A Response Surface Methodology Study for Chlorella vulgaris Mixotrophic Culture Optimization
Abstract
1. Introduction
| Glycerol Concentration (g L−1) | NaNO3 (mg L−1) | MgSO4·7H2O (mg L−1) | Biomass Concentration (g L−1) | Biomass Productivity (g L−1 d−1) | Lipid Content (%) | Lipid Productivity (g L−1 d−1) | Specific Growth Rate (d−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 2.7 | 750 | 75 | 1.56 | 0.17 ± 0.03 | 0.280 ± 0.09 | [28] | ||
| 2 | 1500 | 75 | 1.50 | 0.23 ± 0.02 | 15.91 ± 1.50 | 0.342 ± 0.03 | [29] | |
| 20 | 250 | 75 | 0.66 ± 0.01 | 0.09 ± 0.01 | 34 ± 4.00 | 0.031 ± 0.004 | [30] | |
| 20.4 | 1000 | 500 | 1.17 ± 1.34 | 0.39 ± 0.45 | 40.10 ± 22.06 | 0.16 ± 0.10 | [31] | |
| 5 | 800 | 50 | 1.91 | 0.227 ± 0.007 | 15.91% | 0.342 ± 0.004 | [22] | |
| 5 | 250 | 75 | 2.13 ± 0.34 | 0.53 ± 0.08 | 0.94 ± 0.04 | [17] | ||
| 4 | 100 | 400 | 2.64 ± 0.22 | 0.19 ± 0.01 | 20.36 ± 5.30 | 0.43 ± 0.09 | [32] | |
| 10 | Soil extract | 1.32 ± 0.27 | 26.90 ± 0.21 | [23] | ||||
2. Materials and Methods
2.1. Chlorella vulgaris Growth Conditions
2.2. Experimental Design and Statistical Analysis
2.3. Effects of Organic Carbon Sources on C. vulgaris Nitrogen and Phosphorus Removal and Fatty Acid Profile
2.4. Urea and Glycerol Concentration Measurements
3. Results
3.1. Regression Model and Statistical Analysis
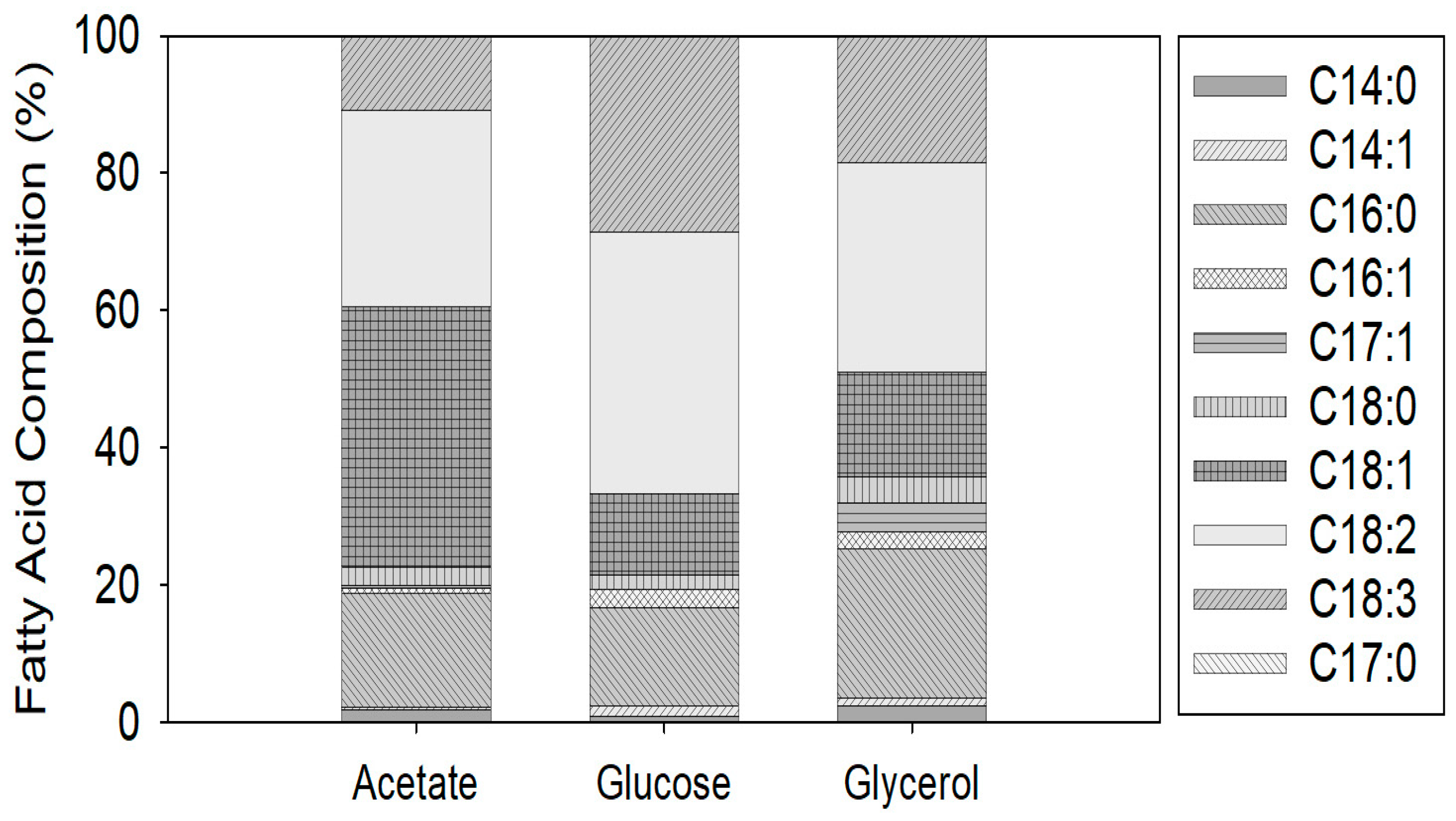
