Exploring the In Vitro Antibacterial Potential of Specific Probiotic Strains against Oral Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain Cultures
2.1.1. Probiotic Strains
2.1.2. Pathogen Strains
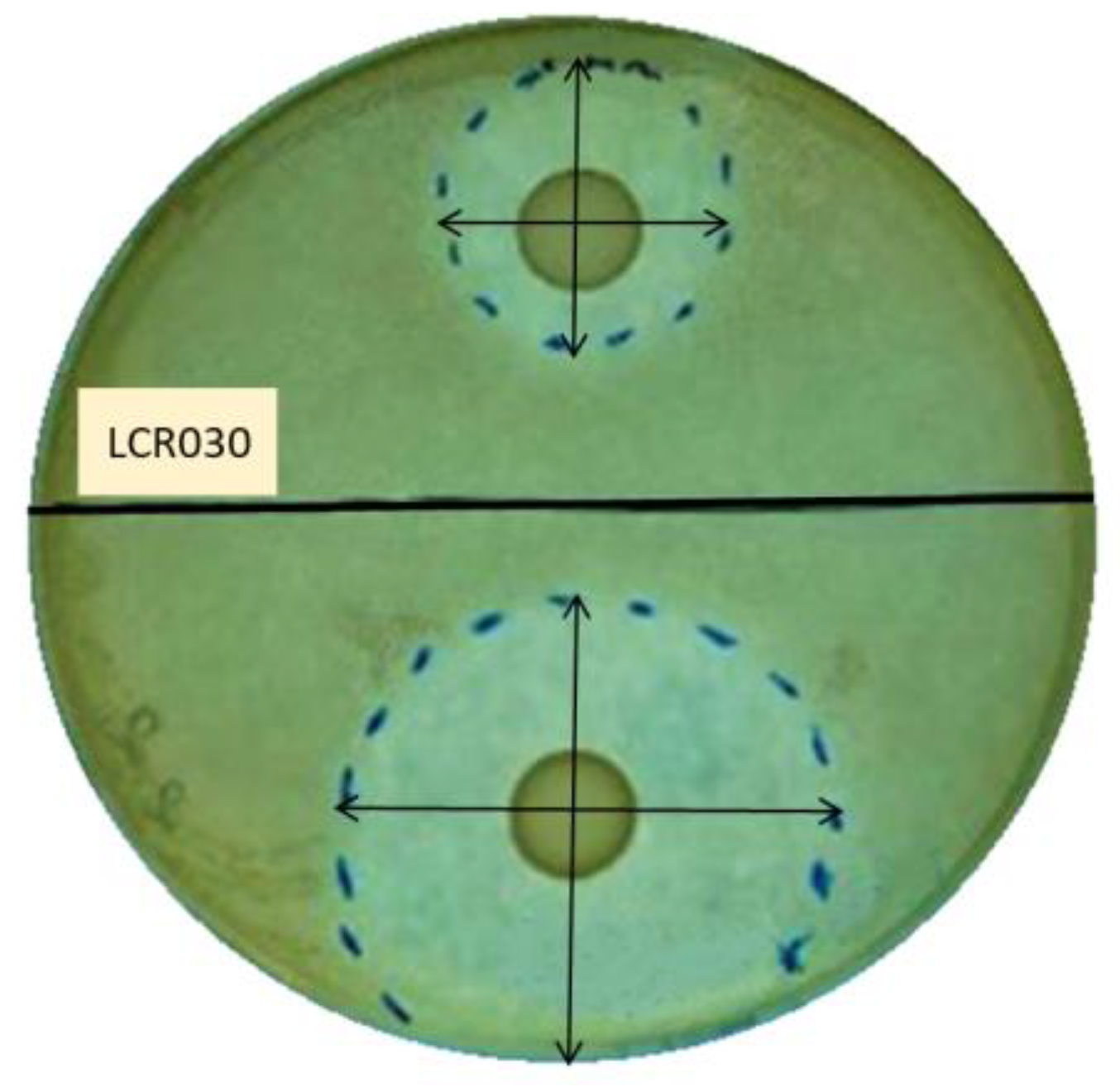
2.2. Screening of Probiotic Antibacterial Effect by Agar Overlay Assay
2.2.1. Preventive Experiments
2.2.2. Treatment Experiments
2.3. Colony Forming Unit (CFU) Count of Adherent Cells in the Preventive Model and Image Acquisition
2.4. Statistical Analysis
3. Results
3.1. Antibacterial Properties of Probiotic Strains
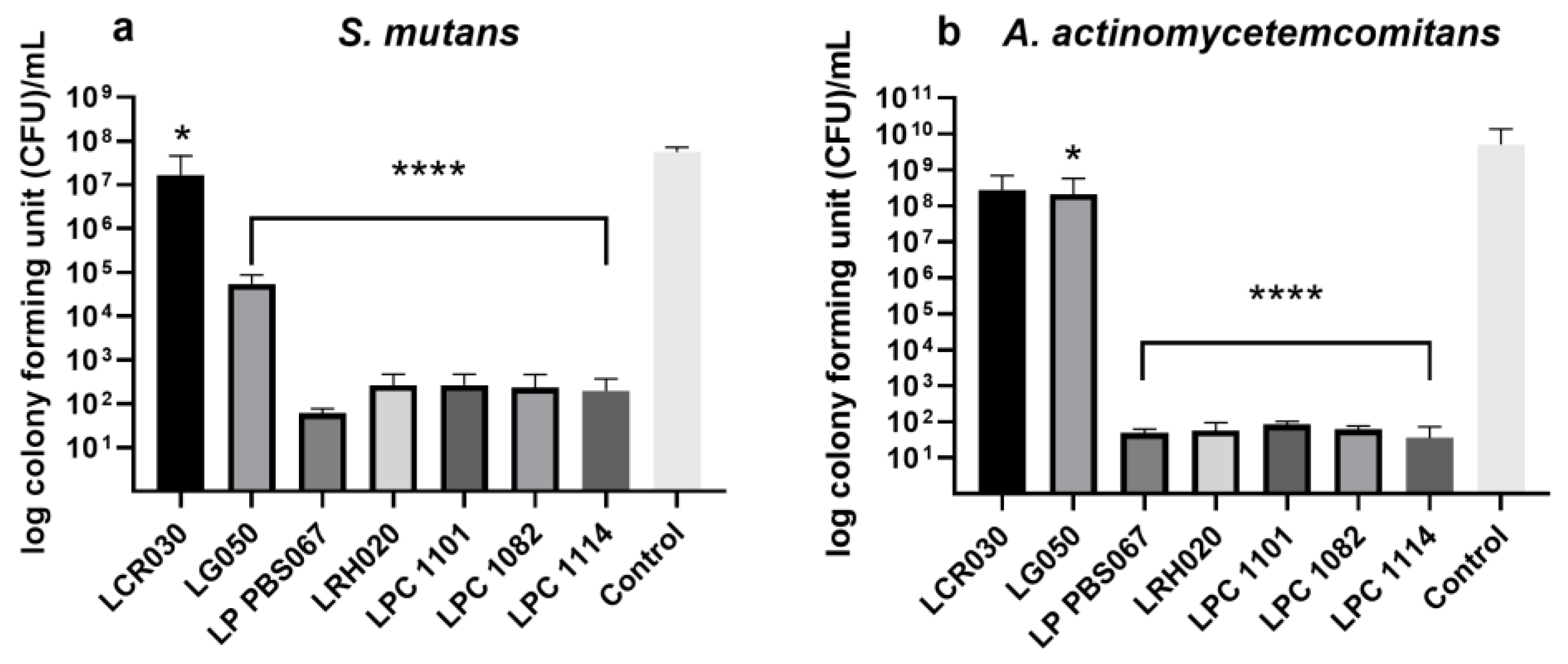
3.2. Inhibition of Pathogen Adhesion and Growth by Probiotic Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deo, P.; Deshmukh, R. Oral Microbiome: Unveiling the Fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.G.; Sarkar, S.; Umar, S.; Lee, S.T.M.; Thomas, S.M. The Contribution of the Human Oral Microbiome to Oral Disease: A Review. Microorganisms 2023, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human Oral Microbiota and Its Modulation for Oral Health. Biomed. Pharmacother. 2018, 99, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Yang, X.; Li, C.; Song, Z. The Oral Microbiota: Community Composition, Influencing Factors, Pathogenesis, and Interventions. Front. Microbiol. 2022, 13, 895537. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The Oral Microbiota: Dynamic Communities and Host Interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, M.; Liu, Y.; Luo, B.; Cui, J.; Huang, L.; Chen, K.; Liu, Y. The Oral Microbiota and Cardiometabolic Health: A Comprehensive Review and Emerging Insights. Front. Immunol. 2022, 13, 1010368. [Google Scholar] [CrossRef]
- Georges, F.M.; Do, N.T.; Seleem, D. Oral Dysbiosis and Systemic Diseases. Front. Dent. Med. 2022, 3, 995423. [Google Scholar] [CrossRef]
- Gross, E.L.; Beall, C.J.; Kutsch, S.R.; Firestone, N.D.; Leys, E.J.; Griffen, A.L. Beyond Streptococcus Mutans: Dental Caries Onset Linked to Multiple Species by 16S rRNA Community Analysis. PLoS ONE 2012, 7, e47722. [Google Scholar] [CrossRef]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus Mutans. Microbiol. Spectr. 2019, 7, 10–1128. [Google Scholar] [CrossRef]
- Forssten, S.D.; Björklund, M.; Ouwehand, A.C. Streptococcus Mutans, Caries and Simulation Models. Nutrients 2010, 2, 290–298. [Google Scholar] [CrossRef]
