]
- Selim, M.T.; Salem, S.S.; Mohamed, A.A.; El-Gamal, M.S.; Awad, M.F.; Fouda, A. Biological treatment of real textile effluent using Aspergillus flavus and Fusarium oxysporium and their consortium along with the evaluation of their phytotoxicity. J. Fungi 2021, 7, 193. [Google Scholar] [CrossRef]
- Kurade, M.B.; Waghmode, T.R.; Xiong, J.Q.; Govindwar, S.P.; Jeon, B.H. Decolorization of textile industry effluent using immobilized consortium cells in upflow fixed bed reactor. J. Clean. Prod. 2019, 213, 884–891. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, C.; Kennes, C.; Ye, J.; Chen, D.; Zhang, S.; Chen, S.; Yu, J. Improved biodegradation potential of chlorobenzene by a mixed fungal-bacterial consortium. Int. Biodeterior. Biodegrad. 2017, 123, 276–285. [Google Scholar] [CrossRef]
- Ajmi, K.; Vismara, E.; Manai, I.; Haddad, M.; Hamdi, M.; Bouallagui, H. Polyvinyl acetate processing wastewater treatment using combined Fenton’s reagent and fungal consortium: Application of central composite design for conditions optimization. J. Hazard. Mater. 2018, 358, 243–255. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rene, E.R.; Lens, P.N.L. Reduction of selenite to elemental Se(0) with simultaneous degradation of phenol by co-cultures of Phanerochaete chrysosporium and Delftia lacustris. J. Microbiol. 2019, 57, 738–747. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Y.; Yao, T.; Ma, Y.; Zhang, H.; Yang, X.; Li, C. Evaluation of seven chemical pesticides by mixed microbial culture (PCS-1): Degradation ability, microbial community, and Medicago sativa phytotoxicity. J. Hazard. Mater. 2020, 389, 121834. [Google Scholar] [CrossRef]
- Tran, K.M.; Lee, H.M.; Thai, T.D.; Shen, J.; Eyun, S.I.; Na, D. Synthetically engineered microbial scavengers for enhanced bioremediation. J. Hazard. Mater. 2021, 419, 126516. [Google Scholar] [CrossRef]
- Lyagin, I.; Efremenko, E. Theoretical evaluation of suspected enzymatic hydrolysis of novichok agents. Catal. Commun. 2019, 120, 91–94. [Google Scholar] [CrossRef]
- Liu, L.; Bilal, M.; Duan, X.; Iqbal, H.M. Mitigation of environmental pollution by genetically engineered bacteria—Current challenges and future perspectives. Sci. Total Environ. 2019, 667, 444–454. [Google Scholar] [CrossRef]
- Meyer, V.; Basenko, E.Y.; Benz, J.P.; Braus, G.H.; Caddick, M.X.; Csukai, M.; de Vries, R.P.; Endy, D.; Frisvad, J.C.; Gunde-Cimerman, N.; et al. Growing a circular economy with fungal biotechnology: A white paper. Fungal Biol. Biotechnol. 2020, 7, 1–23. [Google Scholar] [CrossRef]
- Salazar-Cerezo, S.; de Vries, R.P.; Garrigues, S. Strategies for the development of industrial fungal producing strains. J. Fungi 2023, 9, 834. [Google Scholar] [CrossRef]
- Xue, Y.; Qiu, T.; Sun, Z.; Liu, F.; Yu, B. Mercury bioremediation by engineered Pseudomonas putida KT2440 with adaptationally optimized biosecurity circuit. Environ. Microbiol. 2022, 24, 3022–3036. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Xu, M.; Ahamad, L.; Chaudhary, A.; Kumar, G.; Adeleke, B.S.; Verma, K.K.; Hu, D.-M.; Širić, I.; Kumar, P.; et al. Application of synthetic consortia for improvement of soil fertility, pollution remediation, and agricultural productivity: A Review. Agronomy 2023, 13, 643. [Google Scholar] [CrossRef]
- Xu, C.; Yu, H. Insights into constructing a stable and efficient microbial consortium. Chin. J. Chem. Eng. 2021, 30, 112–120. [Google Scholar] [CrossRef]
- Adamu, K.S.; Bichi, Y.H.; Nasiru, A.Y.; Babangida, A.M.; Umar, M.M.; Usman, G.; Muhammad, R. Synthetic microbial consortia in bioremediation and biodegradation. Int. J. Res. Sci. Innov. Appl. Sci. 2023, 8, 232–241. [Google Scholar] [CrossRef]