3.2. The Fatty Acid Composition Obtained in C. vulgaris Cultured with Glycerol, Acetate, and Glucose
3.3. Nitrogen and Phosphorus Removal by C. vulgaris for Various Organic Carbon Sources
4. Discussion
5. Conclusions
- -
- When glycerol is used as the carbon source, the optimal levels of urea and MgSO4·7H2O for Chlorella vulgaris culture under mixotrophic conditions are 1.7 g L−1 and 1.0 g L−1, respectively.
- -
- At the optimal urea and MgSO4·7H2O concentrations for glycerol consumption, C. vulgaris consumed 3.8 g L−1 glycerol and 0.89 g L−1 urea, with a biomass production of 1.4 g L−1 d−1 after four days of growth.
- -
- The medium’s carbon source (glycerol, glucose, or acetate) did not affect lipid production when the optimal urea and MgSO4·7H2O conditions were used; in this case, the average lipid production was 10%.
- -
- Under the optimal growth conditions for glycerol consumption, the carbon source highly affected the lipid acid profile. With acetate, the major fatty acid was oleic acid (C18:1); followed by linoleic acid (C18:2); with glucose and glycerol, the profile was dominated by linoleic acid (C18:2) and palmitic acid (C16:0)
- -
- The carbon source also affected the bulk solution’s pH variation over time. Glucose acidified the medium, and acetate alkalinized it. However, glycerol maintained the pH at the most suitable value for C. vulgaris growth.