- Sounah, S.A.; Madfa, A.A. Correlation between Dental Caries Experience and the Level of Streptococcus Mutans and Lactobacilli in Saliva and Carious Teeth in a Yemeni Adult Population. BMC Res. Notes 2020, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.; Wilson, N.; Williams, E.; Letner, H.; Bettinger, R.; Bouchendouka, A.; Batagower, J.; Kaspar, J.R. Growth with Commensal Streptococci Alters Streptococcus Mutans Behaviors. J. Dent. Res. 2023, 102, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Krzyściak, W.; Jurczak, A.; Kościelniak, D.; Bystrowska, B.; Skalniak, A. The Virulence of Streptococcus Mutans and the Ability to Form Biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef]
- Khaledi, M.; Sameni, F.; Afkhami, H.; Hemmati, J.; Asareh Zadegan Dezfuli, A.; Sanae, M.-J.; Validi, M. Infective Endocarditis by HACEK: A Review. J. Cardiothorac. Surg. 2022, 17, 185. [Google Scholar] [CrossRef] [PubMed]
- Raja, M. Aggregatibacter Actinomycetemcomitans–A Tooth Killer? JCDR 2014, 8, ZE13–ZE16. [Google Scholar] [CrossRef] [PubMed]
- Nørskov-Lauritsen, N.; Claesson, R.; Jensen, A.B.; Åberg, C.H.; Haubek, D. Aggregatibacter Actinomycetemcomitans: Clinical Significance of a Pathobiont Subjected to Ample Changes in Classification and Nomenclature. Pathogens 2019, 8, 243. [Google Scholar] [CrossRef]
- Herbert, B.A.; Novince, C.M.; Kirkwood, K.L. Aggregatibacter Actinomycetemcomitans, a Potent Immunoregulator of the Periodontal Host Defense System and Alveolar Bone Homeostasis. Mol. Oral Microbiol. 2016, 31, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.N.; Maula, T.; Bao, K.; Lindholm, M.; Bostanci, N.; Oscarsson, J.; Ihalin, R.; Johansson, A. Virulence and Pathogenicity Properties of Aggregatibacter Actinomycetemcomitans. Pathogens 2019, 8, 222. [Google Scholar] [CrossRef]
- Pietiäinen, M.; Kopra, K.A.E.; Vuorenkoski, J.; Salminen, A.; Paju, S.; Mäntylä, P.; Buhlin, K.; Liljestrand, J.M.; Nieminen, M.S.; Sinisalo, J.; et al. Aggregatibacter Actinomycetemcomitans Serotypes Associate with Periodontal and Coronary Artery Disease Status. J. Clin. Periodontol. 2018, 45, 413–421. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between Periodontal Pathogens and Systemic Disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Bahekar, A.A.; Singh, S.; Saha, S.; Molnar, J.; Arora, R. The Prevalence and Incidence of Coronary Heart Disease Is Significantly Increased in Periodontitis: A Meta-Analysis. Am. Heart J. 2007, 154, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, L.L.; Fu, R.; Buckley, D.I.; Freeman, M.; Helfand, M. Periodontal Disease and Coronary Heart Disease Incidence: A Systematic Review and Meta-Analysis. J. Gen. Intern. Med. 2008, 23, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Naka, S.; Nakano, K.; Wada, K.; Endo, H.; Mawatari, H.; Imajo, K.; Nomura, R.; Hokamura, K.; Ono, M.; et al. Involvement of a Periodontal Pathogen, Porphyromonas Gingivalis on the Pathogenesis of Non-Alcoholic Fatty Liver Disease. BMC Gastroenterol. 