- Liu, G.L.; Chi, Z.; Wang, G.Y.; Wang, Z.P.; Li, Y.; Chi, Z.M. Yeast killer toxins, molecular mechanisms of their action and their applications. Crit. Rev. Biotechnol. 2015, 35, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.; Aslanli, A.; Stepanov, N.; Senko, O.; Maslova, O. Various biomimetics, including peptides as antifungals. Biomimetics 2023, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.F.; Silva, L.D.C.; Amaral, A.C. Farnesol: An approach on biofilms and nanotechnology. Med. Mycol. 2021, 59, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Kischkel, B.; Souza, G.K.; Chiavelli, L.U.R.; Pomini, A.M.; Svidzinski, T.I.E.; Negri, M. The ability of farnesol to prevent adhesion and disrupt Fusarium keratoplasticum biofilm. Appl. Microbiol. Biotechnol. 2020, 104, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Greeff-Laubscher, M.R.; Beukes, I.; Marais, G.J.; Jacobs, K. Mycotoxin production by three different toxigenic fungi genera on formulated abalone feed and the effect of an aquatic environment on fumonisins. Mycology 2019, 11, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Loncar, J.; Bellich, B.; Cescutti, P.; Motola, A.; Beccaccioli, M.; Zjalic, S.; Reverberi, M. The effect of mushroom culture filtrates on the inhibition of mycotoxins produced by Aspergillus flavus and Aspergillus carbonarius. Toxins 2023, 15, 177. [Google Scholar] [CrossRef] [PubMed]
- Srinuanpan, S.; Chawpraknoi, A.; Chantarit, S.; Cheirsilp, B.; Prasertsan, P. A rapid method for harvesting and immobilization of oleaginous microalgae using pellet-forming filamentous fungi and the application in phytoremediation of secondary effluent. Int. J. Phytoremed. 2018, 20, 1017–1024. [Google Scholar] [CrossRef]
- Gururani, P.; Bhatnagar, P.; Kumar, V.; Vlaskin, M.S.; Grigorenko, A.V. Algal consortiums: A novel and integrated approach for wastewater treatment. Water 2022, 14, 3784. [Google Scholar] [CrossRef]
- Walls, L.E.; Velasquez-Orta, S.B.; Romero-Frasca, E.; Leary, P.; Noguez, I.Y.; Ledesma, M.T.O. Non-sterile heterotrophic cultivation of native wastewater yeast and microalgae for integrated municipal wastewater treatment and bioethanol production. Biochem. Eng. J. 2019, 151, 107319. [Google Scholar] [CrossRef]
- Céspedes-Bernal, D.N.; Mateus-Maldonado, J.F.; Rengel-Bustamante, J.A.; Quintero-Duque, M.C.; Rivera-Hoyos, C.M.; Poutou-Piñales, R.A.; Diaz-Ariza, L.A.; Castillo-Carvajal, L.C.; Paez-Moralez, A.; Pedroza-Rodríguez, A.M. Non-domestic wastewater treatment with fungal/bacterial consortium followed by Chlorella sp., and thermal conversion of the generated sludge. 3 Biotech 2021, 11, 1–18. [Google Scholar] [CrossRef]
- Djelal, H.; Amrane, A. Biodegradation by bioaugmentation of dairy wastewater by fungal consortium on a bioreactor lab-scale and on a pilot-scale. J. Environ. Sci. 2013, 25, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jeong, S.E.; Hur, M.; Ko, S.; Jeon, C.O. Construction and evaluation of a Korean native microbial consortium for the bioremediation of diesel fuel-contaminated soil in Korea. Front. Microbiol. 2018, 9, 2594. [Google Scholar] [CrossRef] [PubMed]
- Niego, A.G.T.; Lambert, C.; Mortimer, P.; Thongklang, N.; Rapior, S.; Grosse, M.; Schrey, H.; Charria-Girón, E.; Walker, A.; Hyde, K.D.; et al. The contribution of fungi to the global economy. Fungal Divers. 2023, 121, 95–137. [Google Scholar] [CrossRef]
- Bioremediation Market, by Type (In-Situ Bioremediation and Ex-Situ Bioremediation), by Services (Soil, Wastewater, and Oilfield Remediation and Others), by Technology, and by Region Forecast to 2032 (Publ. Febr. 2023, Report ID: ER_00215). Available online: https://www.emergenresearch.com/industry-report/bioremediation-market (accessed on 8 February 2023).