- -
- The nitrogen removal from the liquid medium was 36%, 9%, and 7% when glycerol, acetate, and glucose were used as the carbon source.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shan, S.; Manyakhin, A.Y.; Wang, C.; Ge, B.; Han, J.; Zhang, X.; Zhou, C.; Yan, X.; Ruan, R.; Cheng, P. Mixotrophy, a more promising culture mode: Multi-faceted elaboration of carbon and energy metabolism mechanisms to optimize microalgae culture. Bioresour. Technol. 2023, 386, 129512. [Google Scholar] [CrossRef]
- Yu, H.-C.; Lay, C.-H.; Abdul, P.M.; Wu, J.-Y. Enhancing Lipid Production of Chlorella sp. by Mixotrophic Cultivation Optimization. Processes 2023, 11, 1892. [Google Scholar] [CrossRef]
- Poddar, N.; Sen, R.; Martin, G.J.O. Glycerol and nitrate utilisation by marine microalgae Nannochloropsis salina and Chlorella sp. and associated bacteria during mixotrophic and heterotrophic growth. Algal Res. 2018, 33, 298–309. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating micro-algae into wastewater treatment: A review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.; Eraky, M.; Osman, A.I.; Wang, J.; Farghali, M.; Rashwan, A.K.; Yacoub, I.H.; Hanelt, D.; Abomohra, A. Sustainable valorization of waste glycerol into bioethanol and biodiesel through biocircular approaches: A review. Environ. Chem. Lett. 2023, 1, 1–26. [Google Scholar] [CrossRef]
- Koley, S.; Sonkar, S.; Bagchi, S.K.; Patnaik, R.; Mallick, N. Development of a low-cost cultivation medium for simultaneous production of biodiesel and bio-crude from the chlorophycean microalga Tetradesmus obliquus: A renewable energy prospective. J. Clean. Prod. 2022, 364, 132658. [Google Scholar] [CrossRef]
- Cao, M.; Kang, J.; Gao, Y.; Wang, X.; Pan, X.; Liu, P. Optimization of cultivation conditions for enhancing biomass, polysaccharide and protein yields of Chlorella sorokiniana by response surface methodology. Aquac. Res. 2020, 51, 2456–2471. [Google Scholar] [CrossRef]
- Vimali, E.; Senthil Kumar, A.; Sakthi Vignesh, N.; Ashokkumar, B.; Dhakshinamoorthy, A.; Udayan, A.; Arumugam, M.; Pugazhendhi, A.; Varalakshmi, P. Enhancement of lipid accumulation in microalga Desmodesmus sp. VV2: Response Surface Methodology and Artificial Neural Network modeling for biodiesel production. Chemosphere 2022, 293, 133477. [Google Scholar] [CrossRef] [PubMed]
- Solomon, C.M.; Collier, J.L.; Berg, G.M.; Glibert, P.M. Role of urea in microbial metabolism in aquatic systems: A biochemical and molecular review. Aquat. Microb. Ecol. 2010, 59, 67–88. [Google Scholar] [CrossRef]
- Syrett, P. Uptake and utilization of nitrogen compounds. In Biochemistry of the Algae and Cyanobacteria; Rogers, L.J., Gallon, J.R., Eds.; Oxford: Oxford, UK; Clarendon Press: New York, NY, USA; Oxford University Press: Oxford, UK, 1988. [Google Scholar]
- Hodson, R.C.; Thompson, J.F. Metabolism of Urea by Chlorella vulgaris. Plant Physiol. 1969, 44, 691–696. [Google Scholar] [CrossRef]
- Hodson, R.C.; Williams, S.K.; Davidson, W.R. Metabolic Control of Urea Catabolism in Chlamydomonas reinhardi and Chlorella pyrenoidosa. J. Bacteriol. 1975, 121, 1022–1035. [Google Scholar] [CrossRef]
- Kanamori, T.; Kanou, N.; Kusakabe, S.; Atomi, H.; Imanaka, T. Allophanate hydrolase of Oleomonas sagaranensis involved in an ATP-dependent degradation pathway specific to urea. FEMS Microbiol. Lett. 2005, 245, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Verbruggen, N. Physiological characterization of Mg deficiency in Arabidopsis thaliana. J. Exp. Bot. 2005, 56, 2153–2161. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, K.; Kocot, J.; Horecka, A. Biochemistry of magnesium. J. Elem. 2010, 15, 601–616. [Google Scholar] [CrossRef]
- Rincon, S.M.; Romero, H.M.; Aframehr, W.M.; Beyenal, H. Biomass production in Chlorella vulgaris biofilm cultivated under mixotrophic growth conditions. Algal Res. 2017, 26, 153–160. [Google Scholar] [CrossRef]
- Kong, W.B.; Yang, H.; Cao, Y.T.; Song, H.; Hua, S.F.; Xia, C.G. Effect of Glycerol and Glucose on the Enhancement of Biomass, Lipid and Soluble Carbohydrate Production by Chlorella vulgaris in Mixotrophic Culture. Food Technol. Biotechnol. 2013, 51, 62–69. [Google Scholar]