2012, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Arimatsu, K.; Yamada, H.; Miyazawa, H.; Minagawa, T.; Nakajima, M.; Ryder, M.I.; Gotoh, K.; Motooka, D.; Nakamura, S.; Iida, T.; et al. Oral Pathobiont Induces Systemic Inflammation and Metabolic Changes Associated with Alteration of Gut Microbiota. Sci. Rep. 2014, 4, 4828. [Google Scholar] [CrossRef] [PubMed]
- Chávarry, N.G.M.; Vettore, M.V.; Sansone, C.; Sheiham, A. The relationship between diabetes mellitus and destructive periodontal disease: A meta-analysis. Oral Health Prev. Dent. 2009, 7, 107–127. [Google Scholar] [PubMed]
- Nguyen, T.; Brody, H.; Radaic, A.; Kapila, Y. Probiotics for Periodontal Health—Current Molecular Findings. Periodontology 2000 2021, 87, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Verspecht, T.; Rodriguez Herrero, E.; Khodaparast, L.; Khodaparast, L.; Boon, N.; Bernaerts, K.; Quirynen, M.; Teughels, W. Development of Antiseptic Adaptation and Cross-Adapatation in Selected Oral Pathogens in Vitro. Sci. Rep. 2019, 9, 8326. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Zanetta, P.; Ormelli, M.; Amoruso, A.; Pane, M.; Azzimonti, B.; Squarzanti, D.F. Probiotics as Potential Biological Immunomodulators in the Management of Oral Lichen Planus: What’s New? Int. J. Mol. Sci. 2022, 23, 3489. [Google Scholar] [CrossRef]
- Butt, S.; Sin, M. Can Probiotics Prevent Dental Caries? Evid. Based Dent. 2023, 24, 130–131. [Google Scholar] [CrossRef]
- Kaźmierczyk-Winciorek, M.; Nędzi-Góra, M.; Słotwińska, S.M. The Immunomodulating Role of Probiotics in the Prevention and Treatment of Oral Diseases. Cent. Eur. J. Immunol. 2021, 46, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Alhallak, E.; Kouchaje, C.; Hasan, A.; Makieh, R. Evaluation of the Effectiveness of Probiotic Mouthwashes in Reducing Dental Plaque in Primary and Permanent Teeth: A Randomized Clinical Trial. Cureus 2022, 14, e28125. [Google Scholar] [CrossRef] [PubMed]
- Berezow, A.B.; Darveau, R.P. Microbial Shift and Periodontitis. Periodontology 2000 2011, 55, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Invernici, M.M.; Salvador, S.L.; Silva, P.H.F.; Soares, M.S.M.; Casarin, R.; Palioto, D.B.; Souza, S.L.S.; Taba, M.; Novaes, A.B.; Furlaneto, F.A.C.; et al. Effects of Bifidobacterium Probiotic on the Treatment of Chronic Periodontitis: A Randomized Clinical Trial. J. Clin. Periodontol. 2018, 45, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; You, Y.; Kang, J.; Kim, H.; Kang, M. Weissella Cibaria CMU Exerts an Anti-inflammatory Effect by Inhibiting Aggregatibacter Actinomycetemcomitans-induced NF-κB Activation in Macrophages. Mol. Med. Rep. 2020, 22, 4143–4150. [Google Scholar] [CrossRef] [PubMed]