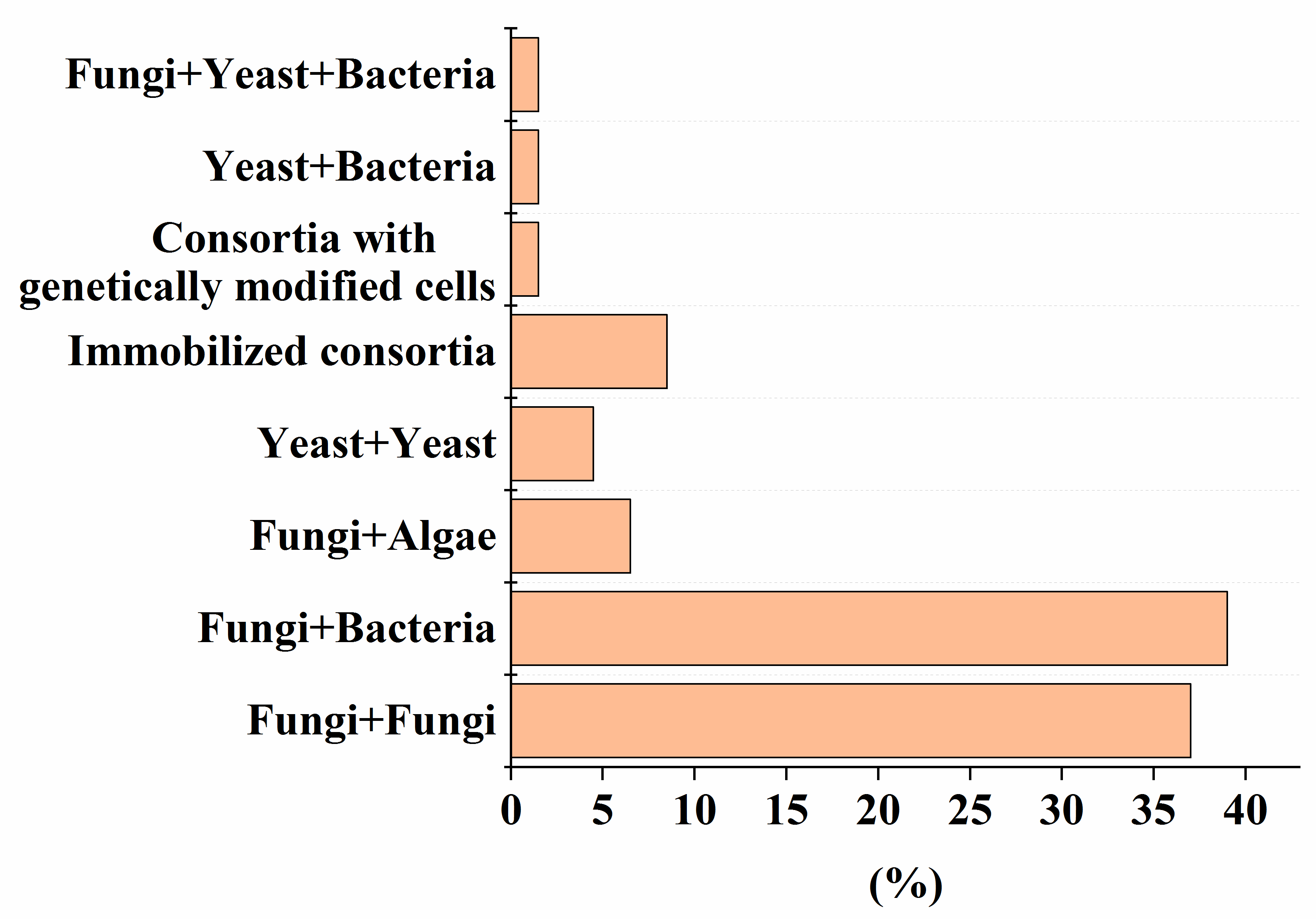
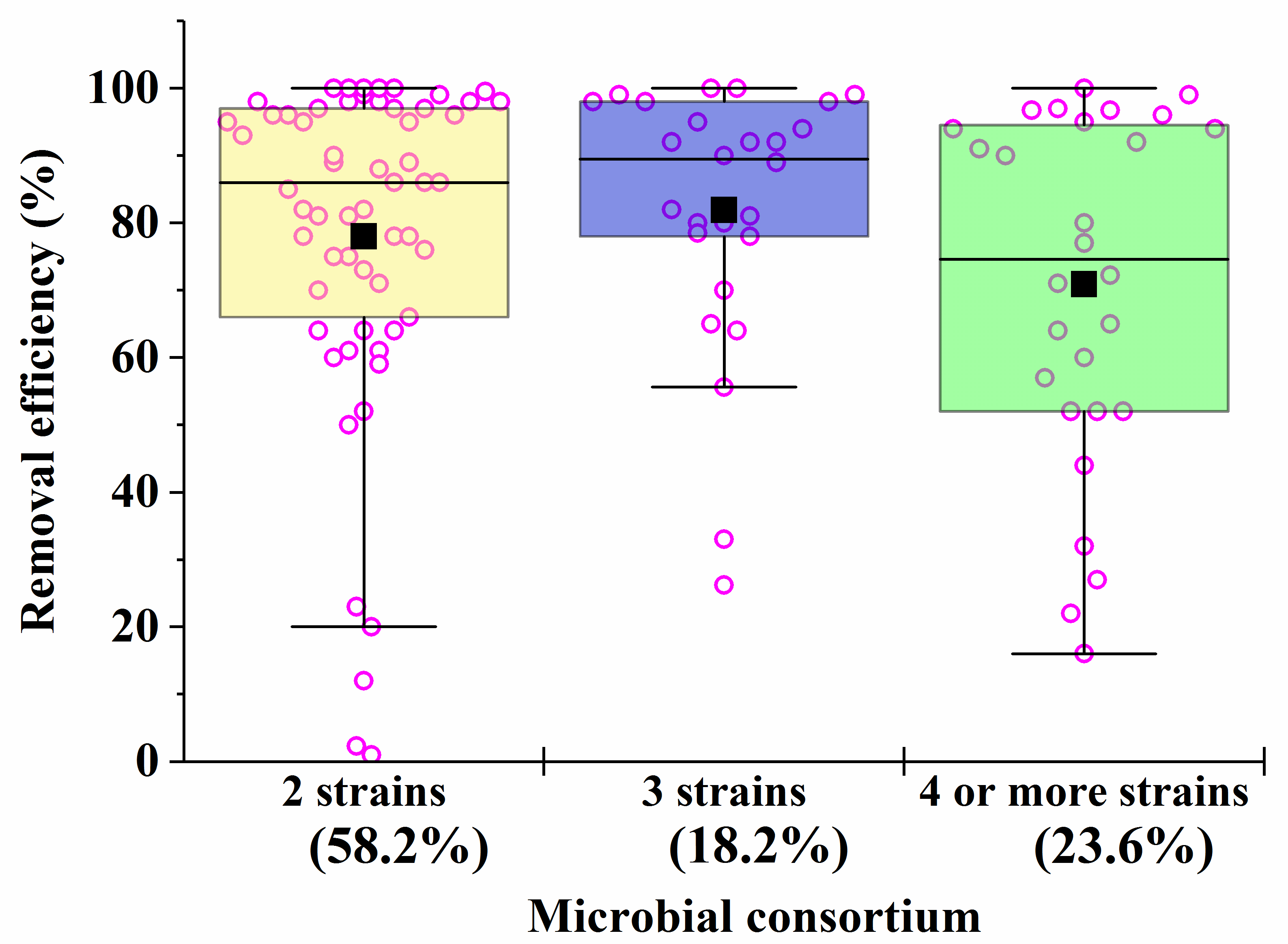
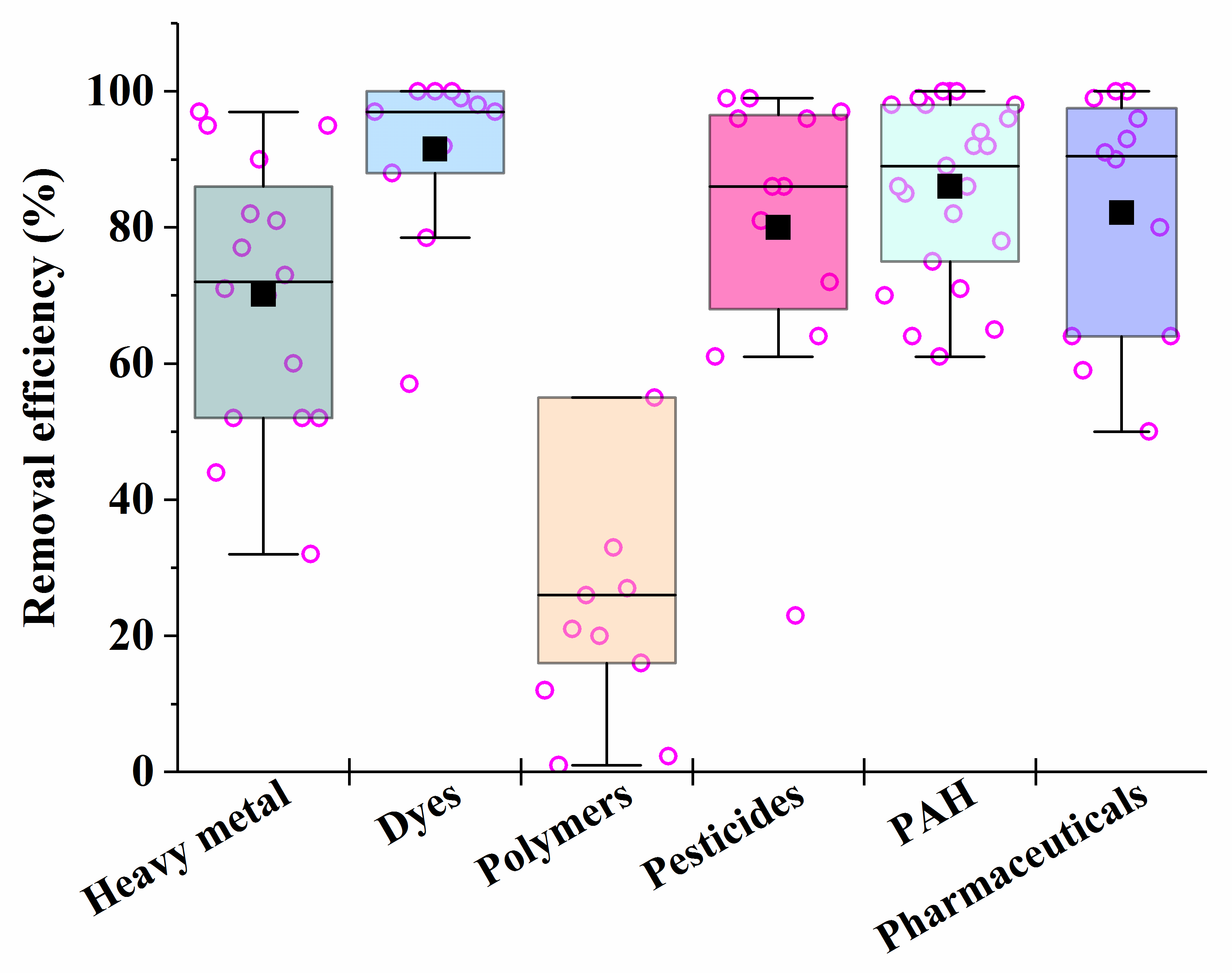
| Consortia [Reference] | Conditions | Pollutant/Process Efficiency |
|---|---|---|
| Aspergillus niveus, A. flavus, A. niger [39] | 1.4 × 106 spore/mL of each strain; pH 5.0, 110 rpm, 30 °C, 96 h | Removal of Cr, Zn, Pb, Cd and Ni—70–90% |
| A. flavus, A. fumigatus [40] | Heavy metal concentration—100 mg/L, 1.2 × 106 spores/mL, pH 5.0, 30 °C, 144 h | Removal of Cr(VI)—81%, Cd(II)—82%, mixture of metals—73% |
| Ascomycota and Basidiomycota fungi [41] | Initial metal concentration (23–2347 mg/kg), pH 7.9, soil moisture 60–65%, 28 °C, 100 days | Removal of As—77%, Cr—60%, Cu—52%, Fe—52%, Mn—71% |
| Ascomycota and Basidiomycota fungi [42] | Initial metal concentration (400–800 mg/kg), pH 7.9, soil moisture 60–65%, 28 °C, 100 days | Removal efficiencies of Ni, Pb, Zn—52%, 44%, 32% respectively |
| A. fumigatus, A. terreus, Paenibacillus dendritiformis [43] | Cd—100 mg/L, pH 5.0, 30 °C, 120 h | Removal of Cd(II)—95% |
| A. terreus, Talaromyces islandicus, Neurospora crassa, Aspergillus flavus [44] | Pb(II)—20.5–293.23 mg/L, Ni(II)—12.1–164.7 mg/L, inoculum 8%, pH 5.0, 30 °C 120 h | Removal of Pb(II) and Ni(II)—95–97% |
| Consortia [Reference] | Conditions | Pollutant/Process Efficiency |
|---|---|---|
| Yarrowia sp., Barnettozyma californica, Sterigmatomyces halophilus [49] | 100 mg/L of dye,30 °C, static conditions, 6–12 h | Degradation of Scarlet GR, Red HE3B, Remazol Brilliant Blue R, Methyl Orange, Rubine GFL and Reactive Red 2—92–100% |
| Daldinia concentrica, Xylaria polymorpha [50] | 50 mg/L of dye, pH 4.5, 30 °C, 150 rpm, 48 h. | Degradation of cibacron brilliant red 3B-A—99% |
| Rhodotorula sp., Raoultella planticola and Staphylococcus xylosus cells immobilized in Ca-alginate beads [51] | 200 mg/L of methylene blue in municipal wastewater and industrial effluent, 144 h | Degradation of methylene blue—100% and 78.5% in municipal wastewater and industrial effluent, respectively |