- Gautam, K.; Pareek, A.; Sharma, D.K. Biochemical composition of green alga Chlorella minutissima in mixotrophic cultures under the effect of different carbon sources. J. Biosci. Bioeng. 2013, 116, 624–627. [Google Scholar] [CrossRef]
- Rincon, S.M.; Urrego, N.F.; Avila, K.J.; Romero, H.M.; Beyenal, H. Photosynthetic activity assessment in mixotrophically cultured Chlorella vulgaris biofilms at various developmental stages. Algal Res. 2019, 38, 101408. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Bashan, Y. Microalgal Heterotrophic and Mixotrophic Culturing for Bio-refining: From Metabolic Routes to Techno-economics. In Algal Biorefineries: Volume 2: Products and Refinery Design; Springer: Berlin/Heidelberg, Germany, 2015; pp. 61–131. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.E.; de-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef]
- Choi, H.-J.; Yu, S.-W. Influence of crude glycerol on the biomass and lipid content of microalgae. Biotechnol. Biotechnol. Equip. 2015, 29, 506–513. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Xie, T.; Xiong, X.; Liu, W.; Liang, B.; Zhang, Y. Enhanced Lipid Accumulation byChlorella vulgarisin a Two-Stage Fed-Batch Culture with Glycerol. Energy Fuels 2014, 28, 3172–3177. [Google Scholar] [CrossRef]
- Bo, Y.; Chu, R.; Sun, D.; Deng, X.; Zhou, C.; Yan, X.; Ruan, R.; Cheng, P. Mixotrophic culture of bait microalgae for biomass and nutrients accumulation and their synergistic carbon metabolism. Bioresour. Technol. 2023, 367, 128301. [Google Scholar] [CrossRef]
- Paranjape, K.; Leite, G.B.; Hallenbeck, P.C. Strain variation in microalgal lipid production during mixotrophic growth with glycerol. Bioresour. Technol. 2016, 204, 80–88. [Google Scholar] [CrossRef][Green Version]
- Yang, C.; Hua, Q.; Shimizu, K. Energetics and carbon metabolism during growth of microalgal cells under photoautotrophic, mixotrophic and cyclic light-autotrophic/dark-heterotrophic conditions. Biochem. Eng. J. 2000, 6, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Sahoo, P.K.; Singhal, S.; Patel, A. Impact of various media and organic carbon sources on biofuel production potential from Chlorella spp. 3 Biotech 2016, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Skorupskaite, V.; Makareviciene, V.; Levisauskas, D. Optimization of mixotrophic cultivation of microalgae Chlorella sp. for biofuel production using response surface methodology. Algal Res. 2015, 7, 45–50. [Google Scholar] [CrossRef]
- Andruleviciute, V.; Makareviciene, V.; Skorupskaite, V.; Gumbyte, M. Biomass and oil content of Chlorella sp., Haematococcus sp., Nannochloris sp. and Scenedesmus sp. under mixotrophic growth conditions in the presence of technical glycerol. J. Appl. Phycol. 2014, 26, 83–90. [Google Scholar] [CrossRef]
- Liang, Y.N.; Sarkany, N.; Cui, Y. Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol. Lett. 2009, 31, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Arroyo, T.; Wei, W.; Ruan, R.; Hu, B. Mixotrophic cultivation of Chlorella vulgaris and its potential application for the oil accumulation from non-sugar materials. Biomass Bioenergy 2011, 35, 2245–2253. [Google Scholar] [CrossRef]
- Abomohra, A.; Li, M.; Faisal, S.; Li, L.; Elsayed, M. Maximizing Nitrogen Removal and Lipid Production by Microalgae under Mixotrophic Growth Using Response Surface Methodology: Towards Enhanced Biodiesel Production. Fermentation 2022, 8, 682. [Google Scholar] [CrossRef]
- Khuri, A.I. Response Surface Methodology and Its Applications in Agricultural and Food Sciences. Biom. Biostat. Int. J. 2017, 5, 155–163. [Google Scholar] [CrossRef]
- ISO 29441:2010; I. Water Quality—Determination of Total Nitrogen after UV Digestion—Method Using Flow Analysis (CFA and FIA) and Spectrometric Detection. International Organization for Standardization: Geneva, Switzerland, 2010. Available online: https://www.iso.org/standard/45480.html (accessed on 8 January 2024).