- Nissen, L.; Sgorbati, B.; Biavati, B.; Belibasakis, G.N. Lactobacillus Salivarius and L. Gasseri down-Regulate Aggregatibacter Actinomycetemcomitans Exotoxins Expression. Ann. Microbiol. 2014, 64, 611–617. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ishikawa, K.H.; Bueno, M.R.; Kawamoto, D.; Simionato, M.R.L.; Mayer, M.P.A. Lactobacilli Postbiotics Reduce Biofilm Formation and Alter Transcription of Virulence Genes of Aggregatibacter Actinomycetemcomitans. Mol. Oral Microbiol. 2021, 36, 92–102. [Google Scholar] [CrossRef]
- Jaffar, N.; Ishikawa, Y.; Mizuno, K.; Okinaga, T.; Maeda, T. Mature Biofilm Degradation by Potential Probiotics: Aggregatibacter Actinomycetemcomitans versus Lactobacillus Spp. PLoS ONE 2016, 11, e0159466. [Google Scholar] [CrossRef]
- Kadkhoda, Z.; Amarlu, Z.; Eshraghi, S.; Samiei, N. Antimicrobial Effect of Chlorhexidine on Aggregatibacter Actinomycetemcomitans Biofilms Associated with Peri-Implantitis. J. Dent. Res. Dent. Clin. Dent. Prospect. 2016, 10, 176–180. [Google Scholar] [CrossRef]
- Rajendiran, M.; Trivedi, H.M.; Chen, D.; Gajendrareddy, P.; Chen, L. Recent Development of Active Ingredients in Mouthwashes and Toothpastes for Periodontal Diseases. Molecules 2021, 26, 2001. [Google Scholar] [CrossRef] [PubMed]
- Ursu, R.G.; Iancu, L.S.; Porumb-Andrese, E.; Damian, C.; Cobzaru, R.G.; Nichitean, G.; Ripa, C.; Sandu, D.; Luchian, I. Host mRNA Analysis of Periodontal Disease Patients Positive for Porphyromonas Gingivalis, Aggregatibacter Actinomycetemcomitans and Tannerella Forsythia. IJMS 2022, 23, 9915. [Google Scholar] [CrossRef] [PubMed]
- Sulistiowati, C.P.; Suhartono, M.; Rahmawati, D.F.; Ulfah, N.; Supandi, S.K.; Wijaksana, I.K.E.; Abullais, S.S.; Dhadse, P. In-Vitro Inhibitory Efficacy of 3 Types of Probiotics on the Growth of Aggregatibacter Actinomycetemcomitans Bacteria. Front. Biosci. (Landmark Ed.) 2023, 28, 106. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Bañuelos, E.; Mukherjee, A.; Darrah, E.; Andrade, F. Rheumatoid Arthritis-Associated Mechanisms of Porphyromonas Gingivalis and Aggregatibacter Actinomycetemcomitans. JCM 2019, 8, 1309. [Google Scholar] [CrossRef] [PubMed]