| A. niger, A. terrus, A. oryzae, A. fumigatus [52] | 20 mg/L of each dye, 150 rpm, 28 °C, 72 h | Degradation of reactive blue 4, fast green, methyl red, crystal violet, alura red AC, tartrazine, naphthol blue black, janus green B, alizarin yellow R, evans blue, brilliant green, pararosaniline, ponceau S, cibacron brilliant red 3B-A, direct violet 51—57–100% |
| Aspergillus sp., Chlorella sorokiniana [53] | Disperse Red—0.1 g/L, pH 6.0, 160 rpm, 25 °C, 4 days | Degradation/adsorption of disperse red 3B—98.1% |
| Daedalea dickinsii, Pseudomonas aeruginosa [54] | Methyl orange—100 mg/L, 30 °C, 7 days | Degradation of methyl orange—98% |
| Sterigmatomyces halophilus, Meyerozyma guilliermondii [55] | Reactive Black 5, Acid Orange 7; Reactive Green 19, Reactive Yellow, ABC, Atlantic Black C—50 mg/L, glucose as co-substrate, pH 7.0, 35 °C, 120 h | Degradation—88–97% |
| Penicillium oxalicum, Aspergillus tubingensis [56] | 100 mg/L of congo red with dextrose (10 g/L), pH 5, 150 rpm, 28 °C, 12 h | Congo red degradation—97.1% |
| Consortia [Reference] | Conditions | Pollutant/Process Efficiency |
|---|---|---|
| Sterigmatomyces halophilus, Meyerozyma guilliermondii, M. caribbica [60] | 30 °C, 45 days | Low-density polyethylene (LDPE) mass reduction—33.2% |
| A. niger, P. aeruginosa [61] | 37 °C, 30 days | Polyurethane weight loss—20% |
| Curvularia lunata, Alternaria alternata, Penicillium simplicissimum, Fusarium sp. [62] | 90 days | Polyethylene weight loss—27% |
| A. niger, A. flavus, A. oryzae [63] | 55 days | Polyethylene weight loss—26.2% |
| Microorganisms isolated from activated sludge and river sediments (Lysinibacillus massiliensis, Bacillus licheniformis, B. indicus, B. megaterium, B. cereus, Pseudomonas alcaligenes, Aspergillus sp., Penicillium sp., Alternaria sp., Candida parapsilosis [64] | 160 rpm, 56 days at room temperature, 10 mL of bacterial and fungi suspension, and one film sample (1 cm2) of polymer materials | Weight loss of sample (LDPE & thermoplastic starch & styrene-ethylene-styrene)—16% |
| Microorganisms isolated from compost (B. sonorensis, B. subtilis, Aspergillus sp. Trichoderma sp., Rhizopus sp.) [64] | Weight loss—21.9% | |