- Dafner, E.V.; Szmant, A.M. A modified segmented continuous flow analysis method for simultaneous determination of total dissolved nitrogen and phosphorus in marine environments. Limnol. Oceanogr. Methods 2014, 12, 577–591. [Google Scholar] [CrossRef]
- Clark, S.; Francis, P.S.; Conlan, X.A.; Barnett, N.W. Determination of urea using high-performance liquid chromatography with fluorescence detection after automated derivatisation with xanthydrol. J. Chromatogr. A 2007, 1161, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Frieler, R.A.; Mitteness, D.J.; Golovko, M.Y.; Gienger, H.M.; Rosenberger, T.A. Quantitative determination of free glycerol and myo-inositol from plasma and tissue by high-performance liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 3667–3672. [Google Scholar] [CrossRef] [PubMed]
- Glibert, P.M.; Wilkerson, F.P.; Dugdale, R.C.; Raven, J.A.; Dupont, C.L.; Leavitt, P.R.; Parker, A.E.; Burkholder, J.M.; Kana, T.M. Pluses and minuses of ammonium and nitrate uptake and assimilation by phytoplankton and implications for productivity and community composition, with emphasis on nitrogen-enriched conditions. Limnol. Oceanogr. 2016, 61, 165–197. [Google Scholar] [CrossRef]
- Wang, J.; Curtis, W.R. Proton stoichiometric imbalance during algae photosynthetic growth on various nitrogen sources: Toward metabolic pH control. J. Appl. Phycol. 2015, 28, 43–52. [Google Scholar] [CrossRef]
- Barros, A.; Guerra, L.T.; Simões, M.; Santos, E.; Fonseca, D.; Silva, J.; Costa, L.; Navalho, J. Mass balance analysis of carbon and nitrogen in industrial scale mixotrophic microalgae cultures. Algal Res. 2017, 21, 35–41. [Google Scholar] [CrossRef]