- Maricic, N.; Dawid, S. Using the Overlay Assay to Qualitatively Measure Bacterial Production of and Sensitivity to Pneumococcal Bacteriocins. JoVE 2014, 91, 51876. [Google Scholar] [CrossRef]
- Hossain, M.L.; Hammer, K.; Lim, L.Y.; Hettiarachchi, D.; Locher, C. Optimisation of an Agar Overlay Assay for the Assessment of the Antimicrobial Activity of Topically Applied Semi-Solid Antiseptic Products Including Honey-Based Formulations. J. Microbiol. Methods 2022, 202, 106596. [Google Scholar] [CrossRef] [PubMed]
- Zawistowska-Rojek, A.; Kośmider, A.; Stępień, K.; Tyski, S. Adhesion and Aggregation Properties of Lactobacillaceae Strains as Protection Ways against Enteropathogenic Bacteria. Arch. Microbiol. 2022, 204, 285. [Google Scholar] [CrossRef] [PubMed]
- Seminario-Amez, M.; Lopez-Lopez, J.; Estrugo-Devesa, A.; Ayuso-Montero, R.; Jane-Salas, E. Probiotics and Oral Health: A Systematic Review. Med. Oral 2017, 22, e282–e288. [Google Scholar] [CrossRef]
- Kriswandini, I.L.; Diyatri, I.; Nuraini, P.; Berniyanti, T.; Putri, I.A.; Tyas, P.N.B.N. The Forming of Bacteria Biofilm from Streptococcus mutans and Aggregatibacter Actinomycetemcomitans as a Marker for Early Detection in Dental Caries and Periodontitis. Infect. Dis. Rep. 2020, 12, 8722. [Google Scholar] [CrossRef]
- Fine, D.H.; Patil, A.G.; Velusamy, S.K. Aggregatibacter Actinomycetemcomitans (Aa) under the Radar: Myths and Misunderstandings of Aa and Its Role in Aggressive Periodontitis. Front. Immunol. 2019, 10, 728. [Google Scholar] [CrossRef]
- Siddiqui, R.; Badran, Z.; Boghossian, A.; Alharbi, A.M.; Alfahemi, H.; Khan, N.A. The Increasing Importance of the Oral Microbiome in Periodontal Health and Disease. Future Sci. OA 2023, 9, FSO856. [Google Scholar] [CrossRef] [PubMed]
- Shetty, B.; Fazal, I.; Khan, S.F.; Nambiar, M.; Irfana, D.K.; Prasad, R.; Raj, A. Association between Cardiovascular Diseases and Periodontal Disease: More than What Meets the Eye. Drug Target Insights 2023, 17, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Schenkein, H.A.; Papapanou, P.N.; Genco, R.; Sanz, M. Mechanisms Underlying the Association between Periodontitis and Atherosclerotic Disease. Periodontology 2000 2020, 83, 90–106. [Google Scholar] [CrossRef] [PubMed]
- Almarhoumi, R.; Alvarez, C.; Harris, T.; Tognoni, C.M.; Paster, B.J.; Carreras, I.; Dedeoglu, A.; Kantarci, A. Microglial Cell Response to Experimental Periodontal Disease. J. Neuroinflamm. 2023, 20, 142. [Google Scholar] [CrossRef] [PubMed]