| Microorganisms of enriched landfill soil (Achromobacter xylosoxidans, Trichosporon chiropterorum, Penicillium chalabudae) [65] | pH 7.2, 150 rpm, 30 °C, 90 days | LDPE weight loss—55.6% |
| Aspergillus sp., Penicillium sp. [66] | 29 °C, 85% humidity, 30 days | Polypropylene/poly (butylene adipate-co-terephthalate)/thermoplastic starch weight loss—1.0–2.3% |
| Bacillus sp., Aspergillus sp. [67] | 30 °C, 150 rpm, 30 days | LDPE weight loss—12% |
| Consortia [Reference] | Conditions | Pollutant/Process Efficiency |
|---|---|---|
| Fomitopsis pinicola, B. subtilis [74] | 30 °C, 7 days | DDT (1,1,1-trichloro-2,2-bis(4-chlorophenyl) ethane) degradation—86% |
| Pleurotus ostreatus, P. aeruginosa [75] | 25 °C, 7 days | DDT degradation—86% |
| A. niger, Chlorella vulgaris [76] | 38 pesticides in mixture—total concentration—72.7µg/L, biomass—181.6 mg dry weight/L, pH 4.0, 100 rpm, 68 h | Degradation—23% |
| Verticilium sp., Metacordyceps sp. [77] | Concentration of each pesticide—50 mg/L, 100 rpm, pH 5.5, 27 °C, 21 days | Degradation of atrazine—81%, iprodione—96%; chlorpyrifos—99% |
| Verticilium sp., Metacordyceps sp. immobilized in Ca-alginate beads [77] | Concentration of each pesticide—50 mg/L, flow rate—90 mL/h, inoculum concentration—30 w/v, 100 rpm, 27 °C | Degradation of atrazine—64%, iprodione—96%; chlorpyrifos—85% (11–15 days) |
| Consortium of microorganisms present in coconut fiber, garden compost and agricultural soil and Trametes versicolor [78] | Mixture of pesticides—30–40 mg/kg, pH 6.4, 25 °C, 16 days | Degradation of atrazine—72.2%, carbendazim—96.7%, carbofuran—98.7%, metalaxyl—96.7% |
| Fomitopsis pinicola, Ralstonia pickettii [79] | DDT—5 mM, 30 °C, 7 days | DDT degradation—61% |
| Consortia [Reference] | Conditions | Pollutant/Process Efficiency |
|---|---|---|
| Acremonium sp, B. subtilis [85] | Concentration of each PAH in mixture—50 mg/L, 28 °C, 160 rpm, 10 days | Degradation of naphthalene—100%, fluorine—89%, phenanthrene—82%, anthracene—71%, fluoranthene—61% |
| Pleurotus ostreatus, Penicillium chrysogenum [86] | 30 °C, 30 days | Degradation of benzo[a]pyrene—86% |