- Eustance, E.; Gardner, R.D.; Moll, K.M.; Menicucci, J.; Gerlach, R.; Peyton, B.M. Growth, nitrogen utilization and biodiesel potential for two chlorophytes grown on ammonium, nitrate or urea. J. Appl. Phycol. 2013, 25, 1663–1677. [Google Scholar] [CrossRef]
- Vasconcelos Fernandes, T.; Shrestha, R.; Sui, Y.; Papini, G.; Zeeman, G.; Vet, L.E.; Wijffels, R.H.; Lamers, P. Closing Domestic Nutrient Cycles Using Microalgae. Environ. Sci. Technol. 2015, 49, 12450–12456. [Google Scholar] [CrossRef]
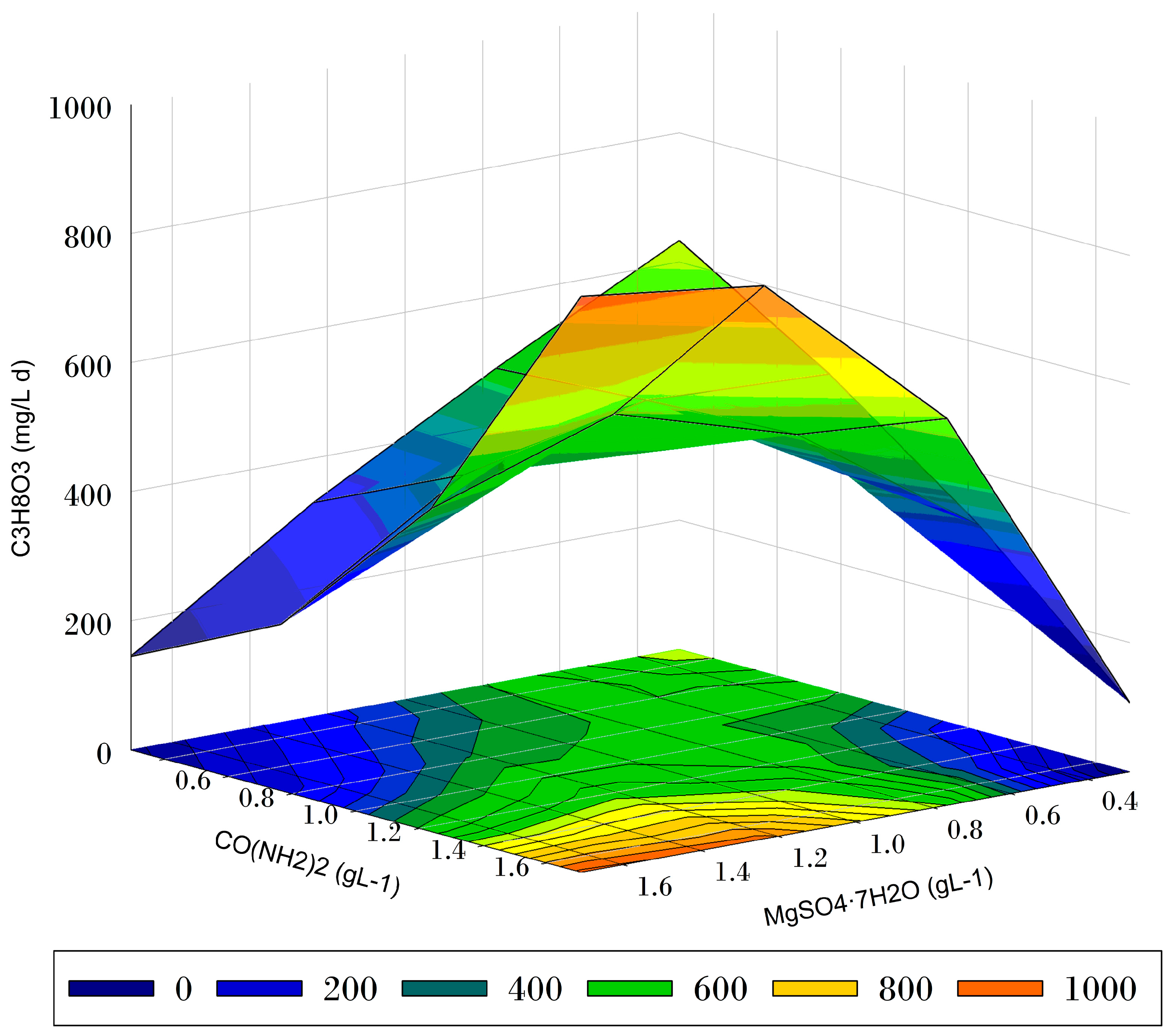
| Coded Values | Real Values | Observed | Predicted | |||
|---|---|---|---|---|---|---|
| Run | X1 | X2 | X1-Urea (g L−1) | X2-MgSO4·7H2O (g L−1) | Glycerol Consumption Rate (mg L−1 d−1) | Glycerol Consumption Rate (mg L−1 d−1) |
| 1 | −1 | −1 | 0.5 | 0.5 | 548.01 | 547.65 |
| 2 | +1 | −1 | 1.5 | 0.5 | 339.70 | 403.13 |
| 3 | −1 | +1 | 0.5 | 1.5 | 276.49 | 223.03 |
| 4 | +1 | +1 | 1.5 | 1.5 | 739.48 | 749.80 |
| 5 | −1.41 | 0 | 0.3 | 1.0 | 409.73 | 449.85 |
| 6 | +1.41 | 0 | 1.7 | 1.0 | 770.23 | 720.15 |
| 7 | 0 | −1.41 | 1.0 | 0.3 | 411.53 | 369.01 |
| 8 | 0 | +1.41 | 1.0 | 1.7 | 352.04 | 384.61 |
| 9 | 0 | 0 | 1.0 | 1.0 | 531.91 | 525.30 |
| 10 | 0 | 0 | 1.0 | 1.0 | 578.12 | 525.30 |
| 11 | 0 | 0 | 1.0 | 1.0 | 555.08 | 525.30 |
| 12 | 0 | 0 | 1.0 | 1.0 | 461.41 | 525.30 |
| 13 | 0 | 0 | 1.0 | 1.0 | 500.00 | 525.30 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 2.35 × 10−5 | 5 | 47,074.16 | 14.70 | 0.0014 * |
| X1 | 73,058.06 | 1 | 73,058.06 | 22.81 | 0.002 * |
| X2 | 243.36 | 1 | 243.36 | 0.076 | 0.7908 |
| X1×2 | 1.13 × 10−5 | 1 | 1.13 × 10−5 | 35.18 | 0.0006 * |
| X12 | 6197.65 | 1 | 6197.65 | 1.94 | 0.2068 |
| X22 | 38,350.52 | 1 | 38,350.52 | 11.98 | 0.0105 * |
| Residual | 22,416.54 | 7 | 3202.36 | ||
| Lack of Fit | 13,974.43 | 3 | 4658.14 | 2.21 | 0.2297 |