- Păunică, I.; Giurgiu, M.; Dumitriu, A.S.; Păunică, S.; Pantea Stoian, A.M.; Martu, M.-A.; Serafinceanu, C. The Bidirectional Relationship between Periodontal Disease and Diabetes Mellitus—A Review. Diagnostics 2023, 13, 681. [Google Scholar] [CrossRef] [PubMed]
- Konig, M.F.; Abusleme, L.; Reinholdt, J.; Palmer, R.J.; Teles, R.P.; Sampson, K.; Rosen, A.; Nigrovic, P.A.; Sokolove, J.; Giles, J.T.; et al. Aggregatibacter Actinomycetemcomitans–Induced Hypercitrullination Links Periodontal Infection to Autoimmunity in Rheumatoid Arthritis. Sci. Transl. Med. 2016, 8, 369ra176. [Google Scholar] [CrossRef] [PubMed]
- Brewer, R.C.; Lanz, T.V.; Hale, C.R.; Sepich-Poore, G.D.; Martino, C.; Swafford, A.D.; Carroll, T.S.; Kongpachith, S.; Blum, L.K.; Elliott, S.E.; et al. Oral Mucosal Breaks Trigger Anti-Citrullinated Bacterial and Human Protein Antibody Responses in Rheumatoid Arthritis. Sci. Transl. Med. 2023, 15, eabq8476. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic Mechanisms of Action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Homayouni Rad, A.; Pourjafar, H.; Mirzakhani, E. A Comprehensive Review of the Application of Probiotics and Postbiotics in Oral Health. Front. Cell. Infect. Microbiol. 2023, 13, 1120995. [Google Scholar] [CrossRef]
- Darmastuti, A.; Hasan, P.N.; Wikandari, R.; Utami, T.; Rahayu, E.S.; Suroto, D.A. Adhesion Properties of Lactobacillus Plantarum Dad-13 and Lactobacillus Plantarum Mut-7 on Sprague Dawley Rat Intestine. Microorganisms 2021, 9, 2336. [Google Scholar] [CrossRef]
- Carvalho, F.M.; Teixeira-Santos, R.; Mergulhão, F.J.M.; Gomes, L.C. The Use of Probiotics to Fight Biofilms in Medical Devices: A Systematic Review and Meta-Analysis. Microorganisms 2020, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Gómez, N.C.; Ramiro, J.M.P.; Quecan, B.X.V.; De Melo Franco, B.D.G. Use of Potential Probiotic Lactic Acid Bacteria (LAB) Biofilms for the Control of Listeria Monocytogenes, Salmonella Typhimurium, and Escherichia Coli O157:H7 Biofilms Formation. Front. Microbiol. 2016, 7, 863. [Google Scholar] [CrossRef] [PubMed]
- Barzegari, A.; Kheyrolahzadeh, K.; Hosseiniyan Khatibi, S.M.; Sharifi, S.; Memar, M.Y.; Zununi Vahed, S. The Battle of Probiotics and Their Derivatives Against Biofilms. Infect. Drug Resist. 2020, 13, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Malfa, P.; Brambilla, L.; Giardina, S.; Masciarelli, M.; Squarzanti, D.F.; Carlomagno, F.; Meloni, M. Evaluation of Antimicrobial, Antiadhesive and Co-Aggregation Activity of a Multi-Strain Probiotic Composition against Different Urogenital Pathogens. Int. J. Mol. Sci. 2023, 24, 1323. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-S.; Kim, M.; Nam, S.