| P. ostreatus, P. aeruginosa [86] | Degradation of benzo[a]pyrene—75% | |
| Consortium (Proteobacteria, Bacteroidota, Fusarium) immobilized on biochar [87] | Mixture of 50 mg/L of phenanthrene and 150 mg/L of Cd2+, 150 rpm, 30 °C, 7 days | Degradation of phenanthrene—92–98%, removing of Cd2+—94–99% |
| Consortium with two genetically modified strains of A. niger [88] | Mixture of pyrene and benzo(a)pyrene—1000 mg/kg soil, pH of 8.4, 30 °C, 14 days | Degradation efficiency of phenanthrene—92%, pyrene—64%, benzo(a)pyrene—65% |
| Kocuria rosea and A. sydowii immobilized in guargum-nanobentonite composite water dispersible granules [89] | Mixture of naphthalene, fluorene, phenanthrene, anthracene, and pyrene—100 µg of each PHA/g of soil, pH 8.3, 27 °C, 30 days | Degradation efficiency—85–100% |
| P. putida, yeast Basidioascus persicus [90] | 800 mg/L of pyrene, rhamnolipid biosurfactant 100 μL, 28 °C, 21 days | Degradation efficiency—78% |
| Ochrobactrum intermedium and white rot fungus Pleurotus ostreatus [91] | Concentrations of different PAH—138.2–268.0 mg/kg of soil, moisture—70%, 30 °C, 110 days | Degradation of fluoranthene, indene[1,2,3-cd]pyrene and benzo[g,h,i]perylene—100%; Anthracene, pyrene, chrysene and benzo[a]anthracene—96%, 86%, 98% and 98%, respectively |
| Pleurotus ostreatus, Azospirillum brasilense [92] | A mixture of anthracene, phenanthrene, fluorene, pyrene, and fluoranthene—50 mg/L, 130 rpm, 24 °C, 14 days | Degradation efficiency > 70% |
| Consortia [Reference] | Conditions | Pollutant/Process Efficiency |
|---|---|---|
| Pycnoporus sanguineus, Phanerochaete chrysosporium [95] | Each antibiotic concentration—10 mg/L, biomass of each strain—0.15 g dry weight/L), pH 4.5, 30 °C, 4 days | Removal efficiency of ciprofloxacin, norfloxacin and sulfamethoxazole in their mixture—100% |
| Pycnoporus sanguineus, Alcaligenes faecalis [96] | Sulfamethoxazole (50 mg/L) and vitamins mixture (VB2, VB6, VB12 and VC), 28 °C, 120 rpm, 24 h | Sulfamethoxazole degradation—93% |
| Ganoderma applanatum, Laetiporus sulphureus [97] | Concentration of each of three pollutants—10 mg/L, pH 6.4, ambient temperature, 150 rpm, 72 h | Degradation (mixture of celecoxib, diclofenac and ibuprofen)—99.5% |