| Pure Error | 8442.11 | 4 | 2110.53 | ||
| Cor Total | 2.58 × 10−5 | 12 |
| Solution 1 of 1 Response | Urea (g L−1) | MgSO4·7H2O (g L−1) | Predicted Mean | Experimentally Observed Glycerol Consumption Rate |
|---|---|---|---|---|
| Glycerol Consumption Rate (mg L−1 d−1) | 1.5 | 1.0 | 718.82 | 723.52 ± 48.60 |
| Carbon Source | Biomass Production Rate (g DW L−1 d−1) | Fatty Acid Content (%) | pH Final | Urea Consumption (mg L−1 d−1) | Nitrogen (mg L−1 d−1) | Phosphorus (mg L−1 d−1) |
|---|---|---|---|---|---|---|
| Acetate | 1.03 ± 0.05 | 11% ± 0.03 | 8.86 ± 0.01 | 144.60 ± 25.1 | 17.82 ± 0.38 | 14.75 ± 1.58 |
| Glucose | 0.70 ± 0.08 | 9.0% ± 0.06 | 5.68 ± 0.01 | 113.50 ± 16.7 | 14.64 ± 0.24 | 1.25 ± 1.189 |
| Glycerol | 0.88 ± 0.07 | 10% ± 0.04 | 6.88 ± 0.01 | 223.58 ± 6.8 | 71.27 ± 0.25 | 4.53 ± 1.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rincon, S.M.; Beyenal, H.; Romero, H.M. A Response Surface Methodology Study for Chlorella vulgaris Mixotrophic Culture Optimization. Microorganisms 2024, 12, 379. https://doi.org/10.3390/microorganisms12020379
Rincon SM, Beyenal H, Romero HM. A Response Surface Methodology Study for Chlorella vulgaris Mixotrophic Culture Optimization. Microorganisms. 2024; 12(2):379. https://doi.org/10.3390/microorganisms12020379
Chicago/Turabian StyleRincon, Sandra Milena, Haluk Beyenal, and Hernán Mauricio Romero. 2024. "A Response Surface Methodology Study for Chlorella vulgaris Mixotrophic Culture Optimization" Microorganisms 12, no. 2: 379. https://doi.org/10.3390/microorganisms12020379
APA StyleRincon, S. M., Beyenal, H., & Romero, H. M. (2024). A Response Surface Methodology Study for Chlorella vulgaris Mixotrophic Culture Optimization. Microorganisms, 12(2), 379. https://doi.org/10.3390/microorganisms12020379