-H.; Kang, M.-S.; Lee, S.-A. Effects of Oral Probiotics on Subjective Halitosis, Oral Health, and Psychosocial Health of College Students: A Randomized, Double-Blind, Placebo-Controlled Study. Int. J. Environ. Res. Public Health 2021, 18, 1143. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Chen, Y.-T.; Ho, H.-H.; Kuo, Y.-W.; Lin, W.-Y.; Chen, J.-F.; Lin, J.-H.; Liu, C.-R.; Lin, C.-H.; Yeh, Y.-T.; et al. Impact of the Food Grade Heat-Killed Probiotic and Postbiotic Oral Lozenges in Oral Hygiene. Aging 2022, 14, 2221–2238. [Google Scholar] [CrossRef] [PubMed]
- Lundtorp Olsen, C.; Massarenti, L.; Vendius, V.F.D.; Gürsoy, U.K.; Van Splunter, A.; Bikker, F.J.; Gürsoy, M.; Damgaard, C.; Markvart, M.; Belstrøm, D. Probiotics Support Resilience of the Oral Microbiota during Resolution after Experimental Gingivitis—A Randomized, Double-Blinded, Placebo-Controlled Trial. Nutrients 2023, 15, 4805. [Google Scholar] [CrossRef]
- Lundtorp Olsen, C.; Massarenti, L.; Vendius, V.F.D.; Gürsoy, U.K.; Van Splunter, A.; Bikker, F.J.; Gürsoy, M.; Damgaard, C.; Markvart, M.; Belstrøm, D. Probiotics Partly Suppress the Impact of Sugar Stress on the Oral Microbiota—A Randomized, Double-Blinded, Placebo-Controlled Trial. Nutrients 2023, 15, 4810. [Google Scholar] [CrossRef]
- Li, X.; Fields, F.R.; Ho, M.; Marshall-Hudson, A.; Gross, R.; Casser, M.E.; Naito, M. Safety Assessment of Streptococcus Salivarius DB-B5 as a Probiotic Candidate for Oral Health. Food Chem. Toxicol. 2021, 153, 112277. [Google Scholar] [CrossRef]
- De Oliveira, G.L.V.; Leite, A.Z.; Higuchi, B.S.; Gonzaga, M.I.; Mariano, V.S. Intestinal Dysbiosis and Probiotic Applications in Autoimmune Diseases. Immunology 2017, 152, 1–12. [Google Scholar] [CrossRef]
- Stene, C.; Röme, A.; Palmquist, I.; Linninge, C.; Molin, G.; Ahrné, S.; Johnson, L.B.; Jeppsson, B. Administration of Probiotics to Healthy Volunteers: Effects on Reactivity of Intestinal Mucosa and Systemic Leukocytes. BMC Gastroenterol. 2022, 22, 100. [Google Scholar] [CrossRef]
- Ghini, V.; Tenori, L.; Pane, M.; Amoruso, A.; Marroncini, G.; Squarzanti, D.F.; Azzimonti, B.; Rolla, R.; Savoia, P.; Tarocchi, M.; et al. Effects of Probiotics Administration on Human Metabolic Phenotype. Metabolites 2020, 10, 396. [Google Scholar] [CrossRef] [PubMed]