| A. niger, Mucor circinelloides, Trichoderma longibrachiatum, Trametes polyzona and Rhizopus microsporus [98] | Pollutants concentration—1 mg/L, pH 4.6, 30 °C, 7 days, consortium concentration—30% (v/v) | Degradation of carbamazepine—90%, diclofenac sodium—96% and ibuprofen—91% |
| A. niger, C. vulgaris [99] | Pharmaceutical substances—8–11 μg/L, microalgae-fungus biomass—75 mg dry weight/L, 72 h | Relative removal of initial ranitidine concentrations—50% |
| Penicillium rastrickii, P. oxalicum, Cladosporium cladosporoides, Micrococcus yunnanensis, Oligella ureolytica, Sphingobacterium jejuense [100] | Mixture of diclofenac, carbamazepine and ketoprofen with 100 μM of each compound, 28 °C, 10 days | Degradation of diclofenac—99%, ketoprofen—80% |
| Chlorella vulgaris, Aspergillus oryzae [101] | Simulated swine wastewater with addition of 0.1–0.5 mg/L Cu (II), 0.4 mg/L of mixture of antimicrobial agents, pH 7.2, 28 °C, 14 days | Removal efficiency of sulfamonomethoxine, sulfamethoxazole and sulfamethazine—58.8%, 63.5%, and 63.9%, respectively |
| Consortia [Reference] | * Conditions | Pollutant/Process Efficiency |
|---|---|---|
| Acinetobacter baumannii, Talaromyces sp. [102] | The initial concentration of petroleum in soil—1220 mg/kg, pH 8.3, 30 °C, 28 days | Degradation of petroleum—65.6% |
| Paraburkholderia sp., Paraburkholderia tropica, Scedosporium boydii [103] | 1% v/v crude oil, 120 rpm, 30 °C, 7 days | Degradation of crude oil—81.5% |
| Scedosporium sp., Acinetobacter sp. [104] | Crude oil—200 mg/L, pH 7.0, 150 rpm, 30 °C, 7 days | Crude oil degradation—58.6% |
| Micrococcus luteus, Rhodococcus equi, A. niger [105] | Greywater—COD—1165.6 mg/L, oil and grease—58 mg/L, sulphate—95.6 mg/L, pH 7, 35 °C, 96 h | Degradation of COD, oil and grease and sulphate were 78.7, 82.6 and 89.7%, respectively |
| Aspergillus versicolor and bacterial species (Pseudomonas, Klebsiella species, B. subtilis) [106] | Greywater with 100 μg/L of carbendazim and thiamethoxam, 80 rpm, 30 °C, 240 h | Degradation of carbendazim and thiamethoxam 94.4 and 93.6%, respectively |
| A. flavus, Fusarium oxysporium [107] | Real textile effluent pH 8.7, COD—611 mg/L, pH 6.0–8.0, 28 °C, 7 days | Degradation—78.1%, COD removal—77.6% |
| Consortium of Brevibacillus laterosporus and Galactomyces geotrichum immobilized into Ca-alginate or polyvinyl alcohol-alginate beads [108] | Textile industry effluent pH 8.8, COD—2400 mg/L, 48–60 h | Degradation during 5 repeated cycles—76–95% |
| Ralstonia pickettii, Trichoderma viride [109] | Chlorobenzene—220 mg/L, 160 rpm, 28 °C, 60 h | Chlorobenzene degradation—100% |
| Chaetomium globosum, A. niger, Rhizopus oryzae [110] | Poly(vinyl acetate) processing wastewater pH 7.1, COD—23.48 g/L; pH 5.5, 150 rpm, 28 °C, 10 days | COD, poly(vinyl acetate) and color removal yields—97.8%, 98.5% and 99.8%, respectively. |
| Phanerochaete chrysosporium, Delftia lacustris [111] | Phenol (1000 mg/L) and selenite concentration—10 mg/L, 180 rpm, pH 6.5, 30 °C, 120 h | Phenol degradation—97.8% with the simultaneous reduction of selenite to Se(0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efremenko, E.; Stepanov, N.; Senko, O.; Aslanli, A.; Maslova, O.; Lyagin, I. Using Fungi in Artificial Microbial Consortia to Solve Bioremediation Problems. Microorganisms 2024, 12, 470. https://doi.org/10.3390/microorganisms12030470
Efremenko E, Stepanov N, Senko O, Aslanli A, Maslova O, Lyagin I. Using Fungi in Artificial Microbial Consortia to Solve Bioremediation Problems. Microorganisms. 2024; 12(3):470. https://doi.org/10.3390/microorganisms12030470
Chicago/Turabian StyleEfremenko, Elena, Nikolay Stepanov, Olga Senko, Aysel Aslanli, Olga Maslova, and Ilya Lyagin. 2024. "Using Fungi in Artificial Microbial Consortia to Solve Bioremediation Problems" Microorganisms 12, no. 3: 470. https://doi.org/10.3390/microorganisms12030470
APA StyleEfremenko, E., Stepanov, N., Senko, O., Aslanli, A., Maslova, O., & Lyagin, I. (2024). Using Fungi in Artificial Microbial Consortia to Solve Bioremediation Problems. Microorganisms, 12(3), 470. https://doi.org/10.3390/microorganisms12030470