- Vong, L.; Lorentz, R.J.; Assa, A.; Glogauer, M.; Sherman, P.M. Probiotic Lactobacillus Rhamnosus Inhibits the Formation of Neutrophil Extracellular Traps. J. Immunol. 2014, 192, 1870–1877. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Zhang, H.; Luo, X.M. SLE: Another Autoimmune Disorder Influenced by Microbes and Diet? Front. Immunol. 2015, 6, 608. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Deng, Y.; He, Q.; Yang, K.; Li, J.; Xiang, W.; Liu, H.; Zhu, X.; Chen, H. Safety and Efficacy of Probiotic Supplementation in 8 Types of Inflammatory Arthritis: A Systematic Review and Meta-Analysis of 34 Randomized Controlled Trials. Front. Immunol. 2022, 13, 961325. [Google Scholar] [CrossRef] [PubMed]
- Jhun, J.; Cho, K.-H.; Lee, D.-H.; Kwon, J.Y.; Woo, J.S.; Kim, J.; Na, H.S.; Park, S.-H.; Kim, S.J.; Cho, M.-L. Oral Administration of Lactobacillus Rhamnosus Ameliorates the Progression of Osteoarthritis by Inhibiting Joint Pain and Inflammation. Cells 2021, 10, 1057. [Google Scholar] [CrossRef] [PubMed]

| Probiotic Strains | S. mutans | A. actinomycetemcomitans | ||
|---|---|---|---|---|
| Model | Model | |||
| Preventive | Treatment | Preventive | Treatment | |
| L. acidophilus PBS066 | +++ | ++ | ++ | ++ |
| L. crispatus LCR030 | ++++ | ++ | +++ | ++ |
| L. gasseri LG050 | ++ | + | ++++ | +++ |
| L. plantarum PBS067 | +++ | ++ | ++++ | +++ |
| L. reuteri PBS072 | ++ | ++ | + | ++ |
| L. rhamnosus LRH020 | +++ | ++ | +++ | ++ |
| B. lactis BL050 | +++ | + | +++ | + |
| L. paracasei LPC 1101 | ++++ | ++ | +++ | ++ |
| L. paracasei LPC 1082 | +++ | ++ | +++ | ++ |
| L. paracasei LPC 1114 | +++ | ++ | ++++ | ++ |
| Control | No zone | No zone | No zone | No zone |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Squarzanti, D.F.; Dell’Atti, F.; Scalia, A.C.; Najmi, Z.; Cochis, A.; Malfa, P. Exploring the In Vitro Antibacterial Potential of Specific Probiotic Strains against Oral Pathogens. Microorganisms 2024, 12, 441. https://doi.org/10.3390/microorganisms12030441
Squarzanti DF, Dell’Atti F, Scalia AC, Najmi Z, Cochis A, Malfa P. Exploring the In Vitro Antibacterial Potential of Specific Probiotic Strains against Oral Pathogens. Microorganisms. 2024; 12(3):441. https://doi.org/10.3390/microorganisms12030441
Chicago/Turabian StyleSquarzanti, Diletta F., Federica Dell’Atti, Alessandro C. Scalia, Ziba Najmi, Andrea Cochis, and Patrizia Malfa. 2024. "Exploring the In Vitro Antibacterial Potential of Specific Probiotic Strains against Oral Pathogens" Microorganisms 12, no. 3: 441. https://doi.org/10.3390/microorganisms12030441
APA StyleSquarzanti, D. F., Dell’Atti, F., Scalia, A. C., Najmi, Z., Cochis, A., & Malfa, P. (2024). Exploring the In Vitro Antibacterial Potential of Specific Probiotic Strains against Oral Pathogens. Microorganisms, 12(3), 441. https://doi.org/10.3390/microorganisms12030441





